Published online Jun 16, 2022. doi: 10.4253/wjge.v14.i6.402
Peer-review started: October 22, 2021
First decision: November 15, 2021
Revised: November 28, 2021
Accepted: May 5, 2022
Article in press: May 5, 2022
Published online: June 16, 2022
Processing time: 233 Days and 11.4 Hours
Pancreatic cystic lesions (PCLs) are common in clinical practice. The accurate classification and diagnosis of these lesions are crucial to avoid unnecessary treatment of benign lesions and missed opportunities for early treatment of potentially malignant lesions.
To evaluate the role of cyst fluid analysis of different tumor markers such as cancer antigens [e.g., cancer antigen (CA)19-9, CA72-4], carcinoembryonic antigen (CEA), serine protease inhibitor Kazal-type 1 (SPINK1), interleukin 1 beta (IL1-β), vascular endothelial growth factor A (VEGF-A), and prostaglandin E2 (PGE2)], amylase, and mucin stain in diagnosing pancreatic cysts and differentiating malignant from benign lesions.
This study included 76 patients diagnosed with PCLs using different imaging modalities. All patients underwent endoscopic ultrasound (EUS) and EUS-fine needle aspiration (EUS-FNA) for characterization and sampling of different PCLs.
The mean age of studied patients was 47.4 ± 11.4 years, with a slight female predominance (59.2%). Mucin stain showed high statistical significance in predicting malignancy with a sensitivity of 87.1% and specificity of 95.56%. It also showed a positive predictive value and negative predictive value of 93.1% and 91.49%, respectively (P < 0.001). We found that positive mucin stain, cyst fluid glucose, SPINK1, amylase, and CEA levels had high statistical significance (P < 0.0001). In contrast, IL-1β, CA 72-4, VEGF-A, VEGFR2, and PGE2 did not show any statistical significance. Univariate regression analysis for prediction of malignancy in PCLs showed a statistically significant positive correlation with mural nodules, lymph nodes, cyst diameter, mucin stain, and cyst fluid CEA. Meanwhile, logistic multivariable regression analysis proved that mural nodules, mucin stain, and SPINK1 were independent predictors of malignancy in cystic pancreatic lesions.
EUS examination of cyst morphology with cytopathological analysis and cyst fluid analysis could improve the differentiation between malignant and benign pancreatic cysts. Also, CEA, glucose, and SPINK1 could be used as promising markers to predict malignant pancreatic cysts.
Core Tip: Nowadays, the awareness of pancreatic cystic lesions has become an essential issue, especially with the increased incidence of asymptomatic pancreatic cysts in the general population. Therefore, the proper diagnosis, meticulous differentiation, and staging of these pancreatic cystic lesions are crucial for proper management and avoiding unnecessary treatment of benign lesions and missing early treatment of the malignant/pre-malignant lesions. Endoscopic ultrasound examination of cyst morphology with cytopathological and chemical analysis and cyst fluid analysis could improve the diagnostic capability. Also, many developed markers are valuable for predicting a malignant pancreatic cyst.
- Citation: Okasha HH, Abdellatef A, Elkholy S, Mogawer MS, Yosry A, Elserafy M, Medhat E, Khalaf H, Fouad M, Elbaz T, Ramadan A, Behiry ME, Y William K, Habib G, Kaddah M, Abdel-Hamid H, Abou-Elmagd A, Galal A, Abbas WA, Altonbary AY, El-Ansary M, Abdou AE, Haggag H, Abdellah TA, Elfeki MA, Faheem HA, Khattab HM, El-Ansary M, Beshir S, El-Nady M. Role of endoscopic ultrasound and cyst fluid tumor markers in diagnosis of pancreatic cystic lesions. World J Gastrointest Endosc 2022; 14(6): 402-415
- URL: https://www.wjgnet.com/1948-5190/full/v14/i6/402.htm
- DOI: https://dx.doi.org/10.4253/wjge.v14.i6.402
Pancreatic cystic lesions (PCLs) are not rare; they vary from a simple benign cyst to a highly malignant one[1]. Awareness of these lesions has increased in recent years, especially with the increased incidence of asymptomatic pancreatic cysts in the general population primarily due to improved detection by different advanced imaging modalities[2,3]. Therefore, the proper diagnosis, meticulous differentiation, and staging of these PCLs are crucial for proper management and avoiding unnecessary treatment of benign lesions and missing early treatment of the malignant/pre-malignant lesions[4,5].
Endoscopic ultrasound (EUS) has become an indispensable tool for diagnosing many pancreatic lesions; it has a benefit for better evaluation of number, location, dimensions, wall thickness, and the content of pancreatic cysts. Also, it is crucial in distinguishing the internal septae and solid areas within the cysts[6].
The morphological features of PCLs are not independent factors in differentiating malignant from nonmalignant lesions. The combination of both EUS-fine needle aspiration (EUS-FNA) findings with cystic fluid tumor marker analysis, along with clinical, radiologic, histologic, genetic, and molecular characteristics, enhances the diagnostic accuracy for PCLs and helps to construct a novel model in the era of PCL diagnosis[4].
Currently, many tumor markers, both in the serum and in pancreatic cyst fluid (CF), have been widely studied as a tool for distinguishing mucinous/malignant and non-mucinous pancreatic cystic lesions, such as carcinoembryonic antigen (CEA), cancer antigen (CA)19-9, CA125, CA15-3, and CA72-4[7].
In this single tertiary referral center prospective study, the samples were collected and stored, and then all markers were detected in the same specimens in the same time. The study aimed primarily to evaluate the role of cyst fluid amylase and tumor markers such as CA 19-9, CEA, serine protease inhibitor Kazal-type 1 (SPINK1), IL1-β, CA 72-4, vascular endothelial growth factor A (VEGF-A), and prostaglandin E2 (PGE2) in addition to mucin stain in diagnosing pancreatic cysts and differentiating malignant from benign lesions.
This prospective study was conducted on 76 patients diagnosed with PCLs using different imaging modalities such as computed tomography (CT), EUS, abdominal ultrasound, or magnetic resonance imaging (MRI). The candidates were recruited over 3 years from the Gastroenterology, Endoscopy, and Hepatology Unit, Internal Medicine Department, Kasr Al-Ainy, Cairo University. Fluid analysis was performed for CA 19-9, CA 72-4, CEA, VEGF-1, SPINK-1, IL1-b, PGE2, amylase, mucin stain, and cytopathology. We compared these data with the final diagnosis based on histopathology after surgical resection, positive cytopathology (positive for malignancy), and a long period of follow-up of the patients for at least 18 mo.
All patients underwent EUS examination for cyst characterization and sampling of the cystic lesions. All included patients were above 18 years of age. Patients included in this study were diagnosed with large pancreatic cysts (larger than 3 cm), suspicious intraductal papillary mucinous neoplasm (IPMN), or pancreatic duct dilatation proved by magnetic resonance cholangiopancreatography. However, patients with small cysts (less than 1 cm), calculous cholecystitis, a potential risk for anesthesia, or a bleeding tendency (international normalized ratio > 1.5, or severe thrombocytopenia, with platelet count < 50000/mm³) and patients who refused to participate were excluded from the study. Also, those who missed the follow-up were ruled out from the study. Our institution’s Research Ethical Committee approved the study, and all patients gave their informed written consent before inclusion in the study, according to the ethical guidelines of the 1975 Declaration of Helsinki.
All the patients, after thorough full history taking and clinical examination, were subjected to: (1) EUS examination using a linear Echoendoscope PENTAX EG3870UTK (HOYA Corporation, PENTAX Life Care Division, Showanomori Technology Center, Tokyo, Japan) connected to an ultrasound unit Hitachi AVIUS machine (Hitachi Medical Systems, Tokyo, Japan). All examinations were performed under deep sedation with IV propofol. For EUS-FNA, we used the Cook 19G and 22G needles (Echotip; Wilson-Cook, Winston Salem, NC). Prophylactic ceftriaxone (1 gm) was administered before the procedure; (2) characterization of the PCLs. All the characteristics of the PCLs were documented, including localization, number, dimensions, wall thickness, presence of septations or mural nodules, calcification, lymph nodes, and cystic dilatation of the main pancreatic duct. The color, transparency, and viscosity of the CF were also recorded; and (3) evacuation of the cystic fluid entirely with a single needle pass. Aspirated material inside the needle was spread over dry slides. Also, a proportion of the fluid sample (at least 2 mL) was sent for cytopathological examination, including mucin staining using alcian blue stain. At least 5 mL of cyst fluid was analyzed for CEA, SPINK1, IL1-β, CA 72-4, VEGF-A, PGE2, and CA-19-9 using two-site immunoassays (Beckman Coulter). Amylase was measured by the enzymatic colorimetric assay on a modular system (Roche).
Cysts were considered malignant when any of the following is present: (1) Cytopathological detection of malignancy; (2) presence of metastasis in the absence of other concomitant malignancies; (3) presence of mural nodules that progress in size within 6 mo; and (4) postoperative pathological diagnosis of malignancy if available. Cysts were considered benign when proved negative for malignancy by cytopathological examination and follow-up for 18 mo without increasing its size, the appearance of mural nodules or metastasis, or occurrence of obstructive jaundice.
The overall complication rate of EUS-FNA in the prospective series ranges from 0% to 2.5%[8]. Such complications include pain, infection, bleeding, acute pancreatitis, perforation of the esophagus or duodenum, bile peritonitis, and seeding of tumorous cells along the needle track[9]. Therefore, a prophylactic antibiotic in the form of 1 gm IM or slow IV third-generation cephalosporin was administered 6 h before the procedure. No major complications occurred in our series. However, self-limiting intracystic bleeding occurred in one patient, and mild pain occurred in three patients. All patients were discharged on the same day, and no hospital admission was needed.
Data management and analysis were performed using Statistical Package for Social Sciences v. 25. Numerical data are summarized using the mean and standard deviation, median, or range, as appropriate. Categorical data are summarized as numbers and percentages. Estimates of the frequency were calculated using the numbers and percentages. Numerical data were explored for normality using the Kolmogorov-Smirnov test and the Shapiro-Wilk test. To measure the association between variables: (1) Chi-square or Fisher’s tests were used to compare independent groups concerning categorical data; (2) kappa statistics were computed to test the agreement between categorical variables. Their values ranged from zero to one; (3) the Mann-Whitney U test implemented comparisons between two groups for non-normally distributed numeric variables; and (4) P value ≤ 0.05 was considered significant.
This study included 76 patients [31 males (40.8%) and 45 females (59.2%)] with a mean age of 47.4 ± 11.4 years (Table 1).
| Gender | Number | Percent (%) |
| Male | 31 | 40.80% |
| Female | 45 | 59.20% |
| Total | 76 | 100% |
EUS evaluation showed that most patients had a unilocular cyst (40 patients, 52.6%), while 36 patients (47.4%) had a multilocular cyst. Mural nodules were found in 24 patients (31.6%). In addition, most cysts had thin walls (77.6%) and clear contents (78.9%). Calcifications and lymph nodes were not found in 92.1% and 82.9% of patients, respectively. The pancreatic duct was dilated in 10 patients (13.2%) (Table 2).
| EUS finding | Number | Percent (%) | |
| Loculation | Unilocular | 40 | 0.526 |
| Multilocular | 36 | 0.474 | |
| Mural nodules | No | 52 | 0.684 |
| Yes | 24 | 0.316 | |
| Wall | Thin Wall | 59 | 0.776 |
| Thick Wall | 17 | 0.224 | |
| Content | Clear | 60 | 0.789 |
| Turbid | 16 | 0.211 | |
| Calcification | No | 70 | 0.921 |
| Yes | 6 | 0.079 | |
| LNs | No | 63 | 0.829 |
| Yes | 13 | 0.171 | |
| Pancreatic duct dilation | No | 66 | 0.868 |
| Yes | 10 | 0.132 | |
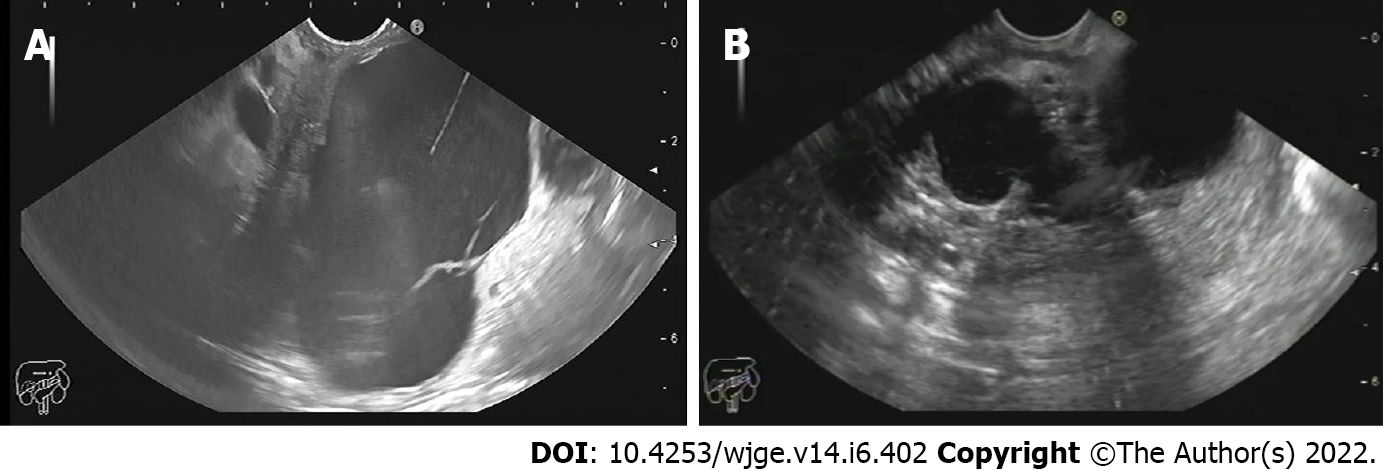
Pancreatic cysts were diagnosed as being malignant/potentially malignant or benign in 38.2% and 61.8% of patients, respectively. Malignant cysts included mucinous cystadenocarcinoma (14.5%) (Figure 1A) and pancreatic adenocarcinoma (5.3%). On the other hand, potentially malignant cysts included IPMN with low (7.9%) and high-grade dysplasia (13.2%) and mucinous cystadenoma. Benign cysts included serous and mucinous cystic neoplasms (17.1%), pseudocysts (39.5%) (Figure 1B), and cystic lymphangioma (1.3%) (Table 3).
| Final diagnosis | Number | Percent (%) |
| Pancreatic pseudocyst | 30 | 39.5 |
| Pancreatic pseudocyst with WOPN | 1 | 1.3 |
| Serous cystadenoma | 13 | 17.1 |
| Mucinous cystadenoma | 11 | 14.5 |
| IPMN (high grade dysplasia) | 10 | 13.2 |
| IPMN (low grade dysplasia) | 6 | 7.9 |
| Pancreatic adenocarcinoma | 4 | 5.3 |
| Cystic lymphangioma | 1 | 1.3 |
| Total | 76 | 100 |
Evaluating PCLs using mucin stain to differentiate between mucinous and non-mucinous pancreatic cystic lesions showed a sensitivity of 100%, specificity of 94%, and accuracy of 96.04% (Table 4). Also, we found that there was high statistical significance for mucin stain in predicting malignancies with a sensitivity of 87.1%, specificity of 95.56%, positive predictive value (PPV) of 93.1%, and negative predictive value (NPV) of 91.49% (P value < 0.001) (Table 5).
| Statistic | Value | 95%CI |
| Sensitivity | 100% | 86.77% to 100% |
| Specificity | 94% | 83.45% to 98.75% |
| Positive likelihood ratio | 16.67 | 5.56 to 49.93 |
| Negative likelihood ratio | 0 | |
| Disease prevalence | 34.21% | 23.71% to 45.99% |
| Positive predictive value | 89.66% | 74.31% to 96.29% |
| Negative predictive value | 100% | |
| Accuracy | 96.05% | 88.89% to 99.18% |
| Statistic | Value | 95%CI |
| Sensitivity | 87.10% | 70.17% to 96.37% |
| Specificity | 95.56% | 84.85% to 99.46% |
| Positive likelihood ratio | 19.60 | 5.02 to 76.47 |
| Negative likelihood ratio | 0.14 | 0.05 to 0.34 |
| Disease prevalence | 40.79% | 29.65% to 52.67% |
| Positive predictive value | 93.10% | 77.58% to 98.14% |
| Negative predictive value | 91.49% | 81.12% to 96.41% |
| Accuracy | 92.11% | 83.60% to 97.05% |
The median CF CEA level was 90 (8.39- 2750) ng/mL. Also, the median CF SPINK1 level was 0.56 (0.35-0.97) ng/mL, and the median CF glucose level was 50 mg/dL (Table 6). When we categorized the CF level of CEA above and below 192 ng/mL, the malignant/potentially malignant cysts were more likely to have a CEA level above 192 ng/mL (P = 0.001), as shown in Table 7.
| Biochemical test | Median (IQR) | Range |
| CEA (ng/ml) | 90 (8.78- 1560) | (5-100000) |
| SPINK1 (ng/ml) | 0.56 (0.35-0.97) | (0.1-2.32) |
| Glucose (mg/dl) | 50 (10-84) | (2-171) |
| Variable | Benign group(n = 45) | Malignant group(n = 31) | P value |
| Mucin stain positivity | 2 (4.4%) | 27 (87.1%) | < 0.0001 |
| Number (%) | |||
| Glucose (mg/dl) | 21.5 (4-45) | 68.5 (47-87) | 0.0001 |
| median (IQR) | |||
| IL1b (pg/mL) | 0.37 (0.58) | 0.34 (0.45) | 0.845 |
| (median, IQR) | |||
| CA 72-4 (U/mL) | 6.36 (9.7) | 7.4 (7.6) | 0.323 |
| (median, IQR) | |||
| VEGF-A (pg/ml) | 707.8 (1056) | 736.9 (2262) | 0.866 |
| (median, IQR) | |||
| VEGFR2 (pg/ml) | 2.5 (5.3) | 1.3 (3) | 0.281 |
| (median, IQR) | |||
| SPINK1 (ng/ml) | 0.91 (0.41-1.45) | 0.47 (0.3-0.72) | 0.001 |
| median (IQR) | |||
| PGE2 (pg/ml) | 307.2 (131) | 409.7 (176) | 0.121 |
| (median, IQR) | |||
| CF amylase (U/L) | 130.5 (353) | 3060 (5191) | 0.034 |
| (median, IQR) | |||
| CF CEA (ng/ml) | 6.4 (234) | 15.8 (2532) | 0.004 |
| (median, IQR) | |||
| CEA (> 192 ng/mL) | 15 | 5 | 0.001 |
As shown in Table 6, CF CEA level and CF amylase were significantly higher in malignant/ potentially malignant cysts than in benign cysts with a median of 15.8 vs 6.4 and 130.5 vs 3060 (P = 0.004 and 0.034, respectively). Also, CF amylase and CF CEA showed statistical significance in predicting malignancy (P = 0.028 and < 0.001, respectively). Furthermore, the SPINK1 level in CF was significantly higher in malignant/potentially malignant cysts compared to benign ones (0.91 vs 0.47, P = 0.001). Meanwhile, glucose was markedly consumed in malignant/potentially malignant cysts than in benign cysts (21.5 vs 68.5, P = 0.0001) (Table 7).
Comparing different CF markers in predicting malignant PCLs among the studied patients revealed that positive Mucin stain, CF glucose, SPINK1, amylase, and CEA showed high statistical significance (P < 0.0001, 0.0001, 0.001, 0.034, and 0.004, respectively). However, IL1-β, CA 72-4, VEGF-A, VEGFR2, and PGE2 did not show any statistical significance (Table 8).
| Variable | Criterion | Specificity | Sensitivity | PPV | NPV | P value | AUC |
| Age | > 35 | 0.244 | 1 | 0.4745 | 1 | 0.605 | 0.534 |
| Mucin stain | 0.9556 | 0.871 | 0.931 | 0.9149 | < 0.001 | 0.913 | |
| Glucose (mg/dL) | ≤ 42 | 0.7353 | 0.8478 | 0.76 | |||
| IL1b (pg/mL) | < 1.13 | 0.209 | 0.9 | 0.4363 | 0.7464 | 0.761 | 0.521 |
| CA 72-4 (U/mL) | > 4.3138 | 0.467 | 0.677 | 0.4657 | 0.678 | 0.32 | 0.567 |
| VEGF-A (pg/mL) | > 1221.7 | 0.844 | 0.29 | 0.561 | 0.634 | 0.87 | 0.511 |
| VEGFR2 (pg/ml) | > 6.601 | 0.933 | 0.29 | 0.7482 | 0.657 | 0.301 | 0.573 |
| SPINK1 (μg/L) | ≥ 0.58 | 0.6533 | 0.7059 | 0.708 | 0.623 | 0.72 | |
| PGE2 (pg/ml) | > 311.77 | 0.556 | 0.8 | 0.5529 | 0.802 | 0.102 | 0.683 |
| CF amylase (U/L) | > 270 | 0.71 | 0.711 | 0.629 | 0.781 | 0.028 | 0.644 |
| CF CEA (ng/ml) | > 8 | 0.742 | 0.689 | 0.622 | 0.795 | < 0.001 | 0.761 |
Univariate regression analysis showed a statistically significant association between malignancy in PCLs and mural nodules, lymph nodes, cyst diameter, mucin stain, CF CEA, SPINK1, and CEA level > 192 ng/mL. In comparison, multivariable regression analysis proved that mural nodules, mucin stain, SPINK1, and CEA level > 192 ng/mL were independent predictors of malignancy in cystic pancreatic lesions (Table 9).
| Variable | Univariate analysis | Multivariate analysis | ||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Age | 1.06 (0.97-1.06) | 0.4312 | ||
| Mural nodules | 6.6 (2.3- 19.3) | 0.0006 | 5.7 (1.37-24.6) | 0.0172 |
| Wall thickness | 1.39 (0.47-4.124) | 0.5514 | ||
| LNs | 11.82 (2.4-58.4) | 0.0024 | 0.14 (0.006-3.3) | 0.2219 |
| Content | 0.59 (0.18-1.923) | 0.3851 | ||
| Loculation | 1.1 (0.43-2.68) | 0.8826 | ||
| Calcification | 1.5 (0.28-7.97) | 0.6342 | ||
| Shortest Diameter | 0.965 (0.94-0.99) | 0.0189 | 1.06 (0.92-1.22) | 0.4044 |
| Longest Diameter | 0.971(0.95-0.99) | 0.0112 | 0.913 (0.81- 1.03) | 0.1326 |
| Mucin Stain | 145 (24.8-847.2) | < 0.0001 | 82.4 (12.1-561) | < 0.0001 |
| Glucose | 0.97 (0.96-0.99) | > 0.001 | 0.99 (0.97-1.01) | 0.48 |
| IL1b (pg/mL) | 0.91 (0.702-1.18) | 0.496 | ||
| CA 72-4 | 1.02 (0.98-1.053) | 0.3017 | ||
| VEGF-A | 1.0001(0.99-1.0005) | 0.5782 | ||
| VEGFR2 | 1.14 (0.99-1.318) | 0.0782 | ||
| SPINK1 | 9.09 (2.62-31.59) | 0.001 | 23.65 (3.10-180.62) | 0.002 |
| PGE2 (pg/mL) | 1.01 (0.999-1.02) | 0.0798 | ||
| CF Amylase | 1 (1-1) | 0.8593 | ||
| CF CEA | 1.0003 (1.0001-1.0005) | 0.0152 | 1.0001 (0.99-1.0006) | 0.5978 |
| CEA > 192 (ng/mL) | 6.47 (2.05-20.42) | 0.001 | 14.12 (2.39-83.22) | 0.003 |
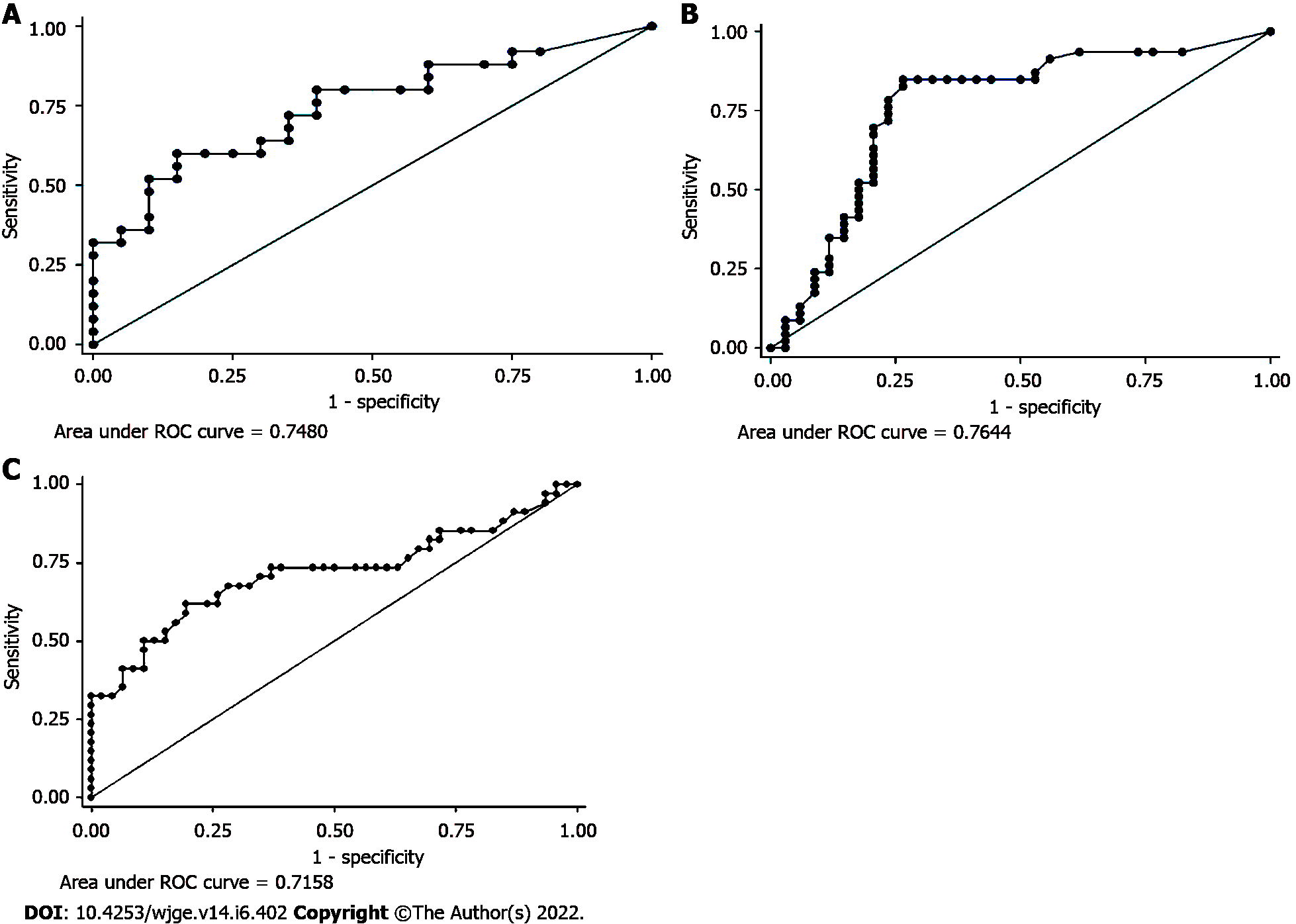
Receiver operating characteristic (ROC) curves were constructed to assess the diagnostic accuracy of CF CEA, SPINK1, IL1-β, CA 72-4, VEGF-A, PGE2, and CA-19-9 in predicting malignant cysts. It revealed that the area under the curve was comparable for CEA, glucose, and SPINK1 (0.75, 0.76, and 0.72, respectively) (Figures 2A-C).
The sensitivity of EUS diagnosis in detecting malignant and premalignant pancreatic cysts was 66.7%, while 69.2% for the specificity, 60% PPV, and 75% NPV with an overall accuracy of 68.2% (Table 10).
| Statistic | Value | 95%CI |
| Sensitivity | 0.6667 | 40.99% to 86.66% |
| Specificity | 0.6923 | 48.21% to 85.67% |
| Positive predictive value | 0.6 | 43.60% to 74.42% |
| Negative predictive value | 0.75 | 59.79% to 85.82% |
| Accuracy | 0.6818 | 52.42% to 81.39% |
Out of 76 patients, two patients died. Both patients had pancreatic adenocarcinoma. Most of the patients showed a stationary course (40 patients, 52.6%), and only three patients (3.9%) ran a regressive course, as demonstrated in Table 11. Two patients with inflammatory pseudocyst underwent a percutaneous pig-tail insertion; one of them was complicated by abscess formation and proceeded to surgery. Most of the patients required no intervention (56 patients, 73.7%). However, some patients were referred to surgeries (17 patients, 22.4%), and only one patient underwent cystogastrostomy, as demonstrated in Table 12.
| Follow-up | Stationary | Regressive | No-recurrence | Progressive | Died |
| Pancreatic pseudocyst (n = 30) | 27 (35.5%) | 3 (3.9%) | 0 | 0 | 0 |
| Pancreatic pseudocyst with WOPN (n = 1) | 0 | 0 | 1 (1.3%) | 0 | 0 |
| Serous cystadenoma (n = 13) | 12 (15.7%) | 0 | 1 (1.3%) | 0 | 0 |
| Mucinous cystadenoma (n = 10) | 9 | 0 | 1 (1.3%) | 0 | 0 |
| Mucinous cystadenocarcinoma (n = 1) | 0 | 0 | 0 | 1 | 0 |
| IPMN (high grade dysplasia) (n = 10) | 3 | 0 | 7 | 0 | 0 |
| IPMN (low grade dysplasia) (n = 6) | 6 | 0 | 0 | 0 | 0 |
| Pancreatic adenocarcinoma (n = 4) | 0 | 0 | 2 (2.6%) | 0 | 2 (2.6%) |
| Cystic lymphangioma (n = 1) | 1 (1.3%) | 0 | 0 | 0 | 0 |
| Total (n = 76) | 40 (52.6%) | 3 (3.9%) | 5 (6.5%) | 0 | 2 (2.6%) |
| Intervention required | No | Surgery | Pig-tail drainage | Cysto-gastrostomy |
| Pancreatic pseudocyst (n = 30) | 26 (34.2%) | 1 (1.3%) | 2 (2.6%) | 1 (1.3%) |
| Pancreatic pseudocyst with WOPN (n = 1) | 0 | 1 (1.3%) | 0 | 0 |
| Serous cystadenoma (n = 13) | 12 (15.8%) | 1 (1.3%) | 0 | 0 |
| Mucinous cystadenoma (n = 10) | 9 (11.7%) | 1 (1.3%) | 0 | 0 |
| Mucinous cystadenocarcinoma (n = 1) | 1 (1.3%) | 0 | 0 | 0 |
| IPMN (high grade dysplasia) (n = 10) | 1 (1.3%) | 9 (11.8%) | 0 | 0 |
| IPMN (low grade dysplasia) (n = 6) | 6 (7.9%) | 0 | 0 | 0 |
| Pancreatic adenocarcinoma (n = 4) | 0 | 4 (5.2%) | 0 | 0 |
| Cystic lymphangioma (n = 1) | 1 (1.3%) | 0 | 0 | 0 |
| Total (n = 76) | 56 (73.7%) | 17 (22.4%) | 2 (2.6%) | 1 (1.3%) |
There are great challenges in diagnosing and managing PCLs that have become a common problem faced by many physicians and surgeons[10]. Some PCLs have a malignant potential with a significant risk of developing invasive cancer[11]. Therefore, the accurate classification and diagnosis of pancreatic cysts provide a potential for preventing and early detection of pancreatic cancer. On the other hand, misdiagnosis or unnecessary surgeries may lead to high cost and harm to the patients[10].
Unfortunately, imaging modalities such as CT and MRI have insufficient sensitivity and specificity to characterize PCLs and provide a suboptimal classification and diagnosis due to poor interobserver variability[12].
EUS is considered the most sensitive tool in delineating the pancreatic cyst characteristics with the capacity to identify the presence of mural nodules and solid components[13]. Also, it has a benefit in enabling EUS-FNA for cytology[14]. Nonetheless, cytology still has a limited diagnostic yield with a pooled sensitivity of 63% and specificity of 88%[15].
Owing to the limited diagnostic accuracy for different pancreatic cysts with the current diagnostic modalities, analysis of the pancreatic CF obtained via EUS-FNA could improve the diagnostic accuracy for pancreatic cysts and help determine the malignant potentiality. Therefore, there is still a growing research interest in discovering and validating novel CF biomarkers that may improve diagnostic accuracy. The present study was designed to determine the role of CF amylase and tumor markers such as CA 19-9, CEA, SPINK1, IL1-β, CA 72-4, VEGF-A, and PGE2 in addition to mucin stain in diagnosing pancreatic cysts and differentiating malignant from benign lesions.
The presence of solid components inside the cyst on imaging could be a significant predictor of malignancy, as reported in many studies[16-18]. Also, we found that the presence of mural nodules was highly predictive of malignancy in univariate and multivariate logistic regression analysis (P = 0.0006 and 0.0172, respectively) along with cyst diameter (P = 0.0189 for shortest diameter and 0.0112 for longest diameter) and lymph node enlargement (P = 0.0024).
In a study conducted by Okasha et al[19] analyzing the CF amylase of 44 patients, they concluded that pancreatic CF amylase level could differentiate between malignant/potentially malignant and benign cysts with a sensitivity of 58%, specificity of 75%, PPV of 73%, NPV of 60%, and accuracy of 66%.
In our study, CF CEA level and CF amylase were significantly higher in malignant/potentially malignant cysts than in benign cysts (P = 0.004 and 0.034, respectively). This finding agrees with other studies stating that pancreatic CF CEA offers the best diagnostic performance than any other single test, especially in differentiating mucinous and non-mucinous cysts[20].
A large multi-institutional study conducted on 1861 patients reported that CEA > 192 ng/mL could differentiate mucinous from non-mucinous cysts with an accuracy of 77%[21]. Their findings are in concordance with our study that reported that the malignant/potentially malignant cysts had CEA levels above 192 ng/mL (P = 0.001).
In CF, positive mucin stain was significantly more frequent in malignant cysts (87.1%) (P < 0.0001). Twenty-seven cysts were positive for mucin stain, with a sensitivity of 87.1% and specificity of 95.56% in differentiating benign from malignant PCLS. Also, mucin staining differentiates mucinous from non-mucinous cysts with a sensitivity and specificity of 100% and 94%, respectively. The results in the current study were more compatible with an Egyptian study by Okasha and his colleagues. They showed that pancreatic CF positive mucin stain was 85% sensitive and 95% specific in detecting mucinous or non-mucinous pancreatic cysts with a 92% PPV, 91% NPV, and 91% accuracy. Also, positive mucin staining was 63% sensitive and 97% specific in differentiating malignant/potentially malignant from benign pancreatic cysts with a PPV of 96%, NPV of 72%, and overall accuracy of 80%. This outcome is in concordance with a recent study by Okasha and his colleagues that showed that a CF positive mucin stain has a sensitivity of 85.5% and specificity of 86.1% for detecting mucinous cystic neoplasm with a 72.3% PPV, 93.3% NPV, and 85.9% accuracy[4]. Many studies also reported that the mucin staining could be complementary to cyst CEA levels and cytology, and when one out of three was found to be positive, this increases the sensitivity to 92% and specificity to 52%, as in a study conducted by Morris-Stiff et al[22].
In our study, CF glucose was markedly consumed in malignant/potentially malignant cysts than in benign cysts (21.5 vs 68.5, P = 0.0001). Since glucose is a simple and cheap biomarker, it could be used as a marker for differentiation between benign and malignant pancreatic cysts with a relatively low cost[23-25].
In 2004, Raty et al[26] were the first to evaluate the role of CF SPINK1 in differentiating potentially malignant from benign cysts. They reported that the SPINK1 level was higher in malignant/potentially malignant than in benign cystic pancreatic lesions (1609 ± 418 vs 46 ± 21 ug/L; P = 0.0001). These findings matched our study that showed that SPINK1 level was higher in malignant/potentially malignant cysts than in benign ones (0.91 vs 0.47, P = 0.001) with a sensitivity and specificity of 70.59% and 65.33%, respectively (Table 8).
In our study, mural nodules, cyst diameter, lymph node enlargement, mucin stain, CF CEA, SPINK1, and glucose measurements in CF were highly predictive of malignancy in univariate analysis. In comparison, only mural nodules, mucin stain, and SPINK1 were highly predictive of malignancy in multivariate analysis.
Of all these markers measured in CF, CEA, glucose, and SPINK1 were independent predictors of malignancy, suggesting that these markers could help differentiate potentially malignant cysts from benign cysts.
The analysis of recent markers - not investigated in this study – such as CF DNA is recommended for future research because it might add more diagnostic value in differentiating benign from malignant cysts.
EUS examination of cyst morphology with cytopathological and chemical analysis and CF analysis could improve the differentiation between malignant and benign pancreatic cysts. Also, CEA, glucose, and SPINK1 are valuable markers for predicting a malignant pancreatic cyst.
Further studies addressing new markers are recommended, which will provide a panel of laboratory data to recognize the malignant and potentially malignant lesions to establish a standard protocol for diagnosis and management. Also, CF DNA is considered a potential diagnostic agent with particular possible use in differentiating between benign and malignant cysts. Further investigation regarding this biomarker is recommended.
Nowadays, the awareness of pancreatic cystic lesions has become an essential issue, especially with the increased incidence of asymptomatic pancreatic cysts in the general population. Therefore, the proper diagnosis, meticulous differentiation, and staging of these pancreatic cystic lesions (PCLs) are crucial for proper management and avoiding unnecessary treatment of benign lesions and missing early treatment of the malignant/pre-malignant lesions. Endoscopic ultrasound (EUS) examination of cyst morphology with cytopathological and chemical analysis and cyst fluid analysis could improve the diagnostic capability. Also, many developed markers are valuable for predicting a malignant pancreatic cyst.
EUS examination of cyst morphology with cytopathological and chemical analysis and cyst fluid analysis could improve the differentiation between malignant and benign pancreatic cysts. Also, carcinoembryonic antigen (CEA), glucose, and the serine protease inhibitor Kazal-type 1 (SPINK1) are valuable markers for predicting a malignant pancreatic cyst.
To evaluate the role of cyst fluid analysis of different tumor markers such as cancer antigens (e.g., CA19-9 and CA72-4), carcinoembryonic antigen (CEA), SPINK1, interleukin 1 beta (IL-1β), vascular endothelial growth factor A (VEGF-A), prostaglandin E2 (PGE2), amylase, and mucin stain in diagnosing pancreatic cysts and differentiating malignant from benign lesions.
This study included 76 patients diagnosed with PCLs using different imaging modalities. All patients underwent EUS and EUS-FNA for characterization and sampling of different PCLs.
The mean age of studied patients was 47.4 ± 11.4 years, with a slight female predominance (59.2%). Mucin stain showed high statistical significance in predicting malignancy with a sensitivity of 87.1% and specificity of 95.56%. It also showed a positive predictive value and negative predictive value of 93.1% and 91.49%, respectively (P < 0.001). We found that positive mucin stain, cyst fluid glucose, SPINK1, amylase, and CEA levels had high statistical significance (P < 0.0001). In contrast, IL-1β, CA 72-4, VEGF-A, VEGFR2, and PGE2 did not show any statistical significance. Univariate regression analysis for prediction of malignancy in PCLs showed a statistically significant positive correlation with mural nodules, lymph nodes, cyst diameter, mucin stain, and cyst fluid CEA. Meanwhile, logistic multivariable regression analysis proved that mural nodules, mucin stain, and SPINK1 were independent predictors of malignancy in PCLs.
EUS examination of cyst morphology with cytopathological analysis and cyst fluid analysis could improve the differentiation between malignant and benign pancreatic cysts. Also, CEA, glucose, and SPINK1 could be used as promising markers to predict malignant pancreatic cysts.
Further studies addressing new markers are recommended, which will provide a panel of laboratory data to recognize the malignant and potentially malignant lesions to establish a standard protocol for diagnosis and management. Also, cyst fluid DNA is considered a potential diagnostic agent with particular possible use in differentiating between benign and malignant cysts. Further investigation regarding this biomarker is recommended.
We would like to acknowledge our great Kasr Al Ainy Hospital, and its workers, nurses, and staff members, for all the support and help in this study and throughout our careers.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, general and internal
Country/Territory of origin: Egypt
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Jin ZD, China; Paiella S, Italy; Poddymova AV, Russia S-Editor: Ma YJ L-Editor: Wang TQ P-Editor: Ma YJ
| 1. | Basturk O, Coban I, Adsay NV. Pancreatic cysts: pathologic classification, differential diagnosis, and clinical implications. Arch Pathol Lab Med. 2009;133:423-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 158] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 2. | Jani N, Bani Hani M, Schulick RD, Hruban RH, Cunningham SC. Diagnosis and management of cystic lesions of the pancreas. Diagn Ther Endosc. 2011;2011:478913. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Laffan TA, Horton KM, Klein AP, Berlanstein B, Siegelman SS, Kawamoto S, Johnson PT, Fishman EK, Hruban RH. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol. 2008;191:802-807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 724] [Cited by in RCA: 658] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 4. | Okasha H, E Behiry M, Ramadan N, Ezzat R, Yamany A, El-Kholi S, Ahmed G. Endoscopic ultrasound-guided fine needle aspiration in diagnosis of cystic pancreatic lesions. Arab J Gastroenterol. 2019;20:86-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Boot C. A review of pancreatic cyst fluid analysis in the differential diagnosis of pancreatic cyst lesions. Ann Clin Biochem. 2014;51:151-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Koito K, Namieno T, Nagakawa T, Shyonai T, Hirokawa N, Morita K. Solitary cystic tumor of the pancreas: EUS-pathologic correlation. Gastrointest Endosc 1997; 45(3): 268-276. [RCA] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 136] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 7. | Sperti C, Pasquali C, Guolo P, Polverosi R, Liessi G, Pedrazzoli S. Serum tumor markers and cyst fluid analysis are useful for the diagnosis of pancreatic cystic tumors. Cancer. 1996;78:237-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 8. | Lee KS, Sekhar A, Rofsky NM, Pedrosa I. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am J Gastroenterol. 2010;105:2079-2084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 436] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 9. | Polkowski M, Larghi A, Weynand B, Boustière C, Giovannini M, Pujol B, Dumonceau JM; European Society of Gastrointestinal Endoscopy (ESGE). Learning, techniques, and complications of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline. Endoscopy. 2012;44:190-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 216] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 10. | Lennon AM, Canto MI. Pancreatic Cysts - Part 2: Should We Be Less Cyst Centric? Pancreas. 2017;46:745-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Matthaei H, Schulick RD, Hruban RH, Maitra A. Cystic precursors to invasive pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2011;8:141-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 144] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 12. | Machicado JD, Koay EJ, Krishna SG. Radiomics for the Diagnosis and Differentiation of Pancreatic Cystic Lesions. Diagnostics (Basel). 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Brugge WR. The use of EUS to diagnose cystic neoplasms of the pancreas. Gastrointest Endosc. 2009;69:S203-S209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Barresi L, Tarantino I, Granata A, Curcio G, Traina M. Pancreatic cystic lesions: How endoscopic ultrasound morphology and endoscopic ultrasound fine needle aspiration help unlock the diagnostic puzzle. World J Gastrointest Endosc. 2012;4:247-259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Thiruvengadam N, Park WG. Systematic Review of Pancreatic Cyst Fluid Biomarkers: The Path Forward. Clin Transl Gastroenterol. 2015;6:e88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 16. | Lee CJ, Scheiman J, Anderson MA, Hines OJ, Reber HA, Farrell J, Kochman ML, Foley PJ, Drebin J, Oh YS, Ginsberg G, Ahmad N, Merchant NB, Isbell J, Parikh AA, Stokes JB, Bauer T, Adams RB, Simeone DM. Risk of malignancy in resected cystic tumors of the pancreas < or =3 cm in size: is it safe to observe asymptomatic patients? J Gastrointest Surg. 2008;12:234-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 144] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 17. | Sarr MG, Carpenter HA, Prabhakar LP, Orchard TF, Hughes S, van Heerden JA, DiMagno EP. Clinical and pathologic correlation of 84 mucinous cystic neoplasms of the pancreas: can one reliably differentiate benign from malignant (or premalignant) neoplasms? Ann Surg. 2000;231:205-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 233] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 18. | Sahani DV, Saokar A, Hahn PF, Brugge WR, Fernandez-Del Castillo C. Pancreatic cysts 3 cm or smaller: how aggressive should treatment be? Radiology. 2006;238:912-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 118] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 19. | Okasha HH, Ashry M, Imam HM, Ezzat R, Naguib M, Farag AH, Gemeie EH, Khattab HM. Role of endoscopic ultrasound-guided fine needle aspiration and ultrasound-guided fine-needle aspiration in diagnosis of cystic pancreatic lesions. Endosc Ultrasound. 2015;4:132-136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Brugge WR, Lewandrowski K, Lee-Lewandrowski E, Centeno BA, Szydlo T, Regan S, del Castillo CF, Warshaw AL. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1016] [Cited by in RCA: 901] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 21. | Gaddam S, Ge PS, Keach JW, Mullady D, Fukami N, Edmundowicz SA, Azar RR, Shah RJ, Murad FM, Kushnir VM, Watson RR, Ghassemi KF, Sedarat A, Komanduri S, Jaiyeola DM, Brauer BC, Yen RD, Amateau SK, Hosford L, Hollander T, Donahue TR, Schulick RD, Edil BH, McCarter M, Gajdos C, Attwell A, Muthusamy VR, Early DS, Wani S. Suboptimal accuracy of carcinoembryonic antigen in differentiation of mucinous and nonmucinous pancreatic cysts: results of a large multicenter study. Gastrointest Endosc. 2015;82:1060-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 22. | Morris-Stiff G, Lentz G, Chalikonda S, Johnson M, Biscotti C, Stevens T, Matthew Walsh R. Pancreatic cyst aspiration analysis for cystic neoplasms: mucin or carcinoembryonic antigen--which is better? Surgery. 2010;148:638-44; discussion 644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Park WG, Wu M, Bowen R, Zheng M, Fitch WL, Pai RK, Wodziak D, Visser BC, Poultsides GA, Norton JA, Banerjee S, Chen AM, Friedland S, Scott BA, Pasricha PJ, Lowe AW, Peltz G. Metabolomic-derived novel cyst fluid biomarkers for pancreatic cysts: glucose and kynurenine. Gastrointest Endosc. 2013;78:295-302.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 24. | Zikos T, Pham K, Bowen R, Chen AM, Banerjee S, Friedland S, Dua MM, Norton JA, Poultsides GA, Visser BC, Park WG. Cyst Fluid Glucose is Rapidly Feasible and Accurate in Diagnosing Mucinous Pancreatic Cysts. Am J Gastroenterol. 2015;110:909-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 25. | Carr RA, Yip-Schneider MT, Simpson RE, Dolejs S, Schneider JG, Wu H, Ceppa EP, Park W, Schmidt CM. Pancreatic cyst fluid glucose: rapid, inexpensive, and accurate diagnosis of mucinous pancreatic cysts. Surgery. 2018;163:600-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 26. | Räty S, Sand J, Alfthan H, Haglund C, Nordback I. Cyst fluid tumor-associated trypsin inhibitor may be helpful in the differentiation of cystic pancreatic lesions. J Gastrointest Surg. 2004;8:569-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |