Published online Oct 16, 2023. doi: 10.4253/wjge.v15.i10.623
Peer-review started: May 10, 2023
First decision: July 9, 2023
Revised: July 21, 2023
Accepted: July 31, 2023
Article in press: July 31, 2023
Published online: October 16, 2023
Processing time: 154 Days and 18.4 Hours
Juvenile polyposis syndrome (JPS) is a rare hereditary polyposis disease freq
We report on the case of a 56-year-old female diagnosed with JPS after genetic testing revealed a rare variant of the BMPR1A gene BMPR1A c.1409T>C (p.Met470Thr). She was initially referred for colonoscopy by her general practitioner after testing positive on a screening faecal immunochemical test and subsequently found to have polyposis throughout the entire colorectum on her index screening colonoscopy. The patient was asymptomatic with a normal physical examination and no related medical or family history. Blood tests revealed only mild iron deficiency without anemia. To date, there has only been one other reported case of JPS with the same genetic variant. Subsequent colonoscopies were organised for complete polyp clearance and the patient was returned for surveillance follow-up.
JPS patients can present with no prior symptoms or family history. Genetic testing plays an important diagnostic role guiding management.
Core Tip: Juvenile polyposis syndrome (JPS) is a hereditary autosomal dominant disease that phenotypically presents with polyposis throughout the colorectum. Detection and diagnosis is important as patients have a high risk of developing gastrointestinal cancer. Symptoms often manifest in childhood and adolescence with most having evidence of an associated family history. We report a case of polyposis found on index screening endoscopy in an asymptomatic female with no prior related family or medical history. Subsequent genetic testing led to the diagnosis of JPS after detecting a rare variant of the BMPR1A gene previously only reported in one other case of JPS.
- Citation: Wu MY, Toon C, Field M, Wong M. Polyposis found on index colonoscopy in a 56-year-old female - BMPR1A variant in juvenile polyposis syndrome: A case report. World J Gastrointest Endosc 2023; 15(10): 623-628
- URL: https://www.wjgnet.com/1948-5190/full/v15/i10/623.htm
- DOI: https://dx.doi.org/10.4253/wjge.v15.i10.623
Hereditary gastrointestinal polyposis syndromes are a rare group of diseases that account for up to approximately 5% of all colorectal cancers[1]. These polyposis syndromes are broadly categorised based on whether polyps demonstrate predominantly adenomatous or hamartomatous changes. Hamartomatous polyposis syndromes include Peutz-Jeghers syndrome, phosphatase and tensin homolog (PTEN) hamartomatous syndromes (Cowden syndrome and PTEN-related Proteus syndromes) and Juvenile Polyposis syndrome (JPS)[2]. Early recognition and detection of these hereditary diseases is important due to the lifetime risk of developing gastrointestinal cancer. JPS often manifests with gastroi
A 56-year-old female was referred in by her general practitioner after she tested positive on screening faecal immunochemical test.
She reported some infrequent constipation but no acute bowel habit changes. Overall, she was constitutionally well with no history of abdominal pain, malaena, haematochezia, or weight loss.
She had a medical history of gastroesophageal reflux disease and asthma. Her only regular medication was a budesonide-formoterol (200 mcg/6 mcg) inhaler.
She had no history of smoking or alcohol use. There was no family history of colorectal cancer or other gastrointestinal diseases.
The patient was fit and well with normal vital signs. There were no significant findings on physical examination such as skin lesions commonly associated with Cowden and other PTEN hamartoma syndromes, mucosal pigmentation associated with Peutz-Jeghers syndrome and macrocephaly associated with JPS[5-7]. There were no features of alopecia, onychodystrophy or hyperpigmentation that may be seen in Cronkhite-Canada syndrome[8]. She had no features of Hereditary Haemorrhagic Telangectasia typically seen in SMAD4 juvenile polyposis.
The only abnormalities on her blood tests were a mild iron deficiency with ferritin level 25 µg/L (reference range 30–300 µg/L) without anaemia.
The patient proceeded to a gastroscopy which found a single medium-sized fundic gland polyp and a colonoscopy demonstrating more than one hundred pedunculated polyps throughout the caecum, ascending colon, transverse colon, descending colon, sigmoid colon, rectosigmoid colon and rectum (Figure 1). Initial biopsies were taken throughout the gastrointestinal tract and several larger polyps were removed for histology. A computed tomography enterography of the small bowel did not show any small bowel polyps.
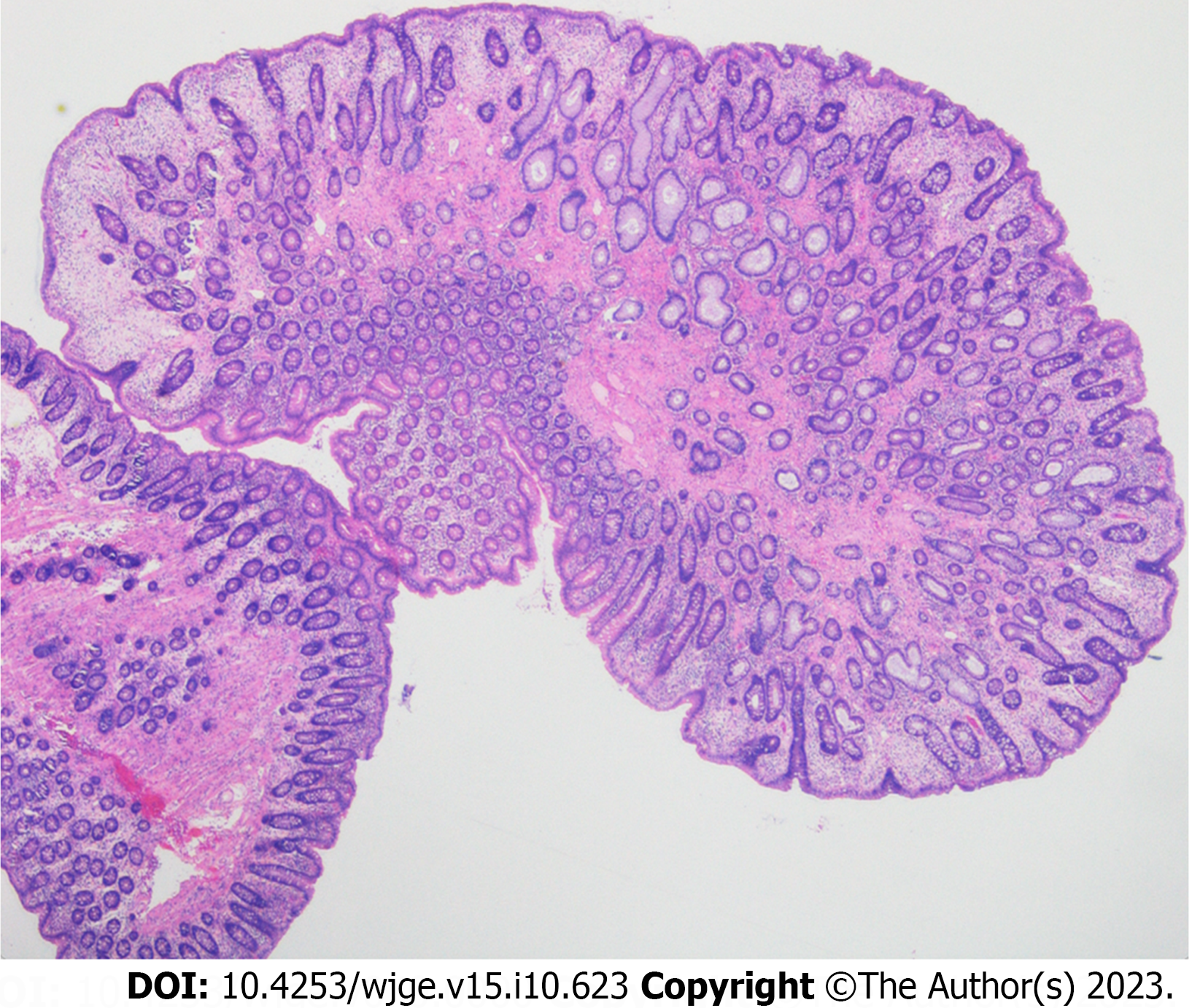
On histology, the polypoid colonic mucosa showed epithelial-stromal hamartomatous features with variable epithelial hyperplasia and subtle myofibroblastic proliferation in the lamina propria (Figure 2). Overall features were consistent with a hamartomatous polyposis syndrome. JPS, Peutz-Jegher syndrome, Cronkhite-Canada syndrome and Cowden syndrome were considered differential diagnoses however sub-classification proved difficult as there were no further distinguishing histological features[9,10].
The patient was referred for multi-gene panel testing which included STK11 associated with Peutz-Jeghers syndrome, PTEN associated with PTEN hamartoma syndromes (Cowden syndrome and PTEN-related Proteus syndromes) and SMAD4 and BMPR1A associated with JPS. Massively Parallel Sequencing of > 99% of the coding sequences including the exon/intron boundaries to a depth of > 200 was performed to generate this result. SOPHiA genetics DDM (Sophia Genetics, Saint-Sulpice, Switzerland) was used to generate aligned reads and call variants against the hg19 human reference genome. A rare variant of BMPR1A written as BMPR1A c.1409T>C (p.Met470Thr) was identified and JPS was diagnosed as the likely cause of her polyposis phenotype. The patient has three siblings and one surviving parent none of whom have a history of colorectal cancer or polyps. All bar one of these relatives lives overseas. The patient’s 35-year-old son subsequently underwent a colonoscopy which showed no polyps. Genetic testing was also offered to the son which returned negative for the BMPR1A variant.
The combination of colonoscopy findings, histopathology and multi-gene panel testing led to the diagnosis of JPS.
Three subsequent colonoscopies were organised for complete polyp clearance and histopathology demonstrated similar hamartomatous polyps with some showing early adenomatous changes.
The patient was recommended to return for yearly colonoscopy surveillance given the initial polyp burden.
JPS is a rare autosomal dominant disease with an estimated incidence around 1/100000–1/160000 and a 39%-68% lifetime risk of colon cancer[11]. Polyp growth occurs primarily in the colorectum but can also appear in the stomach and small bowel. Macroscopically JPS polyps appear as pedunculated, exophytic, shiny and spherical growths[2,12]. Histologically, juvenile polyps typically demonstrate dilated thick mucin-filled glands with inflammatory infiltrates in the lamina propria. Despite these features, polyps in JPS can often still be indistinguishable from other polyposis syndromes. Clinical diagnostic criteria also exist in which a diagnosis can be made with the presence of any of the following: > 5 juvenile polyps in the colorectum, juvenile polyps in other parts of the gastrointestinal tract or any number of juvenile polyps and a positive family history[13]. Confirmatory genetic testing is recommended for all patients meeting clinical diagnostic criteria however the presence of germline mutations may only be present in 20%-60% of individuals[4,12]. Making an accurate diagnosis of JPS can remain a challenge for clinicians due to the similarity of features with other polyposis syndromes and a lack of a clear ‘gold standard’ diagnostic. In our case, a diagnosis was made on the basis of polyp morphology, histology and confirmatory genetic testing.
The heterozygous BMPR1A c.1409T>C (p.Met470Thr) variant is a missense mutation of Methionine to Threonine and identified only in one other patient with JPS[14]. In silico analysis predicted the variant affects protein function. Based on a lack of functional proof and biological information that the variant was damaging, the variant was classified as a variant of unclear significance (class 3) according to the American College of Medical Genetics and Genomics-Association for Molecular Pathology SHERLOC guidelines[15]. Whilst 45% to 60% of JPS cases are attributed to more common diease-causing variants in either the BMPR1A or SMAD4 gene, there are still a number of cases without an identifiable pathogenic variant[16]. Since the variant is absent in a large population control group (gnomAD), has been previously reported in a patient with JPS and showed limited segregation with disease in this family, the BMPR1A c.1409T>C (p.Met470Thr) variant was considered by the authors to be the likely cause of the patients phenotype[17]. Given the rarity of disease, reporting on the polyposis features of this patient diagnosed with JPS contributes to the growing body of knowledge on the pathogenecity of BMPR1A variants.
The role of genetic counselling is invaluable in the management of a patient with JPS. Around 50% of individuals with JPS will have affected parents whilst the remaining half will have no prior family history of polyps and represent a de novo mutation[18]. Children of affected individuals have a 50% chance of inheritance. It is recommended that even asymptomatic relatives of individuals with JPS undergo evaluations with either genetic testing, if the gene variant is known, or endoscopic screening if the variant is unknown[18]. In the case of this patient, genetic screening was performed on the son to ensure early disease surveillance and monitoring.
For patients with polyposis syndrome, current guidelines by the American College of Gastroenterology (ACG) recommend screening gastroscopy and colonoscopy from age 12 or earlier if individuals are symptomatic with repeat surveillance endoscopy every 1–3 years depending on polyp burden[4]. The European Society of Gastrointestinal Endoscopy (ESGE) outline similar colonoscopy age intervals however recommend gastroscopy in asymptomatic individuals start at 18 years for those with a SMAD4 mutation and at 25 years in those with BMPR1A mutation[19]. The ACG recommends removal of all polyps ≥ 5 mm whilst the ESGE recommends removal of those > 10 mm[4,19]. Periodic surveillance of the small bowel is recommended by the ACG however is not recommended by the ESGE given the rarity of small bowel involvement in JPS[4,19]. Surgical management with colectomy and ileo-rectal anastomosis is recommended if cancer, high-grade dysplasia or polyposis cannot be managed endoscopically[4]. In our patient, serial colonoscopies at 3 monthly intervals were adequate for complete polyp clearance and follow-up was organised for yearly surveillance given the significant polyp burden on initial colonoscopy. Current guidelines provide blanket recommendations to all patients diagnosed with JPS regardless of the gene-phenotype. Therefore a better understanding of the pathogenecity of gene variants can provide information that may help individualise clinical surveillance intervals.
This case highlights the presentation of an asymptomatic female found to have a rare potentially de novo variant in the BMPR1A gene leading to the diagnosis of JPS. This case serves as a reminder that many patients may be asymptomatic with no related medical or family history. It demonstrates the importance of referral to a geneticist for multigene panel testing for confirmatory diagnosis, guidance on further management of the patient and cancer surveillance intervals.
JPS is a rare disease that can be a challenging diagnosis to be distinguished from other hereditary polyposis syndromes. This case demonstrates that some patients may present in adulthood with no related symptoms or prior history. We describe the second reported case in literature of a rare potentially de novo variant of the BMPR1A gene in a patient with JPS. This report contributes to the developing body of literature and understanding in the pathogenicity of variants in BMPR1A gene.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Australia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Kishikawa H, Japan; Sahin Y, Turkey; Zhang X, United States; Zhao ZY, China S-Editor: Lin C L-Editor: A P-Editor: Cai YX
| 1. | Patel R, Hyer W. Practical management of polyposis syndromes. Frontline Gastroenterol. 2019;10:379-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Aretz S. The differential diagnosis and surveillance of hereditary gastrointestinal polyposis syndromes. Dtsch Arztebl Int. 2010;107:163-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Manfredi M. Hereditary hamartomatous polyposis syndromes: understanding the disease risks as children reach adulthood. Gastroenterol Hepatol (N Y). 2010;6:185-196. [PubMed] |
| 4. | Syngal S, Brand RE, Church JM, Giardiello FM, Hampel HL, Burt RW; American College of Gastroenterology. ACG clinical guideline: Genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol. 2015;110:223-62; quiz 263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 957] [Cited by in RCA: 1090] [Article Influence: 109.0] [Reference Citation Analysis (0)] |
| 5. | Beggs AD, Latchford AR, Vasen HF, Moslein G, Alonso A, Aretz S, Bertario L, Blanco I, Bülow S, Burn J, Capella G, Colas C, Friedl W, Møller P, Hes FJ, Järvinen H, Mecklin JP, Nagengast FM, Parc Y, Phillips RK, Hyer W, Ponz de Leon M, Renkonen-Sinisalo L, Sampson JR, Stormorken A, Tejpar S, Thomas HJ, Wijnen JT, Clark SK, Hodgson SV. Peutz-Jeghers syndrome: a systematic review and recommendations for management. Gut. 2010;59:975-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 516] [Cited by in RCA: 458] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 6. | Brosens LA, Langeveld D, van Hattem WA, Giardiello FM, Offerhaus GJ. Juvenile polyposis syndrome. World J Gastroenterol. 2011;17:4839-4844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 103] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 7. | Pilarski R, Burt R, Kohlman W, Pho L, Shannon KM, Swisher E. Cowden syndrome and the PTEN hamartoma tumor syndrome: systematic review and revised diagnostic criteria. J Natl Cancer Inst. 2013;105:1607-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 372] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 8. | Sweetser S, Ahlquist DA, Osborn NK, Sanderson SO, Smyrk TC, Chari ST; Boardman LA. Clinicopathologic features and treatment outcomes in Cronkhite-Canada syndrome: support for autoimmunity. Dig Dis Sci. 2012;57:496-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 9. | Kopáčová M, Urban O, Cyrany J, Laco J, Bureš J, Rejchrt S, Bártová J, Tachecí I. Cronkhite-Canada syndrome: review of the literature. Gastroenterol Res Pract. 2013;2013:856873. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Schreibman IR, Baker M, Amos C, McGarrity TJ. The hamartomatous polyposis syndromes: a clinical and molecular review. Am J Gastroenterol. 2005;100:476-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 206] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 11. | Kidambi TD, Kohli DR, Samadder NJ, Singh A. Hereditary Polyposis Syndromes. Curr Treat Options Gastroenterol. 2019;17:650-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Zbuk KM, Eng C. Hamartomatous polyposis syndromes. Nat Clin Pract Gastroenterol Hepatol. 2007;4:492-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 140] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 13. | Latchford AR, Neale K, Phillips RK, Clark SK. Juvenile polyposis syndrome: a study of genotype, phenotype, and long-term outcome. Dis Colon Rectum. 2012;55:1038-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 14. | Kim IJ, Park JH, Kang HC, Kim KH, Kim JH, Ku JL, Kang SB, Park SY, Lee JS, Park JG. Identification of a novel BMPR1A germline mutation in a Korean juvenile polyposis patient without SMAD4 mutation. Clin Genet. 2003;63:126-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Nykamp K, Anderson M, Powers M, Garcia J, Herrera B, Ho YY, Kobayashi Y, Patil N, Thusberg J, Westbrook M; Invitae Clinical Genomics Group, Topper S. Sherloc: a comprehensive refinement of the ACMG-AMP variant classification criteria. Genet Med. 2017;19:1105-1117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 342] [Cited by in RCA: 550] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 16. | Papadopulos ME, Plazzer JP, Macrae FA. Genotype-phenotype correlation of BMPR1a disease causing variants in juvenile polyposis syndrome. Hered Cancer Clin Pract. 2023;21:12. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Karczewski KJ, Francioli LC, Tiao G, Cummings BB, Alföldi J, Wang Q, Collins RL, Laricchia KM, Ganna A, Birnbaum DP, Gauthier LD, Brand H, Solomonson M, Watts NA, Rhodes D, Singer-Berk M, England EM, Seaby EG, Kosmicki JA, Walters RK, Tashman K, Farjoun Y, Banks E, Poterba T, Wang A, Seed C, Whiffin N, Chong JX, Samocha KE, Pierce-Hoffman E, Zappala Z, O'Donnell-Luria AH, Minikel EV, Weisburd B, Lek M, Ware JS, Vittal C, Armean IM, Bergelson L, Cibulskis K, Connolly KM, Covarrubias M, Donnelly S, Ferriera S, Gabriel S, Gentry J, Gupta N, Jeandet T, Kaplan D, Llanwarne C, Munshi R, Novod S, Petrillo N, Roazen D, Ruano-Rubio V, Saltzman A, Schleicher M, Soto J, Tibbetts K, Tolonen C, Wade G, Talkowski ME; Genome Aggregation Database Consortium, Neale BM, Daly MJ, MacArthur DG. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581:434-443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7255] [Cited by in RCA: 6369] [Article Influence: 1273.8] [Reference Citation Analysis (0)] |
| 18. | Larsen Haidle J, MacFarland SP, Howe JR. Juvenile Polyposis Syndrome. In: Adam MP, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, Gripp KW, Amemiya A, editors. GeneReviews®. Seattle (WA): University of Washington, Seattle, 1993–2023. |
| 19. | van Leerdam ME, Roos VH, van Hooft JE, Dekker E, Jover R, Kaminski MF, Latchford A, Neumann H, Pellisé M, Saurin JC, Tanis PJ, Wagner A, Balaguer F, Ricciardiello L. Endoscopic management of polyposis syndromes: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2019;51:877-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 156] [Article Influence: 26.0] [Reference Citation Analysis (0)] |