Published online Mar 16, 2022. doi: 10.4253/wjge.v14.i3.129
Peer-review started: July 27, 2021
First decision: November 11, 2021
Revised: December 15, 2021
Accepted: February 27, 2022
Article in press: February 27, 2022
Published online: March 16, 2022
Processing time: 232 Days and 0.9 Hours
Currently, there is insufficient data about the accuracy in the diagnosing of pancreatic cystic lesions (PCLs), especially with novel endoscopic techniques such as with direct intracystic micro-forceps biopsy (mFB) and needle-based confocal laser-endomicroscopy (nCLE).
To compare the accuracy of endoscopic ultrasound (EUS) and associated techniques for the detection of potentially malignant PCLs: EUS-guided fine needle aspiration (EUS-FNA), contrast-enhanced EUS (CE-EUS), EUS-guided fiberoptic probe cystoscopy (cystoscopy), mFB, and nCLE.
This was a single-center, retrospective study. We identified patients who had undergone EUS, with or without additional diagnostic techniques, and had been diagnosed with PCLs. We determined agreement among malignancy after 24-mo follow-up findings with detection of potentially malignant PCLs via the EUS-guided techniques and/or EUS-guided biopsy when available (EUS malignancy detection).
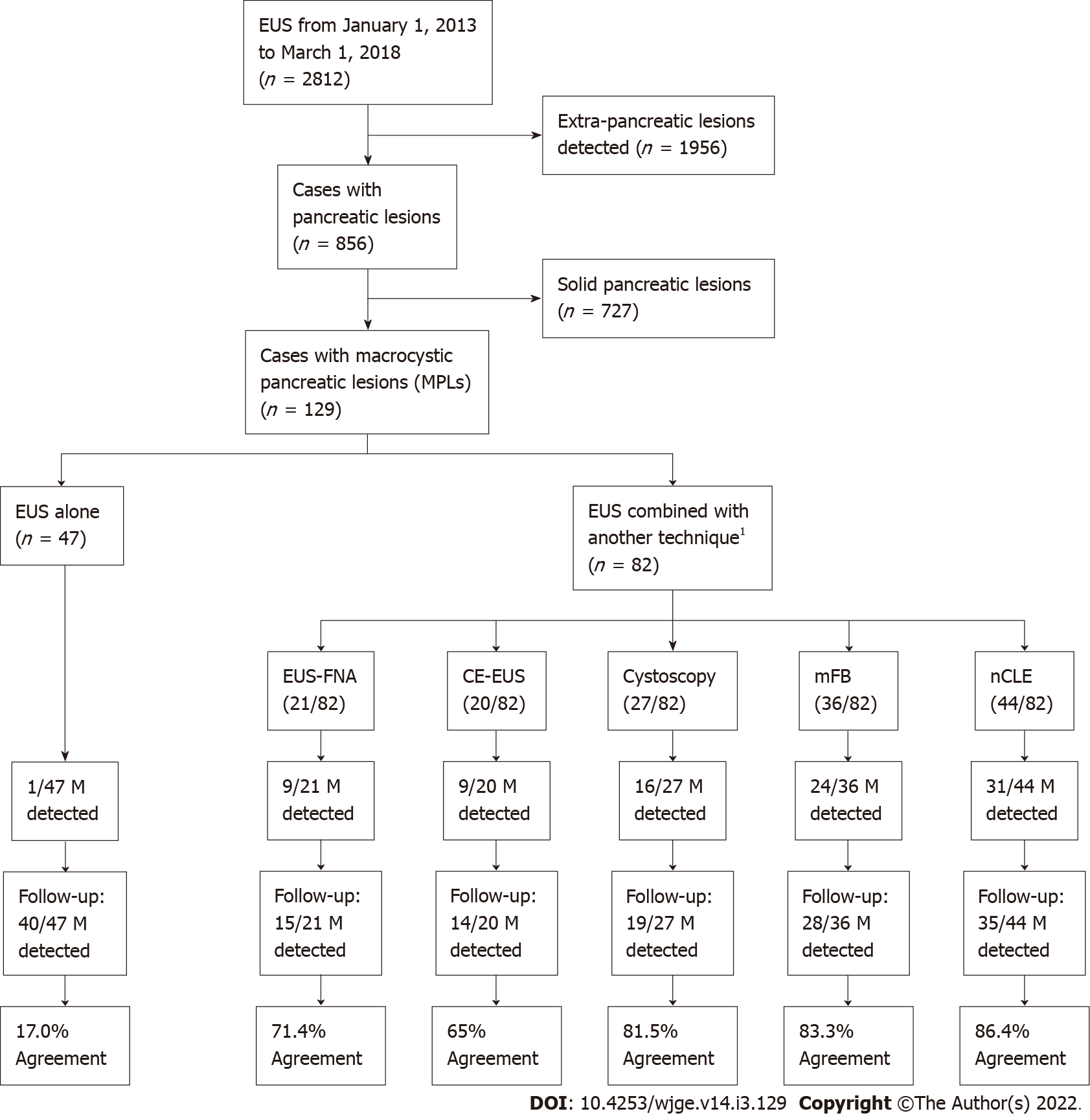
A total of 129 patients were included, with EUS performed alone in 47/129. In 82/129 patients, EUS procedures were performed with additional EUS-FNA (21/82), CE-EUS (20/82), cystoscopy (27/82), mFB (36/82), nCLE (44/82). Agreement between EUS malignancy detection and the 24-mo follow-up findings was higher when associated with additional diagnostic techniques than EUS alone [62/82 (75.6%) vs 8/47 (17%); OR 4.35, 95%CI: 2.70-7.37; P < 0.001]. The highest malignancy detection accuracy was reached when nCLE and direct intracystic mFB were both performed, with a sensitivity, specificity, positive predictive value, negative predictive value and observed agreement of 100%, 89.4%, 77.8%, 100% and 92.3%, respectively (P < 0.001 compared with EUS-alone).
The combined use of EUS-guided mFB and nCLE improves detection of potentially malignant PCLs compared with EUS-alone, EUS-FNA, CE-EUS or cystoscopy.
Core Tip: This retrospective study compared the accuracy of endoscopic ultrasound (EUS) and associated techniques such as EUS-guided fine needle aspiration (EUS-FNA), contrast-enhanced EUS (CE-EUS), EUS-guided fiberoptic probe cystoscopy (cystoscopy), EUS-guided direct intracystic micro-forceps biopsy (mFB), and EUS-guided needle-based confocal laser-endomicroscopy (nCLE) for the detection of potentially malignant pancreatic cystic lesions (PCLs) in 129 patients. Patients were allocated to three cohorts: those evaluated via EUS alone; via EUS-FNA, CE-EUS and/or cystoscopy; and with mFB plus nCLE. We observed that combining EUS, mFB, and nCLE had a statistically significant improved detection of potentially malignant PCLs compared to any of the evaluated techniques alone.
- Citation: Robles-Medranda C, Olmos JI, Puga-Tejada M, Oleas R, Baquerizo-Burgos J, Arevalo-Mora M, Del Valle Zavala R, Nebel JA, Calle Loffredo D, Pitanga-Lukashok H. Endoscopic ultrasound-guided through-the-needle microforceps biopsy and needle-based confocal laser-endomicroscopy increase detection of potentially malignant pancreatic cystic lesions: A single-center study. World J Gastrointest Endosc 2022; 14(3): 129-141
- URL: https://www.wjgnet.com/1948-5190/full/v14/i3/129.htm
- DOI: https://dx.doi.org/10.4253/wjge.v14.i3.129
The incidence of pancreatic cystic lesions (PCLs) is rising mainly in elderly patients[1]. Therefore, early detection of potentially malignant PCLs increases the possibility of a curative approach. Current American Gastroenterological Association guideline recommends magnetic resonance imaging (MRI) or magnetic resonance cholangiopancreatography (MRCP) to assess PCLs[2]. For the same purpose, the revised Fukuoka guideline recommend computerized tomography (CT), MRI or MRCP, keeping endoscopic ultrasound guided fine-needle aspiration (EUS-FNA) for intraductal papillary mucinous neoplasm (IPMN) evaluation[3]. Nevertheless, both guidelines showed an unsatisfactory pooled sensitivity for malignant PCLs of 64% and 59%, respectively[4].
EUS is the most sensitive diagnostic method for detecting potentially malignant pancreatic lesions with an 88.5% sensitivity; yet it holds a 52.9% specificity and a higher inter-observer variability. Thus, EUS alone has very low diagnosability capacity[5-7]. Similarly, a considerable number of PCLs cannot be characterized by CT, MRI or MRCP alone[8,9]. EUS-guided diagnostics techniques increase EUS accuracy for differentiating PCLs, namely: (1) EUS-FNA; (2) Contrast-enhanced EUS (CE-EUS); (3) Fiberoptic probe cystoscopy (cystoscopy); (4) EUS-guided through-the-needle direct intracystic micro forceps biopsy (mFB); and (5) EUS-guided confocal laser endomicroscopy (nCLE)[9].
EUS-FNA allows biopsy of suspicious lesions and cytological and biochemical cystic fluid analysis[7]. Whereas, CE-EUS help to differentiate between solid vs PCLs, by detecting enhanced septa or nodules present within cystic lesions[10]. Through-the-needle fiberoptic probe cystoscopy requires a 19-gauge needle guided by EUS to locate and enter the PCL. Then, the preloaded fiberoptic probe is advanced, allowing visualization of the cyst content as cystic wall features[11]. The microforceps device samples tissue from the cyst’s wall, septations, and/or mural nodules and thus increase cellular yield[12]. Furthermore, nCLE characterizes PCLs type by imaging the intact cyst architecture, targeting abnormal areas and reducing unnecessary sampling of surrounding tissue, with a diagnostic accuracy of 80% to 95%[8].
Given the poor prognosis of malignant pancreatic lesions, determining the best diagnostic approach for early detection of potential malignancy among the variety of newly available EUS-related technology is essential. Therefore, we aimed to compare the accuracy of EUS for detection of potentially malignant PCLs when it is performed alone, EUS-FNA, CE-EUS or cystoscopy and associated with novel EUS-related techniques: mFB and nCLE. We hypothesize that EUS-guided through-the-needle mFB and nCLE may increase malignancy detection during EUS assessment of pancreatic cysts.
The following is an observational, analytic, longitudinal, retrospective cohort and single-center study performed at the Instituto Ecuatoriano de Enfermedades Digestivas (IECED), a tertiary center in Ecuador. The study protocol and informed consent documents were approved by the institutional review board, and the study was conducted in accordance with the Declaration of Helsinki. Selected patients signed corresponding informed written consent for healthcare purposes.
Records from patients older than 18 years of age who underwent EUS at IECED from January 2013 to March 2018 were extracted from the institutional database. Cases with non-pancreatic lesions were excluded. Patients were allocated to three cohorts: (1) Patients who had been evaluated via EUS alone; (2) Patients who had been evaluated with EUS-FNA, CE-EUS and/or cystoscopy; and (3) Those evaluated with novel EUS-related techniques: mFB and nCLE.
Due to sparse cellularity of acquired specimens, several complementary clinical, radiological, and imaging techniques are required to achieve PCLs definitive diagnosis. PCLs with potential to progress to malignancy mainly IPMN, mucinous cystic neoplasms (MCN), and neuroendocrine tumors (c-NET) with cystic degeneration. Identifying malignancy features for these lesions with EUS, CE-EUS, cystoscopy, nCLE, FNA, and mFB include the following:
EUS: Presenting two out of the three following characteristics was considered as increased risk for malignancy criteria: main pancreatic duct dilation between 5-9 mm (10 mm high risk stigmata for malignancy), PCLs size > 3 cm, and mural nodules presence[3,13].
CE-EUS: A thick/hyper-enhancing wall/septum, enhancing solid component within a cyst, or an enhancing mural nodule favors malignancy criterion. Furthermore, there is a radiological correlation between pancreatic duct communication and IPMN diagnosis, but not MCN. Also, main duct type IPMNs hold a higher risk of malignancy transformation than branch duct type IPMNs (up to 68% vs 22%, respectively). MCN may show peripheral calcifications within multilocular septate lesions[3,14].
Cystoscopy: Cloudy fluid and a smooth cyst wall identify MCN, while finger-like projections and a mucin cloud are perceived with IPMN through single-operator cholangioscopy (SOC)[11,14].
nCLE: Prone to malignancy lesions may depict epithelial or vascular patterns in nCLE[5,8,11,13,15]. nCLE Epithelial patterns: MCN show epithelial borders with a flat mosaic appearance (single or multiple layers of epithelial bands). IPMN exhibit dark rings and papillary projections. c-NET portray a trabecular pattern (fibrous bands separating cells nests). nCLE Vascular patterns: MCN, IPMN and cystic-NET may show a branched pattern; IPMN and MCN may also display a rope-ladder pattern[5].
EUS-FNA and EUS-mFB are resources for tissue sample extraction. For these techniques, cytology should be assessed in the context of radiological and clinical findings[3,11,14]. Low and high-grade IPMN dysplasia should be distinguished as the latter may easily become invasive. Low-grade IPMN: may resemble normal gastric epithelium. High-grade IPMN may show a cell size ≤ 12 μm, hypo/hyperchromasia, background necrosis, nuclear irregularity, large single vacuolated cells, and increased nuclear to cytoplasmic ratio[14].
IPMNs histologic examinations exhibit four possible morphologies: gastric (columnar cells lining papillae with basally located nuclei rich in apical mucin), intestinal (similar morphology to colonic villous adenomas with cigar shaped nuclei and variable apical mucin amount), pancreaticobiliary (more complex papillae composed of rounded nuclei cuboidal cells with some prominent nucleoli), and oncocytic (complex papillae lined with round cells with granular eosinophilic cytoplasm and prominent central nucleoli)[3,14].
MCNs also display low and high-grade dysplasia features. While bland mucin-containing epithelium honeycomb sheets are seen with low-grade MCNs, a complex papillary structure with smooth nuclear contour mucin-containing cells, inconspicuous nucleoli, and fine chromatin is found in high-grade MCNs. On histologic examination, MCNs show focally flat o cuboidal lining and tall mucin-containing epithelium, with a densely ovarian-type stroma wall that positively stains for progesterone/estrogen receptors, calretinin, and inhibin[3,14].
C-NET aspirate display classic endocrine morphology (pseudorosettes, isolated, and loosely cohesive groups of round/polygonal cells with finely stippled chromatin round nucleus)[5,11,14,15]. Immunostains (chromogranin, CD10, vimectin, and β-catenin cytoplasmic expression) provide a definitive diagnosis[14].
Three experienced endosonographers (C.R-M., J.O., R.V.) performed all EUS evaluations, under general anesthesia with patients in the supine position and use of antibiotic prophylaxis. EUS procedures were performed with a linear-array video echoendoscope (EG-3870 UTK, Pentax Medical, Montalve, NJ, United States) attached to an ultrasound console (HI VISION Avius®, Hitachi Medical Systems, Steinhaus, Switzerland). Indication of EUS-related techniques was based on endosonographers discretion. Although more techniques are available to perform on larger cysts (> 3 cm).
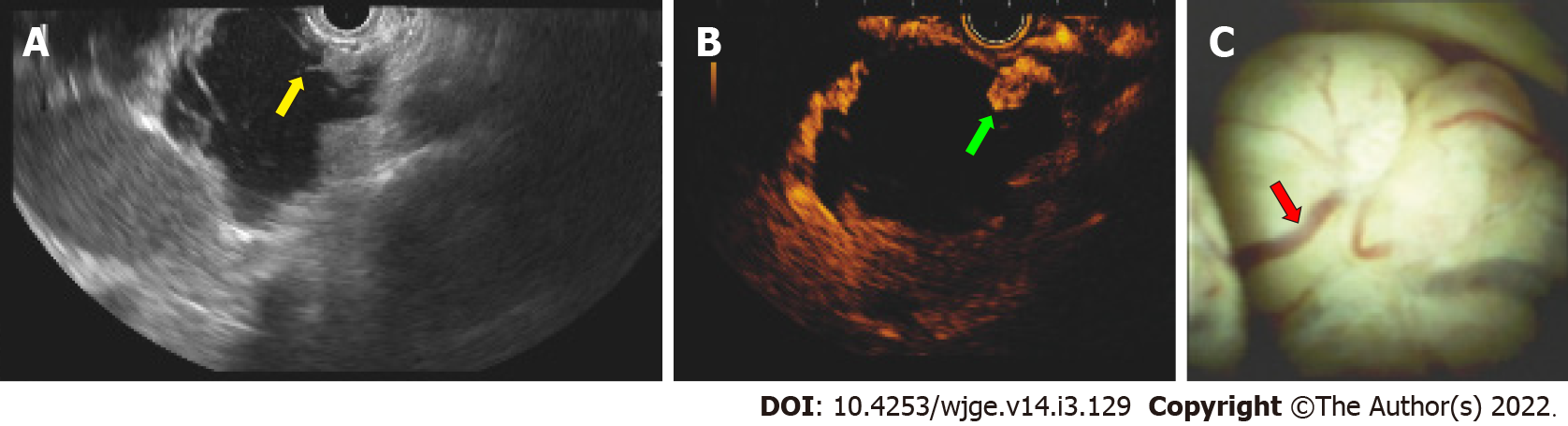
Endoscopic ultrasound fine needle aspiration: EUS-FNA was performed with a 19-gauge needle (Expect™ Slimline, Boston Scientific, Malborough, United States) (Figure 1A). The cystic fluid was examined for tumor markers (amylase, lipase, carcinoembryonic antigen levels).
Contrast enhanced endoscopic ultrasound: To display cystic wall and nodule vascularization, 4.8 mL of SonoVue® (Braccio, Milan, Italy) was used for CE-EUS. Cystic wall and nodule vascularization were defined as visible contrast enhancer bubble movement within the cystic wall, septum, and nodules (Figure 1B), and were referred for further diagnosis with EUS-FNA.
Cystoscopy: Examinations were performed by using a linear-array video echoendoscope attached to an ultrasound console, as previously described. A SOC fiber optic probe (Legacy SpyGlass® fiber optic, Boston Scientific, Marlborough, United States) was inserted through the 19-gauge needle into the cystic cavity to observe the intracystic wall and contents (Figure 1C).
EUS-guided through-the-needle direct intracystic micro forceps biopsy: The target lesion was identified under EUS and punctured with a 19-gauge FNA needle. With the needle inside the lesion, the stylet was removed, and the micro forceps (Moray™ micro forceps, STERIS, Mentor, United States) were inserted through the needle for tissue sampling. Two to three bites of biopsy specimens were taken with each pass of the micro forceps. The tissue acquisition was visually confirmed and directly placed on formalin containers for pathologic evaluation.
EUS-guided confocal laser endomicroscopy: After EUS examination, patients were intravenously injected with 5 mL of 10% fluorescein (BioGlo®, Sofar Productos, Bogota, Colombia) 2 to 3 min before nCLE imaging. CLE was performed using the AQ-Flex nCLE miniprobe (Cellvizio, Mauna Kea Technologies, Paris, France). The probe was advanced through the locking device into the 19-gauge needle. The preloaded needle was advanced under EUS guidance into the PCL. The tip of the nCLE probe was placed in contact with the intracystic epithelium, and intracystic endomicroscopic images were captured (Video 1and Video 2). After image acquisition, the nCLE probe was withdrawn, and the PCL was aspirated.
Demographic, clinic, endoscopic and histopathological and 24-mo follow-up data were obtained from the institutional database and phone calls when necessary. The study endpoint was to determine agreement between detection of potentially malignant in PCLs (EUS malignancy detection) and malignancy after 24-mo follow-up. EUS malignancy detection was defined based on procedure findings (EUS-alone, CE-EUS, cystoscopy and/or nCLE) reported on endoscopic records, as well as EUS-FNA and/or EUS-mFB aquired biopsy results when available. PCLs were classified as malignant (MCN, IPMN and c-NET) according to Fukuoka criteria. This data was recovered by two endoscopists (C.R.M. and H.P-L.). Malignancy after 24-mo follow-up was based on clinical outcomes, endoscopic surveillance, or surgical specimen histopathology when available. This data was recovered by two general practitioners (R.O. and J.B-B.) and a general surgeon (D.C-L.) who were blinded to information concerning to EUS malignancy detection.
An offline interobserver analysis (IOA) of the EUS criteria (EUS borders, lobularity, wall, microcyst component, diagnosis, and level of confidence) was performed by three endoscopists (J.O., R.V. and J.N.) using a randomly selected EUS image set (n = 111 cases) collected by C.R-M.
Technical considerations: Final database was consolidated and encrypted by M.A-M. Data analysis was performed by IECED Institutional Biostatistician (M.P-T.) using R v.4.0 (R Foundation for Statistical Computing, Vienna, Austria). A P-value <0.05 was considered statistically significant.
Sample size calculation: We considered a 100% specificity of EUS + nCLE for the prediction of potentially malignant PCLs, with a 35% disease prevalence (6/31 mucinous cystic neoplasm and 5/31 IPMNs) for defining the sample size (16). We estimated a sample size of 25 patients for each cohort, with an α and β-error of 5% and 20% respectively, and an 80% statistical power.
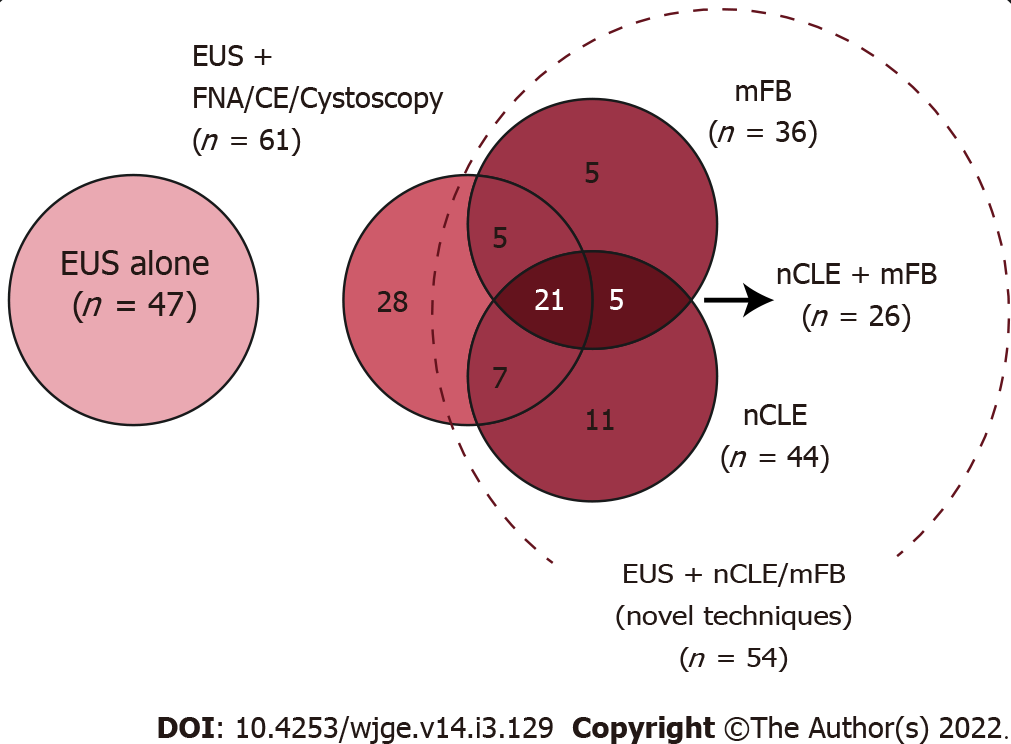
Descriptive analysis: Numeric variables were described through the mean ± SD or median (minimum-maximun range) in accordance with statistical distribution (Kolmógorov-Smirnov test). Categorical variables were described with frequency (%), and 95%CI when corresponding. Descriptions about techniques combination was summarized on a Venn Diagram (17).
Inferential analysis: Observed agreement between EUS malignancy detection and malignancy after 24-mo follow-up was established. The statistical association between EUS alone or EUS with an additional endoscopic technique vs the positive observed agreement described above was determined by binary logistic regression [odds ratio (OR)]. A univariate analysis was performed for each individual technique. Those with a significant association were entered into the multivariate analysis. The overall diagnostic accuracy for malignancy detection was determined for each diagnostic procedure which shown significance on multivariate analysis, considering a 24-mo follow-up as gold standard. Overall diagnostic accuracy comprehended calculation of sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio, negative likelihood ratio, and observed agreement. For multivariate analysis discrimination, we estimated the corresponding area under the receiver operating characteristics (AUROC) curves and contrasting using the DeLong’s test for two ROC curves. The IOA of the EUS criteria was performed using Fleiss’ kappa score (κ) calculation and interpreted based on Landis and Koch criteria.
A total of 2812 patients were referred to our unit for diagnostic EUS along study period. Of these, 856 had pancreatic lesions, of which 129 patients with PCLs were included for analysis (n = 129) (Figure 2).
The median age of the 129 patients with PCLs was 69 years, and 69.8% patients were female. The most frequent pancreatic cyst location was the head of the pancreas (35.7%). Younger patients were significantly evaluated with EUS and an additional novel technique (mFB and/or nCLE) in comparison to those evaluated with EUS alone, EUS-FNA, CE-EUS or cystoscopy (P < 0.001). Cysts size above 30 mm were reported among patients evaluated with EUS and an additional novel technique (46.3%) compared with general cohort (27.1%; P < 0.001). There were no statistically significant differences when comparing gender and PCLs location between patients evaluated with EUS alone and those evaluated with EUS plus additional diagnostic techniques (Table 1).
| Total (n = 129) | EUS alone (n = 47) | EUS + FNA/CE/ Cystoscopy (n = 28) | EUS + mFB/nCLE (novel techniques) (n = 54) | P value | |
| Age (yr), median (range) | 69 (26-97) | 71 (29-97) | 78 (49-92) | 59 (27-97) | < 0.001a |
| Sex (female), n (%) | 90 (69.8) | 33 (70.2) | 19 (67.0) | 38 (70.4) | 0.9694b |
| Pancreatic cyst location, n (%) | 0.6258b | ||||
| Uncinate process | 3 (2.3) | 3 (5.6) | |||
| Head | 46 (35.7) | 17 (36.2) | 9 (32.1) | 20 (37.0) | |
| Neck | 13 (10.1) | 3 (6.4) | 4 (14.3) | 6 (11.1) | |
| Body | 36 (27.9) | 14 (29.8) | 8 (28.6) | 14 (25.9) | |
| Tail | 31 (24.0) | 13 (27.7) | 7 (25.20) | 11 (20.4) | |
| Cyst size (mm), n (%) | |||||
| < 10 mm | 33 (25.6) | 29 (61.7) | 1 (3.6) | 3 (5.6) | < 0.001b |
| 10-30 mm | 61 (47.3) | 16 (34.0) | 19 (67.9) | 26 (48.1) | |
| > 30 mm | 35 (27.1) | 2 (4.3) | 8 (28.6) | 25 (46.3) | |
| Additional endoscopic procedure used for diagnosis1, n (%) | - | ||||
| EUS-FNA | 21 (16.3) | 17 (60.7) | 4 (7.4) | ||
| CE-EUS | 20 (15.5) | 11 (39.3) | 9 (16.7) | ||
| Cystoscopy | 27 (20.9) | 1 (3.6) | 26 (48.1) | ||
| mFB | 36 (27.9) | 36 (66.7) | |||
| nCLE | 44 (34.1) | 44 (81.5) | |||
| Pancreatic cyst diagnosis, n (%) | < 0.001b | ||||
| Malignant2 | 81 (62.8) | 46 (97.9) | 19 (67.9) | 16 (29.6) | |
| Mucinous cystadenocarcinoma | 6 (4.7) | 1 (2.1) | 4 (14.3) | 1 (1.9) | |
| Mucinous cystadenoma | 4 (3.1) | 1 (3.6) | 3 (5.6) | ||
| Intraductal papillary mucinous neoplasm | 70 (54.3) | 45 (95.7) | 14 (50.0) | 11 (20.4) | |
| Neuroendocrine | 1 (0.8) | 1 (1.9) | |||
| Non-malignant2 | 48 (37.2) | 1 (2.1) | 9 (32.1) | 38 (70.4) | |
| Serous cystadenoma | 46 (35.7) | 1 (2.1) | 9 (32.1) | 36 (66.7) | |
| Pseudocysts | 2 (1.6) | 2 (3.7) | |||
| 24-mo follow-up, n (%) | 0.0351b | ||||
| Malignant | 28 (21.7) | 7 (14.9) | 11 (39.3) | 10 (18.5) | |
| Non-malignant | 101 (78.3) | 40 (85.1) | 17 (60.7) | 44 (81.5) | |
| Positive observed agreement between EUS-guided biopsy vs 24-mo follow-up for malignancy detection, n (%) | 70 (54.3) | 8 (17.0) | 18 (64.3) | 44 (81.5) | < 0.001b |
EUS was performed with an additional diagnostic technique in 82/129 patients: EUS-FNA [21/82 (25.6%)], CE-EUS [20/82 (24.4%)], cystoscopy [27/82 (32.9%)], mFB [36/82 (43.9%)], and nCLE [44/82 (53.7%)]. More than one diagnostic technique was performed in a sample proportion (Figure 3). A 100% technical success was reached, with no documented adverse events for any of the performed procedures.
According to the PCLs EUS findings and guided biopsy when available (n = 53), potentially malignant PCLs were detected in 81/129 (62.8%) patients, and the most frequent lesion among this group was IPMN [70/129 (54.3%)]. In the nonmalignant group [48/129 (37.2%)], 46 cases were serous cystadenomas (Table 1). Observed agreement between EUS malignancy detection and malignancy after 24-mo follow-up was higher in patients evaluated with EUS plus at least one additional novel technique (mFB and/or nCLE), followed by EUS-FNA, CE-EUS and or cystoscopy; than in patients evaluated with EUS alone [42/55 (80.0%) vs 18/27 (66.7%) vs 8/47 (17%), respectively; OR 4.35, 95%CI: 2.70-7.37; P < 0.001].
Independently, there was a positive statistical association and observed agreement for EUS malignancy detection with cystoscopy, mFB or nCLE, and 24-mo follow-up. EUS-FNA and CE-EUS exhibited a positive but nonsignificant association; whereas EUS alone only presented a negative significantly association [OR 0.066 (0.025-0.157; P < 0.001)] when considering the agreement between EUS malignancy detection and malignancy after 24-mo follow-up as an outcome.
Through multivariate analysis, we confirmed that malignancy detection was significantly more accurate with nCLE [OR 8.441 (2.698-33.081; P < 0.001)] and mFB [OR 3.425 (1.104-11.682; P = 0.038)] than cystoscopy [OR 0.622 (0.125-2.813; P = 0.541)] (Table 2).
| Univariate analysis1 | Multivariate analysis1 | |
| EUS alone (n = 47) | 0.066 (0.025-0.157; < 0.001) | |
| EUS-FNA (n = 21) | 2.409 (0.905-7.182; 0.091) | |
| CE-EUS (n = 20) | 1.694 (0.642-4.811; 0.298) | |
| Cystoscopy (n = 27) | 4.950 (1.862-15.695; 0.003) | 0.622 (0.125-2.813; 0.541) |
| mFB (n = 36) | 6.625 (2.667-19.024; < 0.001) | 3.425 (1.104-11.682; 0.038) |
| nCLE (n = 44) | 10.489 (4.242-30.125; < 0.001) | 8.441 (2.698-33.081; < 0.001) |
EUS alone was performed in 47 cases and had a sensitivity, specificity, PPV, and NPV of 100%, 3%, 15%, and 100%, respectively. EUS-FNA, CE-EUS, and/or cystoscopy was performed in 28 cases and had a sensitivity, specificity, PPV, and NPV of 91%, 47% 53% and 89%, respectively. EUS with nCLE and mFB yielded similar results for sensitivity (89% vs 88%), specificity (86% vs 82%), PPV (62% vs 58%) and NPV (97% vs 96%). When the three techniques were simultaneously performed (EUS with nCLE and mFB, n = 26), the diagnostic accuracy analysis showed that the sensitivity, specificity, PPV, and NPV were 100%, 89%, 78%, and 100%, respectively. MCC identified a good correlation between EUS malignancy detection and malignancy after the 24-mo follow-up through different techniques. Nonetheless, EUS paired with nCLE and mFB showed the highest agreement (MCC = 0.83) (Table 3).
| EUS alone (n = 47) | EUS + FNA/CE/ Cystoscopy (n = 28) | EUS + mFB (n = 36) | EUS + nCLE (n = 44) | EUS + nCLE + mFB | |
| Sensitivity | 7/7; 100.0% (59.3-100.0) | 10/11; 90.9% (58.7-99.8) | 7/8; 87.5% (47.3-99.7) | 8/9; 88.8%; (51.8-99.7) | 7/7; 100.0% (59.0-100.0) |
| Specificity | 1/40; 2.5% (0.1-13.2) | 8/17; 47.1% (22.9-72.3) | 23/28; 82.1% (63.1-93.9) | 30/35; 85.7% (69.7-95.2) | 17/19; 89.4% (66.9-98.7) |
| PPV | 7/46; 15.2% (6.3-28.9) | 10/19; 52.6% (28.9-75.6) | 7/12; 58.3% (27.7-84.8) | 8/13; 61.5% (31.6-86.1) | 7/9; 77.8% (40.0-97.1) |
| NPV | 1/1; 100.0% (2.5-100.0) | 8/9; 88.9% (51.8-99.7) | 23/24; 95.8% (78.9-99.8) | 30/31; 97% (83-100) | 17/17; 100.0% (80.5-100.0) |
| PLR | 1.03 (0.98-1.08) | 1.72 (1.06-2.79) | 4.90 (2.12-11.31) | 6.22 (2.68-14.47) | 9.50 (2.56-35.24) |
| NLR | n/a | 0.19 (0.03-1.34) | 0.15 (0.02-0.96) | 0.13 (0.02-0.83) | n/a |
| Observed agreement | 8/47 (17%); P = 0.672a | 18/28 (64.3%); P = 0.049a | 30/36 (83.3%); P < 0.001a | 38/44 (86.4%); P < 0.001a | 24/26 (92.3%); P < 0.001a |
| MCC | + 0.06 | + 0.40 | + 0.61 | + 0.66 | + 0.83 |
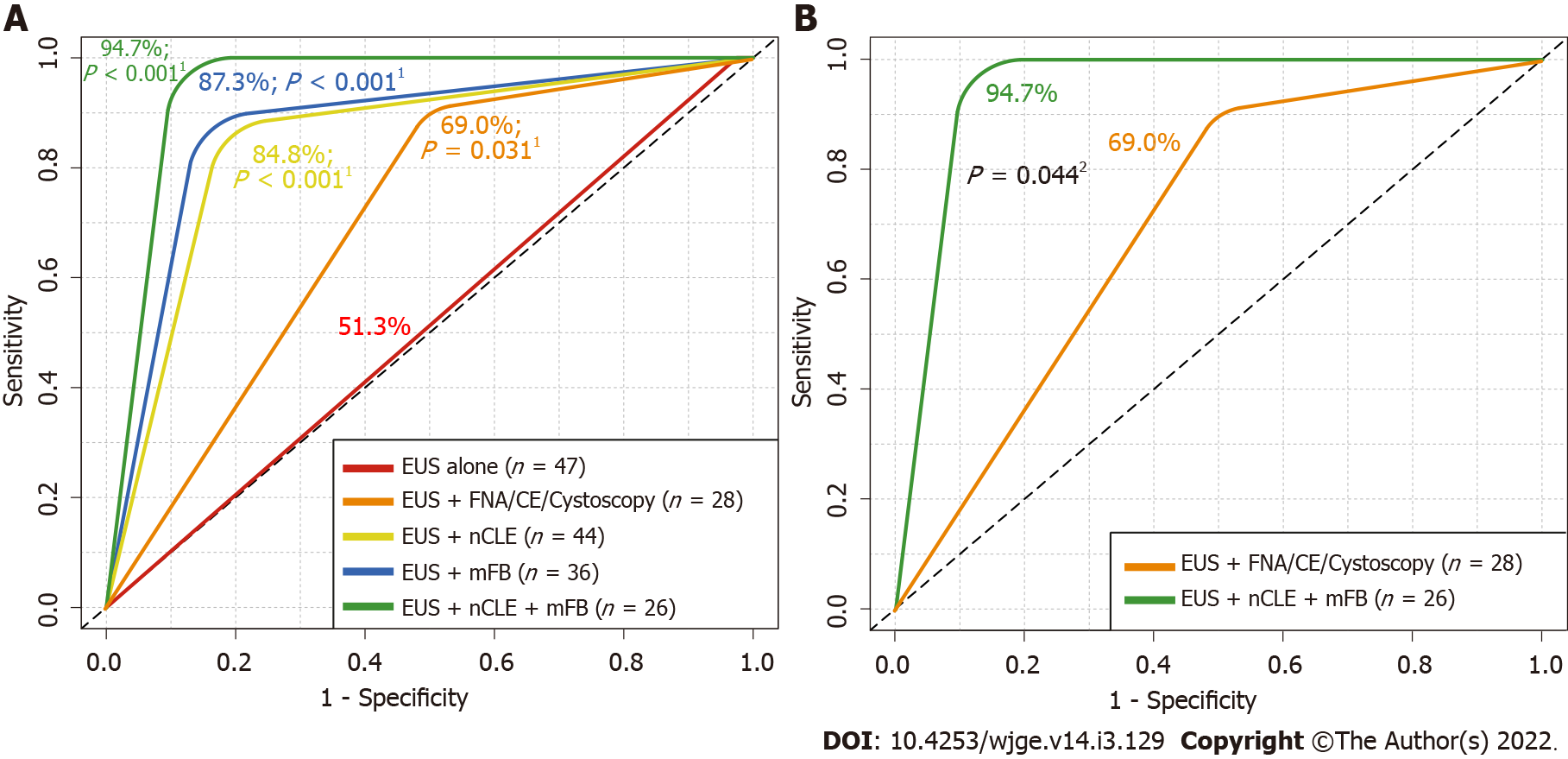
| AU-ROC | 51.3%; P = 0.359b | 69.0%; P = 0.02b | 84.8%; P < 0.001b | 87.3%; P < 0.001b | 94.7%; P < 0.001b |
Detection of potentially malignant PCLs using EUS alone reached a 51.3% AUROC (P = 0.3599; moderate agreement). Meanwhile, EUS-guided mFB, nCLE or/and mFB reached an 87.3% AUROC (P < 0.001), 84.8% (P < 0.001) and 94.7% (P < 0.001), respectively. In addition, nCLE reached a greater AUROC in comparison to EUS alone (P < 0.001) (Figure 4A). Moreover, a significantly higher AUROC was described for combined EUS-guided nCLE and mFB in comparison to EUS-FNA/CE-EUS/cystoscopy (94.7% vs 69%, P = 0.044) (Figure 4B).
In the secondary IOA performed by three experienced endoscopists, the κ values in EUS borders, lobularity, wall, microcyst component, diagnosis, and level of confidence were as follows: 0.12 (poor agreement), 0.08 (poor agreement), 0.04 (poor agreement), 0.29 (fair agreement), 0.21 (fair agreement), and 0.06 (poor agreement) respectively.
Various clinically-available advanced EUS-guided diagnostic techniques have improved the accuracy of malignancy detection among PCLs; however, these techniques are not referenced in current guidelines, with unsatisfactory diagnostic accuracy in the risk stratification of potentially malignant PCLs[4].
To provide guidance on the relative accuracy and effectiveness of these new EUS-related techniques, we compared various additional endoscopic techniques during the EUS evaluation of PCLs. We evaluated the accuracy of EUS alone with more recent EUS-related techniques, namely EUS-FNA, cystoscopy, nCLE, mFB, and CE-EUS and found that the highest level of malignancy detection can be achieved when EUS is combined with both nCLE and direct intracystic mFB.
An increasing number of PCLs have been identified due to the growing use of complementary diagnostic techniques, such as CT and MRI; moreover, the malignancy potential of PCLs vary, and current diagnostic techniques cannot characterize the lesions with precision by their self[18-20]. Due to the malignancy potential, patients with pancreatic neoplasms are recommended to undergo resection therapy; however, for patients with a high risk of postsurgical complications, preoperative determination of malignancy is critical for management guidance.
In our study, EUS alone had a low agreement in comparison to the 24-mo follow-up. Also, in an offline interobserver agreement between three endosonographers, endoscopic criteria showed low agreement between operators, as previously described. Therefore, EUS itself should be complemented with additional endoscopic techniques for a more accurate detection of malignancy in PCLs.
Wang et al[21] demonstrated that EUS-FNA can accurately confirm the presence of malignancy but does not perform well at excluding malignant or premalignant pancreatic lesions. This procedure achieved a pooled sensitivity and specificity of 51%, 94%, respectively, for differentiating malignant lesions. In our study, which included 21/129 patients with pancreatic lesions for whom FNA was performed, we found that EUS-FNA did not achieve statistical significance in detecting malignancy with a modest agreement with the 24-mo follow-up; however, this may be due a limited number of cases in our cohort.
The DETECT trial revealed that a combination of through-the-needle cystoscopy and nCLE for PCLs under EUS was feasible, with a sensitivity of 90% for cystoscopy in the clinical diagnosis of MCNs, an 80%sensitivity for nCLE, and a 100% sensitivity for the combination of both[11]. In our study, we analyzed both techniques (separately and then combined) and obtained similar results – we obtained a sensitivity of 89% for EUS-guided-nCLE and 88% for EUS-guided through-the-needle cystoscopy; however, the sensitivity of EUS-guided nCLE combined with mFB was 78%. Additionally, in our cohort, we had more heterogenic lesions than in the DETECT trial, which was limited to mucinous lesions.
Haghighi et al[8] compared the diagnostic accuracy of nCLE and EUS-FNA, where nCLE was found to have a higher accuracy (87.5%), sensitivity (91.7%), and NPV (93.3%). In our cohort, 44/129 patients underwent nCLE, obtaining similar results (an 86.0% accuracy, an 89% sensitivity, and an NPV of 96%). Konda et al[22] reviewed 31 PCLs that were examined using nCLE, and showed a high specificity (100%) and PPV (100%); and an overall accuracy of 71%. In our study, we obtained a higher sensitivity (89%), NPV (96%) and accuracy (86%) probably owing to a higher number of cases.
EUS-nCLE and mFB exhibited an 86.4% and an 83.3% agreement for PCLs malignancy detection, probably due to a better in vivo cyst component evaluation and guided tissue acquisition. EUS combined with nCLE and mFB reached the highest AUROC (94.7%), in comparison to independent nCLE (87.3%) and mFB (84.8%). We propose that these techniques should be considered for the diagnostic workup of PCLs.
The main limitation of our study lies in its retrospective design and in establishing an agreement of different endoscopic techniques for determining potential malignancy among different types of PCLs. This resulted in a difficulty in the recovery of different size cysts, where the smaller the cyst, the fewer the diagnostic methods at our disposal for use. On the other hand, larger cysts (specially over 30 mm), allowed us to perform a wider array of diagnostic procedures, including novel techniques. Moreover, these novel endoscopic techniques (i.e, nCLE), are costly, limiting their widespread use. Furthermore, these tools require training, which increase the procedure’s startup cost. Despite these limitations, we compared these endoscopic techniques in terms of their ability to detect potential malignancy in patients with PCLs, and not only pancreatic lesions, as with other studies. Finally, as this study was designed in the context of PCLs assessment with EUS, to estimate EUS (and eventual used related techniques) diagnosability of malignancy considering a 24-mo follow-up as gold standard, a prospective diagnostic trial to re-analyse histopathological samples of PCLs after discarding malignancy during follow-up may be warranted to further asses the accuracy in diagnosing high-grade dyspla
In conclusion, new EUS technologies such as through-the-needle techniques (direct intracystic mFB combined with nCLE), improve malignancy detection in patients with PCLs. However, multicenter, and cost-benefit studies are recommended to validate these findings.
Pancreatic cystic lesions (PCLs) incidence is rising mainly in elderly patients. Accurate diagnosing and appropriate management of patients with malignant PCLs, have a positive impact in regards of healthcare expenses and in patients’ quality of life.
Currently, there is insufficient data about the accuracy in the diagnosing of PCLs, especially with novel endoscopic techniques. Furthermore, the early detection of potentially malignant PCLs, increases the possibility of a curative approach in said patients.
Given the poor prognosis of malignant PCLs, attaining early detection, an accurate diagnosis, and determining the best diagnostic approach with newly available endoscopic techniques, was essential to this study.
This was a retrospective, single-center study. Patients were allocated to three evaluation cohorts: (1) Endoscopic ultrasound (EUS) alone; (2) EUS- fine needle aspiration, contrast-enhanced-EUS and/or EUS-guided fiberoptic probe cystoscopy (cystoscopy); and (3) EUS-guided direct intracystic micro-forceps biopsy (mFB) and EUS-guided needle-based confocal laser-endomicroscopy (nCLE); and compared the accuracy of these techniques for the detection of potentially malignant PCLs.
We described that pairing EUS, mFB, and nCLE, had a statistically significant improved detection of potentially malignant PCLs compared to any of the evaluated techniques alone. No adverse events were documented, and a 100% technical success rate was achieved.
In our study, EUS-guided mFB combined with nCLE, improve malignancy detection in patients with PCLs.
To define formal diagnostic and therapeutical guidelines, we encourage researchers to conduct long-term follow-up randomized multicenter and cost-benefit studies, comparing newly available endoscopic techniques for the assessment of PCLs.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Ecuador
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Altonbary AY, Egypt; Ryozawa S, Japan; Sugimoto M, Japan S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Müssle B, Distler M, Wolk S, Shrikhande SV, Aust DE, Arlt A, Weitz J, Hackert T, Welsch T. Management of patients with pancreatic cystic lesions: A case-based survey. Pancreatology. 2017;17:431-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Elta GH, Enestvedt BK, Sauer BG, Lennon AM. ACG Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. Am J Gastroenterol. 2018;113:464-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 423] [Article Influence: 60.4] [Reference Citation Analysis (1)] |
| 3. | Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, Kimura W, Levy P, Pitman MB, Schmidt CM, Shimizu M, Wolfgang CL, Yamaguchi K, Yamao K; International Association of Pancreatology. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1714] [Cited by in RCA: 1614] [Article Influence: 124.2] [Reference Citation Analysis (0)] |
| 4. | Wu J, Wang Y, Li Z, Miao H. Accuracy of Fukuoka and American Gastroenterological Association Guidelines for Predicting Advanced Neoplasia in Pancreatic Cyst Neoplasm: A Meta-Analysis. Ann Surg Oncol. 2019;26:4522-4536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Krishna SG, Brugge WR, Dewitt JM, Kongkam P, Napoleon B, Robles-Medranda C, Tan D, El-Dika S, McCarthy S, Walker J, Dillhoff ME, Manilchuk A, Schmidt C, Swanson B, Shah ZK, Hart PA, Conwell DL. Needle-based confocal laser endomicroscopy for the diagnosis of pancreatic cystic lesions: an international external interobserver and intraobserver study (with videos). Gastrointest Endosc. 2017;86: 644-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 6. | Jang DK, Song BJ, Ryu JK, Chung KH, Lee BS, Park JK, Lee SH, Kim YT, Lee JY. Preoperative Diagnosis of Pancreatic Cystic Lesions: The Accuracy of Endoscopic Ultrasound and Cross-Sectional Imaging. Pancreas. 2015;44:1329-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Lu X, Zhang S, Ma C, Peng C, Lv Y, Zou X. The diagnostic value of EUS in pancreatic cystic neoplasms compared with CT and MRI. Endosc Ultrasound. 2015;4:324-329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Haghighi M, Sethi A, Tavassoly I, Gonda TA, Poneros JM, McBride RB. Diagnosis of Pancreatic Cystic Lesions by Virtual Slicing: Comparison of Diagnostic Potential of Needle-Based Confocal Laser Endomicroscopy versus Endoscopic Ultrasound-Guided Fine-Needle Aspiration. J Pathol Inform. 2019;10:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Durkin C, Krishna SG. Advanced diagnostics for pancreatic cysts: Confocal endomicroscopy and molecular analysis. World J Gastroenterol. 2019;25:2734-2742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Sarno A, Tedesco G, De Robertis R, Marchegiani G, Salvia R, D'Onofrio M. Pancreatic cystic neoplasm diagnosis: Role of imaging. Endosc Ultrasound. 2018;7:297-300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Nakai Y, Iwashita T, Park DH, Samarasena JB, Lee JG, Chang KJ. Diagnosis of pancreatic cysts: EUS-guided, through-the-needle confocal laser-induced endomicroscopy and cystoscopy trial: DETECT study. Gastrointest Endosc. 2015;81:1204-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 134] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 12. | Chen AL, Misdraji J, Brugge WR, Ferrone CR, Pitman MB. Acinar cell cystadenoma: A challenging cytology diagnosis, facilitated by moray® micro-forceps biopsy. Diagn Cytopathol. 2017;45:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Napoleon B, Palazzo M, Lemaistre AI, Caillol F, Palazzo L, Aubert A, Buscail L, Maire F, Morellon BM, Pujol B, Giovannini M. Needle-based confocal laser endomicroscopy of pancreatic cystic lesions: a prospective multicenter validation study in patients with definite diagnosis. Endoscopy. 2019;51:825-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 14. | Abdelkader A, Hunt B, Hartley CP, Panarelli NC, Giorgadze T. Cystic Lesions of the Pancreas: Differential Diagnosis and Cytologic-Histologic Correlation. Arch Pathol Lab Med. 2020;144:47-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (1)] |
| 15. | Napoleon B, Krishna SG, Marco B, Carr-Locke D, Chang KJ, Ginès À, Gress FG, Larghi A, Oppong KW, Palazzo L, Kongkam P, Robles-Medranda C, Sejpal D, Tan D, Brugge WR. Confocal endomicroscopy for evaluation of pancreatic cystic lesions: a systematic review and international Delphi consensus report. Endosc Int Open. 2020;8:E1566-E1581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Yang D, Trindade AJ, Yachimski P, Benias P, Nieto J, Manvar A, Ho S, Esnakula A, Gamboa A, Sethi A, Gupte A, Khara HS, Diehl DL, El Chafic A, Shah J, Forsmark CE, Draganov PV. Histologic Analysis of Endoscopic Ultrasound-Guided Through the Needle Microforceps Biopsies Accurately Identifies Mucinous Pancreas Cysts. Clin Gastroenterol Hepatol. 2019;17:1587-1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 17. |
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK.
limma powers differential expression analyses for RNA-sequencing and microarray studi |
| 18. | Erratum for the Research Article: "Integrated molecular analysis of tumor biopsies on sequential CTLA-4 and PD-1 blockade reveals markers of response and resistance" by W. Roh, P.-L. Chen, A. Reuben, C. N. Spencer, P. A. Prieto, J. P. Miller, V. Gopalakrishnan, F. Wang, Z. A. Cooper, S. M. Reddy, C. Gumbs, L. Little, Q. Chang, W.-S.Chen, K. Wani, M. P. De Macedo, E. Chen, J. L. Austin-Breneman, H. Jiang, J. Roszik, M. T. Tetzlaff, M. A. Davies, J. E. Gershenwald, H. Tawbi, A. J. Lazar, P. Hwu, W.-J. Hwu, A. Diab, I. C. Glitza, S. P. Patel, S. E. Woodman, R. N. Amaria, V. G. Prieto, J. Hu, P. Sharma, J. P. Allison, L. Chin, J. Zhang, J. A. Wargo, P. A. Futreal. Sci Transl Med. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Palazzo M, Sauvanet A, Gincul R, Borbath I, Vanbiervliet G, Bourdariat R, Lemaistre AI, Pujol B, Caillol F, Palazzo L, Aubert A, Maire F, Buscail L, Giovannini M, Marque S, Napoléon B. Impact of needle-based confocal laser endomicroscopy on the therapeutic management of single pancreatic cystic lesions. Surg Endosc. 2020;34:2532-2540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Hashimoto R, Lee JG, Chang KJ, Chehade NEH, Samarasena JB. Endoscopic ultrasound-through-the-needle biopsy in pancreatic cystic lesions: A large single center experience. World J Gastrointest Endosc. 2019;11:531-540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Wang QX, Xiao J, Orange M, Zhang H, Zhu YQ. EUS-Guided FNA for Diagnosis of Pancreatic Cystic Lesions: a Meta-Analysis. Cell Physiol Biochem. 2015;36:1197-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 22. | Konda VJ, Meining A, Jamil LH, Giovannini M, Hwang JH, Wallace MB, Chang KJ, Siddiqui UD, Hart J, Lo SK, Saunders MD, Aslanian HR, Wroblewski K, Waxman I. A pilot study of in vivo identification of pancreatic cystic neoplasms with needle-based confocal laser endomicroscopy under endosonographic guidance. Endoscopy. 2013;45:1006-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 143] [Article Influence: 11.9] [Reference Citation Analysis (0)] |