Published online Jul 16, 2021. doi: 10.4253/wjge.v13.i7.221
Peer-review started: April 5, 2021
First decision: June 7, 2021
Revised: June 17, 2021
Accepted: July 7, 2021
Article in press: July 7, 2021
Published online: July 16, 2021
Processing time: 99 Days and 5.2 Hours
The large majority of gastrointestinal bleedings subside on their own or after endoscopic treatment. However, a small number of these may pose a challenge in terms of therapy because the patients develop hemodynamic instability, and endoscopy does not achieve adequate hemostasis. Interventional radiology supplemented with catheter angiography (CA) and transarterial embolization have gained importance in recent times.
To evaluate clinical predictors for angiography in patients with lower gastro
We compared two groups of patients in a retrospective analysis. One group had been treated for more than 10 years with CA for LGIB (n = 41). The control group had undergone non-endoscopic or endoscopic treatment for two years and been registered in a bleeding registry (n = 92). The differences between the two groups were analyzed using decision trees with the goal of defining clear rules for optimal treatment.
Patients in the CA group had a higher shock index, a higher Glasgow-Blatchford bleeding score (GBS), lower serum hemoglobin levels, and more rarely achieved hemostasis in primary endoscopy. These patients needed more transfusions, had longer hospital stays, and had to undergo subsequent surgery more frequently (P < 0.001).
Endoscopic hemostasis proved to be the crucial difference between the two patient groups. Primary endoscopic hemostasis, along with GBS and the number of transfusions, would permit a stratification of risks. After prospective confirmation of the present findings, the use of decision trees would permit the identification of patients at risk for subsequent diagnosis and treatment based on interventional radiology.
Core Tip: Transarterial embolization enables the clinician to control gastrointestinal bleeding with high rates of technical and clinical success. We still do not know when the clinician should conclude endoscopic procedures to control gastrointestinal bleeding. This retrospective study compared patients with conservative treatment and patients who underwent catheter angiography. Patients in the catheter angiography group had a higher shock index, a higher Glasgow-Blatchford score and more rarely achieved hemostasis in primary endoscopy. These patients needed more transfusions, had longer hospital stays and had to undergo subsequent surgery more frequently. Endoscopic hemostasis proved to be the crucial difference between the two patient groups.
- Citation: Werner DJ, Baar T, Kiesslich R, Wenzel N, Abusalim N, Tresch A, Rey JW. Endoscopic hemostasis makes the difference: Angiographic treatment in patients with lower gastrointestinal bleeding. World J Gastrointest Endosc 2021; 13(7): 221-232
- URL: https://www.wjgnet.com/1948-5190/full/v13/i7/221.htm
- DOI: https://dx.doi.org/10.4253/wjge.v13.i7.221
Flexible endoscopy is the gold standard for the diagnosis and treatment of gastrointestinal bleeding. The majority of lower gastrointestinal bleedings (LGIB) subside spontaneously without intervention. An analysis of 2528 patients revealed that a quarter of the patients received transfusions and 10% needed more than four red cell concentrates[1]. Endoscopy discloses the bleeding in no more than 40% of cases[2]. Diverticular bleeding is the most frequent cause of LGIB, accounting for 30%-65% of all cases. As many as 80% of these subside spontaneously[3]. Further frequent causes of bleeding are angiodysplasia and hemorrhoids, as well as cancer[2,4]. Once the bleeding is identified on endoscopy, more than 90% of these can be treated successfully. The appropriate time point of diagnostic endoscopic investigation is still not clear, because approximately 85% of LGIB can be managed by supportive treatment without any major threat to the patient’s health. Guidelines recommend diagnostic endoscopy within 12-24 h[3-7].
Especially in cases of severe bleeding not amenable to endoscopic treatment, surgery serves an additional invasive therapy option[2,4]. Besides, interventional radiology has emerged as an important alternative in the last few years. A repeated bidirectional endoscopy of flawless quality does not enhance the diagnostic yield. In fact, it delays the course of treatment because the interval between the potential bleeding event and subsequent investigations is prolonged. Thus, further radiological investigation and treatment are obviously needed.
In cases of uncontrollable bleeding or recurrent non-varicose gastrointestinal bleeding, the German guidelines for gastrointestinal bleeding recommend early transfer of the patient to a center that provides the option of interventional radiology[8]. Determining the ideal time point for this measure in the course of a patient’s treatment appears to be of crucial importance.
Currently, radiological diagnostic investigation and treatment are largely oriented to local facilities. These include, in particular, the availability of therapeutic endoscopy and interventional radiology[2]. Interdisciplinary cooperation between gastroenterologists and radiologists is obviously a crucial factor. Prior to catheter angiography (CA), it would be advisable to perform a computed tomography angiography (CTA). The latter is propagated as an effective method for the localization of bleeding, as well as pre-interventional viewing of vascular anatomy and the detection of relevant additional findings[9].
Given the high sensitivity and specificity of CTA for the detection of active gastrointestinal bleeding, this procedure is recommended in the guidelines[10]. Once CTA has provided evidence of bleeding, CA with transarterial embolization (TAE) is currently the method of choice for controlling an acute LGIB[10,11]. TAE enables the clinician to control gastrointestinal bleeding with high rates of technical (90%-100%) and clinical success (50%-90%), low complication rates of 1%-5%, and improved long-term survival rates[4,7,12-16].
We still do not know when the clinician should conclude endoscopic procedures to control gastrointestinal bleeding, whether CTA has an effect on the outcome, and whether patients with no or a negative CTA should also be scheduled to undergo angiography. In view of these facts, the present retrospective study was performed in a large German single-center patient population at a maximum care hospital. We assessed the course of treatment in patients with LGIB who had undergone interventional radiological treatment. We focused on the identification of variables that raised the likelihood of further radiological diagnosis (CTA) and treatment (CA/TAE) in the course of disease.
All patients with LGIB who had undergone a CA (CA-LGIB-group) at a maximum care hospital from 1 January 2007 to 31 March 2018 were included in a retrospective analysis. There were no exclusion criteria. The reference group included patients with suspected LGIB who had undergone treatment from 1 January 2015 to 31 December 2016 (reference group with LGIB, K-LGIB). Patients already recorded in the CA-LGIB registry were excluded from the K-LGIB group. One hundred and twenty variables were registered in the K-LGIB registry, and 110 variables in the CA-LGIB registry. Based on clinical estimates, we selected 20 common variables from both groups for the purposes of the present study. The Glasgow-Blatchford bleeding score (GBS)[17], the course of treatment, and the duration of hospitalization were also registered.
Endoscopic diagnostic investigation and treatment were performed exclusively by investigators who had several years of experience in endoscopic treatment. The data were extracted from a reporting program named E&L (Clinic WinData, Nuremberg) and the hospital information system (SAP, Walldorf). In endoscopic therapy, the absence of hemostasis was defined as persistent bleeding under direct endoscopic visual control, clinically persistent bleeding after the intervention, or persistent clinical bleeding with a drop in hemoglobin levels.
All CTA investigations were performed on a Siemens CT Somatom 128 device. A standardized protocol was not used. Over the entire study period, the CA’s were performed by five radiologists with several years of experience in interventional radiology. In most cases we used a transfemoral access with a 5/6 French sheath, a guiding catheter, and a microcatheter. Embolization was achieved with various materials, such as coils, polyvinyl alcohol particles (PVA), or n-butyl cyanoacrylate (NBCA). The technical success of CA was defined as the visualization of a suspected bleeding vessel without extravasation or localization of the bleeding vessel and performing TAE. Clinical success was defined as the absence of any complication after 30 d. The absence of complications included no repeat angiography, no surgical intervention, or discharge of the patient. Hemodynamic instability was defined as a systolic blood pressure below 100 mmHg, a positive shock index, or transfusion of four or more red cell concentrates in 48 h[18].
Data analysis was performed using R v3.6.1[19]. For two-sample comparisons (Table 1), Wilcoxon's rank sum test was used for continuous data, circumventing the requirements for normality of the t-test. Fisher's exact test was used for categorical data. Variable importance (Figure 1) was determined with the randomForest package v4.6.14[20], and decision trees (Figures 2 and 3) were constructed using the party package v1.3.4[21]. The decision trees were based on the set of all variables, or a reduced set composed of variables with assumed clinical relevance, using conditional inference trees. This algorithm recursively applies binary partitions to the dataset, splitting it by the most informative variable, as determined by Bonferroni-adjusted Monte Carlo p-values. The partitions are applied until further splitting of the dataset would not increase the predictive power of the tree any further (see stop criterion in the package reference manual).
| CA-LGIB | K-LGIB | P value | |
| General data | |||
| Number of patients (n) | 41 | 92 | |
| TAE performed, n (%) | 20 (48.8) | 0 | |
| Age (yr) | 72.8 | 73.2 | 0.42541 |
| Sex (%) | 0.1822 | ||
| Male | 29 (70.7) | 54 (58.2) | |
| Female | 12 (29.3) | 38 (41.8) | |
| Clinical data | |||
| RR sys (mmHg) | 103 | 124 | ≤ 0.00011 |
| HR (bpm) | 97 | 82 | ≤ 0.00011 |
| Shock index | 1 | 0.7 | ≤ 0.00011 |
| Transfusions (n) | 7.44 | 0.55 | ≤ 0.00011 |
| Anticoagulants (%) | 0.122 | ||
| Yes | 22 (53.7) | 63 (68.5) | |
| No | 19 (46.3) | 28 (30.4) | |
| BFS | 11.49 | 8.28 | ≤ 0.00011 |
| Hb (mg/dL) | 7.98 | 10.7 | ≤ 0.00011 |
| Thrombocytes (10³/µL) | 189 | 265 | ≤ 0.00061 |
| Creatinine (mg/dL) | 0.98 | 1.24 | 0.02551 |
| INR | 1.27 | 1.29 | 0.16321 |
| Endoscopic data | |||
| Endoscopies prior to CA (n) | 2.07 | 2.12 | 0.921 |
| Hemostasis achieved in primary endoscopy, n (%) | ≤ 0.00012 | ||
| Yes | 4 (9.8) | 88 (95.7) | |
| No | 37 (90.2) | 3 (3.3) | |
| Location of bleeding, n (%) | ≤ 0.00872 | ||
| Ambiguous | 7 (17.5) | 43 (46.7) | |
| Jejunum/ileum | 4 (10) | 1 (1.1) | |
| Colon | 28 (70) | 45 (50) | |
| Others | 1 (2.5) | 2 (2.2) | |
| Follow up | |||
| Duration of hospitalization (d) | 19.44 | 9.79 | ≤ 0.0011 |
| Discharge, n (%) | 25 (61.0) | 83 (90.2) | ≤ 0.00012 |
| Surgery, n (%) | 13 (31.7) | 4 (4.3) | |
| Death, n (%) | 3 (7.3) | 3 (3.3) |
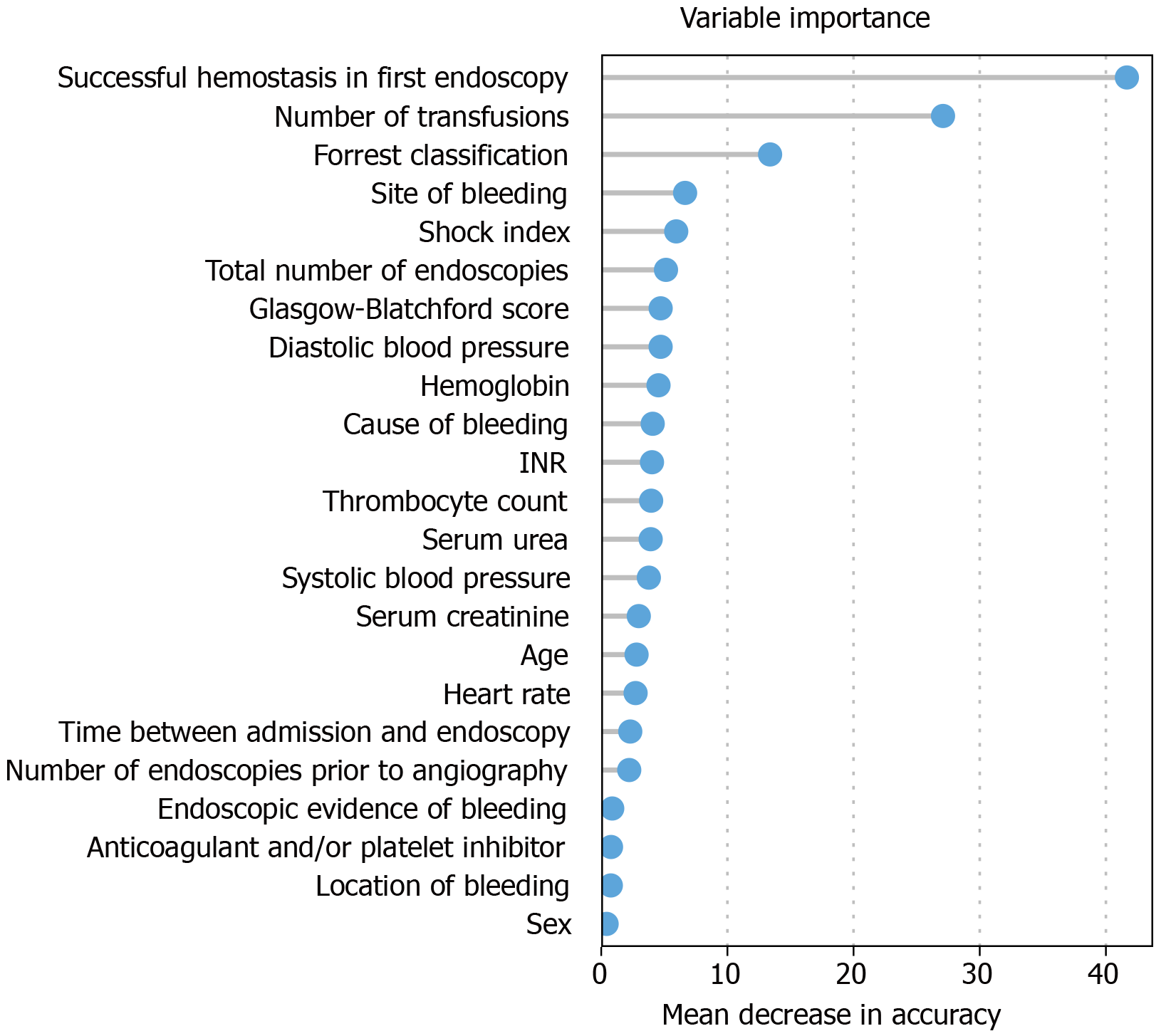
Variable importance (Figure 1): This bar chart shows the variable importance of all features considered for the construction of the decision trees (Figures 2 and 3). Based on the randomForest package for R[20], missing values were first imputed using rfImpute, followed by the construction of a randomForest classifier. The shown metric is the mean decrease in accuracy[22]. Such importance measures serve to identify relevant features and perform variable selection.
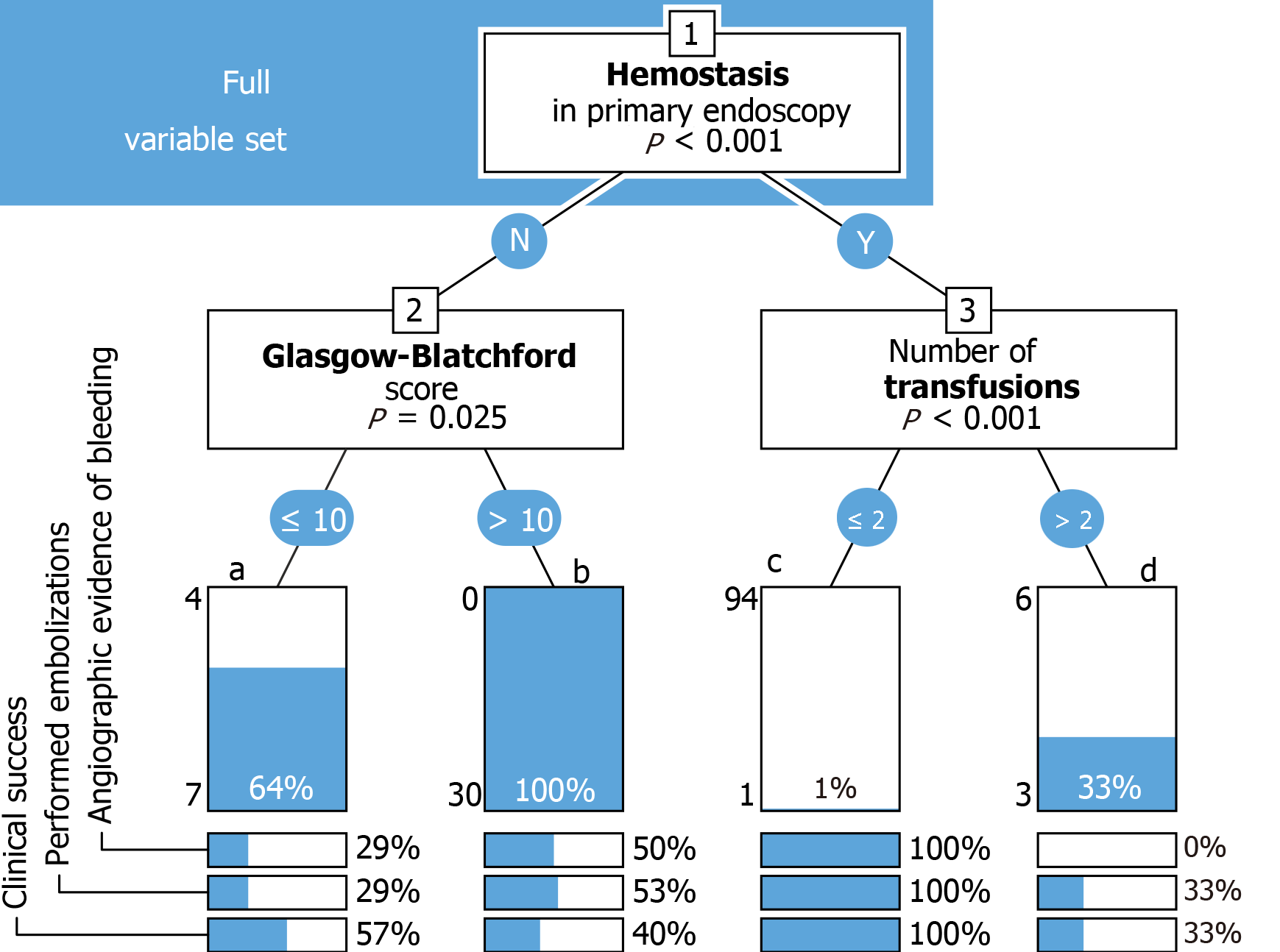
Decision tree (Figures 2 and 3): Decision trees were constructed using the party package for R[21], applying conditional inference trees either to the complete dataset (Figure 2), or to a set of variables selected for assumed clinical relevance (Figure 3). Each binary split (shown as a numbered box) is annotated with its corresponding p-value. Each terminal node (shown as a bar) represents the percentage of angiography-positive cases, with the individual numbers of positive and negative cases to the left. Percentages of cases with angiographic evidence of bleeding, performed embolizations, and clinical success are given below each node.
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki, and was approved by the ethics committee of the Regional Medical Society of Hessen (Landesärztekammer Hessen), approval number 2016/2017, on 31 August 2017. Written informed consent was obtained from each patient included in the registry.
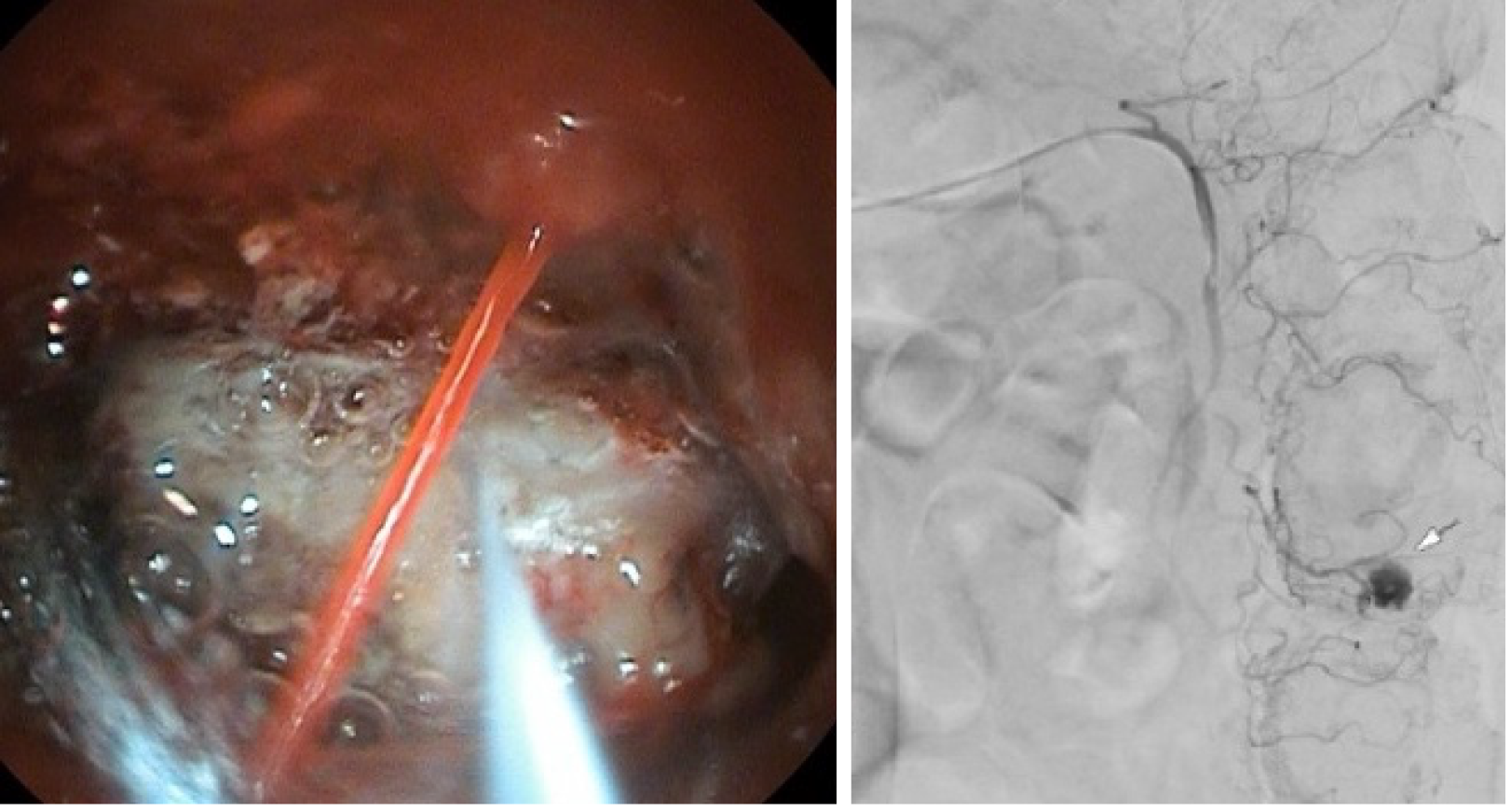
Forty-one patients with LGIB underwent CA between 1 January 2007 and 31 March 2018. Diverticular bleeding (Figure 4) was the most common suspected cause of bleeding (14/41, 34.1%). Endoscopic investigation demonstrated blood in the lower gastrointestinal tract in 17/41 cases (41.5%). The exact site of bleeding could not be localized in endoscopy in 23/41 patients (56.1%). Primary hemostasis in endoscopy was achieved in 4/41 patients (9.8%). In the K-LGIB group, primary endoscopic hemostasis was achieved in 88/92 cases (95.7%).
Seventeen of 41 patients underwent a CTA investigation prior to angiography. CTA revealed extravasation of contrast medium, and therefore a suspected active bleeding, in six cases. CA showed active bleeding in two of the six cases (Table 2). The cross-sectional images yielded significant additional data, especially incidental evidence of tumor, in 13 of 17 cases (76.5%).
| LGIB (n = 17) | CA: Bleeding, y (%) | CA: Bleeding, n (%) |
| CTA: Bleeding y (%) | 2 (11.7) | 4 (23.5) |
| CTA: Bleeding, n (%) | 4 (23.5) | 7 (41.3) |
An average of 2.2 d elapsed from the index endoscopy to the CA (minimum 0 days, maximum 11 d). The time period from admission to the hospital until CA was on average 3.0 d. Twenty-five patients (61.0%) were given anesthesia during the angiography, and 16 (39.0%) were intubated for the intervention. Angiography yielded evidence of bleeding in 18/41 patients (44.0%). In three of these patients, provocative catecholamine therapy was used to demonstrate bleeding. All cases with contrast extravasation received TAE. A superselective embolization could be performed in 16/18 cases (88.9%), and the TAE was successful in 16/18 patients (88.9%). Hemostasis could not be achieved by angiography in two patients. One of these underwent surgical treatment subsequently, and the other was discharged without further treatment.
Coils were the most frequently used material for embolization (13/20). Due to the absence of any evidence of bleeding, no embolization was performed in 21 cases (51.2%). A prophylactic embolization was performed in two cases (4.9%). The average duration of angiography was one hour, and the overall duration of fluoroscopy 22 min. The median dose area product was 24662 cGy/cm². One patient died during the angiography due to hemorrhagic shock. In three cases the investigation was discontinued by the patients.
Twenty-two patients (53.6%) underwent a control endoscopy. Of these, 13 (59.1%) had a normal report. One patient (4.5%) had necrosis due to ischemia, and 5/22 (22.7%) experienced renewed bleeding. In the CA group, 13/41 (31.7%) patients underwent surgery, three (7.3%) died, and 25 (60.1%) could be discharged. Among patients who underwent TAE, the procedure was clinically successful in 11/20 patients (55%).
The K-LGIB group consisted of 415 treated cases, of whom 92 had LGIB. Table 1 summarizes demographic data, laboratory values, endoscopic findings, and the outcome of treatment in both groups.
Weighting of variables for further differentiation was performed with the aid of variable importance (Figure 1). Successful hemostasis in primary endoscopy, the number of transfusions, and the site of bleeding were the major parameters.
All patients with failed primary hemostasis and a GBS >10 in either group underwent angiography (n = 30). The latter investigation yielded evidence of bleeding in 15 patients (50%). Embolization was performed in 16 (53%) patients and was successful in 12 (40%), (Figure 2). Only one patient who achieved hemostasis in primary endoscopy and needed less than two transfusions was scheduled for angiography. Three of nine patients (33%) who needed more than two transfusions underwent angiography, which yielded no evidence of bleeding in any case (Figure 2).
Angiographies were performed in 5/81 patients (6%) who received less than two transfusions regarding both groups (K-LGIB and CA-LGIB), and yielded evidence of bleeding in three cases. Of patients who were given more than two transfusions, angiographies were performed in 36/59 patients (61%), revealed bleeding in 42%, and the treatment was successful in 39% (Figure 3).
Despite high rates of endoscopic hemostasis and spontaneous hemostasis, a small number of patients with severe LGIB require additional treatment after endoscopy[2]. CA and TAE have been established as successful treatment modalities for these patients over the last few years. Surgery is needed in a small number of exceptional cases[7]. In our retrospective analysis, we examined patients with LGIB who had undergone CA over a period of 10 years.
Not surprisingly, endoscopic hemostasis was successful in just a small number of patients in the CA group, but in as many as 88 patients (94.7%) in the reference group. These data confirm the success of endoscopy for the management of bleeding[4,23]. In endoscopic diagnostic investigation, hemostasis is a crucial factor to be considered prior to CA (Figure 2). Our data analysis revealed that the failure to achieve primary hemostasis in endoscopy was a major difference between the investigated groups. In patients who had undergone CA, we also identified other parameters that might justify the involvement of interventional radiology for the purpose of diagnosis and therapy early in the course of the patient’s treatment. Specifically, these parameters are the shock index, GBS, and the number of transfusions.
In accordance with published guidelines, patients in our study underwent endoscopic investigation within a day after admission[8,24]. Diverticular bleeding was suspected in a large number of those who underwent angiography. Localization of bleeding and the achievement of endoscopic hemostasis are both particularly difficult in patients with diverticular bleeding[25]. In cases of severe disease, it would be advisable to consider angiography at an early point in time.
In our patients, pre-interventional diagnostic CTA investigations did not possess sufficient sensitivity or specificity to predict the outflow of contrast medium on CA. This contradicts published data, which consider CTA possibly even superior to colonoscopy for acute diagnostic investigation[26]. The probability of contrast medium outflow in the CTA is maximized in patients who receive a CTA < 60 min earlier. However, the time period between the primary investigation and angiography had no significant impact on the demonstration of contrast medium outflow[27].
In the published literature, CTA has been described as a useful procedure in planning angiography as well[28]. In our retrospective analysis, a non-standardized CTA investigation over a period of 10 years was a limiting factor in regard of the outcome. As Table 2 shows, CTA yielded poor values for the quality criteria (sensitivity, specificity, positive/negative predictive value). A diagnostic CTA examination was only performed in about 40% of patients, and only a third of cases were investigated with the specific aim of achieving morphological evidence of bleeding on radiological investigation.
An adequately performed CTA investigation, as described by Bruce and Erskine[29] (non-contrasted phase, arterial phase and late venous phase, prompt availability of embolization facilities), is essential to ensure the high sensitivity and specificity of CTA. Early diagnostic investigation by radiological procedures appears to be justified in hemodynamically unstable patients with no hemostasis in primary endoscopy. In cases of proven bleeding, a CA should be performed immediately after the CTA[27]. When CTA shows no evidence of bleeding, the decision to perform a CA should be made individually in each patient, because a CTA may yield false-negative findings in rare cases[28]. Especially in clinically unstable patients with bleeding on endoscopy, in whom CA is the last option before definitive surgical treatment, an angiography may be meaningful even in the presence of a negative CTA report. Recommendations issued so far suggest that all options to localize the source of bleeding should be exhausted prior to CA, but the decision to perform a CA should not be dependent on previous evidence of bleeding[11]. In the absence of bleeding on CA, a prophylactic TAE or provocation of bleeding should be performed on an individual basis, and might be justified as a means of preventing recurrence.
Published studies recommend superselective embolization for angiographic localization of bleeding[30]. We used this approach in about 90% of our patients. The choice of embolization material[31] is not important; it depends on the investigator’s preference. We used coils in the large majority of cases. Published reports recommend the use of other materials such as NBCA[30]. Adequate prospective studies on the subject are lacking.
The high degree of technical success we achieved with CA is in line with published data[16]. The detection of bleeding in a little less than a half of the patients has also been confirmed in other studies[1,32]. Finally, our data revealed clinical success in about one half of cases. Retrospective data concerning TAE show similar rates of clinical success (46%-95%)[10,16,33]. Only 3% of patients with LGIB have symptoms of shock and more than 50% have hemoglobin levels in excess of 12 mg/dL[1]. Thus, a positive shock index may be a predictor of angiographic treatment after failed endoscopic therapy. Our analysis revealed that the shock index was a significant variable importance measure. Patients in the CA group had a significantly higher shock index than those who had undergone conservative treatment and were given, on average seven transfusions, which is a predictor of increased 30-d mortality[32,33]. Thus, TAE permitted successful treatment with a minimally invasive procedure in approximately one half of critically ill patients. Surgery and further increases in morbidity and mortality rates could thus be avoided.
Despite primary endoscopic investigation and treatment, angiographies were performed on average within three days. In view of the fact that the patients usually underwent two diagnostic endoscopies, this time interval is indicative of smooth cooperation between the involved specialties, although the published guidelines provide no recommendations about the ideal time point for CA[8]. Interestingly, and analogous to endoscopic investigation, bleeding is detected on angiography more easily when the examination is performed early after the detection of bleeding on CTA[27].
A rising number of transfusions was shown to be a predictor of clinical failure in the treatment of LGIB[11,33]. Furthermore, the probability of detecting bleeding on angiography is significantly higher[27]. Not surprisingly, the number of transfusions is an important parameter of variable importance and was of crucial significance in our results. The GBS is also an extensively investigated factor in the treatment of gastrointestinal bleeding. Although the GBS was actually developed for upper gastrointestinal bleeding, it reduced hospital-based interventions and mortality rates in LGIB as well[34,35]. Besides, we established GBS as a positive predictor in the demonstration of bleeding on angiography.
Our retrospective data analysis served as a basis for the calculation of variable importance. Subject to a prospective multicenter validation, our data provide potential evidence of optimized treatment after failed endoscopic therapy. To our knowledge, such courses of treatment have not been published so far. In addition to previously published flow charts[2], these courses of treatment might serve as a crucial basis for making decisions about CA. Depending on the parameters registered in our courses of treatment (no hemostasis in primary endoscopy, more than two transfusions, BFS > 10), the clinician should consider the option of interventional radiological procedures.
Contrast medium extravasation in TAE should be used as an endpoint in future studies in order to validate the clinical parameters that indicate extravasation. This aspect was not adequately registered in the present study. However, an important point is the changing character of LGIB, which may mask bleeding. Besides, our assumptions need to be validated prospectively. As mentioned earlier, a further limitation of the present study is the use of a non-standardized computed tomography (CT) protocol, which probably led to the selection of patients for angiography on the basis of certain clinical factors. In the future, a CT for the purpose of detecting an LGIB should always be performed in accordance with the above mentioned model and if possible in the acute phase of bleeding in order to ensure adequate selection of patients for CA.
Although LGIB’s do subside spontaneously, or can be reliably and successfully treated by endoscopy, the data reported in the present study are relevant for a small number of patients. Angiography has undoubtedly gained increasing precedence over surgery for the treatment of gastrointestinal bleeding. Further prospective analyses will be needed to answer questions about the appropriate time point and the appropriate radiological procedure for diagnosis and treatment. Following confirmation in prospective investigations, our selected predictors and the retrospective courses of treatment derived from these may contribute to the development of future decision trees.
The large majority of lower gastrointestinal bleedings (LGIB) subside on their own or after endoscopic treatment. A small number of these may pose a challenge in terms of therapy when endoscopy does not achieve hemostasis. Based on what we know, transarterial embolization (TAE) enables the clinician to control gastrointestinal bleeding.
The timing and value of computed tomography angiography (CTA) and catheter angiography (CA) after failed primary hemostasis in endoscopy should be given greater attention in the course of treatment. The use of easily determined diagnostic and treatment parameters for identifying the best time point of escalation therapy in terms of angiography is the principal motivation in this field of science.
The aim was to evaluate clinical predictors for CA in patients with LGIB and create a practical decision-making aid based on these. It was shown that endoscopic hemostasis in primary endoscopy, along with GBS and the number of transfusions, were the most important factors in predicting CA.
We performed a retrospective analysis of all patients with LGIB who received CA over a 10-year period in a maximum-care hospital (CA-LGIB group). A group of patients with LGIB who underwent conservative treatment served as the reference group (K-LGIB group). We used mean decrease in impurity, a random forest-based metric for variable importance, to assess the suitability of the collected data. Conditional inference trees were employed to build decision-making aids based on binary splits.
Most patients with LGIB and no hemostasis received angiography within three days after admission. We designed the treatment on the basis of the most important clinical parameters [Glasgow-Blatchford bleeding score (GBS), shock index, and serum hemoglobin levels]; these should help the clinician in making decisions about early radiological treatment with CA and TAE. Endoscopic hemostasis proved to be the crucial difference between CA and conservative treatment.
Primary endoscopic hemostasis, along with the GBS and the number of transfusions, could permit a stratification of risks. Courses of treatment might serve as a crucial basis for making decisions about scheduling a patient to undergo CA. The present data are intended to enhance the clinician’s awareness of angiographic diagnostic investigation and treatment after or during failed endoscopic treatment.
The timing of the CTA, the procedure for a negative CTA in hemodynamically unstable patients and the benefits of provocative CA should be investigated further. Contrast extravasation in CA and subsequent TAE should be the endpoint of future prospective studies. Hospitals will need strategies to transfer people with failed hemostasis in primary endoscopy to interventional radiology.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Jeong KY, Kamran M, Maglangit SACA S-Editor: Gao CC L-Editor: A P-Editor: Wang LL
| 1. | Oakland K, Guy R, Uberoi R, Hogg R, Mortensen N, Murphy MF, Jairath V; UK Lower GI Bleeding Collaborative. Acute lower GI bleeding in the UK: patient characteristics, interventions and outcomes in the first nationwide audit. Gut. 2018;67:654-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 2. | Werner DJ, Manner H, Nguyen-Tat M, Kloeckner R, Kiesslich R, Abusalim N, Rey JW. Endoscopic and angiographic management of lower gastrointestinal bleeding: Review of the published literature. United European Gastroenterol J. 2018;6:337-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 3. | Yamada A, Niikura R, Yoshida S, Hirata Y, Koike K. Endoscopic management of colonic diverticular bleeding. Dig Endosc. 2015;27:720-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Strate LL, Naumann CR. The role of colonoscopy and radiological procedures in the management of acute lower intestinal bleeding. Clin Gastroenterol Hepatol. 2010;8:333-43; quiz e44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 5. | Jensen DM, Machicado GA, Jutabha R, Kovacs TO. Urgent colonoscopy for the diagnosis and treatment of severe diverticular hemorrhage. N Engl J Med. 2000;342:78-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 493] [Cited by in RCA: 424] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 6. | Green BT, Rockey DC, Portwood G, Tarnasky PR, Guarisco S, Branch MS, Leung J, Jowell P. Urgent colonoscopy for evaluation and management of acute lower gastrointestinal hemorrhage: a randomized controlled trial. Am J Gastroenterol. 2005;100:2395-2402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 201] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 7. | Pannatier M, Duran R, Denys A, Meuli R, Zingg T, Schmidt S. Characteristics of patients treated for active lower gastrointestinal bleeding detected by CT angiography: Interventional radiology vs surgery. Eur J Radiol. 2019;120:108691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Götz M, Anders M, Biecker E, Bojarski C, Braun G, Brechmann T, Dechêne A, Dollinger M, Gawaz M, Kiesslich R, Schilling D, Tacke F, Zipprich A, Trebicka J; Deutsche Gesellschaft für Gastroenterologie; Verdauungs- und Stoffwechselkrankheiten (DGVS) (federführend); Deutschen Morbus Crohn und Colitis ulcerosa Vereinigung (DCCV); Deutsche Röntgengesellschaft (DRG); Deutsche Gesellschaft für interventionelle Radiologie (DeGiR); Deutsche Gesellschaft für Allgemein- und Viszeralchirurgie (DGAV) und Chirurgische Arbeitsgemeinschaft für Endoskopie und Sonographie (CAES) der DGAV; Deutsche Gesellschaft für Internistische Intensivmedizin (DGIIN); Deutsche Gesellschaft für Innere Medizin (DGIM); Deutsche Gesellschaft für Kardiologie (DGK); Akademie für Ethik in der Medizin (AEM); Gesellschaft für Thrombose- und Hämostaseforschung (GTH); Collaborators:. [S2k Guideline Gastrointestinal Bleeding - Guideline of the German Society of Gastroenterology DGVS]. Z Gastroenterol. 2017;55:883-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 9. | García-Blázquez V, Vicente-Bártulos A, Olavarria-Delgado A, Plana MN, van der Winden D, Zamora J; EBM-Connect Collaboration. Accuracy of CT angiography in the diagnosis of acute gastrointestinal bleeding: systematic review and meta-analysis. Eur Radiol. 2013;23:1181-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 116] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 10. | Oakland K, Chadwick G, East JE, Guy R, Humphries A, Jairath V, McPherson S, Metzner M, Morris AJ, Murphy MF, Tham T, Uberoi R, Veitch AM, Wheeler J, Regan C, Hoare J. Diagnosis and management of acute lower gastrointestinal bleeding: guidelines from the British Society of Gastroenterology. Gut. 2019;68:776-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 198] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 11. | Lee HH, Oh JS, Park JM, Chun HJ, Kim TH, Cheung DY, Lee BI, Cho YS, Choi MG. Transcatheter embolization effectively controls acute lower gastrointestinal bleeding without localizing bleeding site prior to angiography. Scand J Gastroenterol. 2018;53:1089-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Tan KK, Wong D, Sim R. Superselective embolization for lower gastrointestinal hemorrhage: an institutional review over 7 years. World J Surg. 2008;32:2707-2715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Evangelista PT, Hallisey MJ. Transcatheter embolization for acute lower gastrointestinal hemorrhage. J Vasc Interv Radiol. 2000;11:601-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 63] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Soh B, Chan S. The use of super-selective mesenteric embolisation as a first-line management of acute lower gastrointestinal bleeding. Ann Med Surg (Lond). 2017;17:27-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Diamantopoulou G, Konstantakis C, Kottorοu A, Skroubis G, Theocharis G, Theopistos V, Triantos C, Nikolopoulou V, Thomopoulos K. Acute Lower Gastrointestinal Bleeding: Characteristics and Clinical Outcome of Patients Treated With an Intensive Protocol. Gastroenterology Res. 2017;10:352-358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Kim PH, Tsauo J, Shin JH, Yun SC. Transcatheter Arterial Embolization of Gastrointestinal Bleeding with N-Butyl Cyanoacrylate: A Systematic Review and Meta-Analysis of Safety and Efficacy. J Vasc Interv Radiol 2017; 28: 522-531. e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 17. | Cheng DW, Lu YW, Teller T, Sekhon HK, Wu BU. A modified Glasgow Blatchford Score improves risk stratification in upper gastrointestinal bleed: a prospective comparison of scoring systems. Aliment Pharmacol Ther. 2012;36:782-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 18. | Kennedy DW, Laing CJ, Tseng LH, Rosenblum DI, Tamarkin SW. Detection of active gastrointestinal hemorrhage with CT angiography: a 4(1/2)-year retrospective review. J Vasc Interv Radiol. 2010;21:848-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Team RC. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2019. |
| 21. | Hothorn T, Hornik K, Zeileis A. Unbiased recursive partitioning: A conditional inference framework. J Comput Graph Stat. 2006;15:651-74. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2216] [Cited by in RCA: 1570] [Article Influence: 82.6] [Reference Citation Analysis (0)] |
| 22. | Breiman L. Random forests. Mach Learn. 2001;45:5-32. [RCA] [DOI] [Full Text] [Cited by in Crossref: 56052] [Cited by in RCA: 34150] [Article Influence: 2845.8] [Reference Citation Analysis (0)] |
| 23. | Strate LL, Gralnek IM. ACG Clinical Guideline: Management of Patients With Acute Lower Gastrointestinal Bleeding. Am J Gastroenterol. 2016;111:459-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 300] [Article Influence: 33.3] [Reference Citation Analysis (2)] |
| 24. | Roshan Afshar I, Sadr MS, Strate LL, Martel M, Menard C, Barkun AN. The role of early colonoscopy in patients presenting with acute lower gastrointestinal bleeding: a systematic review and meta-analysis. Therap Adv Gastroenterol. 2018;11:1756283X18757184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Kaise M, Nagata N, Ishii N, Omori J, Goto O, Iwakiri K. Epidemiology of colonic diverticula and recent advances in the management of colonic diverticular bleeding. Dig Endosc. 2020;32:240-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 26. | Umezawa S, Nagata N, Arimoto J, Uchiyama S, Higurashi T, Nakano K, Ishii N, Sakurai T, Moriyasu S, Takeda Y, Nagase H, Komatsu H, Nakajima A, Mizuki A. Contrast-enhanced CT for Colonic Diverticular Bleeding before Colonoscopy: A Prospective Multicenter Study. Radiology. 2018;288:755-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 27. | Brahmbhatt A, Rao P, Cantos A, Butani D. Time to Catheter Angiography for Gastrointestinal Bleeding after Prior Positive Investigation Does Not Affect Bleed Identification. J Clin Imaging Sci. 2020;10:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Ren JZ, Zhang MF, Rong AM, Fang XJ, Zhang K, Huang GH, Chen PF, Wang ZY, Duan XH, Han XW, Liu YJ. Lower gastrointestinal bleeding: role of 64-row computed tomographic angiography in diagnosis and therapeutic planning. World J Gastroenterol. 2015;21:4030-4037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Bruce G, Erskine B. Analysis of time delay between computed tomography and digital subtraction angiography on the technical success of interventional embolisation for treatment of lower gastrointestinal bleeding. J Med Radiat Sci. 2020;67:64-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Kwon JH, Kim MD, Han K, Choi W, Kim YS, Lee J, Kim GM, Won JY, Lee DY. Transcatheter arterial embolisation for acute lower gastrointestinal haemorrhage: a single-centre study. Eur Radiol. 2019;29:57-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 31. | Ierardi AM, Urbano J, De Marchi G, Micieli C, Duka E, Iacobellis F, Fontana F, Carrafiello G. New advances in lower gastrointestinal bleeding management with embolotherapy. Br J Radiol. 2016;89:20150934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Mohan P, Manov J, Diaz-Bode A, Venkat S, Langston M, Naidu A, Howse R, Narayanan G. Clinical predictors of arterial extravasation, rebleeding and mortality following angiographic interventions in gastrointestinal bleeding. J Gastrointestin Liver Dis. 2018;27:221-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Chan DK, Soong J, Koh F, Tan KK, Lieske B. Predictors for outcomes after super-selective mesenteric embolization for lower gastrointestinal tract bleeding. ANZ J Surg. 2016;86:459-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 34. | Ur-Rahman A, Guan J, Khalid S, Munaf A, Sharbatji M, Idrisov E, He X, Machavarapu A, Abusaada K. Both Full Glasgow-Blatchford Score and Modified Glasgow-Blatchford Score Predict the Need for Intervention and Mortality in Patients with Acute Lower Gastrointestinal Bleeding. Dig Dis Sci. 2018;63:3020-3025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 35. | Chang WC, Tsai SH, Chang WK, Liu CH, Tung HJ, Hsieh CB, Huang GS, Hsu HH, Yu CY. The value of multidetector-row computed tomography for localization of obscure acute gastrointestinal bleeding. Eur J Radiol. 2011;80:229-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |