Published online Jul 16, 2022. doi: 10.4253/wjge.v14.i7.455
Peer-review started: April 6, 2022
First decision: April 28, 2022
Revised: May 18, 2022
Accepted: June 15, 2022
Article in press: June 15, 2022
Published online: July 16, 2022
Pancreatic metastases from squamous cell lung carcinoma (SCLC) are unusual. These lesions are often asymptomatic and detected incidentally or during follow-up investigations, occasionally several years after removal of the primary tumor.
A 56-year-old male with SCLC developed jaundice 1 mo after the cancer diagnosis. An abdominal computed tomography (CT) scan showed a mass in the pancreatic head with distention of both intra- and extrahepatic biliary ducts. Endoscopic retrograde cholangiopancreatography and sphincterotomy were performed first, culminating with plastic biliary stent placement. Cytological examination of the pancreatic mass sample collected by fine-needle aspiration (FNA) under endoscopic ultrasound (EUS) guidance revealed the presence of malignant cells compatible with well-differentiated squamous cell carcinoma. After liver function normalized, chemotherapy was initiated with carboplatin and paclitaxel; however, 4 d later, the patient presented dysphagia. Cervico-thoraco-abdominal CT showed tracheoesophageal fistula and stent migration. After replacement with a 10 cm/10 mm uncovered metallic biliary stent and treatment of the tracheoesophageal fistula with a fully covered esophageal stent, the patient was able to start oral feeding progressively. He died 9 mo after the initial diagnosis.
The diagnosis of pancreatic metastasis from SCLC is challenging for clinicians. EUS-FNA is the primary exam for confirmatory diagnosis.
Core Tip: The pancreatic metastasis of squamous lung carcinoma is a rare disease. There are a few cases in the literature that discuss the modality of diagnosis and the treatment of pancreatic metastasis. In this manuscript, we report our experience in the management of this case and the malignant tracheoesophageal fistula as a rare complication of squamous lung carcinoma.
- Citation: Rais K, El Eulj O, El Moutaoukil N, Kamaoui I, Bennani A, Kharrasse G, Zazour A, Khannoussi W, Ismaili Z. Solitary pancreatic metastasis from squamous cell lung carcinoma: A case report and review of literature. World J Gastrointest Endosc 2022; 14(7): 455-466
- URL: https://www.wjgnet.com/1948-5190/full/v14/i7/455.htm
- DOI: https://dx.doi.org/10.4253/wjge.v14.i7.455
Pancreatic tumors generally have a poor prognosis, and pancreatic cancer ranks as the fourth deadliest type of cancer among men and women[1]. Pancreatic metastases are rare[2]. Their prevalence is estimated at approximately 1%-5%[3]. Renal, lung, colorectal and breast tumors are the main primary tumor sites responsible for pancreatic metastases[2]. We report a case of squamous cell lung carcinoma with pancreatic metastasis in a 56-year-old male patient.
A 56-year-old male presented to the emergency room with complaints of cholestatic jaundice associated with pancreatic epigastralgia and deterioration of his general condition.
The patient reported that his symptoms had started 1 mo prior.
Three months before admission to our department, he had been diagnosed with and followed up for a left hilar lung squamous cell carcinoma, which had been discovered by bronchoscopy with transbronchial biopsy of the lung mass.
The patient self-reported being a 52 pack-year smoker, he had no family history.
The patient had obvious jaundice. The patient was afebrile but had epigastric tenderness.
Blood tests showed a disturbance of liver function based on the following findings: total bilirubin, 5.2 mg/dL (normal range: 0.3-1.9 mg/dL); direct bilirubin, 4.1 mg/dL (normal range: 0-0.3 mg/dL); gamma glutamyl transferase, 1088 UI/L (normal range: 12-64 UI/L); alkaline phosphatase, 450 UI/L (normal range: 40-150 UI/L); aspartate aminotransferase, 102 UI/L (normal range: 5-34 UI/L); alanine aminotransferase, 220 UI/L (normal range: 0-55 UI/L); and carbohydrate antigen (CA) 19-9, 40 U/mL (normal range: 0-33 U/mL).
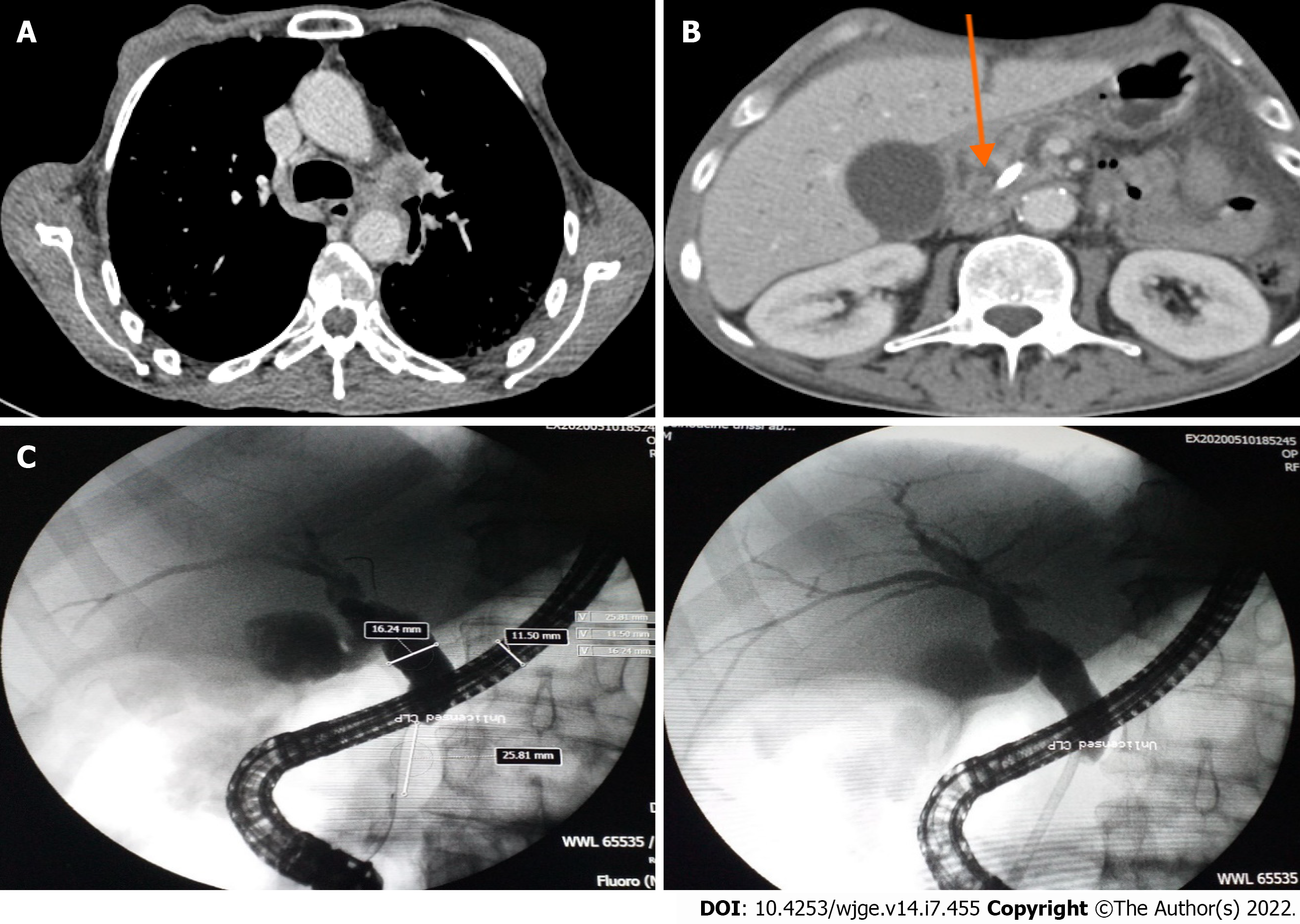
Computed tomographic scanning revealed a tumoral hilar left process, dilation of the intrahepatic bile duct, 11 mm main bile duct and 4 mm Wirsung duct along with a 33 mm × 45 mm pseudotumoral mass of the pancreatic head (Figure 1A and B).
Endoscopic retrograde cholangiopancreatography was performed and showed dilation of the main bile duct (16 mm) among a stricture (extending to 25 mm) located under the cystic duct. Minimal sphincterotomy was performed, and a plastic stent (10 Fr/7 cm) was placed (Figure 1C). Good drainage was ensured. Histological examination of cytological brushing showed atypical cells, namely, category II of Papanicolaou. The patient’s jaundice regressed following these procedures, and his hepatic function blood parameters improved.
A multidisciplinary consultation meeting was held. The clinicians decided to begin chemotherapy for lung squamous cell carcinoma.
The patient received carboplatin and 80 mg/m2 paclitaxel every week; however, the treatment was stopped at the 4th week due to poor therapeutic tolerance.
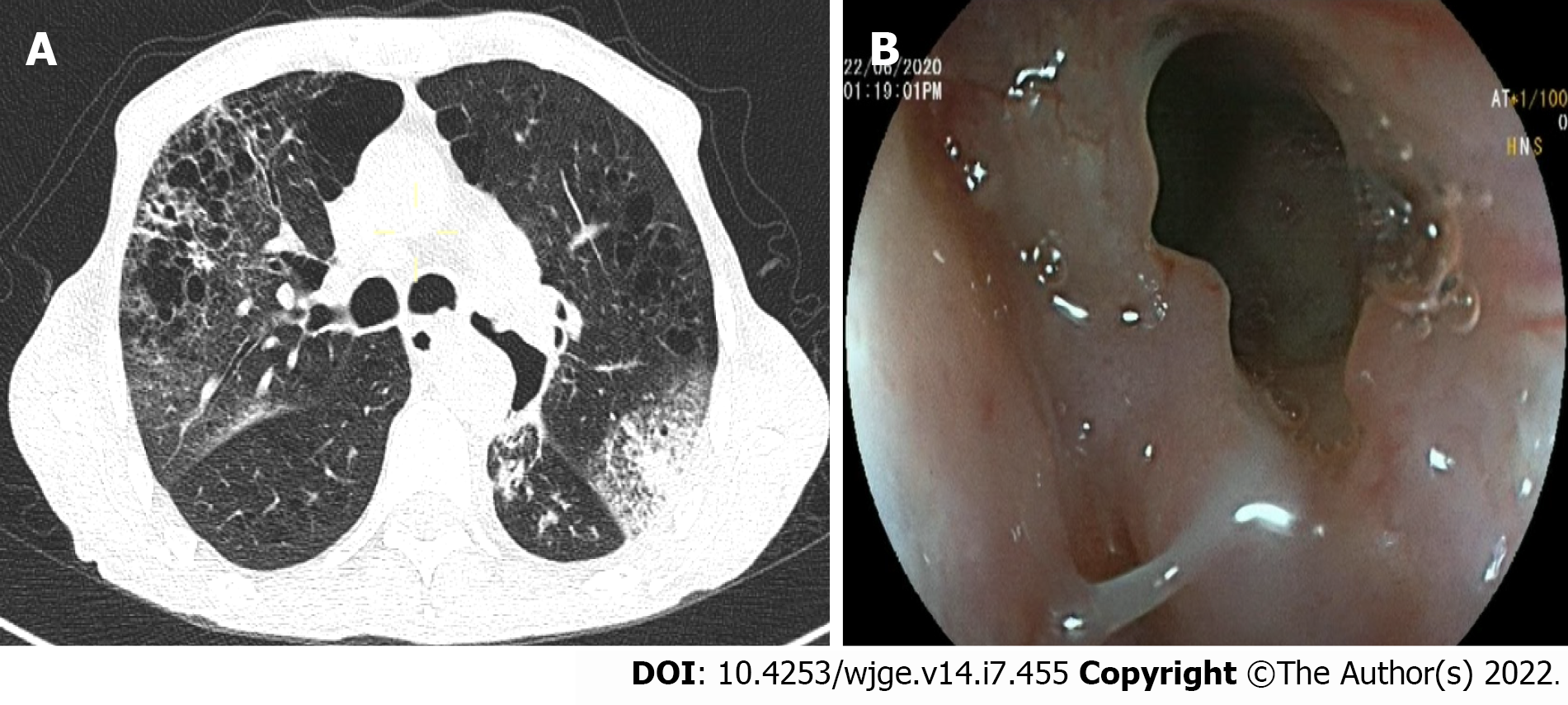
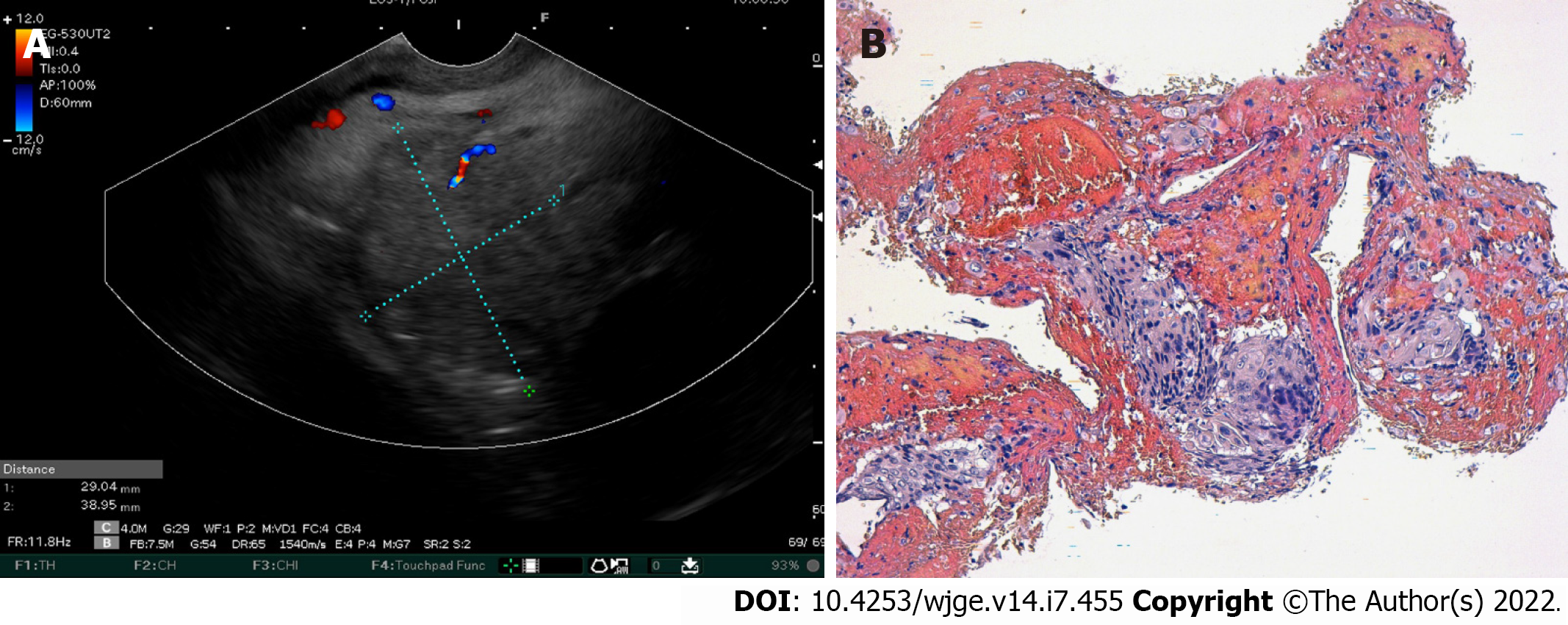
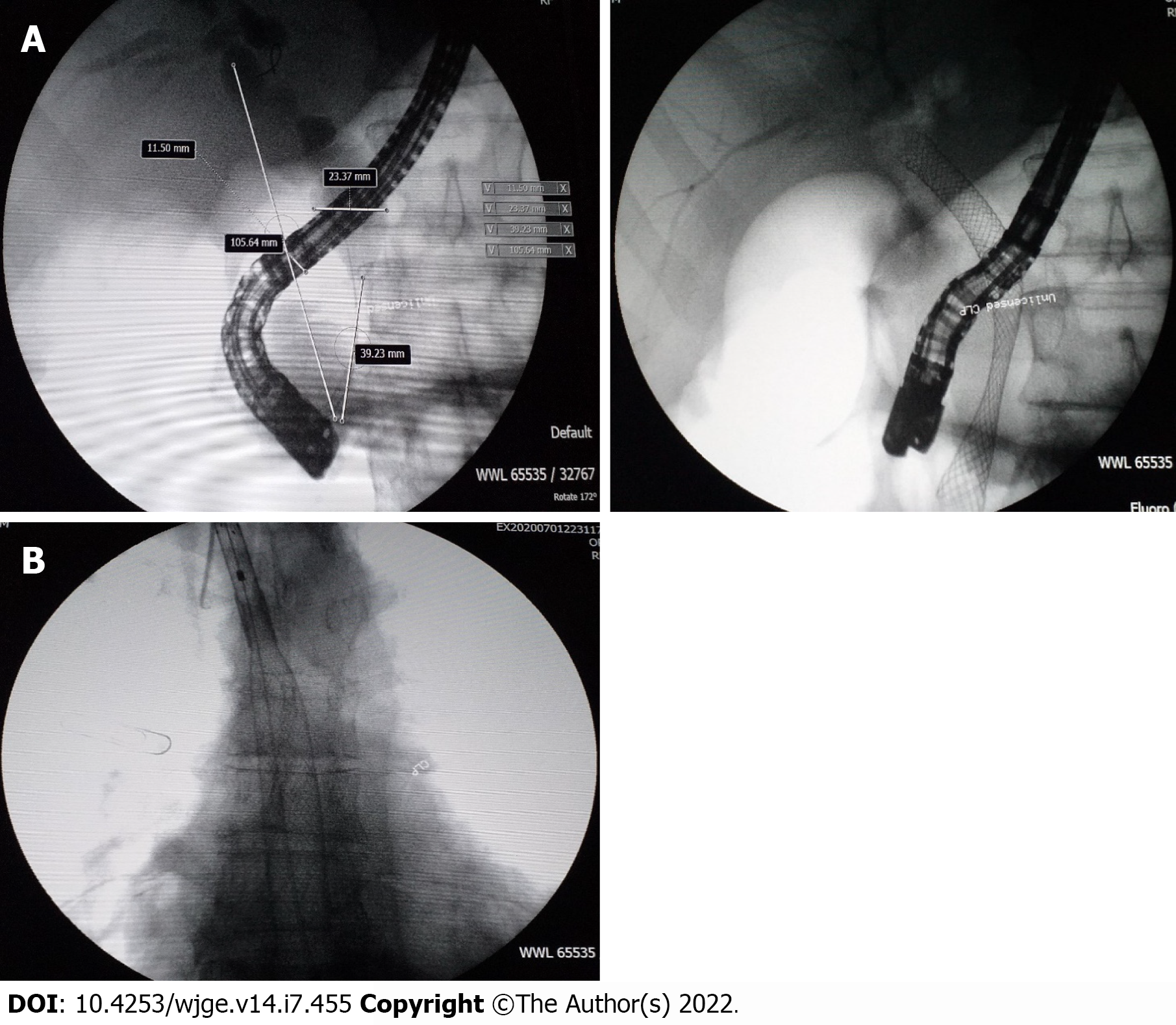
Over the 4-d period after treatment cessation, the patient developed total aphagia associated with dysphonia. He also developed stage 4 New York Heart Association dyspnea and was deemed to be undernourished (nutritional risk index of 64). His performance status was 3. A computed tomography arterial portography scan showed a locally advanced left hilar mass invading the left main bronchus and fistulating into a paraseptal formation with intimate contact within the esophageal wall (Figure 2A). The imaging examination also showed left lobar broncho-alveolitis and a cephalic pancreatic tumor invading the second duodenum and the antropyloric portion with dilation of upstream biliary ducts and no pneumobilia. Esophagogastroduodenoscopy showed a tracheoesophageal fistula located 30 cm from the dental arches that easily crossed (Figure 2B). A biliary stent was observed to partially migrate into the duodenum. EUS showed a 4-cm cephalic pancreatic mass invading the second portion of the duodenum (Figure 3A). Fine-needle (22-G) aspiration of the pancreatic mass was performed and confirmed the presence of a carcinomatous proliferation containing nests and large tumoral polygonal cells with atypical voluminous irregular nuclei surrounded by eosinophils. Focal tumoral necrosis was also present, leading us to conclude that the mass was a well-differentiated keratinizing squamous cell carcinoma. Immunohistochemical examination of the mass showed expression of cytokeratin 5/6 (Figure 3B). On the other hand, the cells did not express TTF1. The final histological report confirmed a poorly differentiated squamous cell lung carcinoma located in the pancreas. To address the migrated biliary stent and to ensure definitive and permanent biliary drainage before treating the tracheoesophageal fistula, endoscopic retrograde cholangiopancreatography was performed first with placement of an uncovered metallic stent measuring 10 cm/10 mm (Figure 4A).
Moulay Zahi Ismaili, Professor and Chief, Department of Hepato-Gastroenterology, Mohammed VI University Hospital Center.
Mohamed Bouziane, Professor and Chief, Department of General Surgery, Mohammed VI University Hospital Center.
Tijani Harroudi, Professor and Chief, Department of Surgical Oncology, Mohammed VI University Hospital Center.
Ghizlane Kharrasse, Professor of Hepato-Gastroenterology, Department of Hepato-Gastroenterology, Mohammed VI University Hospital Center.
Wafaa Khannoussi, Professor of Hepato-Gastroenterology, Department of Hepato-Gastroenterology, Mohammed VI University Hospital Center.
Abdelkrim Zazour, Assistant Professor of Hepato-Gastroenterology, Department of Hepato-Gastroenterology, Mohammed VI University Hospital Center.
The patient’s case was rediscussed in multidisciplinary consultation meetings. The decision was made to retain the diagnosis, and a treatment plan was formulated accordingly (detailed below).
Pancreatic metastasis of squamous cell lung carcinoma, stage IV.
A fully covered metallic esophageal stent was placed as a palliative treatment for the tracheoesophageal fistula. Then, a 12-cm stent was placed, the proximal end of which was 24 cm from the dental arches (Figure 4B).
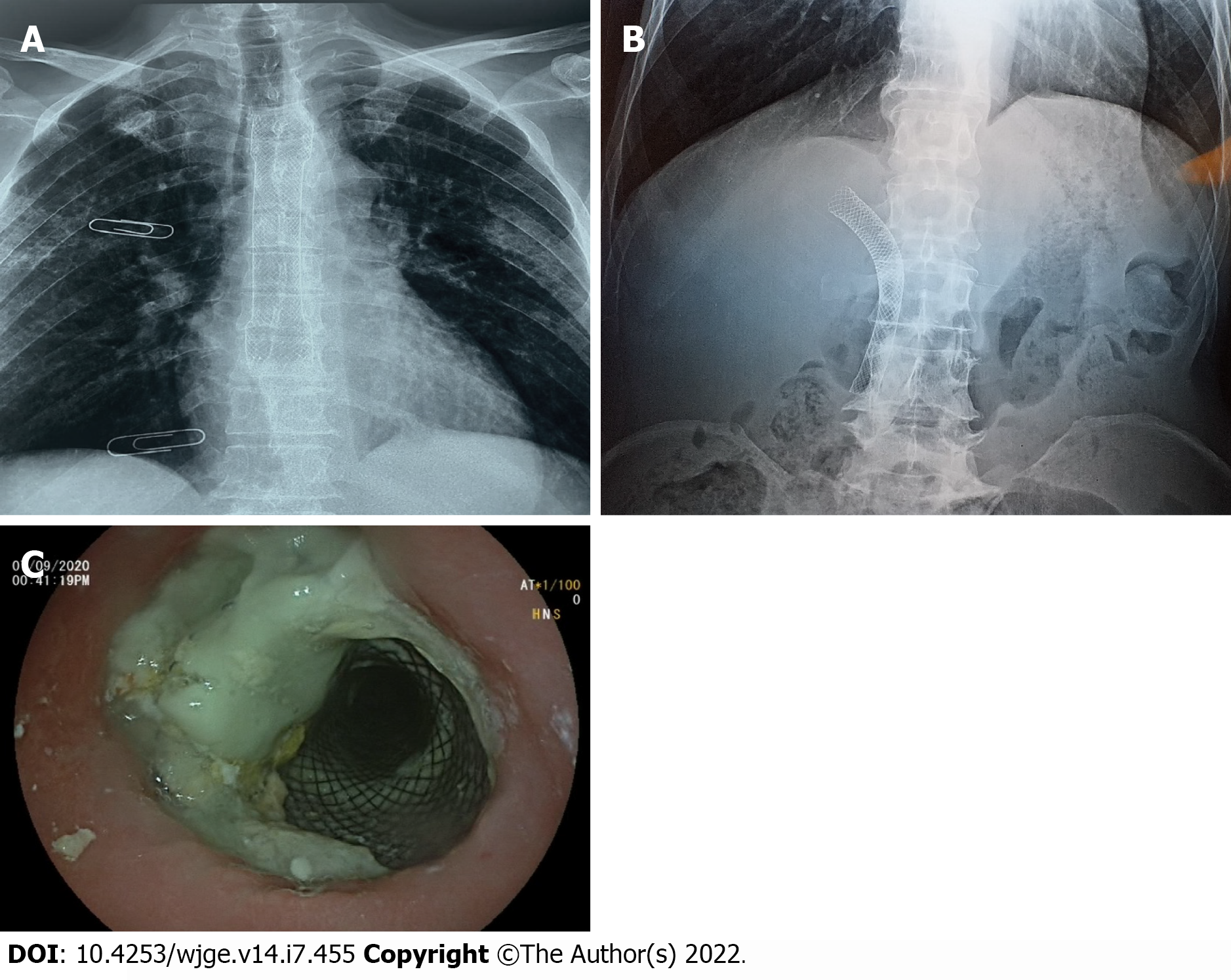
During the following 3 mo, the patient was able to gradually start oral alimentation of a mixed-food diet. However, he lost 5 kg of body weight, and his general state was significantly altered. Thus, palliative chemotherapy was not initiated. Two months later, imaging monitoring using thoracic and abdominal X-rays showed a good position of the esophageal and biliary stents (Figure 5A and 5B), which was confirmed by upper digestive endoscopy (Figure 5C). The patient died 9 mo after the diagnosis.
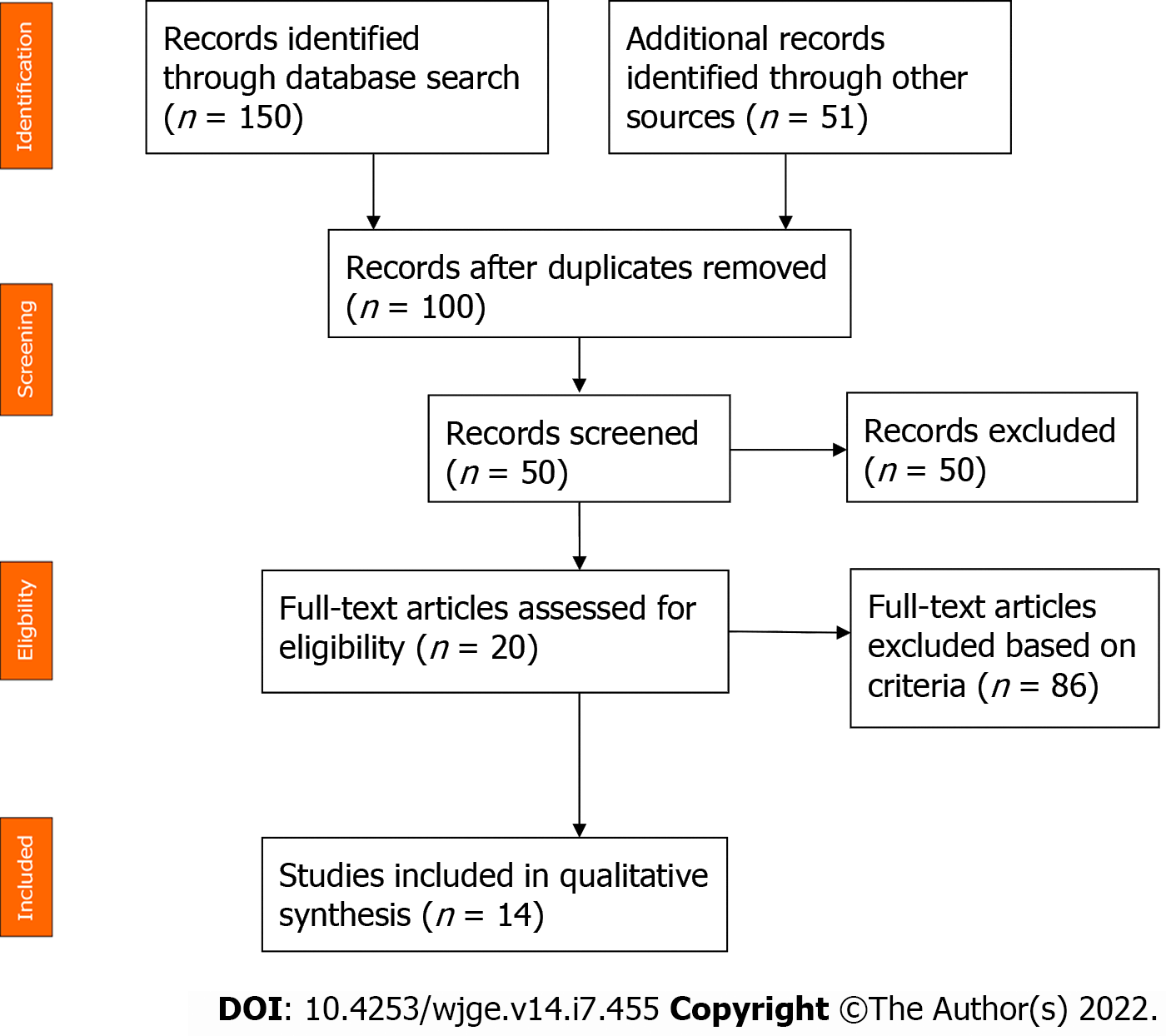
References for this review were identified through searches of the PubMed, Cochrane and Scopus databases using the following Medical Subject Heading terms: (squamous cell lung carcinoma) AND (pancreatic metastasis). Only English-language journals were considered, and only full papers were included. A total of 201 studies were initially identified. After reviewing the abstracts, 14 articles were identified with topical relevance (i.e., pancreatic metastasis of a squamous cell lung carcinoma). Reference lists of the selected studies were checked (cross-referenced), but no additional studies were identified (Figure 6). We followed the Preferred Reporting Items for Systematic reviews and Meta-analysis guidelines for this literature review. Only 23 cases of squamous cell lung carcinoma with pancreatic metastasis were reported in the literature at the time of this review. The mean age of the reported patients was 61.5 years, and 92.3% of the patients were male. The most common symptom was jaundice (55.6%) followed by epigastric pain (44.6%). One patient (11.2%) was asymptomatic. Pancreatic metastasis was located in the head of the pancreas in 60% of the patients and was located equally in the body, tail and uncinated process in the remaining patients. EUS benefitted 50% of the patients. Among these patients, 3 patients underwent EUS with fine-needle aspiration (FNA), and 2 patients underwent EUS with fine-needle biopsy (Table 1). The diagnosis of pancreatic metastasis due to squamous cell lung carcinoma was established by EUS in 4 patients, by surgery in 3 patients, by percutaneous FNA of the pancreatic tumor in 1 patient, and upon autopsy in 4 patients. Three patients were treated with biliary drainage. Seven patients received chemotherapy. Two patients received surgical treatment for pancreatic metastasis. The follow-up period for reported patients varied between a few days and 1 year, with the latter noted for 1 patient who was treated with surgery and adjuvant chemotherapy[4](Table 1).
| Ref. | Yr | Setting | Number | Age in yr | Sex | Symptoms | Imaging | Endoscopy +/- FNA | Diagnostic means | Treatment | Follow-up | Overall survival | Status at time of publication |
| Zhou et al[29] | 2020 | China | 1 | 63 | M | Epigastric pain with jaundice | Hyperintense mass measuring 4.5 cm in the pancreatic head | No | Surgery of the pancreatic mass | Whipple procedure | UNK | UNK | UNK |
| Stoupis et al[30] | 2020 | Greece | 1 | 60 | F | Fatigue, cough and hemoptysis, loss of appetite and 10-kg weight loss | Increased 2-deoxy-2-[F-18] fluoro-D-glucose uptake in the right lung and pancreatic tail | Yes | EUS-FNB of the pancreatic mass using a 22-gauge needle | 7 cycles of anti-PD-L1 antibody pembrolizumab | UNK | UNK | Alive |
| Wang et al[4] | 2020 | China | 1 | 57 | M | Asymptomatic | PET-CT scan showed pancreatic metastasis (1 yr after diagnosis of squamous cell lung carcinoma) | No | Laparoscopic radical pancreatic body tail and splenectomy | 4 cycles of gemcitabine (1000 mg/m2) plus cisplatin (65 mg/m2) due to progression of the lung mass and the appearance of a tumor in the head of the pancreas. He received 3 cycles of pembrolizumab (2 mg/kg) | 1 yr | 21.1 mo | Dead |
| Ishikawa et al[31] | 2017 | Canada | 1 | 70 | M | Abdominal pain and weight loss | 3.8 cm hypodense mass in the pancreatic body with lymphadenopathy in the left supraclavicular region and a 3-cm lung mass posterior to the left main stem bronchus | Yes | EUS-FNB of these two lesions with a 25-G needle | Palliative chemotherapy | UNK | UNK | UNK |
| Fujji et al[32] | 2015 | Japan | 1 | 70 | M | High fever and jaundice 6 mo after left lung inferior lobe resection | Low contrast-enhanced mass with relatively clear border and a size of 40 mm × 33 mm in the head of the pancreas | Yes | FNA via a transgastric approach with linear EUS | 5 cycles of carboplatin plus weekly paclitaxel | 226 d | UNK | Dead |
| Dewanwala et al[33] | 2012 | United States | 1 | 65 | M | Dyspnea and recurrent cough | Left hilar mass with an incidental well-defined mass involving the uncinate process of the pancreas measuring 3.7 cm × 2.2 cm | Yes | Pylorus-preserving pancreaticoduodenectomy | Carboplatin plus gemcitabine and completed 5 cycles | 17 mo | UNK | Dead |
| Layfield et al[34] | 2010 | United States | 1 | UNK | M | UNK | UNK | Yes | EUS + FNA of the pancreatic mass | UNK | UNK | UNK | UNK |
| Liratzopoulos et al[23] | 2006 | Greece | 1 | 53 | M | Jaundice, loss of appetite, nausea and mild abdominal pain | CT scan: carcinoma of the lower lobe of the right lung, a tumor in the pancreatic head measuring 4.0 cm × 4.1 cm × 3.5 cm, dilatation of the biliary tract and multiple enlarged lymph nodes in the cervical area, the mediastinum and the abdomen | No | A percutaneous FNA of the pancreatic tumor under CT guidance | Cholecystojejunostomy + dissection of lymph node near the pancreas | 19 d | UNK | Dead |
| Mesa et al[35] | 2004 | United States | 2 | UNK | UNK | UNK | Mass in the head of the pancreas measuring 3.6 cm and a lung tumor | Yes | EUS-FNA of the pancreatic mass | UNK | UNK | UNK | UNK |
| Volkan et al[36] | 2004 | United States | 5 of 109 autopsy cases | UNK | UNK | UNK | UNK | UNK | Autopsy | UNK | UNK | UNK | Dead |
| Tetsuya et al[37] | 2003 | Japan | 1 | 69 | M | Jaundice | Lung tumor with hilar and mediastinal lymph node swelling and solitary pancreatic head tumor measuring 3 cm | No | Autopsy | Endoscopic nasobiliary drainage and stent drainage therapy prior to chemotherapy using gemcitabine | 4 mo | UNK | Dead |
| Moazzam et al[38] | 2002 | United States | 1 | 54 | M | Anorexia, abdominal pain and jaundice | Mass in right upper lung lobe and mass in the head of pancreas | No | Biopsy of the right upper lobe lung mass | Biliary drainage + carboplatin and paclitaxel | UNK | UNK | Alive: good clinical and radiographic response |
| Nakamura et al[11] | 2001 | Japan | 3 of 103 autopsy cases | UNK | UNK | UNK | UNK | UNK | Autopsy | UNK | UNK | UNK | Dead |
| Matsukuma et al[39] | 1997 | Japan | 3 | 55 | M | UNK | UNK | No | Autopsy | UNK | UNK | UNK | Dead |
| 64 | M | ||||||||||||
| 58 | M |
Lung cancer has a very high rate of morbidity and mortality. In 2018, the World Health Organization reported that lung cancer was responsible for 11.6% of new cancer cases and 18.4% of cancer-related deaths[5]. In total, 20% of non-small-cell lung cancers are classified as squamous cell carcinoma[6]. It has been reported that 40% of cases are already metastatic at diagnosis[7], and the 5-year survival rate is estimated to be only 3.6%[6]. The most common metastatic sites include the bones, lungs, brain, liver and adrenal glands[8]. Pancreatic metastasis is rare, representing only 2% of pancreatic tumors[9]. Primary tumors known to metastasize to the pancreas include renal (25%-48%), lung (15%), breast (8%), colorectal (7%), and bone and melanoma (5%)[9,10]. Through the autopsy of 103 cases of patients with pancreatic metastasis, Nakamura et al[11] determined that metastatic dissemination to the pancreas occurred either via lymphatic (28%), vascular (27%), lymphatic and vascular (19%) or direct invasion (18%) routes. The authors also assumed that the majority of patients with primary lung cancer (66%) had pancreatic metastasis through vascular dissemination. In another report, the most frequent lung cancer histological type with pancreatic metastasis was cited as small cell carcinoma (10%) followed by large cell carcinoma, squamous cell carcinoma (1.1%), and anaplastic bronchial carcinoma[12]. Frequently, pancreatic metastasis is asymptomatic (> 50%) and discovered accidentally through extension and control assessment[13]. It may be expressed by diverse and nonspecific clinical situations, such as asthenia, weight loss, abdominal pain, jaundice, nausea, or vomiting. Pancreatic metastasis can manifest as upper gastrointestinal bleeding or acute pancreatitis, which were reported in 3 cases[14] and 13 cases[12], respectively. According to Deluzio et al[15], 59% of patients with pancreatic metastasis had gastrointestinal symptoms, mostly represented by jaundice and abdominal pain. Jaundice is explained by the obstruction of extrahepatic biliary ducts by pancreatic metastasis, which is essentially observed in small cell lung cancer[16]. The diagnosis of pancreatic metastasis and the differentiation of primary and metastatic tumors represent significant challenges. Pancreatic metastasis shows varied enhancement when imaged. Klein et al[17] reported that 76% of pancreatic metastases showed greater vascular enhancement than normal pancreatic parenchyma or primary pancreatic tumors, which is explained by the richness of metastatic vascularization. EUS is the main exam for pancreatic lesions and their locoregional extension. The sensitivity of EUS is estimated at 100% for tumors < 2 cm, whereas the sensitivity values of ultrasound and abdominal scan are 60% and 50%, respectively[16]. A retrospective study by El Hajj et al[10] included 49 patients with pancreatic metastasis and found that the lesions were hypoechoic in 80% of patients, hyperechoic in 4% of patients, mixed in 4% of patients, and anechoic in 2% of patients. Regular boundaries were observed in 55% of cases. To confirm the diagnosis, cytological analysis was used in 63% of cases, whereas immunohistochemical analysis was added to the former technique in 33% of these cases. Dewitt et al[18] demonstrated that EUS-FNA confirmed the diagnosis of pancreatic metastasis in all patients with a secondary pancreatic tumor. They also deduced that the only ultrasound data that could differentiate between primary and secondary pancreatic tumors involved the lesion margins. Margins were well defined when the tumor was secondary (46% vs 4%) and irregular in 94% of primary pancreatic tumors (94% vs 54%) (P < 0.0001). However, no significant differences were noted between primary and metastatic pancreatic tumors regarding tumor number, size, location, or echogenicity parameters. For metastatic lung cancer, therapeutic care consists of palliative chemotherapy and biliary drainage when the tumor compresses the biliary ducts. According to the National Comprehensive Cancer Network guidelines, metastatic squamous cell carcinoma treatment depends on the patient’s performance status[19]. These options should be discussed during the multidisciplinary expert consultation. Regimens of pembrolizumab, carboplatin and paclitaxel or pembrolizumab, carboplatin, paclitaxel and albumin are used as the first-line treatment for patients whose performance status is 0 to 1. When the performance status is 2, carboplatin, paclitaxel and albumin or carboplatin and gemcitabine or carboplatin and paclitaxel are the recommended therapeutic options. Our patient had a performance status of 2, indicating that he should be treated with carboplatin and paclitaxel. However, this treatment was stopped due to intolerance. Recently, many scientific publications have discussed the surgical treatment of oligometastatic lung cancer in the pancreas. Kageyama et al[3] reported a unique case of a 67-year-old patient who had lung cancer with a pancreatic metastasis that was randomly discovered during follow-up tests 6 years after the primary tumor diagnosis. The patient underwent a distal pancreatectomy and ganglion dissection, which led to survival at 5 years without any recurrence. Ida et al[20] showed a longer survival of 8 years in a 70-year-old male patient with metastatic squamous cell lung carcinoma who underwent a total pancreatectomy and a resection of the portal vein. According to a Japanese retrospective study that evaluated global survival in patients receiving a surgical operation for pancreatic metastasis, 6 of the 9 patients survived for more than 23.5 mo. However, patients with longer survival times had pancreatic tumors secondary to renal cancer[21]. Generally, pancreatic metastasis of squamous cell lung carcinoma is discovered at an advanced stage[22], and only 2% of the tumors are resectable[23], revealing why surgical treatment is rarely utilized. Moreover, this case is unusual given the presence of a malignant tracheoesophageal fistula as a rare complication of squamous cell lung carcinoma. Malignant tracheoesophageal or bronchoesophageal fistula develops in 5%-15% of patients with esophageal cancer, and only 0.2% of lung malignancies have been reported to cause esophageal pulmonary fistulae[24]. In patients with prior lung or esophageal cancer, the presence of symptoms, such as dysphagia, recurrent pneumonia or treatment-resistant pneumonia, should raise concern as to whether an underlying fistula is present. If not detected early or left untreated, the fistulae may lead to pneumonitis and lung abscesses that cause sepsis, acute respiratory distress syndrome, and death. In addition, without treatment, the median survival may be 1-6 wk[25]. There is no cure for malignant tracheoesophageal fistulae, and palliative procedures, such as esophageal stenting, esophageal exclusion, esophageal bypass or surgical repair with fistula resection, may prolong survival and provide immediate symptom relief. Based on a comparative study of the survival time and quality of life of patients who received different treatments for tracheoesophageal fistulae, self-expandable stenting did not significantly prolong the survival time of patients but did remarkably improve health-related quality of life[26]. The European Society of Gastrointestinal Endoscopy recommends esophageal self-expandable metallic stent placement as the preferred treatment for sealing malignant tracheoesophageal fistulae[27]. However, the reported success rates of esophageal stent placement vary from 70% to 100%. In addition, some complications may occur, such as stent migration, bleeding, granulation formation, foreign body sensation, and secondary fistulae, all of which have been reported as late complications of stenting[24]. In our case, the malignant tracheoesophageal fistula was successfully treated by an fully covered esophageal metallic stent. Unfortunately, our patient died 6 mo after the diagnosis of pancreatic metastasis. This was not surprising because stage IV squamous cell lung carcinoma with pancreatic metastasis has a poor prognosis in general with an average reported survival of 8.7 mo after diagnosis[28].
Squamous cell lung carcinoma with pancreatic metastasis is rare, and its diagnosis represents a challenge for clinicians. Radiological, endoscopic and anatomopathological methods are needed for an accurate diagnosis. EUS-FNA is the ideal procedure to diagnose pancreatic metastasis. This disease has a poor prognosis because it is generally detected at an advanced stage. Thus, the treatment is typically palliative.
I am grateful to all of those with whom I have had the pleasure to work during this and other related projects. Each of the members of Hepato-Gastroenterology Department has provided me extensive personal and professional guidance and taught me a great deal about both scientific research and life in general.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Morocco
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Hu B, China; Li T, China S-Editor: Wang LL L-Editor: A P-Editor: Wang LL
| 1. | Tempero MA, Malafa MP, Al-Hawary M, Behrman SW, Benson AB, Cardin DB, Chiorean EG, Chung V, Czito B, Del Chiaro M, Dillhoff M, Donahue TR, Dotan E, Ferrone CR, Fountzilas C, Hardacre J, Hawkins WG, Klute K, Ko AH, Kunstman JW, LoConte N, Lowy AM, Moravek C, Nakakura EK, Narang AK, Obando J, Polanco PM, Reddy S, Reyngold M, Scaife C, Shen J, Vollmer C, Wolff RA, Wolpin BM, Lynn B, George GV. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:439-457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 430] [Article Influence: 143.3] [Reference Citation Analysis (0)] |
| 2. | Bush A, Humes R, Young P. Colon Cancer Metastatic to the Pancreas Presenting as of Diabetic Ketoacidosis. ACG Case Rep J. 2020;7:e00455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Kageyama Y, Yamaguchi R, Watanabe S, Aizu K, Sato F, Fujieda H, Yamada M, Toyoda Y, Iwata T. A long-term survival case after resection of the pancreatic metastasis from lung cancer. Int J Surg Case Rep. 2019;61:222-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Wang W, Huang C, Wu S, Liu Z, Liu L, Li L, Li S. Abscopal effect induced by modulated radiation therapy and pembrolizumab in a patient with pancreatic metastatic lung squamous cell carcinoma. Thorac Cancer. 2020;11:2014-2017. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Namayandeh SM, Khazaei Z, Lari Najafi M, Goodarzi E, Moslem A. GLOBAL Leukemia in Children 0-14 Statistics 2018, Incidence and Mortality and Human Development Index (HDI): GLOBOCAN Sources and Methods. Asian Pac J Cancer Prev. 2020;21:1487-1494. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | Dela Cruz CS, Tanoue LT, Matthay RA. Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med. 2011;32:605-644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 7. | Xu Z, Yang Q, Chen X, Zheng L, Zhang L, Yu Y, Chen M, You Q, Sun J. Clinical associations and prognostic value of site-specific metastases in non-small cell lung cancer: A population-based study. Oncol Lett. 2019;17:5590-5600. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Tamura T, Kurishima K, Nakazawa K, Kagohashi K, Ishikawa H, Satoh H, Hizawa N. Specific organ metastases and survival in metastatic non-small-cell lung cancer. Mol Clin Oncol. 2015;3:217-221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 191] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 9. | Dar FS, Mukherjee S, Bhattacharya S. Surgery for secondary tumors of the pancreas. HPB (Oxford). 2008;10:498-500. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | El Hajj II, LeBlanc JK, Sherman S, Al-Haddad MA, Cote GA, McHenry L, DeWitt JM. Endoscopic ultrasound-guided biopsy of pancreatic metastases: a large single-center experience. Pancreas. 2013;42:524-530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Nakamura E, Shimizu M, Itoh T, Manabe T. Secondary tumors of the pancreas: clinicopathological study of 103 autopsy cases of Japanese patients. Pathol Int. 2001;51:686-690. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 94] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 12. | Woo JS, Joo KR, Woo YS, Jang JY, Chang YW, Lee J 2nd, Chang R. Pancreatitis from metastatic small cell lung cancer successful treatment with endoscopic intrapancreatic stenting. Korean J Intern Med. 2006;21:256-261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Zerbi A, Pecorelli N. Pancreatic metastases: An increasing clinical entity. World J Gastrointest Surg. 2010;2:255-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 21] [Cited by in F6Publishing: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Zheng Y, Gao Q, Fang W, Xu N, Zhou J. Gastrointestinal bleeding due to pancreatic metastasis of non-small cell lung cancer: A report of two cases and a literature review. Oncol Lett. 2015;9:2041-2045. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | DeLuzio MR, Moores C, Dhamija A, Wang Z, Cha C, Boffa DJ, Detterbeck FC, Kim AW. Resection of oligometastatic lung cancer to the pancreas may yield a survival benefit in select patients--a systematic review. Pancreatology. 2015;15:456-462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Chaudhari D, Khanna A, Goenka P, Young M. Lung carcinoma presenting as an obstructive jaundice: case series with literature review. J Gastrointest Cancer. 2014;45 Suppl 1:66-70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Klein KA, Stephens DH, Welch TJ. CT characteristics of metastatic disease of the pancreas. Radiographics. 1998;18:369-378. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 108] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | DeWitt J, Jowell P, Leblanc J, McHenry L, McGreevy K, Cramer H, Volmar K, Sherman S, Gress F. EUS-guided FNA of pancreatic metastases: a multicenter experience. Gastrointest Endosc. 2005;61:689-696. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 100] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 19. | Ettinger DS, Wood DE, Aggarwal C, Aisner DL, Akerley W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac LR, D'Amico TA, Dilling TJ, Dobelbower M, Gettinger S, Govindan R, Gubens MA, Hennon M, Horn L, Lackner RP, Lanuti M, Leal TA, Lin J, Loo BW Jr, Martins RG, Otterson GA, Patel SP, Reckamp KL, Riely GJ, Schild SE, Shapiro TA, Stevenson J, Swanson SJ, Tauer KW, Yang SC, Gregory K; OCN, Hughes M. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 1.2020. J Natl Compr Canc Netw. 2019;17:1464-1472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 360] [Cited by in F6Publishing: 478] [Article Influence: 119.5] [Reference Citation Analysis (0)] |
| 20. | Toshiharu IDA, Masafumi YOSHIDA KN and KI. An eight-year survivor after the resection of a metastatic pancreatic tumor of pulmonary carcinoma. J JPN surg Assoc. 2006;. [DOI] [Cited in This Article: ] |
| 21. | Yagi T, Hashimoto D, Taki K, Yamamura K, Chikamoto A, Ohmuraya M, Beppu T, Baba H. Surgery for metastatic tumors of the pancreas. Surg Case Rep. 2017;3:31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Machairas N, Paspala A, Schizas D, Ntomi V, Moris D, Tsilimigras DI, Misiakos EP, Machairas A. Metastatic squamous cell carcinoma to the pancreas: Report of an extremely rare case. Mol Clin Oncol. 2019;10:144-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Liratzopoulos N, Efremidou EI, Papageorgiou MS, Romanidis K, Minopoulos GJ, Manolas KJ. Extrahepatic biliary obstruction due to a solitary pancreatic metastasis of squamous cell lung carcinoma. Case report. J Gastrointestin Liver Dis. 2006;15:73-75. [PubMed] [Cited in This Article: ] |
| 24. | Buemi L, Stefanelli S, Bichard P, Luscher M, Becker M. Esophageal pulmonary fistula - a rare complication of radiation therapy: a case report. J Med Case Rep. 2018;12:116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Sebastian J, Kirankumar VS, Pappachan JM, Zachariah SA, Radha TR, Sujathan P. Multifactorial dysphagia complicated by esophago-bronchial fistula. J Cancer Res Ther. 2007;3:108-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Hu Y, Zhao YF, Chen LQ, Zhu ZJ, Liu LX, Wang Y, Kou YL. Comparative study of different treatments for malignant tracheoesophageal/bronchoesophageal fistulae. Dis Esophagus. 2009;22:526-531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Spaander MC, Baron TH, Siersema PD, Fuccio L, Schumacher B, Escorsell À, Garcia-Pagán JC, Dumonceau JM, Conio M, de Ceglie A, Skowronek J, Nordsmark M, Seufferlein T, Van Gossum A, Hassan C, Repici A, Bruno MJ. Esophageal stenting for benign and malignant disease: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2016;48:939-948. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 188] [Article Influence: 23.5] [Reference Citation Analysis (1)] |
| 28. | Roland CF, van Heerden JA. Nonpancreatic primary tumors with metastasis to the pancreas. Surg Gynecol Obstet. 1989;168:345-347. [PubMed] [Cited in This Article: ] |
| 29. | Zhou W, Dong H, Dong A. Isolated Pancreatic Metastasis From Squamous Cell Lung Cancer Mimicking Primary Pancreatic Ductal Adenocarcinoma on FDG PET/CT. Clin Nucl Med. 2020;45:420-422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Stoupis I, Voudoukis E, Mastorakis E, Kazamias G, Ieromonachou P, Pappas C. A Rare Pancreatic Tail Metastasis from Squamous Cell Lung Carcinoma Diagnosed by EUS-FNB and a Small Review of the Literature. GE Port J Gastroenterol. 2020;27:29-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Ishikawa T, Hirooka Y, Teman CJ, Goto H, Belletrutti PJ. An Unusual Case of Pancreatic Metastasis from Squamous Cell Carcinoma of the Lung Diagnosed by EUS-Guided Fine Needle Biopsy. Case Rep Gastrointest Med. 2017;2017:3212056. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Fujii M, Watanabe K, Kataoka M, Nose S, Shiode J. A case of a pancreatic tumor that was diagnosed as metastasis from lung cancer by endoscopic ultrasound-guided fine needle aspiration. J Med Ultrason (2001). 2015;42:405-408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 33. | Dewanwala A, Kotowski A, LeVea CM, Ma WW. Secondary Tumors of the Pancreas: Case Report and a Single-Center Experience. J Gastrointest Cancer. 2012;43 Suppl 1:S117-S124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Layfield LJ, Hirschowitz SL, Adler DG. Metastatic disease to the pancreas documented by endoscopic ultrasound guided fine-needle aspiration: a seven-year experience. Diagn Cytopathol. 2012;40:228-233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 35. | Mesa H, Stelow EB, Stanley MW, Mallery S, Lai R, Bardales RH. Diagnosis of nonprimary pancreatic neoplasms by endoscopic ultrasound-guided fine-needle aspiration. Diagn Cytopathol. 2004;31:313-318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 36. | Adsay NV, Andea A, Basturk O, Kilinc N, Nassar H, Cheng JD. Secondary tumors of the pancreas: an analysis of a surgical and autopsy database and review of the literature. Virchows Arch. 2004;444:527-535. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 248] [Cited by in F6Publishing: 224] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 37. | Kubota T, Ikezoe T, Harada R, Nakata H, Kobayashi M, Taguchi H. Pancreatic metastasis from lung cancer: report of an autopsy case. Nihon Kokyuki Gakkai Zasshi. 2003;41:917-921. [PubMed] [Cited in This Article: ] |
| 38. | Moazzam N, Mir A, Potti A. Pancreatic metastasis and extrahepatic biliary obstruction in squamous cell lung carcinoma. Med Oncol. 2002;19:273-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 39. | Matsukuma S, Suda K, Abe H, Ogata S, Wada R. Metastatic cancer involving pancreatic duct epithelium and its mimicry of primary pancreatic cancer. Histopathology. 1997;30:208-213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |