Published online Apr 16, 2022. doi: 10.4253/wjge.v14.i4.205
Peer-review started: May 19, 2021
First decision: June 17, 2021
Revised: June 25, 2021
Accepted: March 14, 2022
Article in press: March 14, 2022
Published online: April 16, 2022
Processing time: 324 Days and 9.3 Hours
Esophageal cancer (ECA) affects 1 in 125 men and 1 in 417 for women and accounts for 2.6% of all cancer related deaths in the United States. The associated survival rate depends on the stage of the cancer at the time of diagnosis, making adequate work up and staging imperative. The 5-year survival rate for localized disease is 46.4%, regional disease is 25.6%, and distant/metastatic disease is 5.2%. Additionally, treatment is stage-dependent, making staging all that much important. For nonmetastatic transmural tumors (T3) and/or those that have locoregional lymph node involvement (N), neoadjuvant therapy is recommended. Conversely, for those who have earlier tumors, upfront surgical resection is reasonable. While positron emission tomography/computed tomography and other cross sectional imaging modalities are exceptional for detecting distant disease, they are inaccurate in staging locoregional disease. Endoscopic ultrasound (EUS) has played a key role in the locoregional (T and N) staging of newly diagnosed ECA and has an evolving role in restaging after neoadjuvant therapy. There is even data to support that the use of EUS facilitates proper triaging of patients and may ultimately save money by avoiding unnecessary or futile treatment. This manuscript will review the current role of EUS on staging and restaging of ECA.
Core Tip: Esophageal cancer (ECA) affects 1 in 125 men and 1 in 417 for women and accounts for 2.6% of all cancer related deaths. The associated survival rate depends on the stage of the cancer when it is first diagnosed; therefore, adequate work up and staging is imperative. Additionally, treatment is stage-dependent, making staging all that much important. Endoscopic ultrasound has played a key role in the locoregional staging of newly diagnosed ECA and has an evolving role in restaging after neoadjuvant therapy. This manuscript will review the current role of endoscopic ultrasound on staging and restaging of ECA.
- Citation: Radlinski M, Shami VM. Role of endoscopic ultrasound in esophageal cancer. World J Gastrointest Endosc 2022; 14(4): 205-214
- URL: https://www.wjgnet.com/1948-5190/full/v14/i4/205.htm
- DOI: https://dx.doi.org/10.4253/wjge.v14.i4.205
There will be an estimated 19260 new cases of esophageal cancer (ECA) in the United States in 2021, which accounts for 1.0% of all new cancer cases. The lifetime risk for development of ECA in the United States is 1 in 125 for men and 1 in 417 for women[1]. Mortality from the disease is significant, with an estimated 15530 deaths in 2021, accounting for 2.6% of all cancer related deaths. When evaluating the data from 2011-2017, the 5-year survival rate was found to be 19.9%[2]. The associated survival rate depends on the stage of the cancer when it is first diagnosed. At the time of diagnosis, a significant subset of patients has either locally advanced or metastatic disease, with 34% of patients having regional spread and 39% of patients having distant or metastatic spread. Unfortunately, only 10% of patients present with localized disease. Five-year survival rates, as expected, vary based on disease extent found on index evaluation. The 5-year survival rate for localized disease is 46.4%, regional disease is 25.6%, and distant/metastatic disease is 5.2%.
The workup for esophageal and esophagogastric junction cancers requires accurate staging as treatment protocols are stage dependent. Upper gastrointestinal endoscopy is essential for the initial evaluation of an esophageal mass. Endoscopy with biopsies is often sufficient to establish the diagnosis of ECA, but in the rare instances that biopsies are nondiagnostic, endoscopic ultrasound (EUS), with fine needle aspiration (FNA) of the esophageal wall, can be utilized for tissue diagnosis[3]. Currently, ECA staging as defined by the American Joint Committee on Cancer staging system utilizes tumor-node-metastasis subclassifications, otherwise known as TNM. The TNM classifications refer to the primary tumor (T stage), regional lymph node status (N stage), and presence or absence of metastatic disease (M classification)[4]. After the initial diagnosis of cancer is made, the National Comprehensive Cancer Network recommends obtaining a computed tomography (CT) of the chest/abdomen/pelvis to assess for metastatic disease (this can also help to define local extent of disease and nodal involvement albeit not as well as EUS in most cases). If there is no overt evidence of M1 disease on cross sectional imaging, then both EUS and positron emission tomography (PET) are indicated at this time for further evaluation[5]. The primary strength of EUS as part of this algorithm is in the ability to establish the extent of locoregional involvement in patients without overt metastatic disease.
Since treatment options for ECA are stage dependent, EUS plays an important role by providing accurate T and N staging. Specifically, EUS helps differentiate patients that should undergo neoadjuvant chemotherapy from patients that would benefit from primary surgical resection.
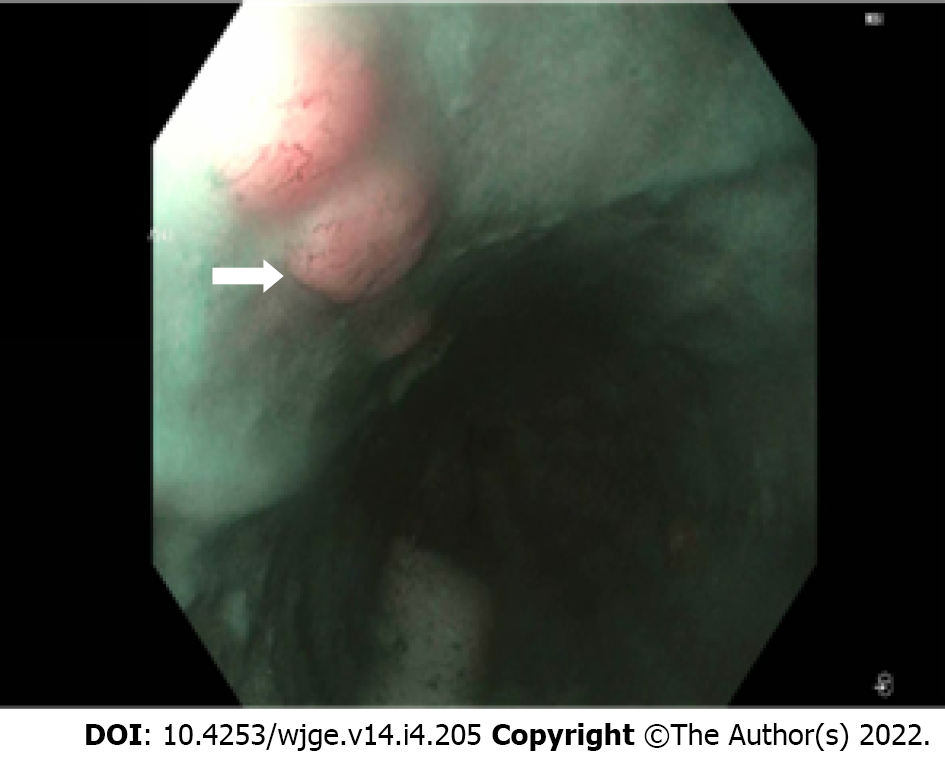
In general, the endoscopic report during the workup for ECA should include several components, including the anatomic landmarks, location of the lesion in question, circumferential extent of the cancer, and the general mucosal appearance. The importance of accurately describing the location of the tumor cannot be overemphasized, as many of the cancers labeled as esophageal are in fact either junctional or primary cardiac/gastric. This distinction is primarily determined by where the bulk of the tumor is. The endoscopist needs carefully to examine and document if the cancer involves the cardia or crosses the junction and how long (in cm) it extends proximal to the esophagogastric junction. Additionally, it is important to look for “skip” lesions (submucosal proximal extension of the cancer) so that the surgeons are aware of the extent of the cancer proximally (Figure 1). Similarly, it is important to document if there is Barrett’s esophagus that extends proximal to the cancer, since ideally this will also be resected if the patient is appropriate for surgery. Additionally, the most stenotic part of the tumor should be documented so that the endoscopist is aware and proceeds with appropriate caution when passing a larger diameter, often oblique viewing, echoendoscope.
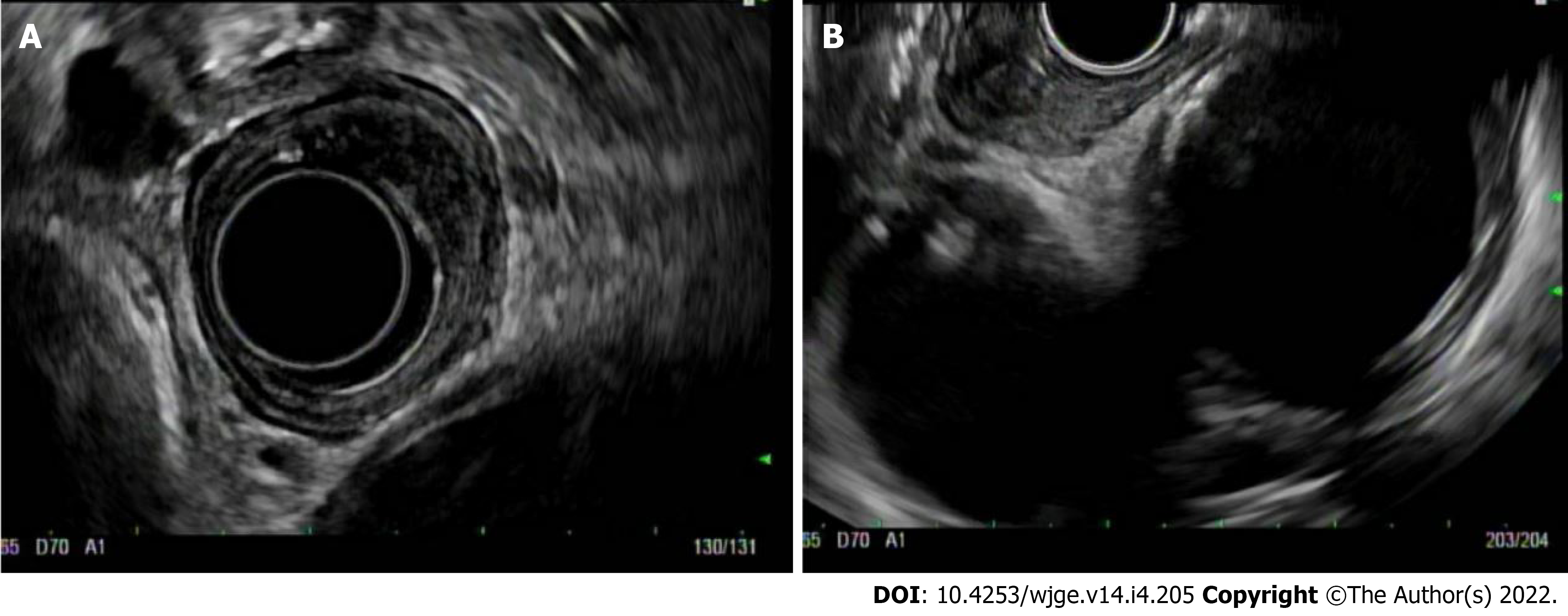
Standard echoendoscopes operate at a frequency of 7.5-12 mHz. EUS can be performed using a radial or linear platform. Radial EUS images at a plane that is perpendicular to the long access of the scope, so the echo ultrasonographer can get a circumferential or 360 view of the ECA. These images are similar to interpreting axial CT slices (Figure 2A). Linear EUS, on the other hand, images parallel to the long access of the scope, and while T-staging is sometimes more challenging, use of this scope allows for performance of FNA or fine needle biopsy (FNB) if needed (Figure 2B). While choice of platform is typically operator dependent, it is common practice that endoscopists start with radial EUS because of the circumferential view. This can be switched to a linear EUS if something is found that needs FNA, such as a lymph node or liver lesion.
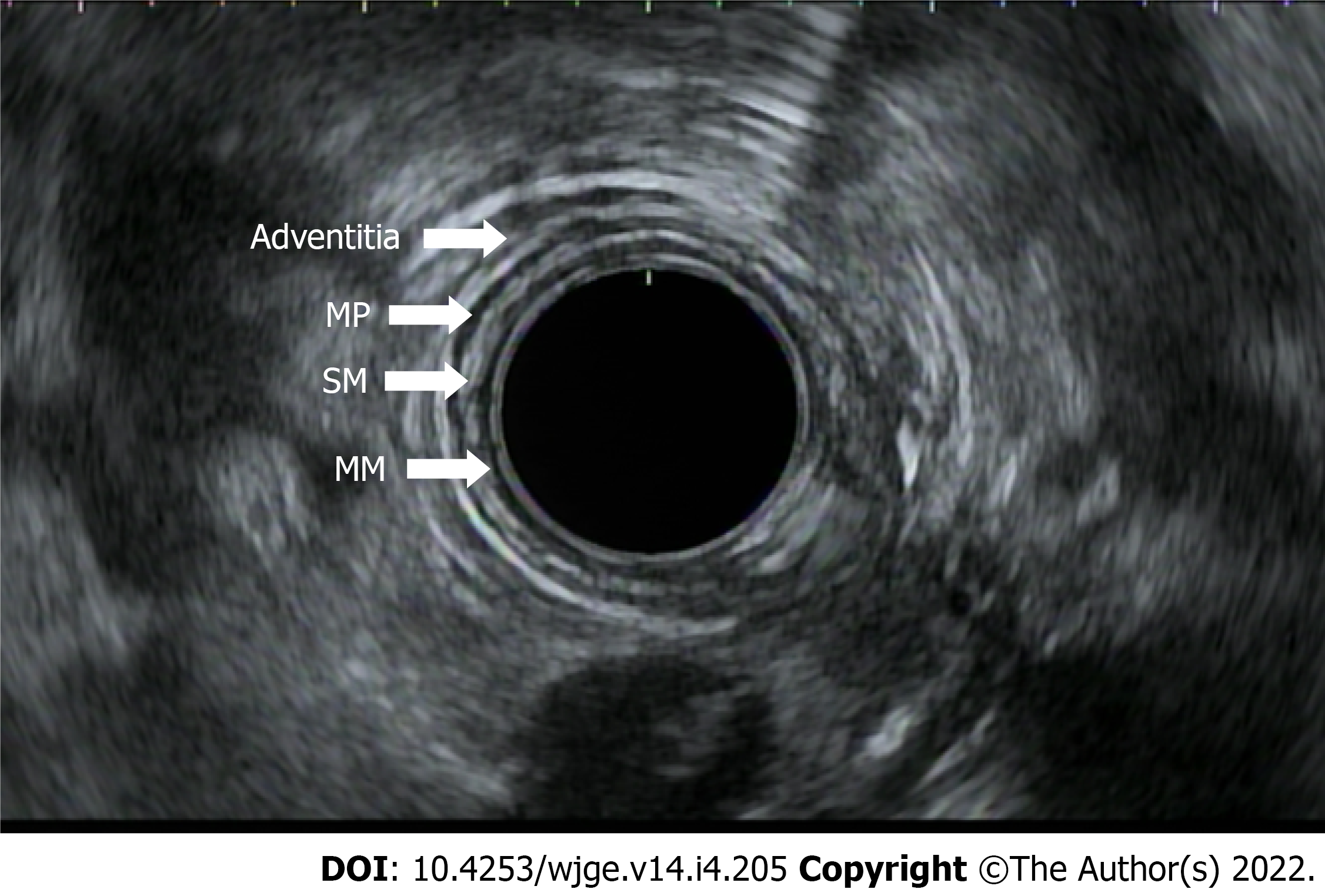
After identifying the distal and proximal extent of the cancer, the T-stage is determined. T staging refers to the depth of tumor invasion with respect to the extent of esophageal wall layer involvement. The esophageal wall is comprised of the mucosa, submucosa, muscularis propria, and adventitia. The mucosal wall layer is further subdivided into the epithelium, lamina propria, and muscularis mucosae. A basement membrane separates the muscularis mucosae from the submucosa. EUS helps to define the esophagus as a five layered structure with the first layer (hyperechoic) representing the superficial mucosa, the second (hypoechoic) representing the deep mucosa, the third (hyperechoic) representing the submucosa, the fourth (hypoechoic) the muscularis propria, and the fifth (hyperechoic) the adventitia (Figure 3). When reporting the T stage, the endosonographic report should also include the maximal wall thickness of the cancer.
EUS is particularly helpful with respect to T staging as we can accurately visualize and delineate the esophageal wall layers. Treatment decisions are partially dependent on T staging since depth of cancer penetration is important in predicting the risk of lymph node metastasis. Treatment for locally advanced disease, defined as stage IIB through IIIC, typically is neoadjuvant chemotherapy, with the goal to proceed with surgical resection following restaging, if appropriate. Neoadjuvant chemotherapy is associated with superior pathologic response and improved outcomes in these patients. For patients with surgically unresectable tumors or patients who are poor surgical candidates, definitive chemotherapy is offered.
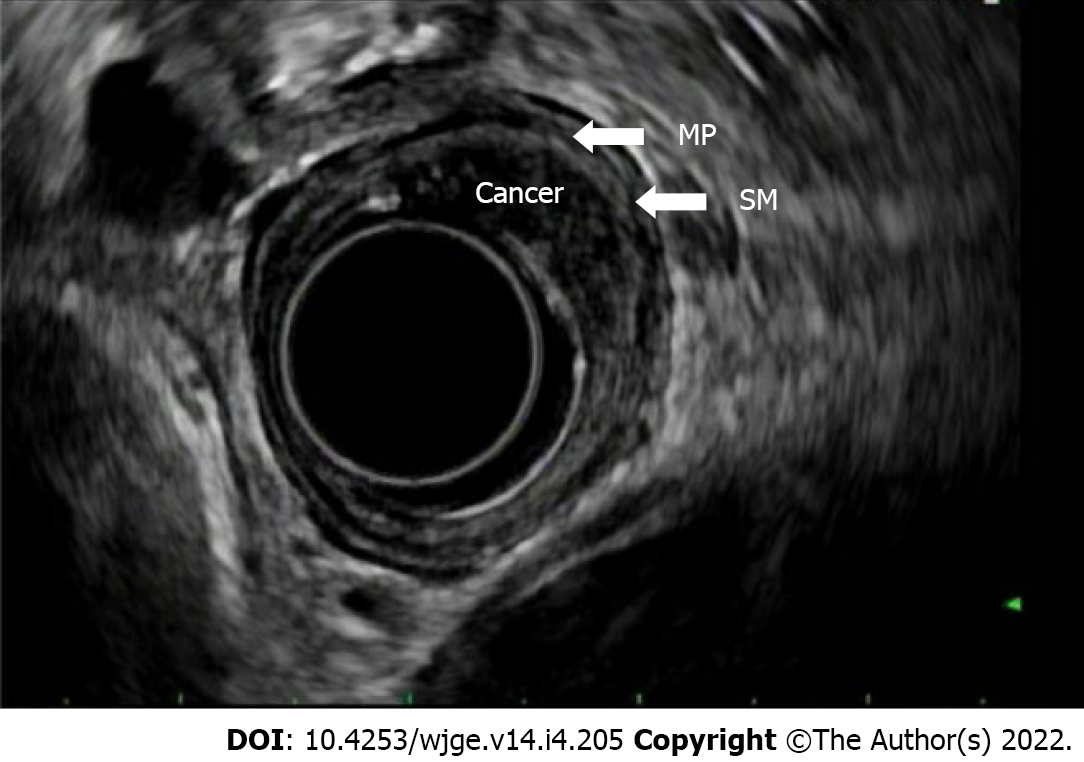
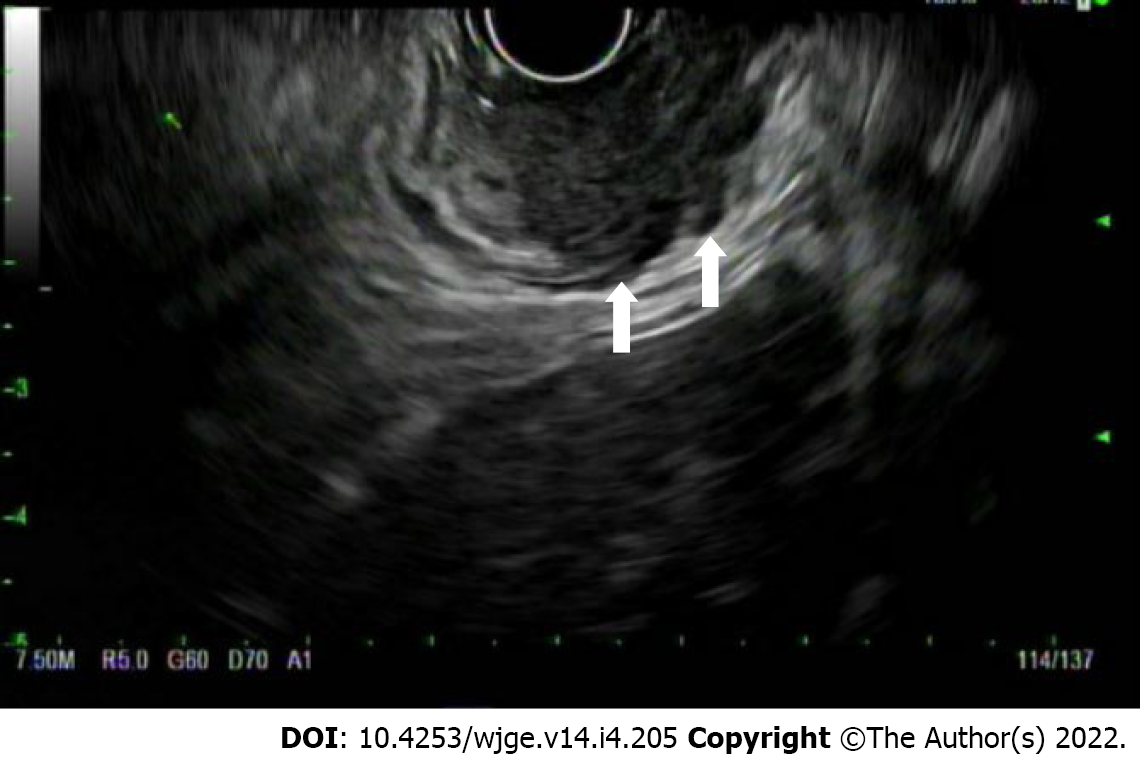
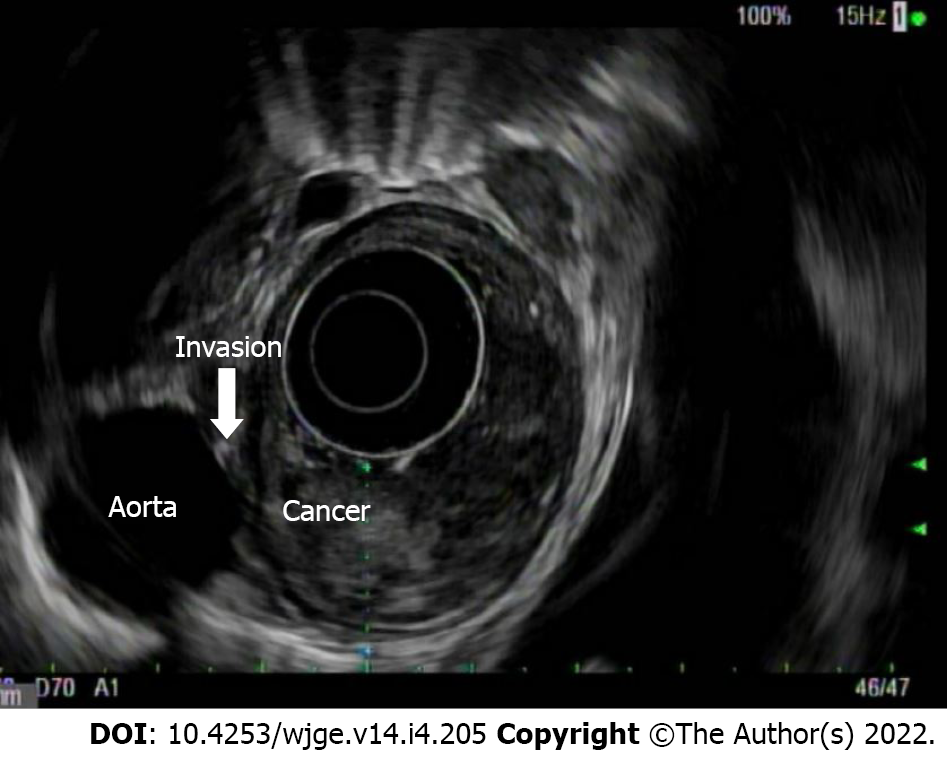
T(is) refers to high grade dysplasia that is limited to the epithelium and does not penetrate the lamina propria. T1a tumors invade the lamina propria and/or muscularis mucosae, whereas T1b lesions invade into (but not through) the submucosa. By EUS, a T1a layer would invade through the first endosonographic, hyperechoic layer and possibly invade into, but not through the second hypoechoic later. T1b lesions would invade into, but not through the third, hyperechoic layer (Figure 4). T2 lesions invade past the submucosa into the muscularis propria (but do not breach the outer border). By EUS, these would invade into, but not through, the fourth (hypoechoic) layer. T3 lesions invade past the muscularis propria into the adventitia (Figure 5). By EUS, this would denote invasion past the fourth endosonographic layer into the fifth (hyperechoic) layer. T4a and T4b both invade structures adjacent to the esophagus, but T4a are considered resectable (invasion of pleura, pericardium, diaphragm), while T4b are considered unresectable (invasion of the aorta, vertebral body, trachea) (Figure 6). The true positive rate for EUS T-staging ranges between 0.89 (0.86-0.92), as gathered by one meta-analysis of 27 primary articles[6].
The accuracy of EUS lessens in staging cancers not on either ends of the spectrum (T1 or T/4). In a study by Tekola et al[7], 38 patients with ECA who were staged as T2N0 underwent surgery. EUS under staged 32% of these tumors. Other data have shown that up to 55% of tumors staged as T2N0 were shown to have nodal disease on resection. For this reason, many patients staged with T2N0 cancers are now undergoing preoperative chemoradiation. This practice is supported by Capovilla et al[11], whose study demonstrated that patients with T2N0 esophageal and squamous cell cancers who underwent neoadjuvant therapy had a statistically higher survival rate than patients who underwent up front surgery. If future studies support this practice, then the importance/ role of EUS in triaging patients to neoadjuvant vs surgery may in fact diminish[7-11].
“Importance of history/ presence of dysphagia in T staging”: In patients with ECA who have dysphagia, the majority have advanced disease. One study showed that dysphagia was noted in 89% of patients having T3-4 ECA, while only 53% without dysphagia had T3-4 disease (P < 0.001). Another study showed similar findings where the presence of dysphagia in the setting of a cancer had a sensitivity 0.89 and sensitivity of 0.88 for at least locally advanced disease. For this reason, in patients with ECA and dysphagia, EUS may be less likely to affect treatment decisions[12,13].
Next, the N-stage is determined. The N stage refers to the presence or absence, along with the total number of regional lymph nodes affected. N0 indicates the absence of lymph node involvement, N1 denotes two involved lymph nodes, N2, three to six involved lymph nodes, and N3, seven or more lymph nodes.
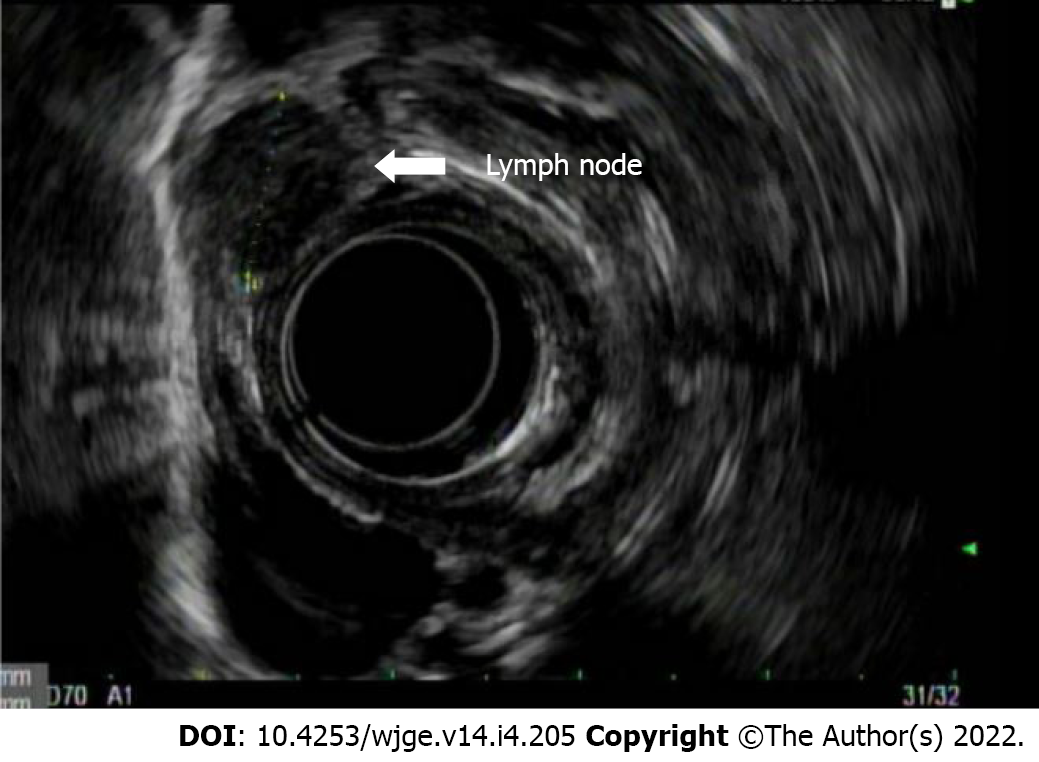
Endosonographic characteristics of lymph nodes that suggest malignant potential include size greater than 1 cm, round shape, sharp and demarcated borders, and hypoechoic echotexture (Figure 7). When a lymph node is found to possess all four of these aforementioned features, the accuracy of predicting a malignant lymph node is 80%-100%[14,15]. The location of the lymph node may also be informative in differentiation of benign and malignant. For example, the presence of celiac lymph nodes usually indicates pathology since they are not usually present. In one study, 89% of endosonographically detectable celiac lymph nodes were confirmed to be malignant on FNA[16]. Another predictor of malignant lymph node status includes association with T3-T4 staged lesions[17].
EUS has a pooled sensitivity of 59.5% to 97.2% sensitivity for N staging (40%-100% specificity). This is compared to a pooled sensitivity of 24% for distinguishing N0 from N1 by CT (with 100% specificity)[6]. Nodal staging is important prognostically since patients with nodal involvement have been found to have worse prognosis as compared to those who do not (N0 disease). Patients with 0, 1-2, and > 2 malignant appearing, peri-esophageal lymph nodes on index EUS were found to have 66 mo, 14.5 mo, and 6.5 mo, respectively, of median survival time[18].
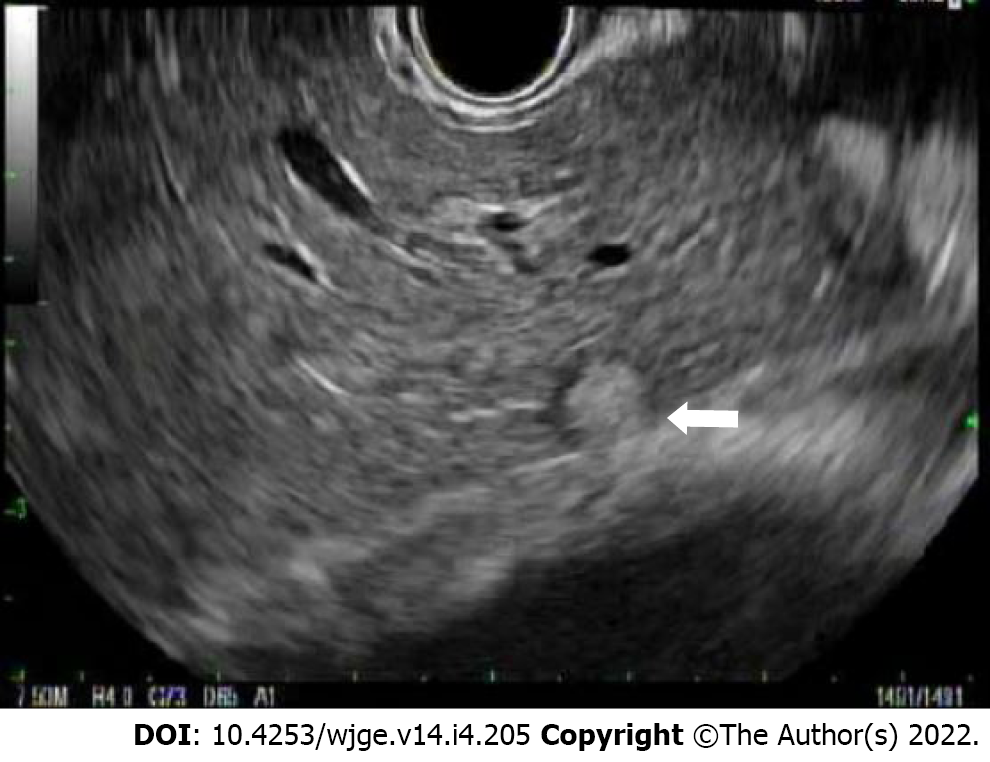
Lastly, distant lymph nodes, the liver, peritoneum, and the left adrenal gland are inspected for lesions. M staging differentiates presence of metastases (M1) vs absence of metastases (M0). As previously discussed, there is a limited role for EUS if M1 disease is established on CT. However, EUS at the position of the antrum or bulb of the duodenum can provide an important means for evaluation of peripancreatic or porta hepatis lymph nodes. In the body of the stomach, EUS can evaluate the liver (Figure 8), and in the fundus and cardia, EUS can evaluate perigastric and peripancreatic lymph nodes as well as evaluate the celiac plexus (though the latter is not considered M1). Additionally, EUS can provide a detailed evaluation of the left adrenal gland and the peritoneum. An important difference between the older classification (American Joint Committee on Cancer) system and the current, affecting the utility of EUS in differentiating M0 from M1 disease, is that the involvement of a celiac lymph node is now considered regional (N) disease and no longer metastatic (M1a).
EUS may not be technically feasible in patients with obstructing cancers. An obstructing tumor can be seen on presentation in up to 30% of cases. There are some risks of dilating a malignant stricture to pass an echo endoscope, including perforation[19]. Additionally, it may be difficult to stage accurately a lesion following esophageal dilation given disruption of normal tissue planes. There is questionable additional benefit of endosonography following the endoscopic finding of a malignant stricture as the presence of a malignant obstruction typically denotes advanced disease (T3-T4)[20]. Patients with malignant obstructions that cannot be traversed have poorer outcomes as compared to patients without evidence of stenosis, with median survivals of 10 mo vs 20 mo, respectively.
One of the benefits of EUS, specifically linear EUS, is the ability to perform FNA and/or FNB of lymph nodes and lesions in adjacent structures. EUS with FNA has 80% sensitivity in distinguishing T4 from T1-T3 disease and 78% accuracy in nodal staging[21]. In patients with T1-T2 disease, FNA can determine lymph node involvement, which in turn determines if these patients would theoretically need neoadjuvant chemotherapy or proceed directly to surgery. When performing FNA, it is important to avoid passing through the main tumor or major blood vessels to avoid both false positives as well as tumor seeding.
In one study, EUS results altered management by guiding the need for neoadjuvant chemotherapy in 34.8% of patients evaluated[22]. In another retrospective study of 56 patients, EUS was superior in the ability to identify locally advanced disease, with 58.9% sensitivity as compared to 26.8% and 37.5% sensitivity for CT and PET, respectively. EUS, however, is less accurate for early-stage lesions (T1 or T2) as compared with more advanced tumors. Additionally, PET is superior for detection of distant metastasis as compared to EUS, with a sensitivity of 81% vs 73% and specificity of 91% vs 86%, respectively[23]. EUS also plays an important role in detecting disease recurrence along with restaging after chemotherapy +/- radiation.
With improvements in imaging such as PET/magnetic resonance imaging (MRI), the overall utility of EUS is controversial. In one study of 74 patients undergoing preoperative staging, MRI outperformed EUS with higher specificity and accuracy in T staging[24]. In patients with dysphagia or an obstructing lesion, EUS has less utility given most of these patients have locally advanced disease and thus would not be definitive surgical resection candidates. In one study evaluating 147 patients with esophageal adenocarcinoma and dysphagia, 133 of these patients had a partially or completely obstructing mass on initial endoscopic evaluation. Overall, 128 of these 133 (96%) patients had locally advanced disease[12].
The utility of EUS is also diminished when evaluating early-stage ECA as there is loss of sensitivity for superficial disease. High frequency probes can help to provide better evaluation of the mucosa and the submucosa. In 75%-82% of cases, high frequency probes (12-20 MHz) can help distinguish T1a from T1b disease in patients without evidence of metastatic disease[25]. This can help determine candidacy for endoscopic resection techniques as a curative option during the same session. In another study, the accuracy of T staging when using a high frequency probe was 64% as compared to a conventional radial EUS, which was 49%[26]. When encountering a more superficial lesion that can be endoscopically resected, performing EUS first is helpful in confirming that the muscularis propria is uninvolved and in ruling out malignant lymphadenopathy. Once the lesion is endoscopically resected, then the true pathologic T stage is confirmed.
We have also found that EUS is challenging when evaluating early to intermediate gastroesophageal junction (GEJ) tumors. In one study evaluating EUS in GEJ tumors prior to surgical resection (in patients that had not undergone prior chemotherapy or radiation), EUS T staging was only accurate in 48% of cases (23% percent were under-staged and 29% were over-staged as correlated with pathologic T staging). This inaccuracy was even more pronounced in short segment tumors at the GEJ[27].
The role of EUS in staging disease following neoadjuvant therapy is evolving. Patients are typically restaged after completion of neoadjuvant therapy to determine if the next most appropriate step is surgical resection vs definitive or palliative chemotherapy. Traditionally, it was thought that EUS is less reliable following neoadjuvant chemotherapy given inflammation and fibrosis sustained during treatment, which affects the ability to interpret reliably an EUS exam. The mucosal changes following neoadjuvant chemotherapy can cause hypoechoic appearance of the esophageal wall and over-staging of tumor invasion, possibly precluding some patients from an appropriate surgical resection. Following neoadjuvant chemotherapy, a recent meta-analysis and systematic review found the sensitivity and specificity of T1 23% and 95%, T2 29% and 84%, T3 81% and 42%, and T4 43% and 96%, respectively. In the same study, the pooled sensitivity and specificity of N staging was found to be 69% and 52%, respectively[28].
Another retrospective study of 103 patients with locoregionally advanced ECA who had undergone neoadjuvant chemotherapy showed that reduced mass size, as determined by EUS (0.7 vs 1.7 cm, P = 0.01), correlated with a pathologic response[29]. However, in this same cohort, fluorodeoxyglucose-PET outperformed EUS in prediction of long-term survival following neoadjuvant chemotherapy (in patients following neoadjuvant chemotherapy but prior to surgical resection).
Even after surgery, EUS can be utilized in determining tumor recurrence, despite post-surgical EUS surveillance not being considered standard of practice at this time. In one small study of 40 patients who had undergone prior surgical resection, 3 recurrences were identified with EUS despite absence of symptoms (no reported dysphagia) and a negative CT[30]. In fact, another study of 43 patients undergoing q6 mo EUS surveillance had a 92% positive predictive value for early recurrence in a population where two-thirds of those with recurrence were asymptomatic[31].
In one meta-analysis, the pooled sensitivity for detecting complete pathologic response following neoadjuvant therapy was 0.35, 0.62, 0.01, and 0.08 for CT, PET-CT, EUS, and MRI, respectively. While the sensitivity of EUS was poor, specificity was 0.99 as compared to 0.83, 0.73, and 0.83 for CT, PET-CT, and MRI, respectively[32].
One multicenter study evaluating 138 patients before and after neoadjuvant therapy showed that EUS was able to detect adequately residual disease in 90% of patients 12 wk following therapy. Specifically, EUS was able to detect residual thickness and residual area of the tumor[33]. Another meta-analysis evaluating EUS for restaging following neoadjuvant chemotherapy found that EUS had a pooled sensitivity and specificity of 81% and 42% in T3 tumors (with markedly lower sensitivities of 23%, 29%, and 43% in T1, T2, and T4 tumors, respectively)[28].
Other considerations when discussing the role of EUS in the staging of ECA include the cost effectiveness. EUS performed prior to treatment decisions has been found to save $3443 per patient in its ability to identify stage 1 or stage 4 disease and avoid inappropriate neoadjuvant chemotherapy or surgery[34]. In patients without metastatic disease, EUS is the least expensive staging modality for ECA ($13811) as compared to CT-guided FNA ($14350) or surgery ($13992). While CT is the most appropriate initial staging test in most cases, EUS can theoretically suffice as a reasonable initial study as demonstrated in one single center study. EUS found advanced disease more frequently than CT (44 % vs 13%) and is cheaper ($804 vs $844) than CT (in cases where the probability of finding advanced disease is less than 20%)[35].
It is also important to note that performing high quality EUS is provider dependent and can vary with skill level and experience. In general, it is believed that at least 100 examinations are needed for a provider to provide T-staging reliably and accurately in ECA. High quality EUS examination also has been shown to improve survival in one randomized control trial of 223 patients with non-metastatic gastroesophageal cancer (hazard ratio of 0.706 with 95% confidence interval from 0.501 to 0.966)[36].
EUS has an important role in the staging of ECA. It is superior to cross sectional imaging in the locoregional staging of ECA. Unlike cross sectional imaging, it also has the added advantage to perform FNA and/or FNB of surrounding lymph nodes and organs and, consequently, alter management. Instances when EUS may not be as beneficial are in patients with dysphagia since they most likely have at least advanced locoregional disease and would undergo neoadjuvant or definitive therapy depending on their M status. While less accurate, EUS has an evolving role in neoadjuvant therapy. Since the performance of EUS is operator dependent, it should ideally be performed by physicians specifically trained in EUS.
Vanessa M Shami is a consultant for Cook Medical, Olympus America and Interpace Diagnostics
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Guo JT, China; Tsou YK, Taiwan; Xi K, China S-Editor: Chang KL L-Editor: Filipodia P-Editor: Chang KL
| 1. | Statistics for Esophageal Cancer: Esophageal Cancer Stats. [cited 19 May 2021]. Available from: www.cancer.org/cancer/esophagus-cancer/about/key-statistics.html. |
| 2. | Cancer Stat Facts: Esophageal Cancer. NIH. [cited 19 May 2021]. Available from: https://seer.cancer.gov/statfacts/html/esoph.html. |
| 3. | Faigel DO, Deveney C, Phillips D, Fennerty MB. Biopsy-negative malignant esophageal stricture: diagnosis by endoscopic ultrasound. Am J Gastroenterol. 1998;93:2257-2260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Rice TW, Patil DT, Blackstone EH. 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: application to clinical practice. Ann Cardiothorac Surg. 2017;6:119-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 529] [Article Influence: 66.1] [Reference Citation Analysis (0)] |
| 5. | Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Corvera C, Das P, Denlinger CS, Enzinger PC, Fanta P, Farjah F, Gerdes H, Gibson M, Glasgow RE, Hayman JA, Hochwald S, Hofstetter WL, Ilson DH, Jaroszewski D, Johung KL, Keswani RN, Kleinberg LR, Leong S, Ly QP, Matkowskyj KA, McNamara M, Mulcahy MF, Paluri RK, Park H, Perry KA, Pimiento J, Poultsides GA, Roses R, Strong VE, Wiesner G, Willett CG, Wright CD, McMillian NR, Pluchino LA. Esophageal and Esophagogastric Junction Cancers, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019;17:855-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 682] [Article Influence: 113.7] [Reference Citation Analysis (0)] |
| 6. | Kelly S, Harris KM, Berry E, Hutton J, Roderick P, Cullingworth J, Gathercole L, Smith MA. A systematic review of the staging performance of endoscopic ultrasound in gastro-oesophageal carcinoma. Gut. 2001;49:534-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 244] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 7. | Tekola BD, Sauer BG, Wang AY, White GE, Shami VM. Accuracy of endoscopic ultrasound in the diagnosis of T2N0 esophageal cancer. J Gastrointest Cancer. 2014;45:342-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Kountourakis P, Correa AM, Hofstetter WL, Lee JH, Bhutani MS, Rice DC, Komaki R, Maru DM, Ross WA, Vaporciyan A, Swisher SG, Ajani JA. Combined modality therapy of cT2N0M0 esophageal cancer: the University of Texas M. D. Anderson Cancer Center experience. Cancer. 2011;117:925-930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Rice TW, Mason DP, Murthy SC, Zuccaro G Jr, Adelstein DJ, Rybicki LA, Blackstone EH. T2N0M0 esophageal cancer. J Thorac Cardiovasc Surg. 2007;133:317-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Stiles BM, Mirza F, Coppolino A, Port JL, Lee PC, Paul S, Altorki NK. Clinical T2-T3N0M0 esophageal cancer: the risk of node positive disease. Ann Thorac Surg. 2011;92:491-6; discussion 496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 11. | Capovilla G, Moletta L, Pierobon ES, Salvador R, Provenzano L, Zanchettin G, Costantini M, Merigliano S, Valmasoni M. Optimal Treatment of cT2N0 Esophageal Carcinoma: Is Upfront Surgery Really the Way? Ann Surg Oncol. 2021;28:8387-8397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Mansfield SA, El-Dika S, Krishna SG, Perry KA, Walker JP. Routine staging with endoscopic ultrasound in patients with obstructing esophageal cancer and dysphagia rarely impacts treatment decisions. Surg Endosc. 2017;31:3227-3233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Ripley RT, Sarkaria IS, Grosser R, Sima CS, Bains MS, Jones DR, Adusumilli PS, Huang J, Finley DJ, Rusch VW, Rizk NP. Pretreatment Dysphagia in Esophageal Cancer Patients May Eliminate the Need for Staging by Endoscopic Ultrasonography. Ann Thorac Surg. 2016;101:226-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Catalano MF, Sivak MV Jr, Rice T, Gragg LA, Van Dam J. Endosonographic features predictive of lymph node metastasis. Gastrointest Endosc. 1994;40:442-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 307] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 15. | Bhutani MS, Hawes RH, Hoffman BJ. A comparison of the accuracy of echo features during endoscopic ultrasound (EUS) and EUS-guided fine-needle aspiration for diagnosis of malignant lymph node invasion. Gastrointest Endosc. 1997;45:474-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 224] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 16. | Eloubeidi MA, Wallace MB, Reed CE, Hadzijahic N, Lewin DN, Van Velse A, Leveen MB, Etemad B, Matsuda K, Patel RS, Hawes RH, Hoffman BJ. The utility of EUS and EUS-guided fine needle aspiration in detecting celiac lymph node metastasis in patients with esophageal cancer: a single-center experience. Gastrointest Endosc. 2001;54:714-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 105] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 17. | Vazquez-Sequeiros E, Wiersema MJ, Clain JE, Norton ID, Levy MJ, Romero Y, Salomao D, Dierkhising R, Zinsmeister AR. Impact of lymph node staging on therapy of esophageal carcinoma. Gastroenterology. 2003;125:1626-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 125] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 18. | Chen J, Xu R, Hunt GC, Krinsky ML, Savides TJ. Influence of the number of malignant regional lymph nodes detected by endoscopic ultrasonography on survival stratification in esophageal adenocarcinoma. Clin Gastroenterol Hepatol. 2006;4:573-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Hancock SM, Gopal DV, Frick TJ, Pfau PR. Dilation of malignant strictures in endoscopic ultrasound staging of esophageal cancer and metastatic spread of disease. Diagn Ther Endosc. 2011;2011:356538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Pfau PR, Ginsberg GG, Lew RJ, Faigel DO, Smith DB, Kochman ML. Esophageal dilation for endosonographic evaluation of malignant esophageal strictures is safe and effective. Am J Gastroenterol. 2000;95:2813-2815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 66] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Cerfolio RJ, Bryant AS, Ohja B, Bartolucci AA, Eloubeidi MA. The accuracy of endoscopic ultrasonography with fine-needle aspiration, integrated positron emission tomography with computed tomography, and computed tomography in restaging patients with esophageal cancer after neoadjuvant chemoradiotherapy. J Thorac Cardiovasc Surg. 2005;129:1232-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 152] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 22. | Pfau PR, Perlman SB, Stanko P, Frick TJ, Gopal DV, Said A, Zhang Z, Weigel T. The role and clinical value of EUS in a multimodality esophageal carcinoma staging program with CT and positron emission tomography. Gastrointest Endosc. 2007;65:377-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Lowe VJ, Booya F, Fletcher JG, Nathan M, Jensen E, Mullan B, Rohren E, Wiersema MJ, Vazquez-Sequeiros E, Murray JA, Allen MS, Levy MJ, Clain JE. Comparison of positron emission tomography, computed tomography, and endoscopic ultrasound in the initial staging of patients with esophageal cancer. Mol Imaging Biol. 2005;7:422-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 107] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 24. | Guo J, Wang Z, Qin J, Zhang H, Liu W, Zhao Y, Lu Y, Yan X, Zhang Z, Zhang T, Zhang S, Dominik NM, Kamel IR, Li H, Qu J. A prospective analysis of the diagnostic accuracy of 3 T MRI, CT and endoscopic ultrasound for preoperative T staging of potentially resectable esophageal cancer. Cancer Imaging. 2020;20:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Shah PM, Gerdes H. Endoscopic options for early stage esophageal cancer. J Gastrointest Oncol. 2015;6:20-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 26. | Wu LF, Wang BZ, Feng JL, Cheng WR, Liu GR, Xu XH, Zheng ZC. Preoperative TN staging of esophageal cancer: comparison of miniprobe ultrasonography, spiral CT and MRI. World J Gastroenterol. 2003;9:219-224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 69] [Cited by in RCA: 63] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 27. | Dhupar R, Rice RD, Correa AM, Weston BR, Bhutani MS, Maru DM, Betancourt SL, Rice DC, Swisher SG, Hofstetter WL. Endoscopic Ultrasound Estimates for Tumor Depth at the Gastroesophageal Junction Are Inaccurate: Implications for the Liberal Use of Endoscopic Resection. Ann Thorac Surg. 2015;100:1812-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Sun F, Chen T, Han J, Ye P, Hu J. Staging accuracy of endoscopic ultrasound for esophageal cancer after neoadjuvant chemotherapy: a meta-analysis and systematic review. Dis Esophagus. 2015;28:757-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 29. | Swisher SG, Maish M, Erasmus JJ, Correa AM, Ajani JA, Bresalier R, Komaki R, Macapinlac H, Munden RF, Putnam JB, Rice D, Smythe WR, Vaporciyan AA, Walsh GL, Wu TT, Roth JA. Utility of PET, CT, and EUS to identify pathologic responders in esophageal cancer. Ann Thorac Surg. 2004;78:1152-60; discussion 1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 215] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 30. | Catalano MF, Sivak MV Jr, Rice TW, Van Dam J. Postoperative screening for anastomotic recurrence of esophageal carcinoma by endoscopic ultrasonography. Gastrointest Endosc. 1995;42:540-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 35] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Fockens P, Manshanden CG, van Lanschot JJ, Obertop H, Tytgat GN. Prospective study on the value of endosonographic follow-up after surgery for esophageal carcinoma. Gastrointest Endosc. 1997;46:487-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | de Gouw DJJM, Klarenbeek BR, Driessen M, Bouwense SAW, van Workum F, Fütterer JJ, Rovers MM, Ten Broek RPG, Rosman C. Detecting Pathological Complete Response in Esophageal Cancer after Neoadjuvant Therapy Based on Imaging Techniques: A Diagnostic Systematic Review and Meta-Analysis. J Thorac Oncol. 2019;14:1156-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 101] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 33. | van der Bogt RD, Noordman BJ, Krishnadath KK, Roumans CAM, Schoon EJ, Oostenbrug LE, Siersema PD, Vleggaar FP, van Lanschot JJB, Spaander MCW. Endoscopic ultrasound measurements for detection of residual disease after neoadjuvant chemoradiotherapy for esophageal cancer. Endoscopy. 2019;51:326-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Shumaker DA, de Garmo P, Faigel DO. Potential impact of preoperative EUS on esophageal cancer management and cost. Gastrointest Endosc. 2002;56:391-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Hadzijahic N, Wallace MB, Hawes RH, VanVelse A, LeVeen M, Marsi V, Hoffman BJ, Sahai AV. CT or EUS for the initial staging of esophageal cancer? Gastrointest Endosc. 2000;52:715-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 36. | Russell IT, Edwards RT, Gliddon AE, Ingledew DK, Russell D, Whitaker R, Yeo ST, Attwood SE, Barr H, Nanthakumaran S, Park KG. Cancer of Oesophagus or Gastricus - New Assessment of Technology of Endosonography (COGNATE): report of pragmatic randomised trial. Health Technol Assess. 2013;17:1-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |