Published online Aug 16, 2021. doi: 10.4253/wjge.v13.i8.336
Peer-review started: February 12, 2021
First decision: May 5, 2021
Revised: June 11, 2021
Accepted: July 13, 2021
Article in press: July 13, 2021
Published online: August 16, 2021
Processing time: 180 Days and 14.5 Hours
Pancreatic endotherapy provides treatment options for the management of chronic pancreatitis-related structural complications such as pancreatic duct stones, strictures, and pancreatic fluid collections. Most studies detailing endotherapy, however, have focused on technical success outcomes such as stone clearance or stricture resolution.
To review the effect of pancreatic endotherapy on patient-centered outcomes.
Systematic review of studies examining pancreatic endotherapy.
A total of 13 studies including 3 randomized clinical trials were included. The majority of studies found an improvement in quality of life with pancreatic endotherapy.
While pancreatic endotherapy does appear to improve quality of life, there are clear gaps in knowledge regarding many pancreatic endotherapy modalities. Furthermore, qualitative analysis is lacking in these studies and further work is needed to elucidate the patient experience with pancreatic endotherapy.
Core Tip: Chronic pancreatitis remains difficult to treat and pancreatic endotherapy offers one option for the management of chronic pancreatitis-related complications. Pancreatic duct decompression via pancreatic duct stone lithotripsy and stenting appears to improve the quality of life of these patients in the short-term. More studies, however, are needed to examine the effect of endotherapy modalities such as endoscopic transmural drainage of pancreatic fluid collections, celiac plexus blocks and more recent innovations on quality of life in these patients.
- Citation: Han SY, Papachristou GI, Shah RJ, Conwell DL. Effect of pancreatic endotherapy on quality of life in chronic pancreatitis patients: A systematic review. World J Gastrointest Endosc 2021; 13(8): 336-344
- URL: https://www.wjgnet.com/1948-5190/full/v13/i8/336.htm
- DOI: https://dx.doi.org/10.4253/wjge.v13.i8.336
Pain, the hallmark feature of chronic pancreatitis (CP), remains difficult to manage effectively and can significantly worsen patients’ quality of life[1-3]. A variety of factors likely play a role in the mechanism of pain, which can include ductal hypertension, inflammation, or neuropathic pain from varying degrees of sensitization of the nervous system[1,4]. Targeted treatment based on the etiology of the pain therefore is challenging and initial treatment will typically consist of medical management.
Pancreatic endotherapy (PET) offers a treatment option for patients with CP-related structural complications such as pancreatic duct (PD) stones, strictures, stones, or pancreatic fluid collections such as pseudocysts. Patients must typically fail medical management before PET is considered with persistent pain being the most common indication. The last decade has ushered in a wave of new PET modalities that have advanced the field beyond standard endoscopic retrograde pancreatography. For PD stones, per-oral pancreatoscopy (POP)-guided lithotripsy using electrohydraulic lithotripsy or laser lithotripsy have dramatically increased the rates of successful PD stone clearance[5,6]. For pancreatic duct strictures, the use of fully covered metal stents, wire-guided cystotomes, and POP-guided laser dissection have greatly expanded the armament of the endoscopist for these refractory stenoses[7-11]. Lastly, the development of lumen-apposing metal stents has revolutionized the drainage of pancreatic fluid collections by facilitating endoscopic transmural drainage in a single step[12,13].
Despite these advances in PET, published studies have largely focused on technical success outcomes such as stricture resolution or stone clearance[5,6,14-16]. Furthermore, the few randomized studies have centered on pain improvement as the primary outcome, which while important, does not capture the holistic impact of PET on patients. As patients and physicians will have different priorities, expectations, and preferences regarding treatment choices, it is critically important to incorporate patient-centered outcomes such as quality of life in the evaluation of these modalities[17]. Therefore, the aim of this review is to detail the effect of PET on quality of life in patients with CP.
We searched PubMed for relevant English-language articles published by January 5, 2021 with no restriction on earliest publication date. The search terms included quality of life and each of the following: endoscopic therapy, endoscopic retrograde cholangiopancreatography (ERCP), celiac plexus block, pancreatic duct stone, pancreatic duct stricture, pancreatic duct stent, pancreatic fluid collection, pseudocyst, pancreatoscopy, lithotripsy, and endoscopic ultrasound.
The relevance of the studies was determined using the hierarchical approach as recommended by the PRISMA statement. We assessed the studies by examining the title, abstract, and/or full text of the studies. We also examined the references of included studies to identify any additional studies. Inclusion criteria included the following: (1) Studies involving PET that included quality of life as an outcome; (2) Publication in the English language; (3) Availability of the full text; and (4) Publication date by January 5th, 2021. Exclusion criteria included the following: (1) Non-original studies including reviews, editorials, commentaries, and study protocols; (2) Insufficient data; and (3) Duplicate studies (i.e., conference abstract and full-text manuscript).
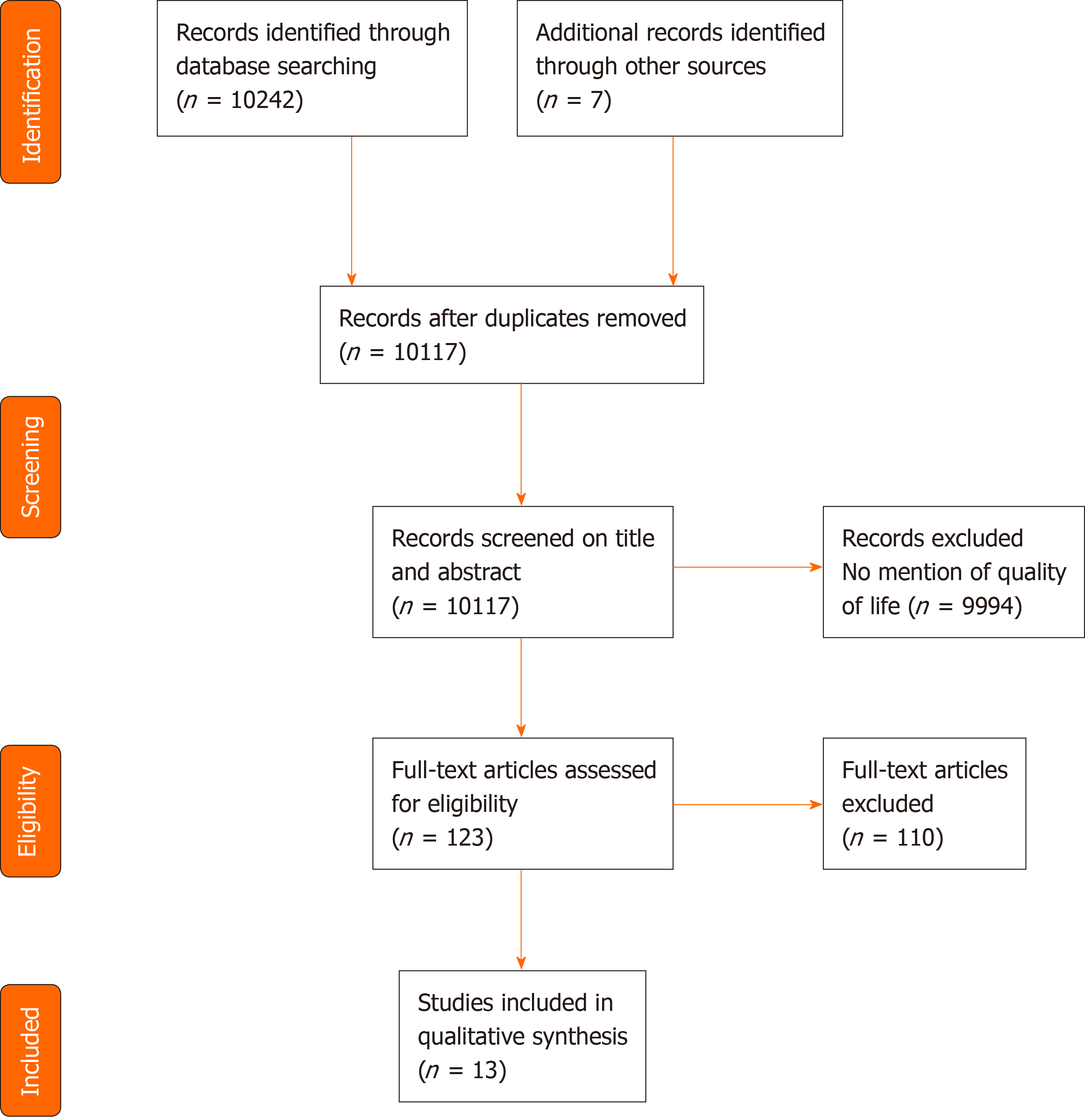
The literature search flow diagram is presented in Figure 1. The initial PubMed database search yielded a total of 10, 242 articles. Upon title and abstract review, the full text of 123 articles were reviewed. Upon excluding 110 of these studies, which were found to be irrelevant, a total of 13 studies, including 3 randomized clinical trials and 10 observational studies were included (Table 1).
| Ref. | Study design | Endoscopic modality | n | Quality of life measurement | Quality of life findings |
| Cahen et al[18,19] | Randomized clinical trial | ERCP ± ESWL | 19 | SF-36 | Physical health: 31 ± 8 to 38 ± 9 (2 yr) and 43 ± 11 (7 yr); Mental health: 33 ± 8 to 40 ± 9 (2 yr) and 46 ± 9 (7 yr) |
| Issa et al[20] | Randomized clinical trial | ERCP ± ESWL | 44 | SF-36 | Physical health: 31 ± 8 to 36 ± 9; Mental health: 36 ± 11 to 41 ± 11 |
| Stevens et al[31] | Randomized study | Celiac plexus block | 40 | SF-12 | Change in physical score: -0.2 ± 7.5 (triamcinolone + bupivacaine), 1.7 ± 8.8 (bupivacaine); Change in mental score: 1.3 ± 10.0 (triamcinolone + bupivacaine), -2.1 ± 12.9 (bupivacaine) |
| Brand et al[22] | Prospective study | ERCP + ESWL | 48 | EORTC | Pain: 37.8 (range 0-81.5) to 18.8 (range 0-83.3); Weight loss: 66.7 (range 0-100) to 0 (range 0-100); Global quality of life: 41.7 (range 16.7-100) to 58.3 (range 8.3-100) |
| Hu et al[24] | Prospective study | ERCP + ESWL | 214 | SF-36 | Physical health: 56.9 ± 18.7 to 59.2 ± 14.8 (no significant difference); Patients with pseudocysts: 95 (range 35-100) to 100 (range 75-100); Mental health: 52.2 ± 21.5 to 58.5 ± 16.4; Patients with pseudocysts: 68 (range 36-100) to 76 (range 28-100) |
| Milovic et al[27] | Prospective study | ERCP + ESWL | 32 | 1-5 scale | 4 (range 2-5) to 2.5 (range 1-4) |
| Basiński et al[32] | Prospective study | Celiac plexus block | 92 | EORTC | Quality of life significantly improved with greatest improvement seen in those with high religiosity |
| Rutter et al[21] | Retrospective study | ERCP | 150 | EORTC | Patients treated with surgery had less nausea/vomiting compared to those treated with endoscopy |
| Tandan et al[23] | Retrospective study | ERCP + ESWL | 636 | 1-10 scale | 252 (92.6%) patients had improved quality of life |
| Seven et al[26] | Retrospective study | ERCP + ESWL | 120 | 1-10 scale | 3.7 ± 2.4 to 7.3 ± 2.7 |
| Gerges et al[28] | Retrospective study | Pancreatoscopy-guided lithotripsy | 20 | Generic quality of life instrument | 89% had no or only mild disability in daily activities, 47% had “excellent” or “very good” general health |
| Vitale et al[29] | Retrospective study | Minor papilla stenting | 32 | Generic quality of life survey | 100% stated improved quality of life, 100% stated satisfaction with treatment |
The major randomized trials comparing endoscopy with surgery focus on pancreatic duct drainage to relieve ductal hypertension. In the landmark trial comparing endoscopic treatment [ERCP with stricture dilation for PD strictures ± extracorporeal shock-wave lithotripsy (ESWL) for concomitant PD stones] with a side-to-side pancreaticojejunostomy, at 2 year follow-up patients who received endotherapy (n = 19) had an improvement in both physical health (31 ± 8 to 38 ± 9) and mental health (33 ± 8 to 40 ± 9) on the 36-Item Short Form Health Survey (SF-36) questionnaire[18]. While this was less than the improvement in quality of life seen in the surgery arm, in the follow-up study examining long-term (mean follow-up of 79 mo) outcomes of both arms, the improvement in both physical and mental quality of life persisted, but there was no longer any difference between the two arms[19]. More recently, the ESCAPE trial from the Dutch pancreatitis study group randomized patients with painful CP and a dilated PD to either early pancreatic drainage surgery (n = 44) or endotherapy (ERCP ± ESWL) first (n = 44)[20]. At 18 mo follow-up, patients in the endotherapy arm did experience an improvement in both physical (31 ± 8 to 36 ± 9) and mental (36 ± 11 to 41 ± 11) health on the SF-36 with no difference seen in quality of life between the two treatment groups. Lastly, in a retrospective study comparing surgery with endotherapy, the European Organization for Research and Treatment of Cancer (EORTC) quality of life instrument and the pancreatic cancer module (PAN26) instrument were utilized with the primary finding that patients treated with surgery had less nausea and vomiting[21].
Internationally, the combination of ESWL with ERCP represents the most common form of treatment for symptomatic PD stones. Starting with a prospective study by Brand et al[22] in 2000, ESWL followed by ERCP was associated with an improvement in pain, weight loss, fevers/chills, jaundice, and global quality of life on the EORTC instrument. Within an Indian patient population, Tandan et al[23] presented a large study (n = 636) of this treatment modality, finding that using a scale of 1-10 (10 representing the best quality of life), quality of life improvement was seen in 92.8% of patients at 2-5 year follow-up and in 92.6% of patients at > 5 year follow-up. In a large Chinese patient cohort using the SF-36, a significant improvement was seen in overall quality of life and physical health, but not in mental health[24,25]. Seven et al[26] presented data on this PET combination in a United States cohort, utilizing a 1-10 quality of life score (10 being the best quality of life), finding a significant improvement in quality of life (3.7 ± 2.4 to 7.3 ± 2.7) after completion of therapy. Similarly, in a study from Germany, Milovic et al[27] reported a significant improvement in quality of life after ESWL and ERCP on a 5-point quality of life scale (2.5 to 4).
In the only study examining pancreatoscopy-guided lithotripsy that included quality of life as a study outcome, Gerges et al[28] utilized both electrohydraulic and laser lithotripsy in 20 patients. They found that post-therapy, 89% of patients had no or only mild disability in daily activities and 47% of patients described their health as “excellent” or “very good.”
Minor papilla endotherapy typically involves performing a minor papilla sphincterotomy and/or stenting. Depending on the presence of strictures or stones, endotherapy can also include dilation or stone lithotripsy. A single-center study examining 32 patients with CP and pancreas divisum-related strictures assessed quality of life through telephone surveys asking about their overall quality of life and their level of satisfaction post-treatment[29]. All subjects treated via endotherapy reported improved quality of life and satisfaction in their treatment.
There were no studies examining transmural drainage of CP-associated pancreatic fluid collections that included quality of life as an outcome. In regards to patients with acute necrotizing pancreatitis, however, Smith et al[30] performed a single-center cross-sectional study examining patients treated with endoscopic ultrasound-guided transmural drainage of walled-off necrosis. Using the SF-36, the authors found that at 2 year follow-up, patients treated with transmural drainage had equivalent scores to a healthy control population in nearly all domains with the exception of the physical role and general health domains, where they had significantly lower scores (physical role: 58.5 ± 40.9 vs 81.0 ± 34.0, general health: 56.9 ± 25.8 vs 72.0 ± 20.3) Notably, these subjects had significantly higher quality of life scores in domains such as pain and vitality compared to patients with irritable bowel syndrome.
In a single-center randomized study comparing celiac plexus block (using bupivacaine) with and without triamcinolone for patients with painful CP, pre and post-therapy quality of life was assessed using the SF-12[31]. The study was stopped prematurely at interim analysis due to no difference between the two treatment arms in improving pain and no significant differences in physical and mental quality of life were seen between the 2 arms. The triamcinolone arm saw a change of -0.2 ± 7.5 for physical health and a change of 1.3 ± 10.0 in mental health while the control arm saw a change of 1.7 ± 8.8 in physical health and a change of -2.1 ± 12.9 in mental health. In a study from Poland, Basiński et al[32] utilized the EORTC quality of life questionnaire, finding improvement in quality of life at 1- and 4-wk follow-up. Stratifying patients on their level of religiosity, the greatest improvement in quality of life was seen in those with high religiosity at both time points.
In this systematic review, while we demonstrate that PET does appear to improve quality of life in patients with CP, the most striking finding is the overall lack of evidence in many of these PET modalities. The majority of evidence comes from endoscopic treatment of pancreatic ductal obstruction secondary to PD stones and strictures with the 2 Landmark trials by Cahen et al[18] and Issa et al[20] comparing surgical with endoscopic drainage. There remain clear gaps in knowledge regarding how endoscopic therapies such as celiac plexus block, pancreatoscopy-guided therapies, endoscopic transmural drainage of pancreatic fluid collections and minor papilla endotherapy affect quality of life in the CP population. This highlights the continued emphasis of endoscopic studies on technical success outcomes rather than patient-centered outcomes and while PET modalities will continue to expand, without understanding the impact of these therapies on patients, choosing the best treatment for each individual patient becomes even more challenging.
As shown in Table 1, studies most often measured quality of life using the SF-36 and the EORTC quality of life instrument, which while validated, are not disease-specific for chronic pancreatitis. The remaining studies assessed quality of life by simply asking about quality of life, speaking to need for more rigorous research in quality of life within this field of endotherapy. The PANcreatitis Quality of Life Instrument is a validated chronic pancreatitis-specific quality of life instrument consisting of 18 items that includes sub-scores for physical function, role function, emotional function, and self-worth domains[33]. Additionally, the National Institute of Health has developed the Patient-Reported Outcomes Measurement Information System instruments to standardize measurement of patient-reported outcomes such as quality of life and pain. Incorporating instruments such as these can facilitate future research in this arena by capturing critical quality of life aspects pertinent to this patient population.
Pain remains the center point of quality of life in patients with CP as constant pain and severe pain, in particular, are associated with worse quality of life[2,34]. Similar to quality of life, pain has been poorly measured in prior PET studies with most reporting a visual analog scale score or the Izbicki pain score, which are simplified assessments of pain[35]. The Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials has recently called for improved phenotyping of pain in an effort to deliver the most appropriate therapy based on an individual patient’s pain characteristics[36]. In line with this, pancreatic quantitative sensory testing (QST) represents a novel method of characterizing sensory processing in the peripheral and central pain pathways[37]. While data has demonstrated how QST can be used to predict the efficacy of pregabalin in CP patients, much work is needed to determine if QST can help predict a priori which patients will respond to PET[38]. Nevertheless, there remains much promise in using tools such as QST to better characterize pain profiles in patients with CP to ultimately develop an algorithm-based approach to the management of this challenging disease.
In addition to the quantitative analysis done in these studies, qualitative studies are needed to truly encapsulate subjects’ experiences with PET and better understand how PET affects their disease. Quantitative assessment of quality of life captures only a portion of the patient’s overall well-being and given the lack of qualitative studies centered around endotherapy, future endeavors are certainly needed to incorporate the patient’s perspective. Understanding factors such as patient expectations, regret, suffering, and coping may help design future randomized sham-controlled trials with patient-centered outcomes to help determine which PET modalities are most effective in which patients.
In summary, given the dearth of treatment options for CP, PET offers a viable therapy for patients with CP-related complications such as PD stones and strictures. Much work is needed, however, to elucidate the patient experience with PET and identify who will respond to PET with the ultimate goal of providing individualized treatment plans for these patients.
While pancreatic endotherapy is frequently performed for the treatment of chronic pancreatitis-related complications, most studies examining endotherapy have focused on technical success outcomes, such as stricture resolution or stone clearance. Studies reporting patient-centered outcomes such as quality of life are lacking, however, making it difficult to determine how endotherapy affects these patients.
The motivation for this systematic review stems from the primary criticism of pancreatic endotherapy on whether endotherapy improves the lives of patients with chronic pancreatitis. While it is well-known that endotherapy can treat the structural complications of chronic pancreatitis, the effect of endotherapy on patient-centered outcomes is poorly studied.
The primary objective of this systematic review was to detail the literature regarding how pancreatic endotherapy affects quality of life in chronic pancreatiits patients.
A systematic review was performed to identify studies reporting on various pancreatic endotherapy modalities and quality of life.
The search yielded 13 studies for review out of 10242 articles. All of the modalities examined found an improvement in quality of life.
Pancreatic endotherapy does appear to improve quality of life, but the assessment of quality of life is very heterogeneous and not disease-specific. Furthermore, there is a lack of evidence regarding many modalities such as transmural fluid drainage, pancreatoscopy-guided therapy and celiac plexus block.
Further studies are clearly needed to elucidate the patient experience with receiving pancreatic endotherapy and future trials will benefit from having patient-centered outcomes as the primary outcome.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dedemadi G S-Editor: Zhang H L-Editor: A P-Editor: Xing YX
| 1. | Singh VK, Yadav D, Garg PK. Diagnosis and Management of Chronic Pancreatitis: A Review. JAMA. 2019;322:2422-2434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 299] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 2. | Mullady DK, Yadav D, Amann ST, O'Connell MR, Barmada MM, Elta GH, Scheiman JM, Wamsteker EJ, Chey WD, Korneffel ML, Weinman BM, Slivka A, Sherman S, Hawes RH, Brand RE, Burton FR, Lewis MD, Gardner TB, Gelrud A, DiSario J, Baillie J, Banks PA, Whitcomb DC, Anderson MA; NAPS2 Consortium. Type of pain, pain-associated complications, quality of life, disability and resource utilisation in chronic pancreatitis: a prospective cohort study. Gut. 2011;60:77-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 231] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 3. | Olesen SS, Juel J, Nielsen AK, Frøkjær JB, Wilder-Smith OH, Drewes AM. Pain severity reduces life quality in chronic pancreatitis: Implications for design of future outcome trials. Pancreatology. 2014;14:497-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 4. | Pasricha PJ. Unraveling the mystery of pain in chronic pancreatitis. Nat Rev Gastroenterol Hepatol. 2012;9:140-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 5. | Brewer Gutierrez OI, Raijman I, Shah RJ, Elmunzer BJ, Webster GJM, Pleskow D, Sherman S, Sturgess RP, Sejpal DV, Ko C, Maurano A, Adler DG, Mullady DK, Strand DS, DiMaio CJ, Piraka C, Sharahia R, Dbouk MH, Han S, Spiceland CM, Bekkali NLH, Gabr M, Bick B, Dwyer LK, Han D, Buxbaum J, Zulli C, Cosgrove N, Wang AY, Carr-Locke D, Kerdsirichairat T, Aridi HD, Moran R, Shah S, Yang J, Sanaei O, Parsa N, Kumbhari V, Singh VK, Khashab MA. Safety and efficacy of digital single-operator pancreatoscopy for obstructing pancreatic ductal stones. Endosc Int Open. 2019;7:E896-E903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 6. | Han S, Shah RJ, Brauer BC, Edmundowicz SA, Hammad HT, Wagh MS, Wani S, Attwell AR. A Comparison of Endoscopic Retrograde Pancreatography With or Without Pancreatoscopy for Removal of Pancreatic Duct Stones. Pancreas. 2019;48:690-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Tringali A, Vadalà di Prampero SF, Landi R, Bove V, Familiari P, Hamanaka J, Attili F, Costamagna G. Fully covered self-expandable metal stents to dilate persistent pancreatic strictures in chronic pancreatitis: long-term follow-up from a prospective study. Gastrointest Endosc. 2018;88:939-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 8. | Sharaiha RZ, Novikov A, Weaver K, Marfatia P, Buscaglia JM, DiMaio CJ, Diehl D, Gabr MM, Gaidhane M, Siddiqui A, Kahaleh M. Fully covered self-expanding metal stents for refractory pancreatic duct strictures in symptomatic chronic pancreatitis, US experience. Endosc Int Open. 2019;7:E1419-E1423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Oh D, Lee JH, Song TJ, Park DH, Lee SK, Kim MH, Lee SS. Long-term outcomes of 6-mm diameter fully covered self-expandable metal stents in benign refractory pancreatic ductal stricture. Dig Endosc. 2018;30:508-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | Rana SS, Shah J, Bush N, Sharma R, Dhalaria L, Gupta R. Endoscopic dilatation of tight difficult pancreatic duct strictures: Soehendra stent retriever or wire guided cystotome. Pancreatology. 2021;21:498-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Han S, Shah RJ. Cholangiopancreatoscopy-guided laser dissection and ablation for pancreas and biliary strictures and neoplasia. Endosc Int Open. 2020;8:E1091-E1096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Brimhall B, Han S, Tatman PD, Clark TJ, Wani S, Brauer B, Edmundowicz S, Wagh MS, Attwell A, Hammad H, Shah RJ. Increased Incidence of Pseudoaneurysm Bleeding With Lumen-Apposing Metal Stents Compared to Double-Pigtail Plastic Stents in Patients With Peripancreatic Fluid Collections. Clin Gastroenterol Hepatol. 2018;16:1521-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 106] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 13. | Bang JY, Navaneethan U, Hasan MK, Sutton B, Hawes R, Varadarajulu S. Non-superiority of lumen-apposing metal stents over plastic stents for drainage of walled-off necrosis in a randomised trial. Gut. 2019;68:1200-1209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 246] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 14. | Ponchon T, Bory RM, Hedelius F, Roubein LD, Paliard P, Napoleon B, Chavaillon A. Endoscopic stenting for pain relief in chronic pancreatitis: results of a standardized protocol. Gastrointest Endosc. 1995;42:452-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 153] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 15. | Tringali A, Bove V, Vadalà di Prampero SF, Boškoski I, Familiari P, Perri V, Costamagna G. Long-term follow-up after multiple plastic stenting for refractory pancreatic duct strictures in chronic pancreatitis. Endoscopy. 2019;51:930-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 16. | Costamagna G, Bulajic M, Tringali A, Pandolfi M, Gabbrielli A, Spada C, Petruzziello L, Familiari P, Mutignani M. Multiple stenting of refractory pancreatic duct strictures in severe chronic pancreatitis: long-term results. Endoscopy. 2006;38:254-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 147] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 17. | Acuna SA, Chesney TR, Baxter NN. Incorporating Patient Preferences in Noninferiority Trials. JAMA. 2019;322:305-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Cahen DL, Gouma DJ, Nio Y, Rauws EA, Boermeester MA, Busch OR, Stoker J, Laméris JS, Dijkgraaf MG, Huibregtse K, Bruno MJ. Endoscopic versus surgical drainage of the pancreatic duct in chronic pancreatitis. N Engl J Med. 2007;356:676-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 579] [Cited by in RCA: 506] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 19. | Cahen DL, Gouma DJ, Laramée P, Nio Y, Rauws EA, Boermeester MA, Busch OR, Fockens P, Kuipers EJ, Pereira SP, Wonderling D, Dijkgraaf MG, Bruno MJ. Long-term outcomes of endoscopic vs surgical drainage of the pancreatic duct in patients with chronic pancreatitis. Gastroenterology. 2011;141:1690-1695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 192] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 20. | Issa Y, Kempeneers MA, Bruno MJ, Fockens P, Poley JW, Ahmed Ali U, Bollen TL, Busch OR, Dejong CH, van Duijvendijk P, van Dullemen HM, van Eijck CH, van Goor H, Hadithi M, Haveman JW, Keulemans Y, Nieuwenhuijs VB, Poen AC, Rauws EA, Tan AC, Thijs W, Timmer R, Witteman BJ, Besselink MG, van Hooft JE, van Santvoort HC, Dijkgraaf MG, Boermeester MA; Dutch Pancreatitis Study Group. Effect of Early Surgery vs Endoscopy-First Approach on Pain in Patients With Chronic Pancreatitis: The ESCAPE Randomized Clinical Trial. JAMA. 2020;323:237-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 160] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 21. | Rutter K, Ferlitsch A, Sautner T, Püspök A, Götzinger P, Gangl A, Schindl M. Hospitalization, frequency of interventions, and quality of life after endoscopic, surgical, or conservative treatment in patients with chronic pancreatitis. World J Surg. 2010;34:2642-2647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Brand B, Kahl M, Sidhu S, Nam VC, Sriram PV, Jaeckle S, Thonke F, Soehendra N. Prospective evaluation of morphology, function, and quality of life after extracorporeal shockwave lithotripsy and endoscopic treatment of chronic calcific pancreatitis. Am J Gastroenterol. 2000;95:3428-3438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 98] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 23. | Tandan M, Reddy DN, Talukdar R, Vinod K, Santosh D, Lakhtakia S, Gupta R, Ramchandani MJ, Banerjee R, Rakesh K, Varadaraj G, Rao GV. Long-term clinical outcomes of extracorporeal shockwave lithotripsy in painful chronic calcific pancreatitis. Gastrointest Endosc. 2013;78:726-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 24. | Hu LH, Ye B, Yang YG, Ji JT, Zou WB, Du TT, Hao JF, Jiang YY, Liao Z, Li ZS. Extracorporeal Shock Wave Lithotripsy for Chinese Patients With Pancreatic Stones: A Prospective Study of 214 Cases. Pancreas. 2016;45:298-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 25. | Li BR, Liao Z, Du TT, Ye B, Chen H, Ji JT, Zheng ZH, Hao JF, Ning SB, Wang D, Lin JH, Hu LH, Li ZS. Extracorporeal shock wave lithotripsy is a safe and effective treatment for pancreatic stones coexisting with pancreatic pseudocysts. Gastrointest Endosc. 2016;84:69-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 26. | Seven G, Schreiner MA, Ross AS, Lin OS, Gluck M, Gan SI, Irani S, Brandabur JJ, Patterson D, Kuhr C, Kozarek R. Long-term outcomes associated with pancreatic extracorporeal shock wave lithotripsy for chronic calcific pancreatitis. Gastrointest Endosc 2012; 75: 997-1004. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | Milovic V, Wehrmann T, Dietrich CF, Bailey AA, Caspary WF, Braden B. Extracorporeal shock wave lithotripsy with a transportable mini-lithotripter and subsequent endoscopic treatment improves clinical outcome in obstructive calcific chronic pancreatitis. Gastrointest Endosc. 2011;74:1294-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Gerges C, Pullmann D, Schneider M, Siersema P, van Geenen E, Neuhaus H, Beyna T. Pancreatoscopy in endoscopic treatment of pancreatic duct stones: a systematic review. Minerva Chir. 2019;74:334-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Vitale GC, Vitale M, Vitale DS, Binford JC, Hill B. Long-term follow-up of endoscopic stenting in patients with chronic pancreatitis secondary to pancreas divisum. Surg Endosc. 2007;21:2199-2202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Smith ZL, Gregory MH, Elsner J, Alajlan BA, Kodali D, Hollander T, Sayuk GS, Lang GD, Das KK, Mullady DK, Early DS, Kushnir VM. Health-related quality of life and long-term outcomes after endoscopic therapy for walled-off pancreatic necrosis. Dig Endosc. 2019;31:77-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Stevens T, Costanzo A, Lopez R, Kapural L, Parsi MA, Vargo JJ. Adding triamcinolone to endoscopic ultrasound-guided celiac plexus blockade does not reduce pain in patients with chronic pancreatitis. Clin Gastroenterol Hepatol 2012; 10: 186-191, 191. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 32. | Basiński A, Stefaniak T, Stadnyk M, Sheikh A, Vingerhoets AJ. Influence of religiosity on the quality of life and on pain intensity in chronic pancreatitis patients after neurolytic celiac plexus block: case-controlled study. J Relig Health. 2013;52:276-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Wassef W, DeWitt J, McGreevy K, Wilcox M, Whitcomb D, Yadav D, Amann S, Mishra G, Alkaade S, Romagnuolo J, Stevens T, Vargo J, Gardner T, Singh V, Park W, Hartigan C, Barton B, Bova C. Pancreatitis Quality of Life Instrument: A Psychometric Evaluation. Am J Gastroenterol. 2016;111:1177-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 34. | Olesen SS, Nøjgaard C, Novovic S, Jensen NM, Nørregaard P, Dahl EE, Waage A, Hauge T, Barauskas G, Parhiala M, Laukkarinen J, Drewes AM. Pain and aetiological risk factors determine quality of life in patients with chronic pancreatitis, but a brick in the puzzle is missing. Pancreatology. 2020;20:1347-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Izbicki JR, Bloechle C, Broering DC, Kuechler T, Broelsch CE. Longitudinal V-shaped excision of the ventral pancreas for small duct disease in severe chronic pancreatitis: prospective evaluation of a new surgical procedure. Ann Surg. 1998;227:213-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 88] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 36. | Edwards RR, Dworkin RH, Turk DC, Angst MS, Dionne R, Freeman R, Hansson P, Haroutounian S, Arendt-Nielsen L, Attal N, Baron R, Brell J, Bujanover S, Burke LB, Carr D, Chappell AS, Cowan P, Etropolski M, Fillingim RB, Gewandter JS, Katz NP, Kopecky EA, Markman JD, Nomikos G, Porter L, Rappaport BA, Rice ASC, Scavone JM, Scholz J, Simon LS, Smith SM, Tobias J, Tockarshewsky T, Veasley C, Versavel M, Wasan AD, Wen W, Yarnitsky D. Patient phenotyping in clinical trials of chronic pain treatments: IMMPACT recommendations. Pain. 2016;157:1851-1871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 277] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 37. | Phillips AE, Faghih M, Kuhlmann L, Larsen IM, Drewes AM, Singh VK, Yadav D, Olesen SS; Pancreatic Quantitative Sensory Testing (P-QST) Consortium. A clinically feasible method for the assessment and characterization of pain in patients with chronic pancreatitis. Pancreatology. 2020;20:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 38. | Olesen SS, Graversen C, Bouwense SA, van Goor H, Wilder-Smith OH, Drewes AM. Quantitative sensory testing predicts pregabalin efficacy in painful chronic pancreatitis. PLoS One. 2013;8:e57963. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 106] [Article Influence: 8.8] [Reference Citation Analysis (0)] |