Published online Feb 16, 2022. doi: 10.4253/wjge.v14.i2.85
Peer-review started: June 14, 2021
First decision: July 27, 2021
Revised: August 2, 2021
Accepted: January 22, 2022
Article in press: January 22, 2022
Published online: February 16, 2022
Processing time: 240 Days and 9.7 Hours
Inflammatory bowel disease (IBD), encompassing Crohn's disease and ulcerative colitis, is a chronic immune-mediated inflammatory disease that primarily affects the gastrointestinal tract and is characterized by periods of activity and remission. The inflammatory activity of the disease involving the colon and rectum increases the risk of colorectal cancer (CRC) over the years. Although prevention strategies are evolving, regular surveillance for early detection of neoplasia as a secondary prevention strategy is paramount in the care of IBD patients. In this review article, we discuss the current evidence of the risks of developing CRC and evaluate the best available strategies for screening and surveillance, as well as future oppor
Core Tip: Colorectal cancer (CRC) is one of the leading causes of death in inflammatory bowel disease (IBD) today. However, subsequent reports have shown lower rates of CRC. The expanding medical options in IBD have substantially improved our ability to control severe inflammation and likely to reduce the risk of CRC in this setting. We discuss the current evidence of the risks of developing CRC, and evaluate the best available strategies for detection and surveillance, as well as future opportunities for cancer prevention.
- Citation: Núñez F P, Quera R, Rubin DT. Endoscopic colorectal cancer surveillance in inflammatory bowel disease: Considerations that we must not forget. World J Gastrointest Endosc 2022; 14(2): 85-95
- URL: https://www.wjgnet.com/1948-5190/full/v14/i2/85.htm
- DOI: https://dx.doi.org/10.4253/wjge.v14.i2.85
Inflammatory bowel disease (IBD) is a chronic, progressive or relapsing and remitting immune-mediated condition of the intestines[1,2]. While the pathogenesis has not been fully elucidated, it is generally considered a consequence of a dysregulated immune response to environmental triggers in genetically predisposed subjects[3,4]. CRC is a major cause of death in IBD, accounting for 10 to 15% of death in IBD[5,6]. CRC risk increases over time after IBD diagnosis. In ulcerative colitis (UC), a prior meta-analysis estimated the CRC risk to be 2%, 8%, and 18% at 10, 20, and 30 years, respectively, after disease diagnosis[7]. This risk is also higher in patients with long-standing and diffuse colonic CD [relative risk (RR) of 4.5 (95%CI: 1.3-4.9)][8]. However, later reports have shown lower rates of left-sided CRC of 2.5%, 7.6%, and 10.8% at 20, 30, and 40 years after diagnosis, respectively[9]. This lower risk may be explained due to successful CRC surveillance programs and better control of mucosal inflammation from early disease stages[10]. The more recent 40-year surveillance experience in the United Kingdom demonstrated decreasing rates of advanced CRC and interval CRC with cumulative incidences of 0.1%, 6.7%, and 10% in the first, third, and fourth decade after diagnosis, respectively[11]. The reasons for decreasing incidences are thought to reflect effective surveillance, access to surgery, and more effective therapies.
Endoscopic surveillance is the primary recommended CRC prevention strategy, with an active search of early-stage cancer or pre-cancerous (dysplastic) lesions[12]. Endoscopic surveillance has been previously suggested to start 8-10 years after IBD diagnosis based on a historical analysis by Eaden et al that showed a CRC risk of 2% 10 years after diagnosis[7]. However, earlier surveillance starting 8 years after diagnosis is modeled to capture an additional 6% of patients developing CRC[13], so newer guidelines embrace this earlier starting time, which may also reflect the emergence of earlier age colorectal cancers described in the population.
Historically, CRC surveillance in patients with IBD has been characterized by extensive four-quadrant non-targeted (random) biopsies to improve the detection of dysplastic mucosa. However, a newer technology that enhances digital mucosal images as high-definition white-light endoscopy (HD-WLE) and dye-assisted chromoendoscopy (CE) with magnification have improved the visualization and detection of early neoplastic lesions, and therefore have increased the diagnostic yield for dysplasia[14,15].
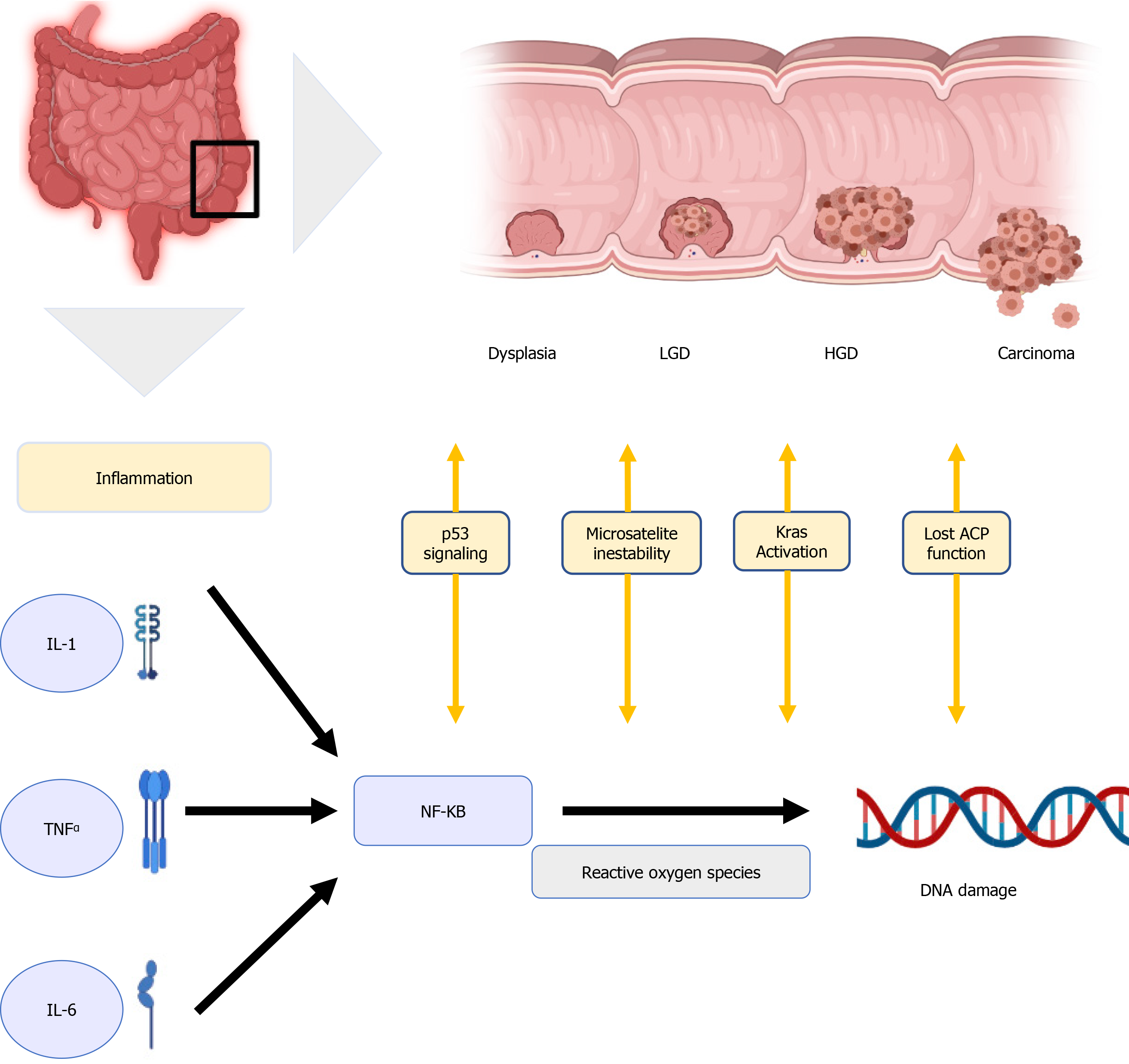
Although the pathogenesis of IBD-related CRC is believed to be different from the pathogenesis of sporadic CRC and CRC that is associated with polyposis and non-polyposis hereditary syndromes, their molecular pathways are similar[16], involving DNA methylation, microsatellite instability, aneuploidy, activation of oncogene Kras, alteration of COX-2 enzymes, and mutation of tumour suppressor genes, with loss of p53 function[17]. One well-known molecular link between cancer and inflammation is the nuclear factor Kappa B (NF-kB)[18]. It can be activated by pro-inflammatory cytokines like interleukin-1 (IL-1), IL-6, and tumor necrosis factor α(TNF-α), ultimately producing reactive oxygen species damaging the DNA and favoring tumor development[19] in Figure 1.
Inflammation plays a central role in carcinogenesis; as a consequence, the severity of flare-ups with accumulated inflammatory damage (persistence of inflammation) predisposes to the development of CCR. Choi et al observed that the accumulative inflammatory burden had a 2-fold increase in the risk of CCR, (95%CI: 1.5 to 2.9; P < 0.001 for endoscopic and 95%CI: 1.4 to 3.0; P < 0.001 for histological) for every 10 years of mild, 5 years of moderate o 3.3 years of severe activity disease[20]. The importance of this finding is that it is based not only on the most recent colonoscopy but also on several colonoscopies in a given time to assess the cumulative effect of inflammation. This persistent inflammation mechanism would explain the predominance of right-sided neoplasia that has been described in PSC patients. In a recent study, UC PSC patients who remain in clinical remission have greater endoscopic and histological activity in the right colon compared to UC patients without PSC[21].
Moreover, chronic inflammation may lead to the development of dysplastic changes in colonic mucosa. These changes can be classified as low-grade dysplasia (LGD), high-grade dysplasia (HGD), or indefinite for dysplasia[22]. LGD is characterized by hyperchromatic enlarged nuclei with preserved cell polarity, decreased mucinous differentiation, and dystrophic goblet cells[23,24]. In contrast, HGD presents as atypical cells with prominent nuclear pleomorphism, hyperchromatic stratified nuclei, and loss of cell polarity, and whenever pathologists cannot distinguish between inflammatory-associated and dysplastic changes, the sample is defined as indefinite for dysplasia[23,24]. This should be distinguished from indeterminate findings, which are usually due to the presence of confounding amounts of histologic inflammation. Given the high inter-observer variability in grading dysplastic changes, guidelines recommend that all cases of suspected dysplasia should be evaluated by two expert pathologists[25,26].
Neoplastic progression can occur multifocally so that dysplasia can be associated with an increased risk of synchronous (simultaneous) or metachronous (six months after diagnosis) dysplasia or carcinoma[25,27].
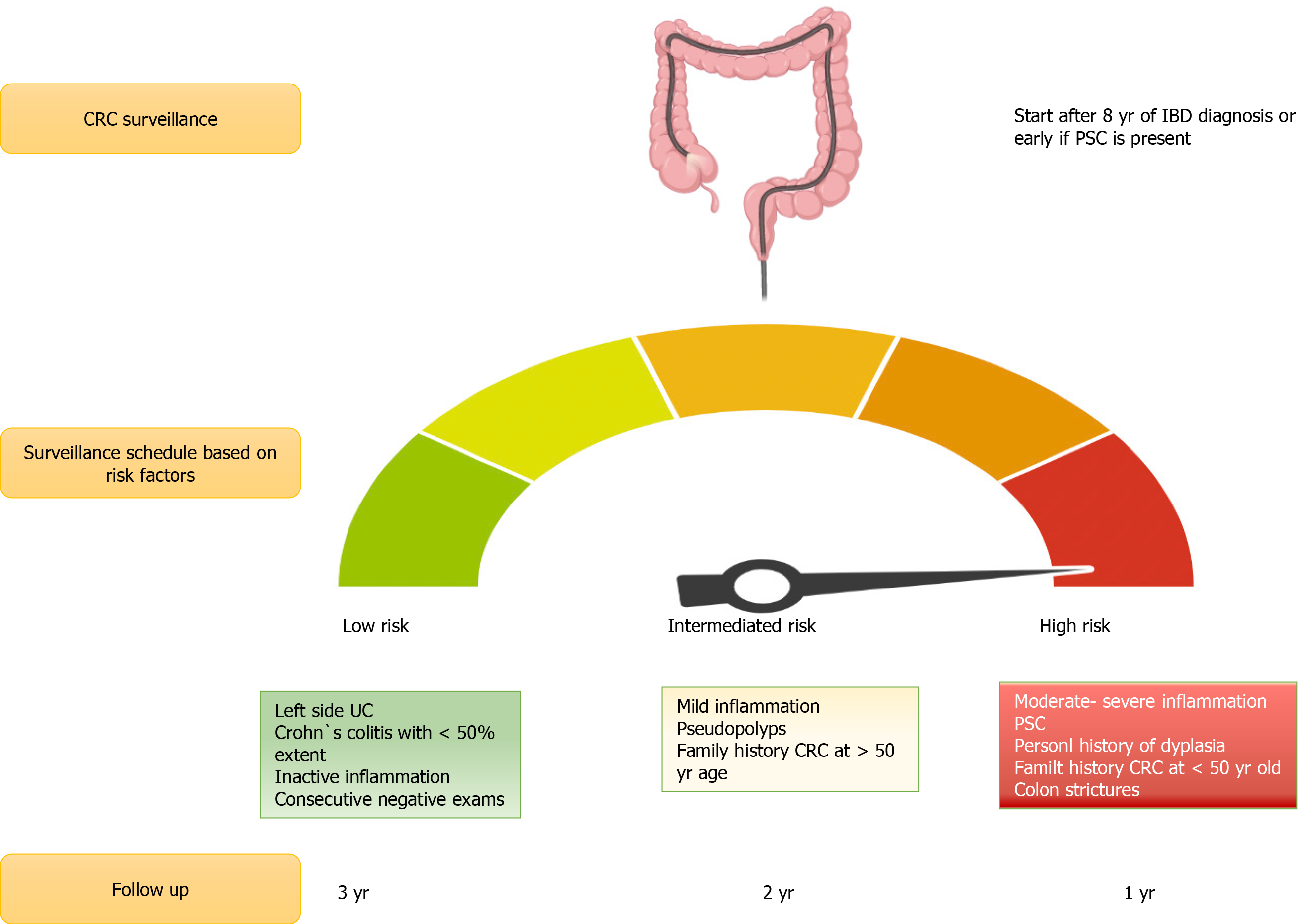
Most relevant CRC risk factors in IBD include longer disease duration, greater disease extent (extensive-pancolitis) and degree of inflammation over time[28,29], family history of CRC[30], personal history of dysplasia or colonic stricture, and diagnosis of primary sclerosing cholangitis (PSC) Table 1[31,32].
| Clinical risk factors | Endoscopic risk factors |
| Disease duration, extension, and severity | Active disease |
| Personal history of dysplasia | Colonic stricture |
| Primary sclerosing cholangitis | Pseudopolys (post-inflammatory polyps) |
| Family history of CRC /dysplasia | Tubular appearance of colon |
Younger age at diagnosis and disease duration have been shown as risk factors for CRC in IBD patients, possibly related to more aggressive phenotypes and longer exposure to mucosal inflammation[33]. A previous meta-analysis showed that patients diagnosed before the age of 30 had a CRC standardized incidence ratio (SIR) of 8.2 (95%CI: 1.8-14.6, I2 82%) compared to patients diagnosed after 30-years-old with an SIR of 1.8 (95%CI: 0.9-2.7, I2 81%)[34]. Also, disease extension in UC has been related to a higher risk of CRC, with SIR of 6.9 (95%CI: 1.9-11.9, I2 84%) for extensive colitis and only 1.7 (CI 95% 0.6-4.5 I2 47%) for left-sided colitis; furthermore, in patients with segmental colitis in CD, there was no higher risk of CRC, with a SIR of 1.7 (95%CI: 0.9-2.6, I2 0%)[35]. There is evidence that IBD patients with a prior family history of CRC have at least a two-fold higher risk of IBD-related CRC (adjusted RR = 2.5; 95%CI: 1.4-4.4); moreover, when CRC family history is associated to first-degree relatives, diagnosed under the age of 50, the risk is even higher (RR = 9.2; 95%CI: 3.7-23)[25,35]. There are some cases of Lynch Syndrome with IBD who develop CRC at a younger age, which are more accelerated and significantly compare with patients without IBD. In this scenario, a colectomy would be necessary due to the high risk of recurrence and multiple CRC[36]. This risk has been seen in UC, and only a few cases in CD, so it does not allow conclusions to be drawn about the risk of CRC[37].
The presence of prior dysplasia or stricture is also associated with an increased risk of neoplasia in IBD[38,39]. Furthermore, colonic strictures in any setting should be considered malignant until proven otherwise.[40] Previous studies have reported variable risk of dysplasia or CRC associated with colonic strictures in UC (from 0% to 86%)[41,42] and there is insufficient data for this risk in CD[43]. Regarding the presence of inflammatory polyps, it is debated if they are related to the development of dysplasia. Historically, case-control studies have reported that patients with inflammatory polyps have 1.9-to-2.5-fold increased risk of CRC[29,44], but recent retrospective cohort studies have suggested that they do not independently predict the development of CRC, nor do they predict progression from LGD to HGD or CRC[20,45].
One major risk factor for CRC in IBD is the presence of concomitant PSC. A previous meta-analysis by Soetikno et al[46] showed that patients with PSC and UC had a higher risk for development of CRC [odds ratio (OR) of 4.09 (95%CI: 2.89-5.76)]. An observational longitudinal cohort study also reported an increased risk for CRC in patients with PSC and UC compared to patients with UC and no PSC with a SIR of 9.8 (95%CI: 1.9-96.6)[47]. Additionally, patients who are in clinical remission have a higher chance of endoscopic and histological inflammation in the right colon compared to UC patients without PSC, being the place where the CCR is most frequently found[21] in Figure 2.
Recommendations for CRC surveillance in IBD vary according to the type of IBD, comorbidities, and previous family history of CRC. According to the current SCENIC consensus statements and ACG guidelines, surveillance colonoscopies should start 8 years after diagnosis in patients with left-sided or extensive UC, and in patients with a colonic CD that comprise more than 30% of the colonic surface or > 1 colonic segment[48,49]. Patients with a first-degree family history of CRC should start surveillance colonoscopies 10 years before the age their relative was diagnosed with CRC or 8 years after IBD diagnosis, whichever occurs first[50]. In patients with IBD and PSC, surveillance colonoscopies should start at diagnosis and be repeated on an annual basis[51]. Surveillance colonoscopy intervals are every 1-3 years, according to each patient risk-stratification[27,52]. Patients with isolated proctitis do not need surveillance colonoscopies[51].
Despite the greater surveillance efforts for early detection of CRC in IBD patients, CRC risk remains significant, and the incidence of interval cases may be due to rapid progression and unclear pathogenesis[53]. In order to perform an optimal evaluation of the colonic mucosa, optimum bowel preparation is essential[54,55].
Several advanced imaging techniques have been developed to improve visualization of mucosal defects, enhancing dysplasia and early CRC detection. High-definition white light endoscopy (HD-WLE) has demonstrated higher adenoma detection than standard definition colonoscopy in patients undergoing screening colonoscopy in non-IBD patients[56]. Chromoendoscopy uses optical or computer/bas
The National Institute for Health and Care Excellence (NICE) and the European Crohn’s and Colitis Organization (ECCO) have recommended the routine use of CE with targeted biopsies in IBD-CRC surveillance in their society guidelines[49]. In 2015 an international expert consensus, SCENIC (Surveillance for Colorectal Endoscopic Neoplasia Detection and Management in Inflammatory Bowel Disease Patients: International Consensus) recommended a surveillance study with high-definition colonoscopy or else the use of dye spray chromoendoscopy if a standard definition white-light exam is performed[20]. Prior to HD- WLE, the standard of care for CRC surveillance included four-quadrant non-targeted (random) biopsies every 10 cm from the cecum to the rectum, with a minimum of 32 biopsies, with the goal of detecting “invisible” dysplasia[64]. This technique intended to sample the mucosa in order to identify “invisible” lesions; we now understand that newer imaging technology, if used by experienced endoscopists, has likely made this approach unnecessary in many patients[65].
Virtual chromoendoscopy (VCE) is an optical imaging technique that uses filters to enhance the contrast of both the mucosa and the superficial vasculature, allowing a better evaluation. In a multicenter study with UC patients comparing DCE vs NBI, no significant difference was reported between these techniques in detecting neoplastic lesions (OR: 1.02 (95%CI: 0.44-2.35, P = 0.964)[66]. A recent randomized controlled trial comparing DCE, VCE, and HD-WLE found that both techniques were non-inferior to DCE[67]. The 2019 ACG guidelines recommend the use of DCE or NBI for the surveillance of dysplasia (conditional recommendation, low quality of evidence)[50].
Despite their low yield, random biopsies may have a role when performed in association with CE in IBD patients with a personal history of neoplasia, an appearing tubular colon, or concomitant PSC. A French multicenter study performed quadrantic random biopsies every 10 cm in patients with a personal history of neoplasia, showing that 12.8% of neoplasia can be detected[68]. Saravia et al[69] consider that random biopsies should be performed when CE is not available or when WLE is used in the presence of inflammation or high-risk factors.
Artificial intelligence (AI) is evolving as a topic of interest in the field of gastrointestinal endoscopy. AI has been used in endoscopic polyp detection; no studies on AI in IBD surveillance have been published so far[70].
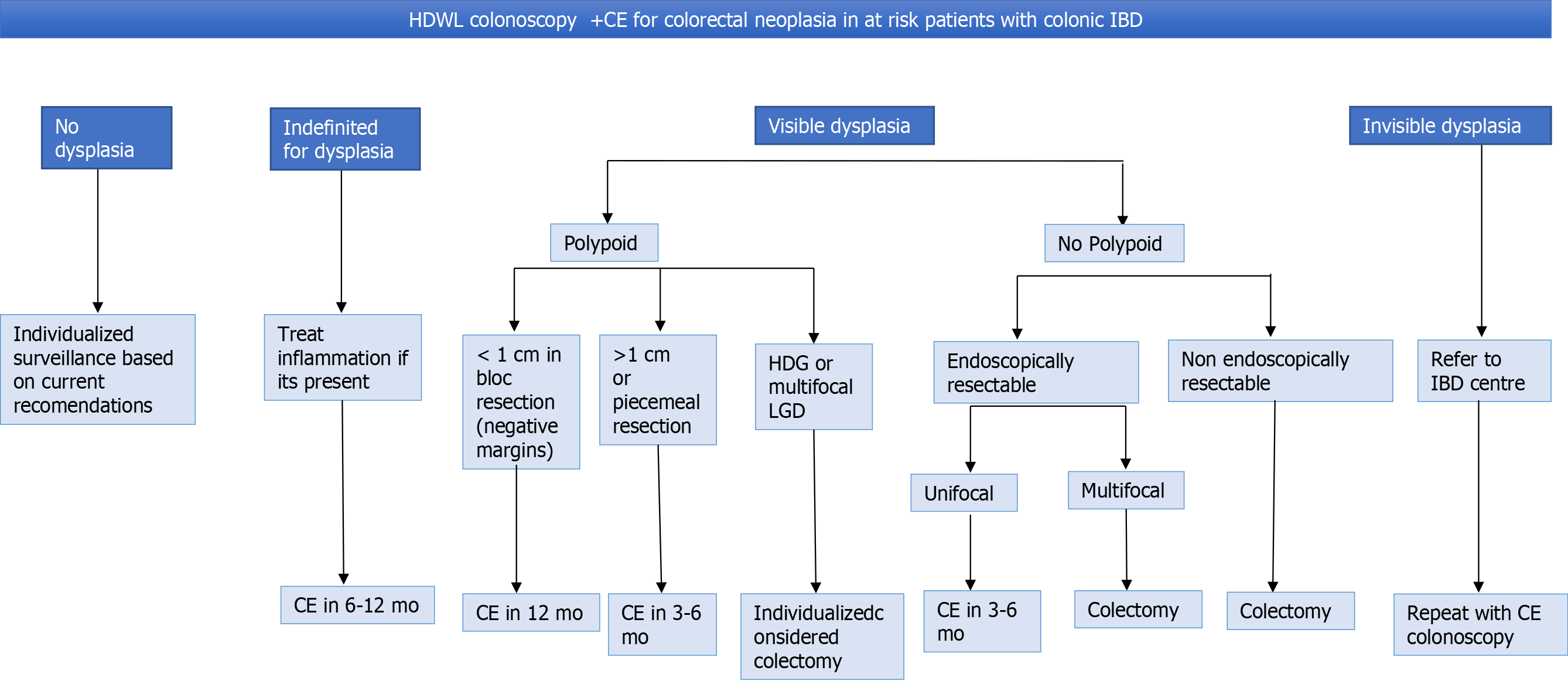
It is important to distinguishing polypoid from non-polypoid lesions, due to their different management, prognosis, and follow-up[71]. A meta-analysis performed by Wanders et al showed that patients with polypoid lesions had a lower incidence of CRC compared to patients with non-polypoid lesions, which was attributed to the complete endoscopic resection of the first type of lesions[72].
Less than 1 cm polypoid lesions (with negative margins) should be followed up with colonoscopy at 12 mo. For lesions greater than 1 cm or lesions that have been removed piecemeal, surveillance colonoscopy should be performed within 3-6 mo[49]. LGD had a low risk of progression to HGD or CRC from an incomplete resection if it is unifocal. In contrast, multifocal LGD carries substantial risk[73]. The rate of progression from LGD vs HGD to adenocarcinoma was significantly greater for HGD (P < 0.001)[74]. Although most dysplasias were found in the right colon, being higher in UC, the rate of progression of LGD and HGD dysplasia or adenocarcinoma was not significantly different in CD vs UC[75]. A Dutch nationwide cohort study observed that the cumulative incidence of advanced neoplasia was 21.7% after 15 years of follow-up. Male sex, older age at LDG (> 55 years), and follow-up by a tertiary IBD referral center were independent risk factors for advanced neoplasia[76]. The management of HGD in a visible lesion with complete resection is controversial. The decision should be made case by case between colectomy vs shorter follow-up[77].
In cases of non-polypoid dysplasia, classically, these were sent to colectomy. However, if there is complete resection, it can be followed up instead of colectomy but, always evaluating progression factors[78].
For endoscopically invisible LGD (found only on random biopsy), it should be referred to an IBD Centre or endoscopist with experience at high-risk surveillance. Surveillance endoscopy using CE with HD-WLE is required in an attempt to identify the neoplastic lesion (or others) and to remove it endoscopically[79]. In Figure 3, the management of dysplasia/LGD and HGD is summarized.
It is essential to know which risk factors affect the CRC risk in every IBD patient, allowing to identify the subgroups of patients who need closer surveillance and more intensive treatment. The risk of CRC is increased in IBD but not as high as previously reported. The expanding medical options in IBD have substantially improved our ability to control severe inflammation and likely to reduce the risk of CRC. The advance of new technologies allows us a better characterization of lesions and treat them on time.
Prospective studies to monitor the rate of interval cancer, the cost-effectiveness of surveillance programs are needed.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Batyrbekov K, KAMADA Y, Triantafillidis J, Yoshimatsu K S-Editor: Wang LL L-Editor: A P-Editor: Wang LL
| 1. | Ungaro R, Mehandru S, Allen PB, Peyrin-Birolet L, Colombel JF. Ulcerative colitis. Lancet. 2017;389:1756-1770. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2199] [Cited by in RCA: 2480] [Article Influence: 310.0] [Reference Citation Analysis (2)] |
| 2. | Loftus EV Jr. Crohn's Disease: Etiology, Complications, Assessment, Therapy, and Management. Gastroenterol Clin North Am. 2017;46:xiii-xixv. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 3. | Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol. 2015;12:205-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 930] [Cited by in RCA: 1238] [Article Influence: 123.8] [Reference Citation Analysis (0)] |
| 4. | Cosnes J, Beaugerie L, Carbonnel F, Gendre JP. Smoking cessation and the course of Crohn's disease: an intervention study. Gastroenterology. 2001;120:1093-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 279] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 5. | Dyson JK, Rutter MD. Colorectal cancer in inflammatory bowel disease: what is the real magnitude of the risk? World J Gastroenterol. 2012;18:3839-3848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 147] [Cited by in RCA: 161] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 6. | Munkholm P. Review article: the incidence and prevalence of colorectal cancer in inflammatory bowel disease. Aliment Pharmacol Ther. 2003;18 Suppl 2:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 395] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 7. | Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1985] [Cited by in RCA: 2077] [Article Influence: 86.5] [Reference Citation Analysis (1)] |
| 8. | Canavan C, Abrams KR, Mayberry J. Meta-analysis: colorectal and small bowel cancer risk in patients with Crohn's disease. Aliment Pharmacol Ther. 2006;23:1097-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 424] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 9. | Rutter MD, Saunders BP, Wilkinson KH, Rumbles S, Schofield G, Kamm MA, Williams CB, Price AB, Talbot IC, Forbes A. Thirty-year analysis of a colonoscopic surveillance program for neoplasia in ulcerative colitis. Gastroenterology. 2006;130:1030-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 451] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 10. | Herrinton LJ, Liu L, Levin TR, Allison JE, Lewis JD, Velayos F. Incidence and mortality of colorectal adenocarcinoma in persons with inflammatory bowel disease from 1998 to 2010. Gastroenterology. 2012;143:382-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 246] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 11. | Choi CH, Rutter MD, Askari A, Lee GH, Warusavitarne J, Moorghen M, Thomas-Gibson S, Saunders BP, Graham TA, Hart AL. Forty-Year Analysis of Colonoscopic Surveillance Program for Neoplasia in Ulcerative Colitis: An Updated Overview. Am J Gastroenterol. 2015;110:1022-1034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 211] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 12. | Bye WA, Ma C, Nguyen TM, Parker CE, Jairath V, East JE. Strategies for Detecting Colorectal Cancer in Patients with Inflammatory Bowel Disease: A Cochrane Systematic Review and Meta-Analysis. Am J Gastroenterol. 2018;113:1801-1809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 13. | Lutgens MW, Vleggaar FP, Schipper ME, Stokkers PC, van der Woude CJ, Hommes DW, de Jong DJ, Dijkstra G, van Bodegraven AA, Oldenburg B, Samsom M. High frequency of early colorectal cancer in inflammatory bowel disease. Gut. 2008;57:1246-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 148] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 14. | Gasia MF, Ghosh S, Panaccione R, Ferraz JG, Kaplan GG, Leung Y, Novak KL, Seow CH, Iacucci M. Targeted Biopsies Identify Larger Proportions of Patients With Colonic Neoplasia Undergoing High-Definition Colonoscopy, Dye Chromoendoscopy, or Electronic Virtual Chromoendoscopy. Clin Gastroenterol Hepatol. 2016;14:704-12.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | Ananthakrishnan AN. Chromoendoscopy Is Better: So Why Am I Not (yet) Using it for Routine Inflammatory Bowel Disease Surveillance? Clin Gastroenterol Hepatol. 2016;14:720-722. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Fogt F, Vortmeyer AO, Goldman H, Giordano TJ, Merino MJ, Zhuang Z. Comparison of genetic alterations in colonic adenoma and ulcerative colitis-associated dysplasia and carcinoma. Hum Pathol. 1998;29:131-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 77] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Axelrad JE, Lichtiger S, Yajnik V. Inflammatory bowel disease and cancer: The role of inflammation, immunosuppression, and cancer treatment. World J Gastroenterol. 2016;22:4794-4801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 285] [Cited by in RCA: 343] [Article Influence: 38.1] [Reference Citation Analysis (4)] |
| 18. | Kosinsky RL, Chua RL, Qui M, Saul D, Mehlich D, Ströbel P, Schildhaus HU, Wegwitz F, Faubion WA, Johnsen SA. Loss of RNF40 Decreases NF-κB Activity in Colorectal Cancer Cells and Reduces Colitis Burden in Mice. J Crohns Colitis. 2019;13:362-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Nadeem MS, Kumar V, Al-Abbasi FA, Kamal MA, Anwar F. Risk of colorectal cancer in inflammatory bowel diseases. Semin Cancer Biol. 2020;64:51-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 171] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 20. | Choi CR, Al Bakir I, Ding NJ, Lee GH, Askari A, Warusavitarne J, Moorghen M, Humphries A, Ignjatovic-Wilson A, Thomas-Gibson S, Saunders BP, Rutter MD, Graham TA, Hart AL. Cumulative burden of inflammation predicts colorectal neoplasia risk in ulcerative colitis: a large single-centre study. Gut. 2019;68:414-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 128] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 21. | Krugliak Cleveland N, Rubin DT, Hart J, Weber CR, Meckel K, Tran AL, Aelvoet AS, Pan I, Gonsalves A, Gaetano JN, Williams KM, Wroblewski K, Jabri B, Pekow J. Patients With Ulcerative Colitis and Primary Sclerosing Cholangitis Frequently Have Subclinical Inflammation in the Proximal Colon. Clin Gastroenterol Hepatol. 2018;16:68-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 22. | Greenson JK. Dysplasia in inflammatory bowel disease. Semin Diagn Pathol. 2002;19:31-37. [PubMed] |
| 23. | Itzkowitz SH, Harpaz N. Diagnosis and management of dysplasia in patients with inflammatory bowel diseases. Gastroenterology. 2004;126:1634-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 311] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 24. | Feakins RM; British Society of Gastroenterology. Inflammatory bowel disease biopsies: updated British Society of Gastroenterology reporting guidelines. J Clin Pathol. 2013;66:1005-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 129] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 25. | Mattar MC, Lough D, Pishvaian MJ, Charabaty A. Current management of inflammatory bowel disease and colorectal cancer. Gastrointest Cancer Res. 2011;4:53-61. [PubMed] |
| 26. | Cairns SR, Scholefield JH, Steele RJ, Dunlop MG, Thomas HJ, Evans GD, Eaden JA, Rutter MD, Atkin WP, Saunders BP, Lucassen A, Jenkins P, Fairclough PD, Woodhouse CR; British Society of Gastroenterology; Association of Coloproctology for Great Britain and Ireland. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut. 2010;59:666-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 897] [Cited by in RCA: 808] [Article Influence: 53.9] [Reference Citation Analysis (2)] |
| 27. | Ibraheim H, Dhillon AS, Koumoutsos I, Gulati S, Hayee B. Curriculum review: colorectal cancer surveillance and management of dysplasia in IBD. Frontline Gastroenterol. 2018;9:271-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Rubin DT, Turner JR. Surveillance of dysplasia in inflammatory bowel disease: The gastroenterologist-pathologist partnership. Clin Gastroenterol Hepatol. 2006;4:1309-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Jess T, Rungoe C, Peyrin-Biroulet L. Risk of colorectal cancer in patients with ulcerative colitis: a meta-analysis of population-based cohort studies. Clin Gastroenterol Hepatol. 2012;10:639-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 660] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 30. | Jess T, Horváth-Puhó E, Fallingborg J, Rasmussen HH, Jacobsen BA. Cancer risk in inflammatory bowel disease according to patient phenotype and treatment: a Danish population-based cohort study. Am J Gastroenterol. 2013;108:1869-1876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 189] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 31. | Velayos FS, Loftus EV Jr, Jess T, Harmsen WS, Bida J, Zinsmeister AR, Tremaine WJ, Sandborn WJ. Predictive and protective factors associated with colorectal cancer in ulcerative colitis: A case-control study. Gastroenterology. 2006;130:1941-1949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 254] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 32. | Wang R, Leong RW. Primary sclerosing cholangitis as an independent risk factor for colorectal cancer in the context of inflammatory bowel disease: a review of the literature. World J Gastroenterol. 2014;20:8783-8789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 33. | Loftus EV Jr, Harewood GC, Loftus CG, Tremaine WJ, Harmsen WS, Zinsmeister AR, Jewell DA, Sandborn WJ. PSC-IBD: a unique form of inflammatory bowel disease associated with primary sclerosing cholangitis. Gut. 2005;54:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 518] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 34. | Wijnands AM, de Jong ME, Lutgens MWMD, Hoentjen F, Elias SG, Oldenburg B; Dutch Initiative on Crohn and Colitis (ICC). Prognostic Factors for Advanced Colorectal Neoplasia in Inflammatory Bowel Disease: Systematic Review and Meta-analysis. Gastroenterology. 2021;160:1584-1598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 152] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 35. | Lutgens MW, van Oijen MG, van der Heijden GJ, Vleggaar FP, Siersema PD, Oldenburg B. Declining risk of colorectal cancer in inflammatory bowel disease: an updated meta-analysis of population-based cohort studies. Inflamm Bowel Dis. 2013;19:789-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 376] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 36. | Askling J, Dickman PW, Karlén P, Broström O, Lapidus A, Löfberg R, Ekbom A. Family history as a risk factor for colorectal cancer in inflammatory bowel disease. Gastroenterology. 2001;120:1356-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 283] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 37. | McNamara KL, Aronson MD, Cohen Z. Is there a role for prophylactic colectomy in Lynch syndrome patients with inflammatory bowel disease? Int J Colorectal Dis. 2016;31:9-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 38. | Derikx LAAP, Simits LJT, van Vliet S, Dekker E, Aalf CM, van Kouwen MCA, Nagengast FM, Nagtegaal ID, Hoogerbrugge N, Hoentjen F. Colorectal Cancer risk in patients with Lynch Syndrome and Inflamatory bowel disease. Clin Gastroenterol Hepatol. 2017;15:454-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 39. | Coviello LC, Stein SL. Surgical management of nonpolypoid colorectal lesions and strictures in colonic inflammatory bowel disease. Gastrointest Endosc Clin N Am. 2014;24:447-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | Kristo I, Riss S, Argeny S, Maschke S, Chitsabesan P, Stift A. Incidental adenocarcinoma in patients undergoing surgery for structuring Crohn`s disease. World J Gastroenterol. 2017;23:472-477. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 41. | Gumaste V, Sachar DB, Greenstein AJ. Benign and malignant colorectal strictures in ulcerative colitis. Gut. 1992;33:938-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 141] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 42. | Rutter MD, Saunders BP, Wilkinson KH, Rumbles S, Schofield G, Kamm MA, Williams CB, Price AB, Talbot IC, Forbes A. Cancer surveillance in longstanding ulcerative colitis: endoscopic appearances help predict cancer risk. Gut. 2004;53:1813-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 299] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 43. | Lashner BA, Turner BC, Bostwick DG, Frank PH, Hanauer SB. Dysplasia and cancer complicating strictures in ulcerative colitis. Dig Dis Sci. 1990;35:349-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 63] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 44. | Yamazaki Y, Ribeiro MB, Sachar DB, Aufses AH Jr, Greenstein AJ. Malignant colorectal strictures in Crohn's disease. Am J Gastroenterol. 1991;86:882-885. [PubMed] |
| 45. | Baars JE, Looman CW, Steyerberg EW, Beukers R, Tan AC, Weusten BL, Kuipers EJ, van der Woude CJ. The risk of inflammatory bowel disease-related colorectal carcinoma is limited: results from a nationwide nested case-control study. Am J Gastroenterol. 2011;106:319-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 46. | Mahmoud R, Shah SC, Ten Hove JR, Torres J, Mooiweer E, Castaneda D, Glass J, Elman J, Kumar A, Axelrad J, Ullman T, Colombel JF, Oldenburg B, Itzkowitz SH; Dutch Initiative on Crohn and Colitis. No Association Between Pseudopolyps and Colorectal Neoplasia in Patients With Inflammatory Bowel Diseases. Gastroenterology. 2019;156:1333-1344.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 47. | Soetikno RM, Lin OS, Heidenreich PA, Young HS, Blackstone MO. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis: a meta-analysis. Gastrointest Endosc. 2002;56:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 383] [Article Influence: 16.7] [Reference Citation Analysis (1)] |
| 48. | Boonstra K, Weersma RK, van Erpecum KJ, Rauws EA, Spanier BW, Poen AC, van Nieuwkerk KM, Drenth JP, Witteman BJ, Tuynman HA, Naber AH, Kingma PJ, van Buuren HR, van Hoek B, Vleggaar FP, van Geloven N, Beuers U, Ponsioen CY; EpiPSCPBC Study Group. Population-based epidemiology, malignancy risk, and outcome of primary sclerosing cholangitis. Hepatology. 2013;58:2045-2055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 504] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 49. | Laine L, Kaltenbach T, Barkun A, McQuaid KR, Subramanian V, Soetikno R; SCENIC Guideline Development Panel. SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastroenterology. 2015;148:639-651.e28. [PubMed] |
| 50. | Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. ACG Clinical Guideline: Ulcerative Colitis in Adults. Am J Gastroenterol. 2019;114:384-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 870] [Cited by in RCA: 1039] [Article Influence: 173.2] [Reference Citation Analysis (0)] |
| 51. | Axelrad JE, Shah SC. Diagnosis and management of inflammatory bowel disease-associated neoplasia: considerations in the modern era. Therap Adv Gastroenterol. 2020;13:1756284820920779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 52. | Eaden JA, Mayberry JF; British Society for Gastroenterology; Association of Coloproctology for Great Britain and Ireland. Guidelines for screening and surveillance of asymptomatic colorectal cancer in patients with inflammatory bowel disease. Gut. 2002;51 Suppl 5:V10-V12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 53. | Magro F, Gionchetti P, Eliakim R, Ardizzone S, Armuzzi A, Barreiro-de Acosta M, Burisch J, Gecse KB, Hart AL, Hindryckx P, Langner C, Limdi JK, Pellino G, Zagórowicz E, Raine T, Harbord M, Rieder F; European Crohn’s and Colitis Organisation [ECCO]. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. J Crohns Colitis. 2017;11:649-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1446] [Cited by in RCA: 1294] [Article Influence: 161.8] [Reference Citation Analysis (0)] |
| 54. | Wang YR, Cangemi JR, Loftus EV Jr, Picco Michael F. Rate of early/missed colorectal cancers after colonoscopy in older patients with or without inflammatory bowel disease in the United States. Am J Gastroenterol. 2013;108:444-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 55. | Megna B, Weiss J, Ley D, Saha S, Pfau P, Grimes I, Li Z, Caldera F. Clear liquid diet before bowel preparation predicts successful chromoendoscopy in patients with inflammatory bowel disease. Gastrointest Endosc. 2019;89:373-379.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 56. | Sanduleanu S, Kaltenbach T, Barkun A, McCabe RP, Velayos F, Picco MF, Laine L, Soetikno R, McQuaid KR. A roadmap to the implementation of chromoendoscopy in inflammatory bowel disease colonoscopy surveillance practice. Gastrointest Endosc. 2016;83:213-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 57. | Buchner AM, Shahid MW, Heckman MG, McNeil RB, Cleveland P, Gill KR, Schore A, Ghabril M, Raimondo M, Gross SA, Wallace MB. High-definition colonoscopy detects colorectal polyps at a higher rate than standard white-light colonoscopy. Clin Gastroenterol Hepatol. 2010;8:364-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 58. | American Society for Gastrointestinal Endoscopy Standards of Practice Committee. , Shergill AK, Lightdale JR, Bruining DH, Acosta RD, Chandrasekhara V, Chathadi KV, Decker GA, Early DS, Evans JA, Fanelli RD, Fisher DA, Fonkalsrud L, Foley K, Hwang JH, Jue TL, Khashab MA, Muthusamy VR, Pasha SF, Saltzman JR, Sharaf R, Cash BD, DeWitt JM. The role of endoscopy in inflammatory bowel disease. Gastrointest Endosc. 2015;81:1101-21.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 259] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 59. | Mooiweer E, van der Meulen-de Jong AE, Ponsioen CY, Fidder HH, Siersema PD, Dekker E, Oldenburg B. Chromoendoscopy for Surveillance in Inflammatory Bowel Disease Does Not Increase Neoplasia Detection Compared With Conventional Colonoscopy With Random Biopsies: Results From a Large Retrospective Study. Am J Gastroenterol. 2015;110:1014-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 60. | Tontini GE, Rath T, Neumann H. Advanced gastrointestinal endoscopic imaging for inflammatory bowel diseases. World J Gastroenterol. 2016;22:1246-1259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 61. | Mönkemüller K, Fry LC, Zimmermann L, Mania A, Zabielski M, Jovanovic I. Advanced endoscopic imaging methods for colon neoplasia. Dig Dis. 2010;28:629-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 62. | East JE, Vleugels JL, Roelandt P, Bhandari P, Bisschops R, Dekker E, Hassan C, Horgan G, Kiesslich R, Longcroft-Wheaton G, Wilson A, Dumonceau JM. Advanced endoscopic imaging: European Society of Gastrointestinal Endoscopy (ESGE) Technology Review. Endoscopy. 2016;48:1029-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 140] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 63. | Feuerstein JD, Rakowsky S, Sattler L, Yadav A, Foromera J, Grossberg L, Cheifetz AS. Meta-analysis of dye-based chromoendoscopy compared with standard- and high-definition white-light endoscopy in patients with inflammatory bowel disease at increased risk of colon cancer. Gastrointest Endosc. 2019;90:186-195.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 64. | Clarke K, Kang M, Gorrepati VS, Stine JG, Tinsley A, Williams E, Moyer M, Coates M. Dysplasia detection is similar between chromoendoscopy and high-definition white-light colonoscopy in inflammatory bowel disease patients: a US-matched case-control study. Int J Colorectal Dis. 2020;35:2301-2307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 65. | Eaden JA, Ward BA, Mayberry JF. How gastroenterologists screen for colonic cancer in ulcerative colitis: an analysis of performance. Gastrointest Endosc. 2000;51:123-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 148] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 66. | Kabir M, Fofaria R, Arebi N, Bassett P, Tozer PJ, Hart AL, Thomas-Gibson S, Humphries A, Suzuki N, Saunders B, Warusavitarne J, Faiz O, Wilson A. Systematic review with meta-analysis: IBD-associated colonic dysplasia prognosis in the videoendoscopic era (1990 to present). Aliment Pharmacol Ther. 2020;52:5-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 67. | Iacucci M, Furfaro F, Matsumoto T, Uraoka T, Smith S, Gosh S, Kliesslich. Advanced endoscopic techniques in the assessment of inflammatory bowel disease: new technology, new era. Gut. 2019;68:562-572.. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 68. | Bisschops R, Bessissow T, Joseph JA, Baert F, Ferrante M, Ballet V, Willekens H, Demedts I, Geboes K, De Hertogh G, Vermeire S, Rutgeerts P, Van Assche G. Chromoendoscopy vs narrow band imaging in UC: a prospective randomised controlled trial. Gut. 2018;67:1087-1094. [RCA] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 69. | Moussata D, Allez M, Cazals-Hatem D, Treton X, Laharie D, Reimund JM, Bertheau P, Bourreille A, Lavergne-Slove A, Brixi H, Branche J, Gornet JM, Stefanescu C, Moreau J, Marteau P, Pelletier AL, Carbonnel F, Seksik P, Simon M, Fléjou JF, Colombel JF, Charlois AL, Roblin X, Nancey S, Bouhnik Y, Berger F, Flourié B; the GETAID. Are random biopsies still useful for the detection of neoplasia in patients with IBD undergoing surveillance colonoscopy with chromoendoscopy? Gut. 2018;67:616-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 70. | Saraiva S, Rosa I, Moleiro J, Pereira da Silva J, Fonseca R, Dias Pereira A. Dysplasia Surveillance in Inflammatory Bowel Disease: A Cohort Study. GE Port J Gastroenterol. 2021;28:97-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 71. | van der Laan JJH, van der Waaij AM, Gabriëls RY, Festen EAM, Dijkstra G, Nagengast WB. Endoscopic imaging in inflammatory bowel disease: current developments and emerging strategies. Expert Rev Gastroenterol Hepatol. 2021;15:115-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 72. | Voorham QJ, Rondagh EJ, Knol DL, van Engeland M, Carvalho B, Meijer GA, Sanduleanu S. Tracking the molecular features of nonpolypoid colorectal neoplasms: a systematic review and meta-analysis. Am J Gastroenterol. 2013;108:1042-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 73. | Wanders LK, Dekker E, Pullens B, Bassett P, Travis SP, East JE. Cancer risk after resection of polypoid dysplasia in patients with longstanding ulcerative colitis: a meta-analysis. Clin Gastroenterol Hepatol. 2014;12:756-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 133] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 74. | Zisman TL, Bronner MP, Rulyak S, Kowdley KV, Saunders M, Lee SD, Ko C, Kimmey MB, Stevens A, Maurer J, Brentnall TA. Prospective study of the progression of low-grade dysplasia in ulcerative colitis using current cancer surveillance guidelines. Inflamm Bowel Dis. 2012;18:2240-2246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 75. | Lightner AL, Vogler S, McMichael J, Jia X, Regueiro M, Qazi T, Steele SR. Dysplastic Progression to Adenocarcinoma is Equivalent in Ulcerative Colitis and Crohn's Disease. J Crohns Colitis. 2021;15:24-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 76. | De Jong ME, Van Tilburg SB, Nissen LHC, Kievit W, Nagtegaal ID, Horjus CS, Römkens TEH, Drenth JPH, Hoentjen F, Derikx LAAP. Long-term Risk of Advanced Neoplasia After Colonic Low-grade Dysplasia in Patients With Inflammatory Bowel Disease: A Nationwide Cohort Study. J Crohns Colitis. 2019;13:1485-1491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 77. | Krugliak Cleveland N, Ollech JE, Colman RJ, Rodriquez D, Hirsch A, Cohen RD, Hanauer SB, Hart J, Hurst R, Rubin DT. Efficacy and Follow-up of Segmental or Subtotal Colectomy in Patients With Colitis-Associated Neoplasia. Clin Gastroenterol Hepatol. 2019;17:205-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 78. | Melcher AH, Chan J. The relationship between section thickness and the ultrastructural visualization of collagen fibrils: importance in studies on resorption of collagen. Arch Oral Biol. 1978;23:231-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 79. | Rubin DT, Krugliak Cleveland N, Rodriquez DM. Outcomes of colitis-associated dysplasia after referral from the community to a tertiary center. Gastrointest Endosc. 2016;84:1078-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |