Published online Jan 16, 2022. doi: 10.4253/wjge.v14.i1.35
Peer-review started: May 5, 2021
First decision: June 17, 2021
Revised: July 3, 2021
Accepted: December 21, 2021
Article in press: December 21, 2021
Published online: January 16, 2022
Processing time: 253 Days and 0.4 Hours
Endoscopic ultrasound (EUS) has emerged as an invaluable tool for the diagnosis, staging and treatment of pancreatic ductal adenocarcinoma (PDAC). EUS is currently the most sensitive imaging tool for the detection of solid pancreatic tumors. Conventional EUS has evolved, and new imaging techniques, such as contrast-enhanced harmonics and elastography, have been developed to improve diagnostic accuracy during the evaluation of focal pancreatic lesions. More recently, evaluation with artificial intelligence has shown promising results to overcome operator-related flaws during EUS imaging evaluation. Currently, an appropriate diagnosis is based on a proper histological assessment, and EUS-guided tissue acquisition is the standard procedure for pancreatic sampling. Newly developed cutting needles with core tissue procurement provide the pos
Core Tip: Endoscopic ultrasound (EUS) is currently an essential tool in the diagnostic work-up and treatment of pancreatic cancer. Contrast-enhanced harmonics, elastography and artificial intelligence provide additional information in the evaluation of focal pancreatic lesions to improve diagnostic accuracy during EUS evaluation. Interventional EUS has dramatically improved the palliative treatment of patients with pancreatic cancer, basically for local ablation therapies, adequate pain control with celiac plexus neurolysis and EUS-guided biliary drainage for the treatment of biliary obstruction.
- Citation: Salom F, Prat F. Current role of endoscopic ultrasound in the diagnosis and management of pancreatic cancer. World J Gastrointest Endosc 2022; 14(1): 35-48
- URL: https://www.wjgnet.com/1948-5190/full/v14/i1/35.htm
- DOI: https://dx.doi.org/10.4253/wjge.v14.i1.35
Pancreatic cancer is a serious oncological condition with a very poor outcome and survival. Pancreatic ductal adenocarcinoma (PDAC) is the most frequent pancreatic cancer, which represents 85% of the pathological diagnoses[1]. It is the 14th most common cancer and has the 7th highest cancer-related mortality in the world[2], and it has the fourth highest mortality in the United States[3]. The incidence is increasing, mainly in the Western world. It is predicted to increase to the second most common cause of cancer-related death in the United States and Western Europe by 2030[4]. The 5-year survival rate is very low, ranging from 2% to 9%. The most important factor that influences survival is tumor stage at diagnosis, although only 20% of patients are candidates for surgical resection at the time of diagnosis[5,6]. Its indolent clinical presentation, proximity to major vessels and absence of accurate serum markers and imaging modalities for early diagnosis are features that complicate early detection and screening for this severe disease. However, an accurate histological diagnosis and proper staging are essential in the treatment strategy of pancreatic cancer.
Multidetector computed tomography (MDCT) is the mainstay imaging technique for the evaluation of solid pancreatic lesions suggestive of potential PDAC, not so much for adequate characterization of the lesion as for accurate staging of potential malignant disease[7]. Preoperative evaluation for surgical resectability is currently based on MDCT staging[8]. Magnetic resonance imaging (MRI) is also an interesting imaging modality, but it does not reach the accuracy of MDCT with regard to resectability and particular vascular involvement[9].
Endoscopic ultrasound (EUS) was introduced in the 1980s as a high-precision tool for the analysis of the gastrointestinal wall and adjacent structures. High-quality images that have dramatically improved over time and the proximity of the transducer to the pancreatic parenchyma make EUS an invaluable tool for the description of pancreatic parenchyma and, thus, for pancreatic cancer diagnosis and staging.
The performance of EUS has been compared with that of computed tomography (CT) for pancreatic cancer staging. A meta-analysis did not find any difference in determining tumor resectability when these two techniques were compared[10]. However, rapid and recent progress in CT technology and the ability to review CT scan imaging studies during multidisciplinary meetings for treatment planning make CT the method of choice for initial staging and subsequent follow-up. In contrast, EUS has a higher sensitivity for the detection of solid pancreatic tumors, mainly for lesions under 2 cm in diameter, when compared with CT and MRI[11]. Hence, EUS is the preferred imaging technique for the screening of pancreatic cancer in high-risk populations[12]. Due to the benefits of EUS imaging provides in pancreatic cancer evaluation, many additional technological tools have been developed in recent years to try to improve the quality of EUS imaging and increase the diagnostic accuracy of this technique. In addition, the availability of large working channel linear array probes, or “therapeutic EUS scopes”, has opened a new range of possibilities beyond tissue acquisition for an accurate pathological diagnosis. It is also highly useful for therapeutic interventions, mainly for the palliation of pancreatic cancer-associated symptoms or to deliver targeted local treatment. The role of EUS in the evaluation and treatment of pancreatic cancer will be thoroughly discussed.
Contrast-enhanced (CE) harmonic EUS is an ultrasonographic technique that uses a microbubble-based contrast agent (Sonovue™, Sonazoid™ or Definity™, depending on local market availability) to visualize vascularization and perfusion patterns in the liver, pancreatic parenchyma or lymph nodes. This technique was made available for EUS during the late 2000s. Harmonic components of the signal generated by intravenously injected microbubbles improve the evaluation of the microcirculation without Doppler-related artifacts[13]. Two main features are evaluated during contrast evaluation: one is the enhancement of the lesion with the contrast agent, which can be non-, hypo-, iso- or hyperenhancement, and the second is the contrast distribution, which can be classified as homogeneous or heterogeneous. Regarding focal pancreatic lesions, contrast is a useful tool to differentiate pancreatic adenocarcinoma from other focal lesions. Whereas pancreatic adenocarcinoma has a hypoenhanced pattern, other focal lesions, such as neuroendocrine tumors, metastatic lesions and inflammatory diseases, are either iso- or hyperenhanced[14,15]. Two different meta-analyses have shown a pooled sensitivity between 92% and 93% and a pooled specificity between 87% and 88% for the differential diagnosis between pancreatic cancer and other focal pancreatic lesions[16,17]. CE-EUS also plays a role in patients with suspected pancreatic adenocarcinoma, but negative results after EUS fine needle aspiration (FNA), mainly in the setting of chronic pancreatitis, improve biopsy targeting at a second attempt[18,19]. Finally, CE-EUS is an important tool in deciding between surgery or surveillance of focal lesions with a negative or inconclusive histological diagnosis after EUS FNA or FNB. Being an operator-dependent procedure is one of the pitfalls of CE-EUS, but this disadvantage has been counterbalanced by an optimized technique of quantification analysis including a time-intensity curve for the region of interest[20,21].
Elastography is an ancillary technique for the endosonographic evaluation of solid pancreatic lesions that evaluates tissue stiffness. There are two different types of elastography, namely, strain and shear wave elastography. However, only strain elastography is available for EUS, which measures tissue distortion after applying a predetermined pressure. Three different elastography measurements are available: The pattern of recognition in which the stiffness is defined by colors in which green represents the normal pancreatic tissue stiffness, blue stands for hard tissue and red represents softer tissue. This measurement is highly operator-dependent and does not provide objective information. The second measure, called the strain ratio, is a method of stiffness comparison between the target area and a reference area in a grayscale image. The distance and the selected area of reference can induce some bias with this technique[22]. Finally, the strain histogram is a computer-enhanced method for dynamic analysis, where color images are transformed into a grayscale of 256 tones. These two latter quantitative measurements provide more objective information than the pattern of recognition color evaluation. Interestingly, a meta-analysis did not show any difference in accuracy between qualitative and quantitative evaluations. It showed a pooled sensitivity of 98% and specificity of 63% for qualitative measurement and a pooled sensitivity of 95% and specificity of 61% for quantitative endoscopic ultrasound elastrography measurement for correct differentiation between malignant and benign solid pancreatic lesions[23]. However, the low specificity of elastography suggests that the stiffness of a lesion is not perfectly correlated with the presence of neoplastic tissue.
Few studies have addressed this comparison. One of the first studies compared CE power Doppler EUS and EUS elastography[24]. No difference was found between the two techniques regarding sensitivity, specificity or accuracy. A more recent pros
It is well known that the performance of EUS for an accurate diagnosis depends highly on the technical capacity, knowledge and experience of the endoscopist. To overcome this flaw, a strong effort has been made in the development of artificial intelligence (AI) in the evaluation and differential diagnosis of pancreatic lesions[26]. AI is a mathematical prediction technique that recognizes patterns after analyzing data in computer-based programs, performing tasks supposedly mimicking some of the processes of human intelligence. Computer-aided diagnosis (CAD) refers to diagnoses based on image processing by computer programs[27].
The first study using CAD for pancreatic endoscopic ultrasound was reported 20 years ago by Norton et al[28], who concluded that digital image analysis of the pancreas is feasible and at least comparable to human interpretation, setting the basis for future AI studies in the field of pancreatic diseases[28]. Subsequent studies have evaluated the performance of AI for the differential diagnosis of pancreatic lesions, with a reported accuracy of 94%[29].
Deep learning techniques refer to more advanced AI algorithms that use deep neural networks to provide high-performance predictions in which computers improve their own performance by taking advantage of previous success and error without further human intervention[30]. Deep learning is used in computer vision for imaging classification. Automatic image feature detection is its most prominent advantage[31]. Few studies have described the use of deep learning for EUS image analysis since its introduction in 2019. One study was designed for IPMN malignancy diagnosis with an accuracy of 94%[32], and another study by Tonozuka et al[33] was the first deep learning AI study that evaluated the ability of AI to detect pancreatic cancer. This study showed promising results with a sensitivity of 92.4%, specificity of 84.1%, positive predictive values of 86.8% and negative predictive values of 90.7%[33].
In the future, AI can probably help in the treatment strategy ahead of tissue acqu
The mainstay for an accurate diagnosis of pancreatic cancer is based on tissue acquisition. EUS FNA has been the standard method to acquire pancreatic tissue for more than 25 years. Great effort has been made to improve the diagnostic accuracy of FNA. Different changes in the standard technique have been adapted to improve FNA performance. Regarding technical issues, the fanning technique, which involves sampling different areas of the lesion during a single needle pass, can decrease the number of passes needed for an adequate diagnosis and increase the number of patients in which the diagnosis can be achieved at the first attempt. The use of suction during FNA has been reported in a randomized controlled trial to improve diagnostic accuracy[35], but the slow-pull technique in which no suction is applied has also been shown to yield equivalent results with less blood contamination[36]. Finally, the number of passes recommended for a better diagnostic yield is 3 or 4. More than 4 passes have no proven additional benefit[37]. Other technical variations, such as puncture with or without the use of the stylet or the availability of an on-site cytologic evaluation, have provided no significant improvements in the diagnostic yield to ensure adequate EUS tissue acquisition.
A variety of needles with modifications in the type of tip and needle size (diameter) have been manufactured, and their diagnostic performance has been evaluated. Different sizes, from 25G to 19G, were produced to try to improve the sample size and ease of manipulation. No significant difference was seen in sample quality when different needle sizes were compared for solid pancreatic lesions[38,39].
Recently, FNB needles have been made available. One can differentiate two types of FNB needles, namely, fenestrated needles, introduced in approximately 2010, and more recently, “cutting” needles with a bevelless, dented tip. Both types aim to provide core tissue samples. The performance of regular FNA needles with reverse bevel needles was compared. A randomized controlled trial reported that fewer passes are needed to obtain an adequate sample and better histological diagnosis with reverse bevel needles[40]. Nevertheless, a different meta-analysis showed no significant difference in diagnostic accuracy between these two different needle types[41].
“Cutting” needles provide core biopsy tissue and permit the preservation of cellular architecture, allowing FNB molecular profiles of pancreatic samples to be obtained for personalized oncological treatment. Two different types of “cutting” needles are available: A Franseen needle and a fork-tip needle.
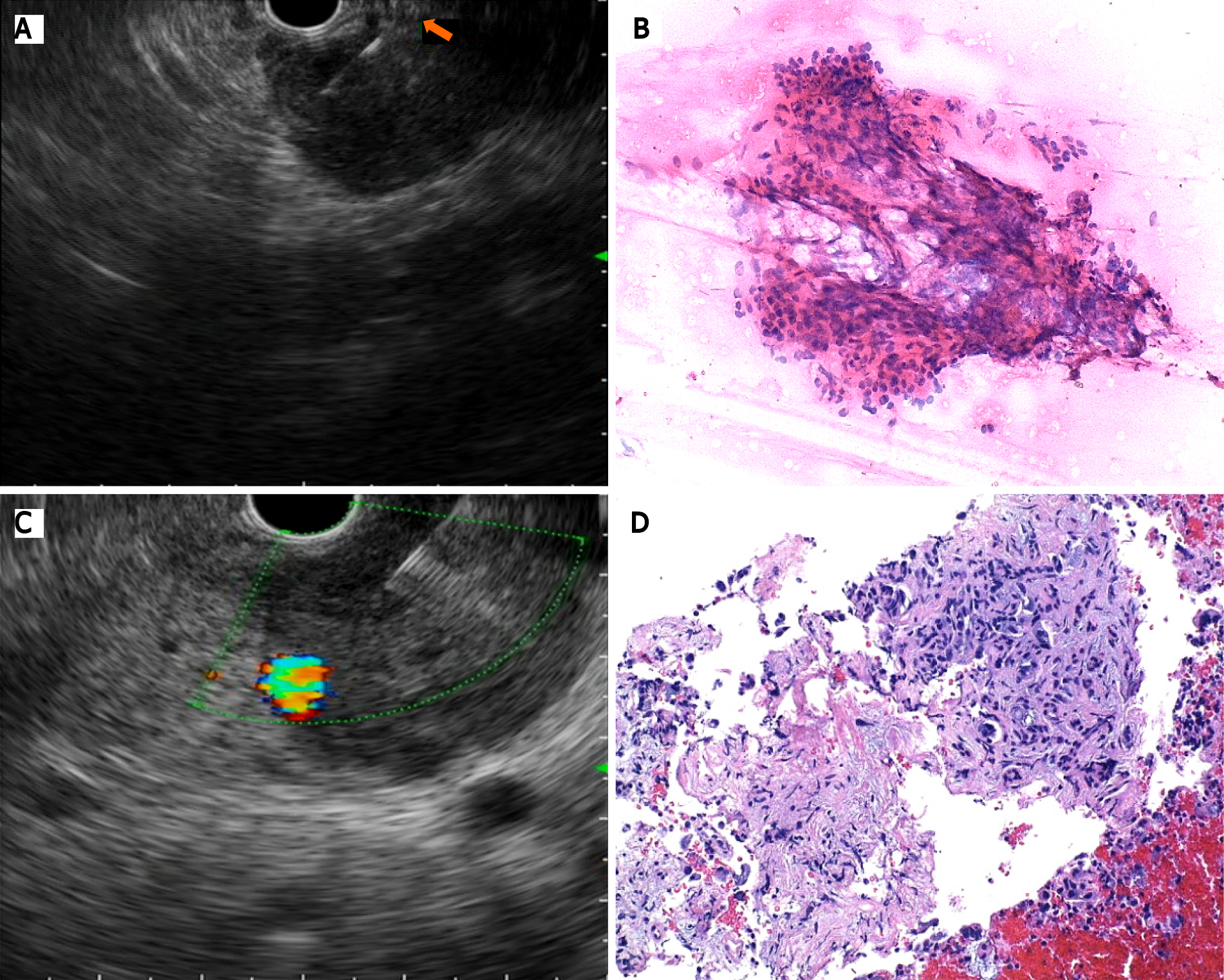
A recent meta-analysis including only randomized controlled trials comparing FNA and FNB for solid pancreatic needles showed comparable results regarding sample adequacy and diagnostic accuracy, with similar sensitivity for both needles (93.1% for FNB and 90.4% for FNA)[42]. One of these studies yielded a higher quality histological sample with the FNB needle when compared with the standard FNA needle, with the former achieving better histological architecture retainment[43] (Figure 1).
Complications due to EUS-guided tissue acquisition have been described in 0.5%-3% of cases, including acute pancreatitis, infection, perforation, and bleeding[44]. Although less frequently, needle tract seeding has also been described. This complication has a prevalence of 0.003%-0.009% with FNA needles, and to our knowledge, only one case of needle tract seeding has been reported with FNB needles[45]. Even though the risk is low, we should be aware of this risk mainly for cases in which surgery is performed, but the needle site of puncture is not within the scope of surgical resection[44,45].
The only curative option in patients with pancreatic cancer is surgical resection. Unfortunately, only 20% of patients are surgical candidates after adequate diagnostic evaluation and staging[46]. In advanced stages, chemotherapy and radiotherapy can improve survival and quality of life[47]. Image-guided radiotherapy (IGRT) can precisely deliver radiation to the target lesion through real-time advanced imaging guidance to decrease toxicity to surrounding tissue. Stereotactic body radiotherapy (SBRT) is a form of IGRT in which multiple beam radiation allows high-dose radiation therapy to a select location for a precise target treatment[48]. This technique allows adequate control of local disease with a significant decrease in radiation toxicity[49]. To achieve this goal, implantable markers (fiducials) are needed as landmarks for precise radiation delivery. Fiducials are radiopaque markers, usually made of gold, placed in the target lesion to ease accurate radiation treatment. Originally, fiducials were placed either percutaneously or surgically. The former has the limitation of intervening structures in the needle tract, and the latter requires a more invasive procedure. EUS fiducial placement has emerged as a potential alternative to avoid these hurdles. Initially, they were placed with a 19G FNA needle, but due to the stiffness of these needles, smaller fiducials were developed for 22G FNA needle placement. Recently, preloaded needles became available to ease this procedure. A recent meta-analysis evaluated technical aspects of EUS-guided fiducial placement specifically for pancreatic cancer. This study showed an overall technical success rate of 96.27%, a migration rate of 4.33% and an adverse event rate of 4.85%[50].
Radiofrequency ablation (RFA) is a local procedure that generates tissue coagulative necrosis induced by high temperature[51]. This is a well-established treatment for solid tumors of the kidney, lung and liver. Recently, an EUS RFA device composed of a specifically designed 19G needle and a purpose-built RF generator was developed to perform RFA treatment under EUS guidance. This technique produces local ablation through thermal coagulation and is also assumed by some authors to stimulate the immune response by the release of antitumoral-specific antigens (also known as the abscopal effect), thus potentially offering two different therapeutic mechanisms[52]. It is important to point out that this latter effect has been adequately described in many reports, but it is a rarely recognized clinical event[53].
As with every invasive procedure, there are potential adverse events, including pancreatitis, pancreatic duct strictures, bowel perforation, bleeding and peritonitis[54]. EUS FRA has recently been evaluated for two indications: one for the local treatment of unresectable pancreatic cancer and the other for neuroendocrine pancreatic tumors unsuitable for surgical resection.
RFA for unresectable pancreatic cancer is a safe and feasible procedure. A recent study that enrolled 10 patients with unresectable pancreatic cancer reported a technical feasibility of 100% and no major adverse events[55]. To date, none of the published studies have reported any significant efficacy data.
Pancreatic neuroendocrine tumors (NETs) are infrequent tumors (1% of all pancreatic neoplasms) usually exhibiting indolent behavior that occur sporadically or in the context of hereditary multiple endocrine neoplasia (MEN) type 1[56]. Small nonfunctional NETs (diameter under 20 mm) are usually followed with CT, MRI and/or positron emission tomography[57], whereas surgical resection is advised in larger or hormone-producing NETs. Adverse events, such as pancreatic fistula, have been reported in 45% of cases after tumor enucleation and 14% after pancreatectomy[58]. RFA has emerged as a potential treatment option for these cases. Some data have been published in recent years regarding the usefulness of RFA for NET treatment. In a prospective study that evaluated the efficacy of EUS RFA in 12 patients bearing a total of 14 treated tumors, the 1-year complete resolution rate was 86%[59]. The role of RFA has also been described for functional NETs[60]. In a recent meta-analysis, the role of RFA in pancreatic neuroendocrine tumors demonstrated an overall effectiveness of 96% without differences between functional and nonfunctional NETs[61].
Another meta-analysis evaluated this technique for the treatment of different types of pancreatic tumors and showed a technical success of 100%, a clinical success of 91.5% and an overall adverse event rate of 14.6%, where abdominal pain was the most frequently reported[62]. Most available studies that have evaluated this technique are small-sized studies with fewer than 10 patients and uncontrolled protocols. Many different settings of ablation time and energy delivery were used in each study, but this had no impact on the final results. One prospective study evaluated EUS RFA plus chemotherapy vs chemotherapy alone for unresectable pancreatic cancer. Even though there was a decrease in the morphine dose requirement for pain control, no difference was seen regarding survival[63]. Larger multicentric prospective and controlled trials are needed to determine the utility of this potential therapeutic resource in the treatment of pancreatic cancer.
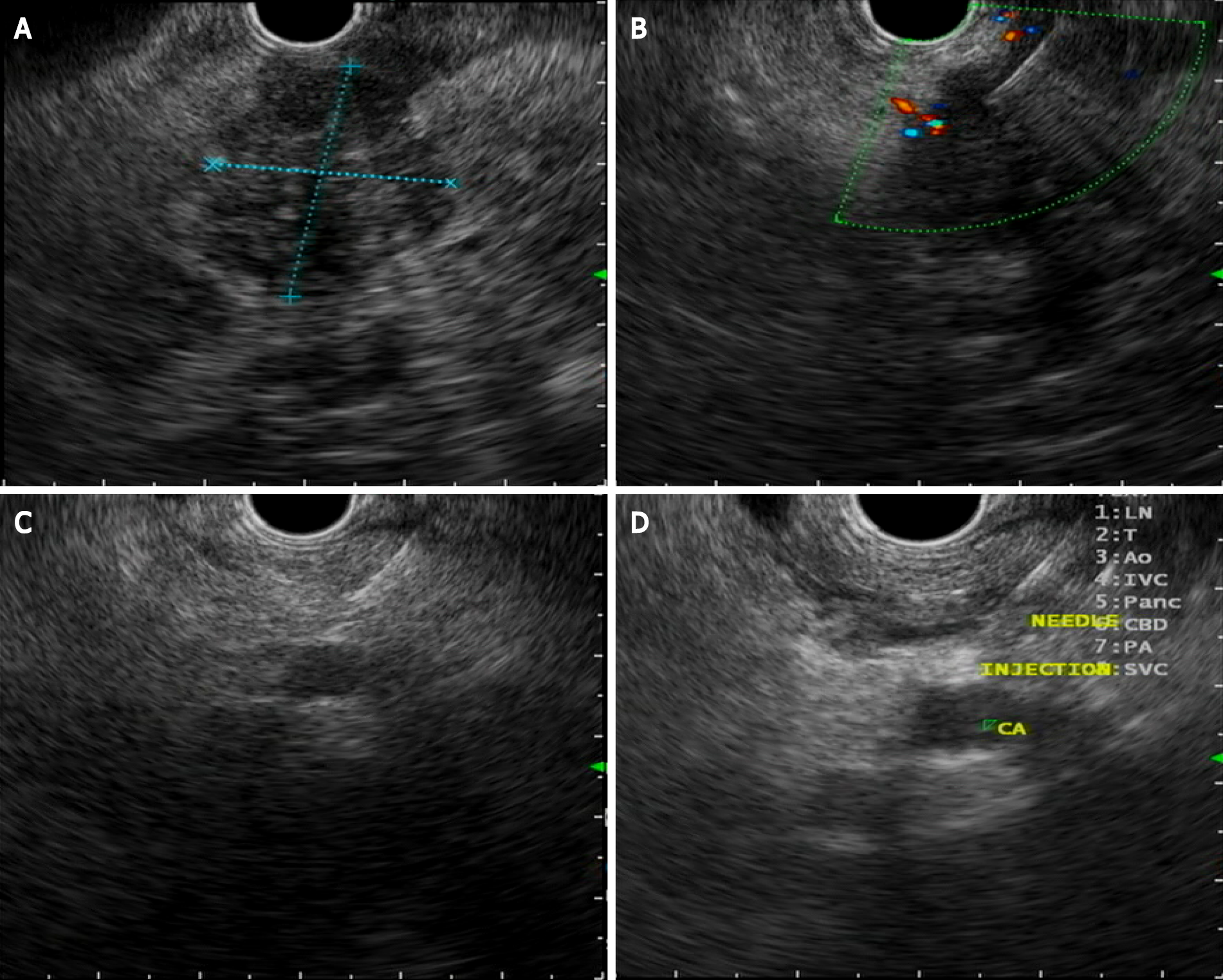
Endoscopic ultrasound celiac plexus neurolysis was introduced in 1996 for the management of pain caused by pancreatic cancer[64], which is the most common symptom in pancreatic cancer and the main impairment in quality of life of this group of patients. Pain is present in 60% of patients at presentation and in 80% of patients with advanced pancreatic cancer[65]. During celiac plexus neurolysis, absolute alcohol is injected as a neurolytic agent directly into the celiac plexus area to disrupt the transmission of pain signals. Bupivacaine 0.25% is additionally injected as an analgesic agent (Figure 2).
Three techniques have been described: A central technique in which the total amount of the agent is injected at the origin of the celiac artery, a bilateral technique in which the injection is done on both sides of the celiac artery with an equal distribution, and the most recently described direct celiac ganglia neurolysis. A meta-analysis evaluated the efficacy of this procedure, with pain relief being obtained in 72% of patients[66]. Conflicting results have been obtained regarding the best EUS neurolysis technique, but visibility and direct injection of the celiac ganglia substantially increase the response to treatment[67]. Regarding the timing of neurolysis, a randomized controlled trial concluded that early CPN reduces pain and decreases morphine consumption in patients with advanced pancreatic adenocarcinoma[68]. A systematic review described CPN having minimal superiority over analgesic drugs but with fewer adverse effects than opioids[69]. The most commonly described complications associated with CPN are transient and include diarrhea (23%), hypotension (33%) and pain exacerbation (36%)[70]. A mildly higher risk of retroperitoneal bleeding has been described with the bilateral technique[71]. EUS-guided celiac plexus neurolysis is a good option for pain treatment in patients needing high doses of opioids or with important adverse events related to these medications.
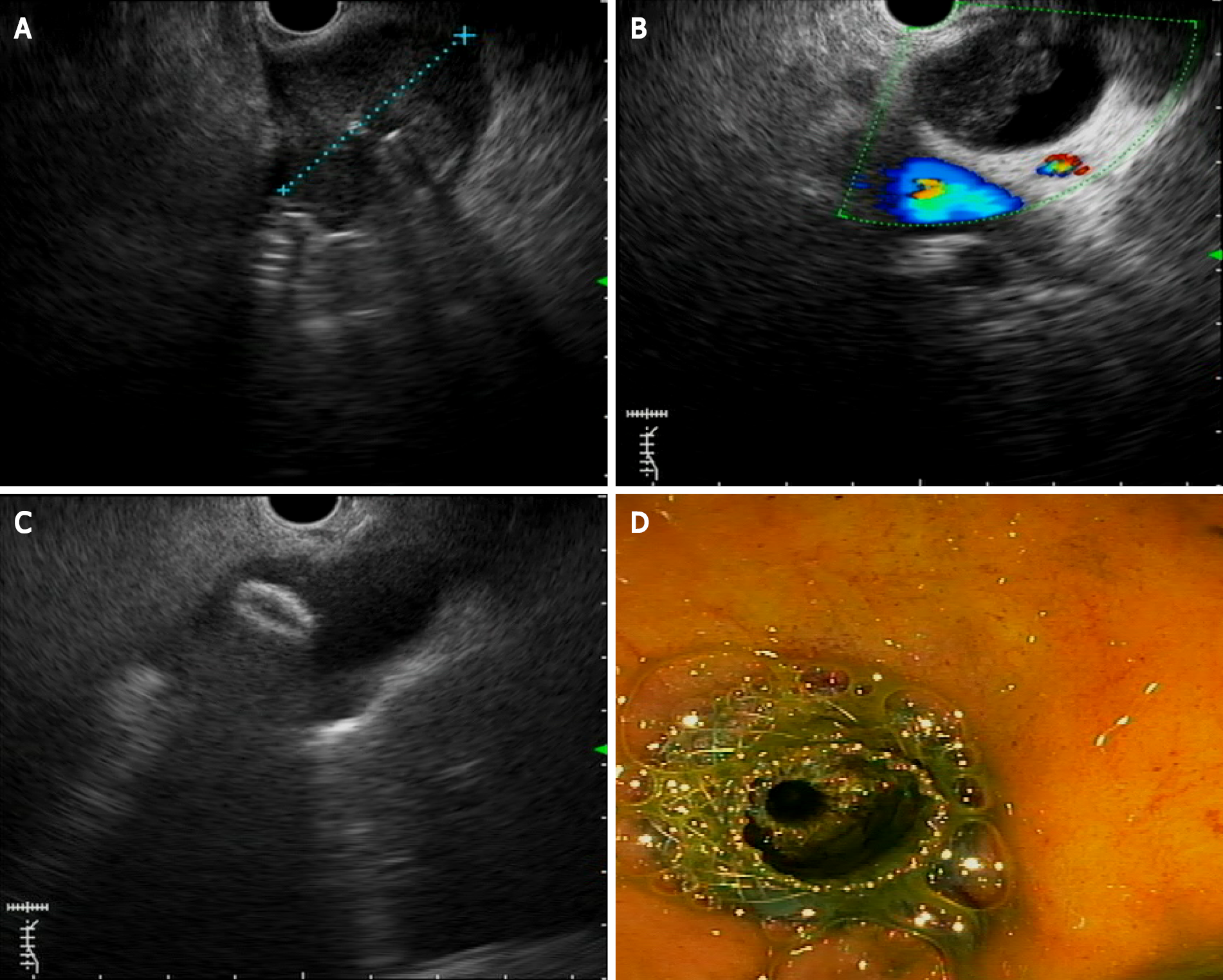
Biliary duct obstruction is one of the main complications related to pancreatic cancer. Endoscopic retrograde cholangiopancreatography (ERCP) with stent placement is the standard treatment to drain biliary duct obstruction. Nevertheless, ERCP fails in 5-7% of the cases[72]. Until recently, percutaneous transhepatic biliary drainage (PTBD) was the most frequent approach for biliary drainage after ERCP failures. Although PTBD has significant morbidity, it is uncomfortable and generally requires more than one procedure[73]. This is why EUS biliary drainage emerged as an option for obstructive jaundice in patients with pancreatic cancer where ERCP fails with similar technical and clinical success compared with PTBD, with a lower incidence of adverse events. The first EUS biliodigestive anastomosis was described in 2001[74]. Since then, many advances in this endoscopic technique have been developed. A meta-analysis reported a technical success rate of 90% and adverse event rate in 17% of patients treated by EUS BD[75]. EUS biliary drainage can be divided into two distinct approaches, namely, gastrohepatic (or EUS-guided hepaticogastrostomy) and extrahepatic (or EUS-guided choledocoduodenostomy) approaches (Figure 3). Each approach can be divided into direct drainage and the Rendez-vous technique. The latter has been preferred by some for benign diseases, but it is important to note that it is technically challenging, with a higher risk of failure and complications. We consider this technique to be discouraged. When the duodenum is accessible, choledocoduodenostomy can be attempted, and the development of lumen-appossable metallic stents (LAMSs) has simplified this approach. Recently, EUS BD has been evaluated as a first-line treatment instead of ERCP for malignant biliary obstruction, mainly due to the high technical success rate and the absence of papilla manipulation, which can decrease the risk of pancreatitis. A recent meta-analysis evaluated EUS BD as the primary palliation option for distal biliary obstruction, describing equivalent technical and clinical success, with no difference in adverse events between EUS BD and ERCP[76]. Further high-quality multicenter and controlled studies are clearly needed to determine the right place for EUS-guided BD techniques beyond ERCP failures. Choledocoduodenostomy, equivalent to side-to side biliodigestive anastomosis, is prone to alimentary biliary reflux, causing cholangitis, and may thus be preferred for short-term drainage. For a nonaccessible duodenum, the gastrohepatic approach with hepatogastrostomy is the best approach, which can also be considered in benign conditions and in cases of biliodigestive anastomosis dysfunction after Whipple resection. A dilated left intrahepatic duct is needed to succeed in this route. A partially covered metallic stent (uncovered intrahepatic portion) has been developed for this approach, with promising results. A systematic review that evaluated the efficacy and safety of EUS BD found no difference in technical success and adverse event rates between transgastric and transduodenal approaches[77].
Even though LAMSs are highly useful for the EUS BD approach, they are a regionally limited device. Regarding the risk of recurrent biliary obstruction, EUS BD has a lower risk of tumor ingrowth but a higher risk of food impaction than ERCP BD. Stent patency for EUS BD is comparable to ERCP BD. A study by Park et al[78] described a cumulative stent patency of 379 d for EUS BD[78].
Gastric outlet obstruction (GOO) is present in 15%-25% of patients with PDAC[79] and has a severe impact on quality of life. Traditionally, this complication is treated either surgically or with self-expandable metallic stents (SEMSs) placed by the endoscopic route. Recently, EUS-guided gastroenterostomy has emerged as a successful alter
Another application of interventional EUS is for the treatment of afferent limb syndrome (ALS). This is a rare late postsurgical complication of PDAC pancreaticoduodenectomy, most frequently due to local cancer recurrence and mechanical obstruction, with dilation of the afferent limb and accumulation of biliopancreatic fluid. EUS-guided drainage with a LAMS has been described, which provides an adequate therapeutic approach to decompress the limb for palliative and symptomatic treatment[82]. Most of the evidence for these two EUS therapeutic applications is primarily retrospective. Even though they seem to be promising techniques, well-designed multicentric, prospective, controlled trials are needed to validate these resources.
Since its introduction as an endoscopic technique, EUS has evolved from a diagnostic imaging device toward a therapeutic tool, primarily for palliative cancer management. Considerable progress has been made, particularly in the diagnosis and management of PDAC. New imaging techniques can improve the differential diagnosis of focal pancreatic lesions and can decrease the bias of human imaging interpretation. EUS is the standard method for tissue acquisition, and the development of new “cutting” needles allows the procurement of core tissue for molecular profiling and personalized oncological treatment. Outstanding progress has been made in EUS interventional procedures, mainly for biliary drainage and local tumor ablation, with good technical and clinical success and fewer complications compared to other techniques. Future randomized controlled trials should be directed to evaluate the role of EUS-guided treatment, such as RFA, for unresectable pancreatic cancer or patients unsuitable for surgery. Diagnostic and interventional EUS have become essential in the workup and management of PDAC.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Costa Rica
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kuraoka N S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Ferlay J, Colombet M, Soerjomataram I, Dyba T, Randi G, Bettio M, Gavin A, Visser O, Bray F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. 2018;103:356-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1625] [Cited by in RCA: 1660] [Article Influence: 237.1] [Reference Citation Analysis (0)] |
| 2. | International Agency for Research on Cancer. World Health Organization. Global Cancer Observatory 2018. [cited 5 May 2021]. Available from: http://gco.iarc.fr. |
| 3. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13300] [Cited by in RCA: 15475] [Article Influence: 2579.2] [Reference Citation Analysis (2)] |
| 4. | Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913-2921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5379] [Cited by in RCA: 5140] [Article Influence: 467.3] [Reference Citation Analysis (0)] |
| 5. | Society TAC. Key Statistics for Pancreatic Cancer. 2017. [cited 5 May 2021]. Available from: https://www.cancer.org/cancer/pancreatic-cancer/about/key-statistics.html. |
| 6. | Vicent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607-620. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2129] [Cited by in RCA: 2115] [Article Influence: 151.1] [Reference Citation Analysis (3)] |
| 7. | Kinney T. Evidence-based imaging of pancreatic malignancies. Surg Clin North Am. 2010;90:235-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Bockhorn M, Uzunoglu FG, Adham M, Imrie C, Milicevic M, Sandberg AA, Asbun HJ, Bassi C, Büchler M, Charnley RM, Conlon K, Cruz LF, Dervenis C, Fingerhutt A, Friess H, Gouma DJ, Hartwig W, Lillemoe KD, Montorsi M, Neoptolemos JP, Shrikhande SV, Takaori K, Traverso W, Vashist YK, Vollmer C, Yeo CJ, Izbicki JR; International Study Group of Pancreatic Surgery. Borderline resectable pancreatic cancer: a consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2014;155:977-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 654] [Cited by in RCA: 652] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 9. | Bipat S, Phoa SS, van Delden OM, Bossuyt PM, Gouma DJ, Laméris JS, Stoker J. Ultrasonography, computed tomography and magnetic resonance imaging for diagnosis and determining resectability of pancreatic adenocarcinoma: a meta-analysis. J Comput Assist Tomogr. 2005;29:438-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 166] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 10. | Nawaz H, Fan CY, Kloke J, Khalid A, McGrath K, Landsittel D, Papachristou GI. Performance characteristics of endoscopic ultrasound in the staging of pancreatic cancer: a meta-analysis. JOP. 2013;14:484-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 11. | Müller MF, Meyenberger C, Bertschinger P, Schaer R, Marincek B. Pancreatic tumors: evaluation with endoscopic US, CT, and MR imaging. Radiology. 1994;190:745-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 304] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 12. | Lu C, Xu CF, Wan XY, Zhu HT, Yu CH, Li YM. Screening for pancreatic cancer in familial high-risk individuals: A systematic review. World J Gastroenterol. 2015;21:8678-8686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 67] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 13. | de Jong N, Frinking PJ, Bouakaz A, Ten Cate FJ. Detection procedures of ultrasound contrast agents. Ultrasonics. 2000;38:87-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 93] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Yamashita Y, Shimokawa T, Napoléon B, Fusaroli P, Gincul R, Kudo M, Kitano M. Value of contrast-enhanced harmonic endoscopic ultrasonography with enhancement pattern for diagnosis of pancreatic cancer: A meta-analysis. Dig Endosc. 2019;31:125-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 15. | Fusaroli P, Spada A, Mancino MG, Caletti G. Contrast harmonic echo-endoscopic ultrasound improves accuracy in diagnosis of solid pancreatic masses. Clin Gastroenterol Hepatol. 2010;8:629-34.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 153] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 16. | He XK, Ding Y, Sun LM. Contrast-enhanced endoscopic ultrasound for differential diagnosis of pancreatic cancer: an updated meta-analysis. Oncotarget. 2017;8:66392-66401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Mei S, Wang M, Sun L. Contrast-Enhanced EUS for Differential Diagnosis of Pancreatic Masses: A Meta-Analysis. Gastroenterol Res Pract. 2019;2019:1670183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Iordache S, Costache MI, Popescu CF, Streba CT, Cazacu S, Săftoiu A. Clinical impact of EUS elastography followed by contrast-enhanced EUS in patients with focal pancreatic masses and negative EUS-guided FNA. Med Ultrason. 2016;18:18-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Sugimoto M, Takagi T, Hikichi T, Suzuki R, Watanabe K, Nakamura J, Kikuchi H, Konno N, Waragai Y, Watanabe H, Obara K, Ohira H. Conventional vs contrast-enhanced harmonic endoscopic ultrasonography-guided fine-needle aspiration for diagnosis of solid pancreatic lesions: A prospective randomized trial. Pancreatology. 2015;15:538-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | Matsubara H, Itoh A, Kawashima H, Kasugai T, Ohno E, Ishikawa T, Itoh Y, Nakamura Y, Hiramatsu T, Nakamura M, Miyahara R, Ohmiya N, Ishigami M, Katano Y, Goto H, Hirooka Y. Dynamic quantitative evaluation of contrast-enhanced endoscopic ultrasonography in the diagnosis of pancreatic diseases. Pancreas. 2011;40:1073-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 21. | Seicean A, Badea R, Stan-Iuga R, Mocan T, Gulei I, Pascu O. Quantitative contrast-enhanced harmonic endoscopic ultrasonography for the discrimination of solid pancreatic masses. Ultraschall Med. 2010;31:571-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Havre RF, Waage JR, Gilja OH, Ødegaard S, Nesje LB. Real-Time Elastography: Strain Ratio Measurements Are Influenced by the Position of the Reference Area. Ultraschall Med. 2012;33:559-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 23. | Zhang B, Zhu F, Li P, Yu S, Zhao Y, Li M. Endoscopic ultrasound elastography in the diagnosis of pancreatic masses: A meta-analysis. Pancreatology. 2018;18:833-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 24. | Figueiredo FA, da Silva PM, Monges G, Bories E, Pesenti C, Caillol F, Delpero JR, Giovannini M. Yield of Contrast-Enhanced Power Doppler Endoscopic Ultrasonography and Strain Ratio Obtained by EUS-Elastography in the Diagnosis of Focal Pancreatic Solid Lesions. Endosc Ultrasound. 2012;1:143-149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Iglesias-Garcia J, Lindkvist B, Lariño-Noia J, Abdulkader-Nallib I, Dominguez-Muñoz JE. Differential diagnosis of solid pancreatic masses: contrast-enhanced harmonic (CEH-EUS), quantitative-elastography (QE-EUS), or both? United European Gastroenterol J. 2017;5:236-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 26. | Kuwahara T, Hara K, Mizuno N, Haba S, Okuno N, Koda H, Miyano A, Fumihara D. Current status of artificial intelligence analysis for endoscopic ultrasonography. Dig Endosc. 2021;33:298-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 27. | Doi K. Computer-aided diagnosis in medical imaging: historical review, current status and future potential. Comput Med Imaging Graph. 2007;31:198-211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1345] [Cited by in RCA: 737] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 28. | Norton ID, Zheng Y, Wiersema MS, Greenleaf J, Clain JE, Dimagno EP. Neural network analysis of EUS images to differentiate between pancreatic malignancy and pancreatitis. Gastrointest Endosc. 2001;54:625-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 29. | Zhu M, Xu C, Yu J, Wu Y, Li C, Zhang M, Jin Z, Li Z. Differentiation of pancreatic cancer and chronic pancreatitis using computer-aided diagnosis of endoscopic ultrasound (EUS) images: a diagnostic test. PLoS One. 2013;8:e63820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (2)] |
| 30. | LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521:436-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36149] [Cited by in RCA: 20058] [Article Influence: 2005.8] [Reference Citation Analysis (0)] |
| 31. | Rawat W, Wang Z. Deep Convolutional Neural Networks for Image Classification: A Comprehensive Review. Neural Comput. 2017;29:2352-2449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1407] [Cited by in RCA: 822] [Article Influence: 102.8] [Reference Citation Analysis (0)] |
| 32. | Kuwahara T, Hara K, Mizuno N, Okuno N, Matsumoto S, Obata M, Kurita Y, Koda H, Toriyama K, Onishi S, Ishihara M, Tanaka T, Tajika M, Niwa Y. Usefulness of Deep Learning Analysis for the Diagnosis of Malignancy in Intraductal Papillary Mucinous Neoplasms of the Pancreas. Clin Transl Gastroenterol. 2019;10:1-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 130] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 33. | Tonozuka R, Itoi T, Nagata N, Kojima H, Sofuni A, Tsuchiya T, Ishii K, Tanaka R, Nagakawa Y, Mukai S. Deep learning analysis for the detection of pancreatic cancer on endosonographic images: a pilot study. J Hepatobiliary Pancreat Sci. 2021;28:95-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 34. | Tonozuka R, Mukai S, Itoi T. The Role of Artificial Intelligence in Endoscopic Ultrasound for Pancreatic Disorders. Diagnostics (Basel). 2020;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 35. | Tarantino I, Di Mitri R, Fabbri C, Pagano N, Barresi L, Granata A, Liotta R, Mocciaro F, Maimone A, Baccarini P, Fabio T, Curcio G, Repici A, Traina M. Is diagnostic accuracy of fine needle aspiration on solid pancreatic lesions aspiration-related? Dig Liver Dis. 2014;46:523-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 36. | El Haddad R, Barret M, Beuvon F, Grabar S, Leblanc S, Terris B, Coriat R, Chaussade S, Prat F. The slow-pull capillary technique increases the quality of endoscopic ultrasound fine needle biopsy samples in solid pancreatic lesions. Eur J Gastroenterol Hepatol. 2016;28:911-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | Mohamadnejad M, Mullady D, Early DS, Collins B, Marshall C, Sams S, Yen R, Rizeq M, Romanas M, Nawaz S, Ulusarac O, Hollander T, Wilson RH, Simon VC, Kushnir V, Amateau SK, Brauer BC, Gaddam S, Azar RR, Komanduri S, Shah R, Das A, Edmundowicz S, Muthusamy VR, Rastogi A, Wani S. Increasing Number of Passes Beyond 4 Does Not Increase Sensitivity of Detection of Pancreatic Malignancy by Endoscopic Ultrasound-Guided Fine-Needle Aspiration. Clin Gastroenterol Hepatol. 2017;15:1071-1078.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 38. | Lee JK, Lee KT, Choi ER, Jang TH, Jang KT, Lee JK, Lee KH. A prospective, randomized trial comparing 25-gauge and 22-gauge needles for endoscopic ultrasound-guided fine needle aspiration of pancreatic masses. Scand J Gastroenterol. 2013;48:752-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 39. | Fabbri C, Polifemo AM, Luigiano C, Cennamo V, Baccarini P, Collina G, Fornelli A, Macchia S, Zanini N, Jovine E, Fiscaletti M, Alibrandi A, D'Imperio N. Endoscopic ultrasound-guided fine needle aspiration with 22- and 25-gauge needles in solid pancreatic masses: a prospective comparative study with randomisation of needle sequence. Dig Liver Dis. 2011;43:647-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 40. | Alatawi A, Beuvon F, Grabar S, Leblanc S, Chaussade S, Terris B, Barret M, Prat F. Comparison of 22G reverse-beveled vs standard needle for endoscopic ultrasound-guided sampling of solid pancreatic lesions. United European Gastroenterol J. 2015;3:343-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 41. | Oh HC, Kang H, Lee JY, Choi GJ, Choi JS. Diagnostic accuracy of 22/25-gauge core needle in endoscopic ultrasound-guided sampling: systematic review and meta-analysis. Korean J Intern Med. 2016;31:1073-1083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 42. | Facciorusso A, Bajwa HS, Menon K, Buccino VR, Muscatiello N. Comparison between 22G aspiration and 22G biopsy needles for EUS-guided sampling of pancreatic lesions: A meta-analysis. Endosc Ultrasound. 2020;9:167-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 43. | Bang JY, Hebert-Magee S, Navaneethan U, Hasan MK, Hawes R, Varadarajulu S. EUS-guided fine needle biopsy of pancreatic masses can yield true histology. Gut. 2018;67:2081-2084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 109] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 44. | Katanuma A, Maguchi H, Yane K, Hashigo S, Kin T, Kaneko M, Kato S, Kato R, Harada R, Osanai M, Takahashi K, Nojima M. Factors predictive of adverse events associated with endoscopic ultrasound-guided fine needle aspiration of pancreatic solid lesions. Dig Dis Sci. 2013;58:2093-2099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 45. | Gao RY, Wu BH, Shen XY, Peng TL, Li DF, Wei C, Yu ZC, Luo MH, Xiong F, Wang LS, Yao J. Overlooked risk for needle tract seeding following endoscopic ultrasound-guided minimally invasive tissue acquisition. World J Gastroenterol. 2020;26:6182-6194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 46. | Gillen S, Schuster T, Meyer Zum Büschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7:e1000267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1243] [Cited by in RCA: 1170] [Article Influence: 78.0] [Reference Citation Analysis (1)] |
| 47. | Yip D, Karapetis C, Strickland A, Steer CB, Goldstein D. Chemotherapy and radiotherapy for inoperable advanced pancreatic cancer. Cochrane Database Syst Rev. 2006;CD002093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 48. | Timmerman RD, Kavanagh BD, Cho LC, Papiez L, Xing L. Stereotactic body radiation therapy in multiple organ sites. J Clin Oncol. 2007;25:947-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 319] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 49. | Tchelebi LT, Lehrer EJ, Trifiletti DM, Sharma NK, Gusani NJ, Crane CH, Zaorsky NG. Conventionally fractionated radiation therapy vs stereotactic body radiation therapy for locally advanced pancreatic cancer (CRiSP): An international systematic review and meta-analysis. Cancer. 2020;126:2120-2131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 50. | Patel JB, Revanur V, Forcione DG, Bechtold ML, Puli SR. Endoscopic ultrasound-guided fiducial marker placement in pancreatic cancer: A systematic review and meta-analysis. World J Gastrointest Endosc. 2020;12:231-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 51. | Tatli S, Tapan U, Morrison PR, Silverman SG. Radiofrequency ablation: technique and clinical applications. Diagn Interv Radiol. 2012;18:508-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 52. | Slovak R, Ludwig JM, Gettinger SN, Herbst RS, Kim HS. Immuno-thermal ablations - boosting the anticancer immune response. J Immunother Cancer. 2017;5:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 143] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 53. | Kaminski JM, Shinohara E, Summers JB, Niermann KJ, Morimoto A, Brousal J. The controversial abscopal effect. Cancer Treat Rev. 2005;31:159-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 167] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 54. | Rossi S, Viera FT, Ghittoni G, Cobianchi L, Rosa LL, Siciliani L, Bortolotto C, Veronese L, Vercelli A, Gallotti A, Ravetta V. Radiofrequency ablation of pancreatic neuroendocrine tumors: a pilot study of feasibility, efficacy, and safety. Pancreas. 2014;43:938-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 55. | Scopelliti F, Pea A, Conigliaro R, Butturini G, Frigerio I, Regi P, Giardino A, Bertani H, Paini M, Pederzoli P, Girelli R. Technique, safety, and feasibility of EUS-guided radiofrequency ablation in unresectable pancreatic cancer. Surg Endosc. 2018;32:4022-4028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 56. | Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A, Evans DB. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063-3072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3022] [Cited by in RCA: 3246] [Article Influence: 190.9] [Reference Citation Analysis (0)] |
| 57. | Rosenberg AM, Friedmann P, Del Rivero J, Libutti SK, Laird AM. Resection vs expectant management of small incidentally discovered nonfunctional pancreatic neuroendocrine tumors. Surgery. 2016;159:302-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 58. | Jilesen AP, van Eijck CH, in't Hof KH, van Dieren S, Gouma DJ, van Dijkum EJ. Postoperative Complications, In-Hospital Mortality and 5-Year Survival After Surgical Resection for Patients with a Pancreatic Neuroendocrine Tumor: A Systematic Review. World J Surg. 2016;40:729-748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 59. | Barthet M, Giovannini M, Lesavre N, Boustiere C, Napoleon B, Koch S, Gasmi M, Vanbiervliet G, Gonzalez JM. Endoscopic ultrasound-guided radiofrequency ablation for pancreatic neuroendocrine tumors and pancreatic cystic neoplasms: a prospective multicenter study. Endoscopy. 2019;51:836-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 140] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 60. | Choi JH, Seo DW, Song TJ, Park DH, Lee SS, Lee SK, Kim MH. Endoscopic ultrasound-guided radiofrequency ablation for management of benign solid pancreatic tumors. Endoscopy. 2018;50:1099-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 61. | Imperatore N, de Nucci G, Mandelli ED, de Leone A, Zito FP, Lombardi G, Manes G. Endoscopic ultrasound-guided radiofrequency ablation of pancreatic neuroendocrine tumors: a systematic review of the literature. Endosc Int Open. 2020;8:E1759-E1764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 62. | Dhaliwal A, Kolli S, Dhindsa BS, Choa J, Mashiana HS, Ramai D, Chandan S, Bhogal N, Sayles H, Bhat I, Singh S, Adler DG. Efficacy of EUS-RFA in pancreatic tumors: Is it ready for prime time? Endosc Int Open. 2020;8:E1243-E1251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 63. | Tiankanon K, Kongkam P, Orprayoon T, Luangsukrerk T, Seo DW, Sriuranpong V, Nantavithya C, Jantarattana T, Cañones A, Angsuwatcharakon P, Ridtitid W, Kullavanijaya P, Rerknimitr R. EUS-guided radiofrequency ablation plus chemotherapy vs chemotherapy alone for unresectable pancreacreatic cancer (ERAP): preliminary results of a prospective comparative study. Endoscopy. 2019;51:59. |
| 64. | Faigel DO, Veloso KM, Long WB, Kochman ML. Endosonography-guided celiac plexus injection for abdominal pain due to chronic pancreatitis. Am J Gastroenterol. 1996;91:1675. [PubMed] |
| 65. | Caraceni A, Portenoy RK. Pain management in patients with pancreatic carcinoma. Cancer. 1996;78:639-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 66. | Kaufman M, Singh G, Das S, Concha-Parra R, Erber J, Micames C, Gress F. Efficacy of endoscopic ultrasound-guided celiac plexus block and celiac plexus neurolysis for managing abdominal pain associated with chronic pancreatitis and pancreatic cancer. J Clin Gastroenterol. 2010;44:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 181] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 67. | Minaga K, Kitano M, Sakamoto H, Miyata T, Imai H, Yamao K, Kamata K, Omoto S, Kadosaka K, Sakurai T, Nishida N, Chiba Y, Kudo M. Predictors of pain response in patients undergoing endoscopic ultrasound-guided neurolysis for abdominal pain caused by pancreatic cancer. Therap Adv Gastroenterol. 2016;9:483-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 68. | Wyse JM, Carone M, Paquin SC, Usatii M, Sahai AV. Randomized, double-blind, controlled trial of early endoscopic ultrasound-guided celiac plexus neurolysis to prevent pain progression in patients with newly diagnosed, painful, inoperable pancreatic cancer. J Clin Oncol. 2011;29:3541-3546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 165] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 69. | Arcidiacono PG, Calori G, Carrara S, McNicol ED, Testoni PA. Celiac plexus block for pancreatic cancer pain in adults. Cochrane Database Syst Rev. 2011;CD007519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 70. | Yasuda I, Wang HP. Endoscopic ultrasound-guided celiac plexus block and neurolysis. Dig Endosc. 2017;29:455-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 71. | Sahai AV, Lemelin V, Lam E, Paquin SC. Central vs. bilateral endoscopic ultrasound-guided celiac plexus block or neurolysis: a comparative study of short-term effectiveness. Am J Gastroenterol. 2009;104:326-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 72. | Coté GA, Singh S, Bucksot LG, Lazzell-Pannell L, Schmidt SE, Fogel E, McHenry L, Watkins J, Lehman G, Sherman S. Association between volume of endoscopic retrograde cholangiopancreatography at an academic medical center and use of pancreatobiliary therapy. Clin Gastroenterol Hepatol. 2012;10:920-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 73. | Khashab MA, Valeshabad AK, Afghani E, Singh VK, Kumbhari V, Messallam A, Saxena P, El Zein M, Lennon AM, Canto MI, Kalloo AN. A comparative evaluation of EUS-guided biliary drainage and percutaneous drainage in patients with distal malignant biliary obstruction and failed ERCP. Dig Dis Sci. 2015;60:557-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 158] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 74. | Giovannini M, Moutardier V, Pesenti C, Bories E, Lelong B, Delpero JR. Endoscopic ultrasound-guided bilioduodenal anastomosis: a new technique for biliary drainage. Endoscopy. 2001;33:898-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 480] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 75. | Khan MA, Akbar A, Baron TH, Khan S, Kocak M, Alastal Y, Hammad T, Lee WM, Sofi A, Artifon EL, Nawras A, Ismail MK. Endoscopic Ultrasound-Guided Biliary Drainage: A Systematic Review and Meta-Analysis. Dig Dis Sci. 2016;61:684-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 141] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 76. | Han SY, Kim SO, So H, Shin E, Kim DU, Park DH. EUS-guided biliary drainage vs ERCP for first-line palliation of malignant distal biliary obstruction: A systematic review and meta-analysis. Sci Rep. 2019;9:16551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (1)] |
| 77. | Hedjoudje A, Sportes A, Grabar S, Zhang A, Koch S, Vuitton L, Prat F. Outcomes of endoscopic ultrasound-guided biliary drainage: A systematic review and meta-analysis. United European Gastroenterol J. 2019;7:60-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 78. | Park JK, Woo YS, Noh DH, Yang JI, Bae SY, Yun HS, Lee JK, Lee KT, Lee KH. Efficacy of EUS-guided and ERCP-guided biliary drainage for malignant biliary obstruction: prospective randomized controlled study. Gastrointest Endosc. 2018;88:277-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 167] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 79. | Tendler DA. Malignant gastric outlet obstruction: bridging another divide. Am J Gastroenterol. 2002;97:4-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 80. | Tyberg A, Perez-Miranda M, Sanchez-Ocaña R, Peñas I, de la Serna C, Shah J, Binmoeller K, Gaidhane M, Grimm I, Baron T, Kahaleh M. Endoscopic ultrasound-guided gastrojejunostomy with a lumen-apposing metal stent: a multicenter, international experience. Endosc Int Open. 2016;4:E276-E281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 179] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 81. | Iqbal U, Khara HS, Hu Y, Kumar V, Tufail K, Confer B, Diehl DL. EUS-guided gastroenterostomy for the management of gastric outlet obstruction: A systematic review and meta-analysis. Endosc Ultrasound. 2020;9:16-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 106] [Article Influence: 21.2] [Reference Citation Analysis (1)] |
| 82. | El Bacha H, Leblanc S, Bordacahar B, Brieau B, Barret M, Savier E, Soubrane O, Dousset B, Prat F. Endoscopic Ultrasound-Guided Enteroenterostomy for Afferent Limb Syndrome. ACG Case Rep J. 2020;7:e00442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |