Published online May 16, 2022. doi: 10.4253/wjge.v14.i5.291
Peer-review started: July 2, 2021
First decision: January 10, 2022
Revised: January 22, 2022
Accepted: April 21, 2022
Article in press: April 21, 2022
Published online: May 16, 2022
Processing time: 317 Days and 18.1 Hours
The differential diagnosis between benign and malignant biliary strictures is challenging and requires a multidisciplinary approach with the use of serum biomarkers, imaging techniques, and several modalities of endoscopic or percutaneous tissue sampling. The diagnosis of biliary strictures consists of laboratory markers, and invasive and non-invasive imaging examinations such as computed tomography (CT), contrast-enhanced magnetic resonance cholangiopancreatography, and endoscopic ultrasonography (EUS). Nevertheless, invasive imaging modalities combined with tissue sampling are usually required to confirm the diagnosis of suspected malignant biliary strictures, while pathological diagnosis is mandatory to decide the optimal therapeutic strategy. Although EUS-guided fine-needle aspiration biopsy is currently the standard procedure for tissue sampling of solid pancreatic mass lesions, its diagnostic value in intraductal infiltrating type of cholangiocarcinoma remains limited. Moreover, the “endobiliary approach” using novel slim biopsy forceps, transpapillary and percutaneous cholangioscopy, and intraductal ultrasound-guided biopsy, is gaining ground on traditional endoscopic retrograde cholangiopancreatography and percutaneous transhepatic cholangiography endobiliary forceps biopsy. This review focuses on the available endobiliary techniques currently used to perform biliary strictures biopsy, comparing the diagnostic performance of endoscopic and percutaneous approaches.
Core Tip: Invasive imaging modalities combined with tissue sampling are almost always required to confirm the diagnosis of suspected malignant biliary strictures. The “endobiliary approach” using novel slim biopsy forceps, transpapillary and percutaneous cholangioscopy, and intraductal ultrasound-guided biopsy is gaining ground over traditional endoscopic retrograde cholangiopancreatography and percutaneous endobiliary forceps biopsy. Nevertheless, both endoscopic and percutaneous interventional radiology modalities are today considered safe and effective tissue sampling options, providing histologic identification of biliary strictures with satisfactory sensitivity and specificity rates.
- Citation: Inchingolo R, Acquafredda F, Posa A, Nunes TF, Spiliopoulos S, Panzera F, Praticò CA. Endobiliary biopsy. World J Gastrointest Endosc 2022; 14(5): 291-301
- URL: https://www.wjgnet.com/1948-5190/full/v14/i5/291.htm
- DOI: https://dx.doi.org/10.4253/wjge.v14.i5.291
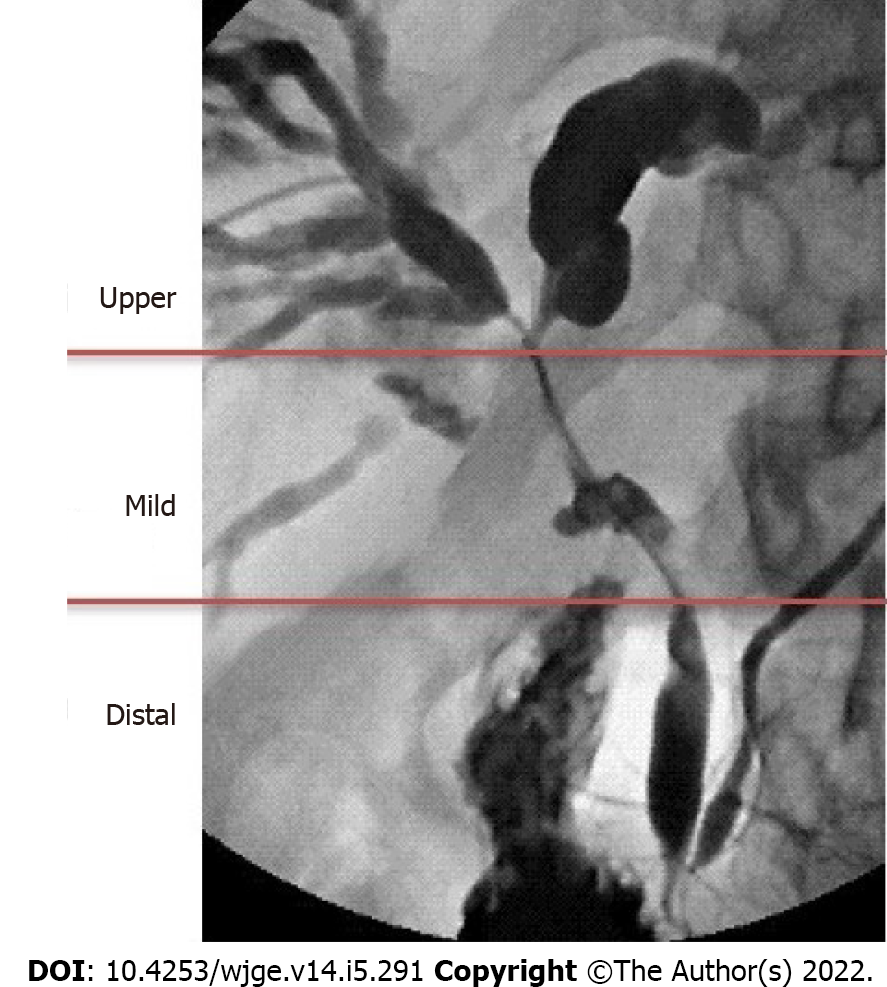
The diagnosis of biliary strictures remains a challenge, even in an era of considerable technologic advances regarding our current diagnostic tools. A biliary stricture is an area of stenosis in the intrahepatic or extrahepatic biliary tree (Figure 1). It can be the result of either malignant or benign pathologies, with a high prevalence of malignancy (two-third of cases)[1]. Malignant strictures of the biliary system (MBS) are commonly divided into distal strictures (involving the common bile duct) and proximal strictures (involving the hepatic hilum and right and left hepatic ducts). Pancreatic ductal adenocarcinoma is the most common cause of distal malignant stenosis, followed by cholangiocarcinoma, and, less commonly, ampullary or metastatic cancer. Proximal malignant strictures are due to cholangiocarcinoma, hepatocellular and gallbladder cancer or lymphoproliferative disorders, and metastatic lesions. The most common causes of a benign stricture include iatrogenic injury, chronic pancreatitis, primary sclerosing cholangitis, autoimmune diseases, and others. Biliary strictures are defined indeterminate when a clear diagnosis cannot be obtained after a non-invasive diagnostic work-up and an endoscopic retrograde cholangiopancreatography (ERCP) with biliary sampling. Their evaluation should be extremely careful given the noteworthy false-positive preoperative diagnosis of cancer, resulting in a 13%-24% resection rate of benign lesions[2].
Differentiating between the nature of strictures and diagnosing the relative aetiology often require a complex diagnostic approach. The evaluation of biliary strictures consists of laboratory markers and invasive and non-invasive imaging examinations including focused abdominal ultrasound (US), computed tomography (CT), contrast-enhanced magnetic resonance cholangiopancreatography, and endoscopic ultrasonography (EUS).
Nevertheless, invasive imaging modalities combined with tissue sampling are almost always required to support the diagnosis of a suspected MBS. If a histological diagnosis is obtained through the first procedure, further invasive diagnostic modalities can be avoided and appropriate treatment can be started. Both endoscopic retrograde cholangiography (ERC) and percutaneous transhepatic cholangiography endobiliary forceps biopsy (PTHC-EFB) have been valid procedures for a while for histological assessment of intrahepatic and/or extrahepatic biliary strictures.
EUS-guided fine-needle aspiration biopsy (FNAB) is nowadays the standard procedure for tissue sampling of solid pancreatic lesions because of its high diagnostic rate: In this setting, previous meta-analyses reported that the sensitivity rates of EUS-FNAB ranged from 85% to 89%[3]. However, EUS-FNAB has some limitations in cases of MBS other than pancreatic lesions, such as the frequent intraductal infiltrating type of cholangiocarcinoma. Furthermore, over the past 20 years, the technique of EUS-guided biliopancreatic lesion sampling has not gained widespread availability.
Currently, other endobiliary techniques for biliary tissue acquisition are increasing the possibility to obtain a definitive diagnosis: In fact, the “endobiliary approach” to suspect MBS is expanding past the more traditional ERCP and PTHC, through the use of novel slim biopsy forceps, to include trans-papillary and percutaneous cholangioscopy, and intraductal ultrasound-guided biopsy (IDUS-G biopsy).
ERCP is a diagnostic and therapeutic invasive imaging modality that provides an “indirect” radiological visualization of the biliopancreatic ductal system. ERCP with endobiliary brushing and/or forceps biopsy is often the first endoscopic approach for tissue sampling of biliary strictures because of its wide availability. According to several studies, the forceps biopsy sampling method has slightly better performance in comparison to brush cytology: A systematic review and a meta-analysis (9 studies; n = 730 patients) by Navaneethan et al[4] reported a pooled diagnostic odds ratio in detecting malignant biliary strictures of 43.18 (95% confidence interval [CI]), with a 48.1% pooled sensitivity and 99.2% pooled specificity, for intraductal biopsies, compared to a pooled diagnostic odds ratio of 33.43 (95%CI), with a 45% pooled sensitivity and 99% pooled specificity, for brushing. Combining the two sampling methods only modestly increased the sensitivity to 59.4%.
Theoretically, sufficient biliary tissue sampling provides adequate identification of the tissue’s specific features such as superficial intraductal spread and/or wall invasion, details that cannot be obtained by brush cytology. Despite a low-diagnostic sensitivity, brush cytology is still the first line ERCP sampling modality, because of its feasibility and safety. However, as trans-papillary forceps biopsy has got a higher sensitivity rate in comparison to brush cytology, it may play an important role in the pathological confirmation of MBS.
Several series reported malignancy detection rates with ERCP endobiliary forceps biopsy ranging from 33% to 71 % for pancreatic cancer and 44% to 89 % for cholangiocarcinoma[5]. A more recent review by Korc and Sherman[6] reported detection rates for pancreatic cancers and cholangiocarcinoma of 37% and 63%, respectively. The poor sensitivity of endobiliary forceps biopsy is likely due to the blind modality of sampling under fluoroscopic guidance. In addition, MBS that mainly infiltrate the wall of the duct or incite extrinsic compression are challenging to be targeted through the ERCP tissue sampling modality. ERCP with trans-papillary biopsies are performed using forceps designed for standard endoscopes[6] that should provide an adequate sample of bile duct tissue deep to the epithelium. The biopsy forceps are introduced into the bile duct after sphincterotomy of the papilla, even though some studies described the forceps insertion modality without previous sphincterotomy[7]. The forceps are pushed under fluoroscopic guidance to the level of the stricture to grasp specimens from the lower part of the stricture. The ideal number of specimens to perform has not been standardized, although several studies[5-8] suggest that at least three specimens should be obtained.
To optimize the unsatisfying sensitivity of trans-papillary forceps biopsy, in 2011 Wright et al[9] proposed a method of rapid on-site cytopathological evaluation (ROSE) through the cytologic preparation and analysis of forceps biopsy sampling made by an onsite cytopathologist (Smash protocol). In total, 133 patients were enrolled in the study. A “smash” specimen sensibility of 72% was reported.
Another work[10] valued the yield of ERCP biliary biopsy sampling subjected to ROSE and reported that sensitivity for cancer diagnosis increased to 76%-97%. This gain suggests that ROSE modality may improve the sensitivity of ERCP forceps biopsy sampling. However, this resource is available only to a few tertiary referral centres. Adverse events related to endobiliary forceps biopsy sampling are rare: To date, the same minor and only a few major cases of haemobilia[8] and perforation of the common hepatic duct[11] have been described.
To overcome the difficulty of common bile duct cannulation that is related to the thickness and the hardness of the standard biopsy forceps, some novel biopsy forceps have been developed. In 2017, Inoue et al[11] published a study about the diagnostic yield of controllable biopsy-forceps (C-BF) in MBS. C-BF (MTW Endoskopie, Wesel, Germany) allows the tip’s angle to be adjusted by up to 90°. In that study, 110 patients with biliary strictures were retrospectively evaluated. A high technical success rate (99%) of biliary biopsies sampled was reported.
That study reported different performances of the biopsies performed with C-BF depending on the target site: Adequate samples were respectively obtained in 96% (22/23) of specimens from the intrapancreatic common bile ducts, 92% (11/12) of those from the upper common bile ducts, 80% (12/15) from the carrefour of the hepatic ducts, 75% (9/12) from the right intrahepatic bile ducts, and 31% (5/16) from the left intrahepatic bile ducts.
Moreover, the diagnostic sensitivity for biliary strictures reported was just 60%, which is similar to those reported from studies carried out on conventional forceps biopsy. The benefits of using C-BF may be limited because of its lack of rotation torque ability; thus, only a curvature to the patient’s right-hand side can be performed: This feature leads to an adequate sampling of lesions located to the right intrahepatic bile duct (75%), in contrast to a poor success rate in procedures that involved selecting the left intrahepatic bile duct (31%).
Another novel slim biopsy forceps, with a soft and thinner shaft of 1.8 mm (Radial Jaw 4P, Boston Scientific, Boston, MA, United States), has been developed to enable the jaws to pivot onto the targeted biopsy site for better tissue grasping. To evaluate the feasibility and efficacy of this novel biopsy device in the diagnosis of MBS, in 2017, Yamamoto et al[12] tested it on a cohort of 360 patients who underwent ERCP for biliary strictures. That study showed a higher sensitivity than previous studies of trans-papillary bile duct biopsies: In fact, the overall sensitivity and accuracy were 69.6% and 78.8%, respectively. The sensitivity was 75.6% in cholangiocarcinoma, 64% in pancreatic cancer, and 57.1% in metastasis. In cholangiocarcinoma, a lower sensitivity was observed for perihilar lesions (68.7%) rather than for distal stricture (83.1%). A better sensitivity has been reported for longer stenosis of pancreatic cancer and metastasis. These results suggest that trans-papillary forceps biopsy should be performed in consideration of the stricture level, stricture length, and cancer type. Actually, a lower sensitivity was observed for the perihilar MBS rather than for the distal one. This may be due to the features of the strictures: Narrow, smooth, and angled lesions could lower the biopsy forceps ability to hit the targeted area. Moreover, the distance of the MBS from the papilla could reduce the possibility of precisely grasping the lesion. In contrast, a better sensitivity was observed for the distal MBS. Regarding the lower bile duct, a better sensitivity was observed for the strictures in which an adequate space to open enough the biopsy forceps jaws was present.
In 2017, Kwon et al[13] reported a single experience of MBS sampling with the use of a custom-made prototype guide-wire assisted endobiliary forceps biopsy: Targeted sampling from the central area of the mass was easy and successful.
Peroral cholangioscopy (POCS) modalities provide direct visualization of the biliary ductal system. Those procedures are important diagnostic tools in cases of suspect MBS in which other available invasive/non-invasive imaging modalities (e.g., EUS, CT, MRI, and ERCP with transpapillary biopsy sampling) cannot provide a definitive diagnosis. Three different cholangioscopic techniques are currently available: The “mother-baby” dual-operator cholangioscopy (DOC), the “mother-baby” single-operator cholangioscopy (SOC), and the direct cholangioscopy[14]. DOC is necessarily performed by two endoscopists with the use of a very slim endoscope passed through the working channel of a duodenoscope up to cannulating the common bile duct, usually over a guide-wire. POCS with optical image manipulation using narrow-band imaging (NBI) allows emphasizing the imaging of certain features of the bile duct tissue, such as mucosal structures and capillary vessels (e.g., irregular and tortuous vessels, papillogranular or nodular elevated surface), enabling to target biopsy onto the suspect lesion.
A prospective multicentre study on indeterminate bile duct lesions and preoperative mucosal cancerous extension diagnosis by DOC plus NBI was conducted by Osanai et al[15] in 2013. This work was conducted on a cohort of 87 patients of whom only 35 underwent endobiliary forceps biopsy sampling via DOC for indeterminate lesions. In 34/35 patients, NBI was useful in differentiating benign from malignant lesions. Collected data showed an accuracy rate of 85.7 % for indeterminate biliary lesion diagnosis using endobiliary forceps biopsy via DOC. That study also reported additional accuracy for detection of mucosal cancerous extension in the bile duct with POCS: In fact, the accuracy rate of ERCP alone in verifying the presence or absence of mucosal cancerous extension was 73.5%, in comparison to an accuracy rate of 92.9% for ERCP with POCS plus biopsy. However, as the authors acknowledged, that prospective study had the same bias concerning the non-randomized selection of patients and the fact that most of the targeted patients had already a bile duct cancer diagnosis: Those aspects could explain the high rate of accurate diagnosis of the study. A video endoscope and a disposable access catheter using fiberoptics (SpyGlass system; Boston Scientific, MA, United States) enable the SOC modality[16].
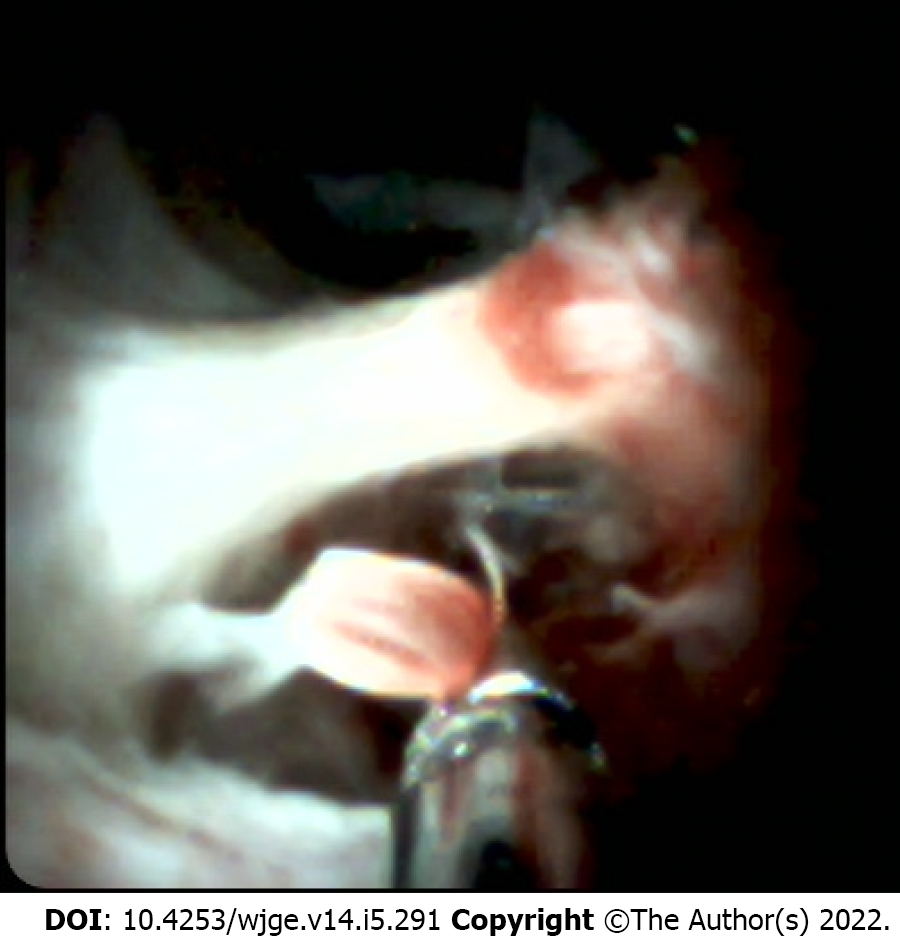
Since the launch of the first-generation SpyGlass system, in 2007, several studies have reported increasing sensitivity and accuracy with the addition of its direct endoscopic visualization of the bile duct to ERCP or tissue sampling [17-19]. However, the mean sensitivity of biliary sampling, using the dedicated biopsy forceps (SpyByte), for discriminating between malignant and benign biliary lesions was only slightly superior (68%) to that of the other conventional sampling modalities (Figure 2).
The initial version of SpyGlass was fiberoptic and the optical probe was reusable. Since 2015, a new digital single-operator/single-use instrument (SpyGlass DS; Boston Scientific, MA, United States) has been available. This 2nd generation system does not require to be reprocessed to avoid the issue of potential image degradation with repeated use. In 2016, a prospective multicenter study in Japan enrolled 148 patients with a collection of pancreaticobiliary diseases (124 with biliary disease). This work reported a SpyGlass targeted biopsy sensitivity of 81.4% and an accuracy of histologic diagnosis in indeterminate biliary strictures of 70.7%[20].
Direct cholangioscopy employing is questionable because of the same safety issue related to the occurrence of rare but life-threatening adverse events such as stroke caused by leakage of air into the portal or hepatic venous system[21], biliary perforation, and slightly higher incidence of postprocedural cholangitis[22].
IDUS involves the insertion into the bile duct of a high-frequency ultrasound ultrathin probe, generally over a wire. It provides high-resolution images of the ductal wall and periductal tissues[23]. Potentially, IDUS could be an important diagnostic tool in the evaluation of the indeterminate biliary strictures in whom is not possible to obtain a diagnosis despite previous evaluations. ERCP with IDUS examination, if performed by an expert endoscopist trained in both EUS and ERCP, helps to identify patients with a high suspicious of MBS[1] better than EUS does, particularly for lesions located at the hilum or mid-bile duct[23,24]. Several studies[25-28] reported high diagnostic sensitivity and specificity of IDUS during ERCP in differentiating malignant from benign strictures. Since IDUS provides real-time, high-resolution images of the bile duct wall and the adjacent structures[27], it is an ideal tool to use before biliary stenting. Unfortunately, this modality is not widely used because of the lack of ERCP operators who are also skilled in EUS. IDUS is also limited by the lack of a specific sampling modality.
Consequently, based on those aspects, two studies have investigated the performance of IDUS-guided biopsy sampling[29,30]. In these two works the ultrasonic probe is inserted into the bile duct over the wire after endoscopic sphincterotomy until IDUS recognize the suspected MBS. While maintaining the ultrasonic probe on the narrowest position to the stricture, a conventional biopsy forceps is inserted into the orifice of the papilla to the tip of the placed ultrasonic probe under fluoroscopic guidance. During the trans-papillary biopsy forceps sampling the scanning ultrasonic probe is keep at the nearest intraductal position.
Jong et al[29] reported a higher sensitivity for cancer diagnosis of indeterminate biliary strictures (87% with IDUS-guided biopsy in comparison to 67% with fluoroscopically trans-papillary guided biopsy).
Similarly, Kim et al[30] designed a prospective randomized study on the accuracy of IDUS-guided trans-papillary biopsy and conventional biopsy on fluoroscopy in suspected MBS and 65 out of 72 patients enrolled in the study underwent ERCP with IDUS.
The accuracy of IDUS-guided trans-papillary biopsy for MBS is significantly higher than conventional trans-papillary biopsy (90.8% vs 76.9%) in cases with intraductal infiltrating lesions, which were the most common findings on IDUS (47.5%). There was no significant difference in cancer detection rate according to the location of the stricture, as well as any significant improvement of cancer detection rates was reported in cases with extrinsic compressed lesions. This study reported no significant procedure-related adverse events (only two mild cases of hemobilia after trans-papillary forceps biopsy).
However, to date, there are no dedicated accessories that combine IDUS and forceps biopsy, thus IDUS-guided trans-papillary forceps biopsy is more challenging than conventional sampling modalities for the risks of bile-duct trauma. New types of IDUS probes or accessories for IDUS-guided trans-papillary forceps biopsy, as well as larger studies for validation, are expected.
In cases in which the endoscopic approach to biliary strictures has failed or is deemed difficult or impossible due to unfavourable anatomy (e.g., in cases of surgical interventions as hepatico-jejunostomy), their cyto-histological assessment can be performed with percutaneous transhepatic endobiliary brushing and/or forceps biopsy (PTEFB)[31].
Percutaneous transhepatic endobiliary sampling of biliary strictures/obstructions is usually performed after local anaesthesia and during conscious sedation, under fluoroscopic guidance, through a biliary drainage access, before drainage positioning, both from the right or left liver lobe based on stricture/obstruction location, even though right intercostal approach is preferred for positional advantage and operator easiness. Periprocedural broad-spectrum antibiotic coverage is recommended. In cases of occurrence of hemobilia or cholangitis after percutaneous transhepatic biliary access, the sampling should be delayed 24-48 h[32,33]. Cholangiography-guided detection of the stenosis/ obstruction is obtained and, after passing through the stricture with a guide-wire and positioning a 6-8F introducer sheath in the biliary ducts, the sampling procedure can be performed.
In cases of brushing, a flexible probe with a brush on an atraumatic tip is introduced through the sheath up to the stricture and then is pushed and pulled and rotated under fluoroscopic guidance multiple times[34].
In case of PTEFB, a careful and accurate forceps biopsy is performed advancing the forceps through the introducer sheath. Patel et al[35] described a variant of this technique, the so-called “cross and push”, in which the introducer sheath is advanced on a guidewire into the stricture/obstruction and is used to push the biopsy forceps granting greater stability of the forceps and allowing to obtain a larger lesion sample. Multiple samples should be taken, if possible, to obtain greater true-positive rates[36]. A bile sample after the brushing/biopsy (as much as 10 milliliters) should be always taken for bile cytology, as it demonstrated to have up to a 34% of sensitivity, which increases to 52% in case of multiple and seriate samplings[37,38]. In the case of forceps biopsy, a transhepatic cholangiography should be always performed to evaluate contrast medium leak from the bioptic site.
Cyto-histologic diagnosis of the sample obtained with the biopsy must always be confirmed after the surgical excision or, in case of benign disease diagnosis or non-specific findings, after dimensional stability of the lesion at a close follow-up. Redo-sampling should be performed in cases of a negative histological result, particularly in patients with high suspicion of malignancy, and in cases in which the operator deemed the first histological specimen inadequate for evaluation, as the fibrotic and scirrhous tissue which associates to cholangiocarcinoma and pancreatic carcinoma, in addition to necrotic and inflammatory changes, can hinder a correct diagnosis, even though Rabinovitz et al436] reported that biopsies repeated three or more times yielding only negative results should reduce the probability of malignancy to 0%; it is mandatory, however, to perform a strict imaging and laboratory follow-up in these patients.
Percutaneous transhepatic endobiliary brushing demonstrates sensitivity rates ranging from 26 to 67%, and low negative predictive values (around 12.5%). Noticeably, Xing et al[39] reported a superior sensitivity value of 75% with greater sensitivity in cases of cholangiocarcinoma vs other strictures (P < 0.05) while stricture location had no effect on brushing sensitivity[32,34,40-43].
Overall percutaneous biliary forceps biopsy sensitivity has been attested between 55.8 and 93.3%, with a higher sensitivity for cholangiocarcinoma (up to 94%)[33,35,40,41,44-47]. Augustin et al[44] performed PTEFB in 13 patients, with at least 3 samples of 1-2 mm per patient, and in 92.3% of cases the material was deemed sufficient for histological analysis; PTEFB had sensitivity and accuracy rates of 88.9% and 92.3% respectively.
Jung et al[33] performed 130 PTEFB obtaining a 78.4% sensitivity rate. Park et al[48] retrospectively reviewed 271 PTEFB, finding 77.2% of sensitivity and 78.9% of accuracy. Patel et al[35] with their abovementioned “cross and push” technique performed in 52 patients obtained a sensitivity of 93.3%. Inchingolo et al[47] prospectively performed 30 PTEFB in 29 patients, with the “cross and push” technique, obtaining a sensitivity rate of 91.67% and an accuracy rate of 92.59%. Boos et al[40] described better sensitivity rates when forceps biopsy and brush cytology were combined in a tandem approach (55.8% vs 40.6% of forceps biopsy alone); while this procedure can be considered expensive when compared to the use of forceps biopsy alone, it is cost-effective when compared to performing two separate procedures in case of an initial negative histological sample; however randomized studies comparing the sensitivity of the two approaches (single and tandem) should be performed. The tandem approach must be distinguished from obtaining a smear from forceps biopsy for cytological analysis[41].
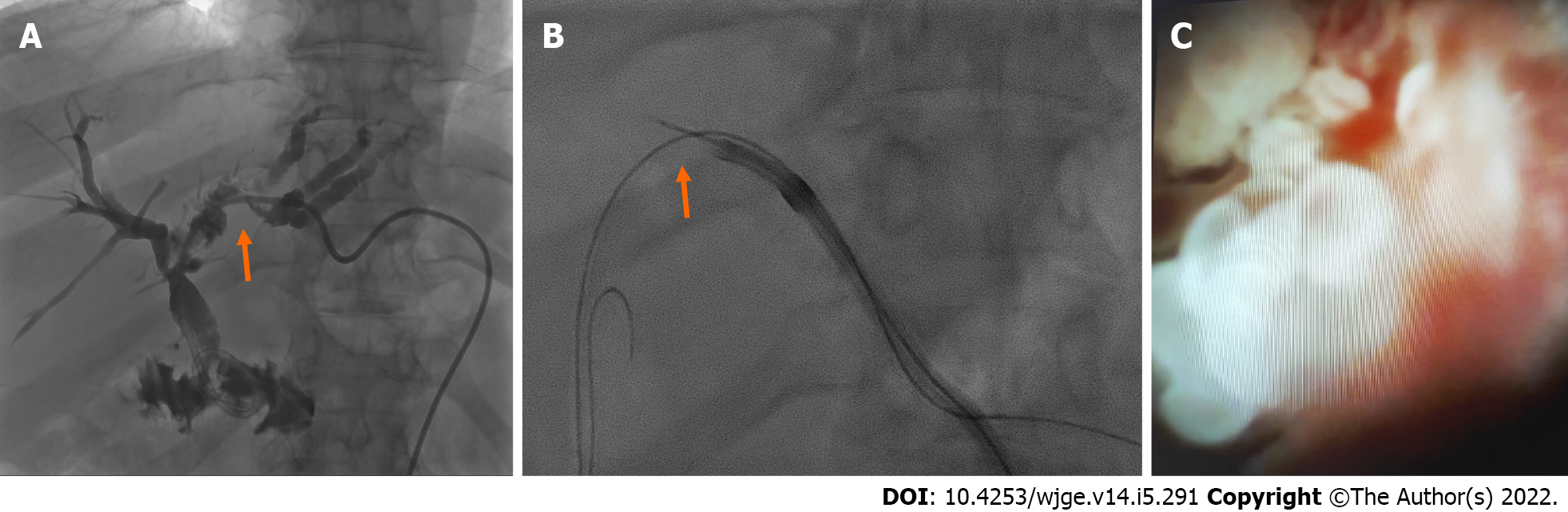
PTEFB can be also performed under cholangioscopic/choledochoscopic guidance, which gives the operator the ability to directly visualize and target the pathologic tissue (Figure 3). After adequate sequential dilation of the transhepatic tract (with an introducer sheath of up to 11-16 F vs 7-8 F of fluoroscopy-guided PTEFB) a scope is positioned over a stiff guidewire and the forceps are inserted through its working channel. This approach has sensitivity and specificity exceeding 95% for diagnosing biliary malignancies despite its greater costs when compared to fluoroscopy-guided PTEFB and the need for specialized equipment and expertise[32,42,49,50]. Due to the diameter of the cholangioscope and the risk of hemobilia after first puncture of the biliary ducts, percutaneous tract “maturation” for one week or more after placement of a 8-10 French biliary drainage is recommended to avoid hemorrhage and prevent peritonitis due to extra-hepatic bile leak, as well as progressive oversizing of the biliary tube reduces the subsequent trauma from cholangioscope insertion[51]. Flexible endoscopes are preferred over the rigid ones due to their smaller diameter, better control and wider view; in addition, long endoscopes should be preferred, particularly in case of lesions in the distal common bile duct or in the contralateral ducts. Complication of transhepatic cholangioscopy include cholangitis, hemobilia, biloma or abscess formation, but in half of cases are related to the initial access and tract dilation, and can be avoidable with tract maturation[52].
Among percutaneous transhepatic biopsy approaches, Schechter et al[55] reported the use of the Simpson atherectomy catheter, with a sensitivity of 79% but 11% of hemorrhages, high costs, and difficulties in passing through angled transhepatic tracts.
On the other hand, Rossi et al[34] described the diagnostic yield of sampling the balloon surface in patients with strictures which needed bilioplasty, reporting a sensitivity of 87.5%.
Various authors reported great diagnostic sensitivity of PTEFB in strictures of the upper biliary tree (up to 92%), whereas Ierardi et al[56] reported lower sensitivity for lesions of the hilum and common bile duct as compared to the common hepatic bile duct and ampulla[33,35,42,54,55]. Overall, the PTEFB procedure does not have severe technical difficulties, therefore the learning curve is reported to be steep, with only a few cases needed to master the technique[47].
In terms of safety, PTEFB yielded low rates of complications, the most common being transient hemobilia, postprocedural cholangitis, transient bile leakage, and less often, the formation of biloma in the bioptic site, which were promptly treated with percutaneous drainage[33,35,44,45,47].
Other complications were related to the percutaneous puncture and not to the sampling procedure itself, ranging from subcapsular biloma to hepatic hematoma to pseudoaneurysm formation[35,56].
The main limitation of PTEFB is linked to the diagnosis of extra-biliary neoplasms determining biliary obstruction and which have not infiltrated yet the biliary duct walls (e.g., hepatic hilum lymph-nodal metastasis, tumor infiltration/compression), due to the limited tissue samples, determining false-negative results both during surgical inspection or at follow-up[57]. Among metastatic tumor-related extrinsic biliary compression, the prospective analysis from Estrella et al[58] demonstrated that metastases from colorectal cancer more commonly present with intrabiliary growth when compared to other tumors (10.6 vs 1.9%). Another limitation is represented by the intrinsic characteristics of the forceps, which can cause “crush” artifacts of the bioptic specimen, represented by the degradation of the specimen during the bioptic maneuver, that can hinder the diagnosis[35].
The diagnostic approach (Table 1) and correct histologic identification of a biliary stricture can be a demanding issue, while first-line non-invasive diagnostic methods alone cannot confirm the diagnosis of MBS in most of the cases. Moreover, pathological diagnosis is mandatory for the decision on the therapeutic approach. Therefore, it is crucial to establish the optimal sampling modality to confirm the diagnosis. According to current literature, both PTC and ERCP forceps biopsy are sensitive and accurate sampling modalities for suspected MBS.
| Endoscopic techniques | ||
| Advantage | Disadvantage | |
| ERC + TPB | Safeness, feasibility and large availability; better sensibility for MBS versus brushing | Low sensitivity for MBS (48%), difficulty of cannulation with standard biopsy forceps, not easy targeting of the lesion |
| ERC + TPB with C-BF | Slight better sensibility (60%) for MBS respect to conventional biopsy forceps | Sampling benefits limited to lesions located to the right intrahepatic bile duct (75%) |
| Cholangioscopy + endobiliary biopsy | Gain in accuracy for diagnosis of malignancy in indeterminate lesions (85-92%) versus ERCP + TPB | Same safety; issue with direct cholangioscopy related to rare adv events (leakege of air in to portal vein) |
| IDUS + TPB | Higher sensitivity for malignancy in indeterminate intraductal lesiones (87-91%) versus ERCP + TPB | Advanced experience in both ERCP/EUS requested, lack of standardized procedure and specific devices, time-consuming technique |
| Interventional radiology techniques | ||
| Advantage | Disadvantage | |
| PTE endobiliary brushing | Safe, cheap and large availability; | Low sensitivity for MBS |
| PTE endobiliary biopsy | High sensitivity; Larger biopsy cup comapred to ERC + TPB | Indirect visualization of the lesion |
| Colangioscopy + PTEFB | Direct visualization of the lesion; | Combined procedure with endoscopist; Expensive procedure; small size specimen |
Chang et al[45] retrospectively compared a group of 38 patients undergoing PTEFB and brushing with a group of patients undergoing endoscopic trans-papillary biopsy; PTEFB had a sensitivity of 86.7% compared to the 77.1% of endoscopic biopsy, especially for biliary strictures located at the hilum. Mohkam et al[46] retrospectively compared 75 PTEFB with patients who underwent endoscopic trans-papillary biopsy and PTEFB demonstrated sensitivity rate of 69%, similar to endoscopic biopsy (75%, P = 0.45). The choice of biliary strictures that more suitable for endoscopic rather than a percutaneous biopsy seems to mainly depend on the anatomical location and type of stricture.
Several studies[45,54] demonstrated that PTEFB is correlated with high diagnostic sensitivity for strictures located in the upper biliary tree, distant from the papilla – where endoscopic biopsy has better sensitivity. Particularly, Chang et al[45], reported higher sensitivity for PTEFB in hilum lesions than those located within the common bile duct. According to the authors, sensitivity was higher for strictures located close to the hilum. On the contrary, compared to PTC, ERCP resulted in higher accuracy for lower strictures. In this setting, the distance between the site of biliary stricture and the device used to push and maneuver the biopsy forceps seems to play a key role: the greater the distance, the lesser the precision of sampling. Therefore, specimen sampling of the biliary strictures located proximal to the hilum should ideally be performed via PTEFB, while for strictures located at the hilum or more distally, ERCP should be preferred. Other factors influencing the effectiveness of endobiliary biopsy are insufficient space for forceps opening noted in cases of severe strictures, lesions located at sites with marked angulation, lesion shape, and of course local expertise, and device availability.
Both ERCP and PTC endobiliary biopsy remain valid methods for tissue identification demonstrating satisfactory diagnostic accuracy, especially in properly selected lesions. Novel slim biopsy forceps and new endobiliary sampling modalities such as POCS, and IDUS-guided biopsy, currently under investigation, seem to improve the efficacy of histologic characterization.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Costache RS, Romania; Mohamed SY, Egypt; Okasha H, Egypt S-Editor: Ma YJ L-Editor: Wang TQ P-Editor: Ma YJ
| 1. | Singh A, Gelrud A, Agarwal B. Biliary strictures: diagnostic considerations and approach. Gastroenterol Rep (Oxf). 2015;3:22-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 131] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 2. | Gerhards MF, Vos P, van Gulik TM, Rauws EA, Bosma A, Gouma DJ. Incidence of benign lesions in patients resected for suspicious hilar obstruction. Br J Surg. 2001;88:48-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 132] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 3. | Hewitt MJ, McPhail MJ, Possamai L, Dhar A, Vlavianos P, Monahan KJ. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: a meta-analysis. Gastrointest Endosc. 2012;75:319-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 509] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 4. | Navaneethan U, Njei B, Lourdusamy V, Konjeti R, Vargo JJ, Parsi MA. Comparative effectiveness of biliary brush cytology and intraductal biopsy for detection of malignant biliary strictures: a systematic review and meta-analysis. Gastrointest Endosc. 2015;81:168-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 340] [Article Influence: 34.0] [Reference Citation Analysis (1)] |
| 5. | Kimura H, Matsubayashi H, Sasaki K, Ito H, Hirosawa K, Uesaka K, Kanemoto H, Ono H. Factors affecting the yield of endoscopic transpapillary bile duct biopsy for the diagnosis of pancreatic head cancer. Pancreatology. 2013;13:524-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Korc P, Sherman S. ERCP tissue sampling. Gastrointest Endosc. 2016;84:557-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 7. | Sugiyama M, Atomi Y, Wada N, Kuroda A, Muto T. Endoscopic transpapillary bile duct biopsy without sphincterotomy for diagnosing biliary strictures: a prospective comparative study with bile and brush cytology. Am J Gastroenterol. 1996;91:465-467. [PubMed] |
| 8. | Schoefl R, Haefner M, Wrba F, Pfeffel F, Stain C, Poetzi R, Gangl A. Forceps biopsy and brush cytology during endoscopic retrograde cholangiopancreatography for the diagnosis of biliary stenoses. Scand J Gastroenterol. 1997;32:363-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 129] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Wright ER, Bakis G, Srinivasan R, Raju R, Vittal H, Sanders MK, Bernadino K, Stefan A, Blaszyk H, Howell DA. Intraprocedural tissue diagnosis during ERCP employing a new cytology preparation of forceps biopsy (Smash protocol). Am J Gastroenterol. 2011;106: 294-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Adhya AK, Mohanty R. Utility of touch imprint cytology in the preoperative diagnosis of malignancy in low resource setting. Diagn Cytopathol. 2017 Jun;45(6):507-512. [PMID: 28267274 DOI: 10.1002/dc.23699. |
| 11. | Inoue T, Kitano R, Kobayashi Y, Ishii N, Sakamoto K, Ohashi T, Nakade Y, Sumida Y, Ito K, Nakao H, Yoneda M. Assessing the diagnostic yield of controllable biopsy-forceps for biliary strictures. Scand J Gastroenterol. 2018;53:598-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Yamamoto K, Tsuchiya T, Itoi T, Tsuji S, Tanaka R, Tonozuka R, Honjo M, Mukai S, Kamada K, Fujita M, Asai Y, Matsunami Y, Nagakawa Y, Yamaguchi H, Sofuni A. Evaluation of novel slim biopsy forceps for diagnosis of biliary strictures: Single-institutional study of consecutive 360 cases (with video). World J Gastroenterol. 2017;23:6429-6436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Kwon CI, Kim TH, Kim KA. Guide-Wire Assisted Endobiliary Forceps Biopsy Sampling. Clin Endosc. 2017;50:404-405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Tringali A, Lemmers A, Meves V, Terheggen G, Pohl J, Manfredi G, Häfner M, Costamagna G, Devière J, Neuhaus H, Caillol F, Giovannini M, Hassan C, Dumonceau JM. Intraductal biliopancreatic imaging: European Society of Gastrointestinal Endoscopy (ESGE) technology review. Endoscopy. 2015;47:739-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 15. | Osanai M, Itoi T, Igarashi Y, Tanaka K, Kida M, Maguchi H, Yasuda K, Okano N, Imaizumi H, Itokawa F. Peroral video cholangioscopy to evaluate indeterminate bile duct lesions and preoperative mucosal cancerous extension: a prospective multicenter study. Endoscopy. 2013;45:635-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 16. | Igarashi Y, Ukita T, Inoue H, Ishiguro J, Ogawa S, Satou M, Maetani I, Sakai Y. Clinical evaluation of the peroral cholangioscopy using a new videoscope. Diagn Ther Endosc. 1999;5:231-237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Lenze F, Bokemeyer A, Gross D, Nowacki T, Bettenworth D, Ullerich H. Safety, diagnostic accuracy and therapeutic efficacy of digital single-operator cholangioscopy. United European Gastroenterol J. 6 (6):902-909.. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 18. | Ogura T, Imanishi M, Kurisu Y, Onda S, Sano T, Takagi W, Okuda A, Miyano A, Amano M, Nishioka N, Yamada T, Masuda D, Takenaka M, Kitano M, Higuchi K. Prospective evaluation of digital single-operator cholangioscope for diagnostic and therapeutic procedures (with videos). Dig Endosc. 29 (7):782-789.. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 19. | Karagyozov P, Boeva I, Tishkov I. Role of digital single-operator cholangioscopy in the diagnosis and treatment of biliary disorders. World J Gastrointest Endosc. 11(1):31-40.. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | Kurihara T, Yasuda I, Isayama H, Tsuyuguchi T, Yamaguchi T, Kawabe K, Okabe Y, Hanada K, Hayashi T, Ohtsuka T, Oana S, Kawakami H, Igarashi Y, Matsumoto K, Tamada K, Ryozawa S, Kawashima H, Okamoto Y, Maetani I, Inoue H, Itoi T. Diagnostic and therapeutic single-operator cholangiopancreatoscopy in biliopancreatic diseases: Prospective multicenter study in Japan. World J Gastroenterol. 22:1891-1901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 90] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 21. | Finsterer J, Stöllberger C, Bastovansky A. Cardiac and cerebral air embolism from endoscopic retrograde cholangio-pancreatography. Eur J Gastroenterol Hepatol. 2010;22:1157-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Ogura T, Takagi W, Kurisu Y, Higuchi K. Technical tips for peroral transluminal cholangioscopy using novel single-operator cholangioscope (with videos). J Hepatobiliary Pancreat Sci. 23:E25-E29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Tamada K, Tomiyama T, Wada S, Ohashi A, Satoh Y, Ido K, Sugano K. Endoscopic transpapillary bile duct biopsy with the combination of intraductal ultrasonography in the diagnosis of biliary strictures. Gut. 50:326-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Frossard JL, Dumonceau JM. The Role of EUS in the Biliary System. In: Shami VM, Kahaleh M (eds) Endoscopic Ultrasound. Clinical Gastroenterology. 2010;Humana Press, Totowa, NJ. |
| 25. | Heinzow HS, Kammerer S, Rammes C, Wessling J, Domagk D, Meister T. Comparative analysis of ERCP, IDUS, EUS and CT in predicting malignant bile duct strictures. World J Gastroenterol. 2014;20:10495-10503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 49] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 26. | Domagk D, Wessling J, Reimer P, Hertel L, Poremba C, Senninger N, Heinecke A, Domschke W, Menzel J. Endoscopic retrograde cholangiopancreatography, intraductal ultrasonography, and magnetic resonance cholangiopancreatography in bile duct strictures: a prospective comparison of imaging diagnostics with histopathological correlation. Am J Gastroenterol. 2004;99:1684-1689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 84] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. |
Domagk D, Poremba C, Dietl KH, Senninger N, Heinecke A, Domschke W, Menzel J.
Endoscopic transpapillary biopsies and intraductal ultrasonography in the diagnostics of bile duct strictures: a prospective study |
| 28. | Menzel J, Poremba C, Dietl KH, Domschke W. Preoperative diagnosis of bile duct strictures--comparison of intraductal ultrasonography with conventional endosonography. Scand J Gastroenterol. 2000;35:77-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 99] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 29. | Jong HM. The usefulness of IDUS-guided trans-papillary bile duct biopsy for the diagnosis of malignant biliary strictures. Endoscopy. 2011;43-A53. [DOI] [Full Text] |
| 30. | Kim HS, Moon JH, Lee YN, Choi HJ, Lee HW, Kim HK, Lee TH, Choi MH, Cha SW, Cho YD, Park SH. Prospective Comparison of Intraductal Ultrasonography-Guided Transpapillary Biopsy and Conventional Biopsy on Fluoroscopy in Suspected Malignant Biliary Strictures. Gut Liver. 2018;12:463-470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Elyaderani MK, Gabriele OF. Brush and forceps biopsy of biliary ducts via percutaneous transhepatic catheterization. Radiology. 1980;135:777-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Savader SJ, Prescott CA, Lund GB, Osterman FA. Intraductal biliary biopsy: comparison of three techniques. J Vasc Interv Radiol. 1996;7:743-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 33. | Jung GS, Huh JD, Lee SU, Han BH, Chang HK, Cho YD. Bile duct: analysis of percutaneous transluminal forceps biopsy in 130 patients suspected of having malignant biliary obstruction. Radiology. 2002;224:725-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 34. | Rossi M, Cantisani V, Salvatori FM, Rebonato A, Greco L, Giglio L, Guido G, Pagliara E, David V. Histologic assessment of biliary obstruction with different percutaneous endoluminal techniques. BMC Med Imaging. 2004;4:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 35. | Patel P, Rangarajan B, Mangat K. Improved Accuracy of Percutaneous Biopsy Using "Cross and Push" Technique for Patients Suspected with Malignant Biliary Strictures. Cardiovasc Intervent Radiol. 2015;38:1005-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 36. | Rabinovitz M, Zajko AB, Hassanein T, Shetty B, Bron KM, Schade RR, Gavaler JS, Block G, Van Thiel DH, Dekker A. Diagnostic value of brush cytology in the diagnosis of bile duct carcinoma: a study in 65 patients with bile duct strictures. Hepatology. 1990;12:747-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 125] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 37. | Muro A, Mueller PR, Ferrucci JT Jr, Taft PD. Bile cytology. A routine addition to percutaneous biliary drainage. Radiology. 1983;149:846-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 38. | Tsuchiya T, Yokoyama Y, Ebata T, Igami T, Sugawara G, Kato K, Shimoyama Y, Nagino M. Randomized controlled trial on timing and number of sampling for bile aspiration cytology. J Hepatobiliary Pancreat Sci. 2014;21:433-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 39. | Xing GS, Geng JC, Han XW, Dai JH, Wu CY. Endobiliary brush cytology during percutaneous transhepatic cholangiodrainage in patients with obstructive jaundice. Hepatobiliary Pancreat Dis Int. 2005;4:98-103. [PubMed] |
| 40. | Boos J, Yoo RJ, Steinkeler J, Ayata G, Ahmed M, Sarwar A, Weinstein J, Faintuch S, Brook OR. Fluoroscopic percutaneous brush cytology, forceps biopsy and both in tandem for diagnosis of malignant biliary obstruction. Eur Radiol. 2018;28:522-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 41. | Tapping CR, Byass OR, Cast JE. Cytological sampling versus forceps biopsy during percutaneous transhepatic biliary drainage and analysis of factors predicting success. Cardiovasc Intervent Radiol. 2012;35:883-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 42. | Rossi M, Lemos A, Bonaiuti P, Amoruso M, Petrone A, Petrozza V, Benvenuto A, Rossi P. [Instrumental diagnosis of obstructive jaundice: brushing versus biopsy]. Radiol Med. 1997;93:230-235. [PubMed] |
| 43. | Mendez G Jr, Russell E, Levi JU, Koolpe H, Cohen M. Percutaneous brush biopsy and internal drainage of biliary tree through endoprosthesis. AJR Am J Roentgenol. 1980;134:653-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 44. | Augustin AM, Steingrüber M, Fluck F, Goetze O, Bley TA, Kickuth R. Percutaneous endobiliary forceps biopsy of biliary strictures for histopathologic examination. Diagn Interv Radiol. 2020;26:339-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 45. | Chang HY, Liu B, Wang YZ, Wang WJ, Wang W, Li D, Li YL. Percutaneous transhepatic cholangiography versus endoscopic retrograde cholangiography for the pathological diagnosis of suspected malignant bile duct strictures. Medicine (Baltimore). 2020;99:e19545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 46. | Mohkam K, Malik Y, Derosas C, Isaac J, Marudanayagam R, Mehrzad H, Mirza DF, Muiesan P, Roberts KJ, Sutcliffe RP. Percutaneous transhepatic cholangiographic endobiliary forceps biopsy versus endoscopic ultrasound fine needle aspiration for proximal biliary strictures: a single-centre experience. HPB (Oxford). 2017;19:530-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 47. | Inchingolo R, Spiliopoulos S, Nestola M, Nardella M. Outcomes of percutaneous transluminal biopsy of biliary lesions using a dedicated forceps system. Acta Radiol. 2019;60:602-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 48. | Park JG, Jung GS, Yun JH, Yun BC, Lee SU, Han BH, Ko JH. Percutaneous transluminal forceps biopsy in patients suspected of having malignant biliary obstruction: factors influencing the outcomes of 271 patients. Eur Radiol. 2017;27:4291-4297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 49. | Colombi D, Aragona G, Bodini FC, Zangrandi A, Morelli N, Michieletti E. SpyGlass percutaneous transhepatic cholangioscopy-guided diagnosis of adenocarcinoma of the ampullary region in a patient with bariatric biliopancreatic diversion. Hepatobiliary Pancreat Dis Int. 2019;18:291-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 50. | Jung JY, Lee SK, Oh HC, Lee TY, Kwon SH, Lee SS, Seo DW, Kim MH. The role of percutaneous transhepatic cholangioscopy in patients with hilar strictures. Gut Liver. 2007;1:56-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 51. | Ahmed S, Schlachter TR, Hong K. Percutaneous Transhepatic Cholangioscopy. Tech Vasc Interv Radiol. 2015;18:201-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 52. | Darcy M, Picus D. Cholangioscopy. Tech Vasc Interv Radiol. 2008;11:133-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 53. | Schechter MS, Doemeny JM, Johnson JO. Biliary ductal shave biopsy with use of the Simpson atherectomy catheter. J Vasc Interv Radiol. 1993;4:819-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 54. | Fohlen A, Bazille C, Menahem B, Jegonday MA, Dupont B, Le Pennec V, Lubrano J, Guiu B, Pelage JP. Transhepatic forceps biopsy combined with biliary drainage in obstructive jaundice: safety and accuracy. Eur Radiol. 2019;29:2426-2435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 55. | Eloubeidi MA, Chen VK, Jhala NC, Eltoum IE, Jhala D, Chhieng DC, Syed SA, Vickers SM, Mel Wilcox C. Endoscopic ultrasound-guided fine needle aspiration biopsy of suspected cholangiocarcinoma. Clin Gastroenterol Hepatol. 2004;2:209-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 188] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 56. | Ierardi AM, Mangini M, Fontana F, Floridi C, De Marchi G, Petrillo M, Capasso R, Chini C, Cocozza E, Cuffari S, Segato S, Rotondo A, Carrafiello G. Usefulness and safety of biliary percutaneous transluminal forceps biopsy (PTFB): our experience. Minim Invasive Ther Allied Technol. 2014;23:96-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 57. | Inchingolo R, Nestola M, Nunes TF, Spiliopoulos S, Nardella M. Biliary involvement in liver metastases: long-term experience with biliary biopsy from a single center. Radiol Bras. 2021;54:15-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 58. | Estrella JS, Othman ML, Taggart MW, Hamilton SR, Curley SA, Rashid A, Abraham SC. Intrabiliary growth of liver metastases: clinicopathologic features, prevalence, and outcome. Am J Surg Pathol. 2013;37:1571-1579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |