Published online Aug 16, 2020. doi: 10.4253/wjge.v12.i8.241
Peer-review started: March 31, 2020
First decision: June 7, 2020
Revised: June 12, 2020
Accepted: July 18, 2020
Article in press: July 18, 2020
Published online: August 16, 2020
Processing time: 135 Days and 3.6 Hours
Patients with cirrhosis frequently require sedation for elective endoscopic procedures. Several sedation protocols are available, but choosing an appropriate sedative in patients with cirrhosis is challenging.
To conduct a systematic review and meta-analysis to compare propofol and midazolam for sedation in patients with cirrhosis during elective endoscopic procedures in an attempt to understand the best approach.
This systematic review and meta-analysis was conducted using the PRISMA guidelines. Electronic searches were performed using MEDLINE, EMBASE, Central Cochrane, LILACS databases. Only randomized control trials (RCTs) were included. The outcomes studied were procedure time, recovery time, discharge time, and adverse events (bradycardia, hypotension, and hypoxemia). The risk of bias assessment was performed using the Revised Cochrane Risk-of-Bias tool for randomized trials (RoB-2). Quality of evidence was evaluated by GRADEpro. The meta-analysis was performed using Review Manager.
The search yielded 3,576 records. Out of these, 8 RCTs with a total of 596 patients (302 in the propofol group and 294 in the midazolam group) were included for the final analysis. Procedure time was similar between midazolam and propofol groups (MD: 0.25, 95%CI: -0.64 to 1.13, P = 0.59). Recovery time (MD: -8.19, 95%CI: -10.59 to -5.79, P < 0.00001). and discharge time were significantly less in the propofol group (MD: -12.98, 95%CI: -18.46 to -7.50, P < 0.00001). Adverse events were similar in both groups (RD: 0.02, 95%CI: 0-0.04, P = 0.58). Moreover, no significant difference was found for bradycardia (RD: 0.03, 95%CI: -0.01 to 0.07, P = 0.16), hypotension (RD: 0.03, 95%CI: -0.01 to 0.07, P = 0.17), and hypoxemia (RD: 0.00, 95%CI: -0.04 to 0.04, P = 0.93). Five studies had low risk of bias, two demonstrated some concerns, and one presented high risk. The quality of the evidence was very low for procedure time, recovery time, and adverse events; while low for discharge time.
This systematic review and meta-analysis based on RCTs show that propofol has shorter recovery and patient discharge time as compared to midazolam with a similar rate of adverse events. These results suggest that propofol should be the preferred agent for sedation in patients with cirrhosis.
Core tip: Patients with cirrhosis often require elective endoscopic procedures, but choosing an appropriate sedative is challenging. We performed a systematic review and meta-analysis of randomized controlled trials to compare propofol and midazolam for sedation in patients with cirrhosis during elective endoscopic procedures. We concluded propofol has shorter recovery and patient discharge time as compared to midazolam with a similar rate of adverse events, suggesting that propofol should be the preferred agent for sedation in patients with cirrhosis.
- Citation: Guacho JAL, de Moura DTH, Ribeiro IB, da Ponte Neto AM, Singh S, Tucci MGB, Bernardo WM, de Moura EGH. Propofol vs midazolam sedation for elective endoscopy in patients with cirrhosis: A systematic review and meta-analysis of randomized controlled trials. World J Gastrointest Endosc 2020; 12(8): 241-255
- URL: https://www.wjgnet.com/1948-5190/full/v12/i8/241.htm
- DOI: https://dx.doi.org/10.4253/wjge.v12.i8.241
Cirrhosis is an advanced form of fibrosis that affects the liver with the destruction of the organ's lobular and vascular architecture[1]. The progression of liver disease causes portal hypertension, which can lead to complications such as esophagogastric varices, portal hypertensive gastropathy, and gastric antral vascular ectasia (GAVE)[2-11]. These patients often undergo diagnostic or therapeutic upper gastrointestinal endoscopy, and choosing an appropriate sedative is challenging. Sedation in this group of patients with underlying liver disease and their complications presents increased risks even when performed by well-trained personnel, mainly due to drug metabolism and interactions, baseline hemodynamics, and increased risk of adverse events. The recommended sedation level for elective endoscopies in patients with cirrhosis is mild to moderate that can be administered by anesthesiologists, endoscopists, or registered nurses[12].
The most commonly used sedatives are usually benzodiazepine midazolam and short duration hypnotic agent propofol, while synthetic opioids can be added for their analgesic effect in some cases. Midazolam is the preferred benzodiazepine because of its short induction, recovery time, and amnesic properties[13]. However, the half-life of midazolam can be prolonged in patients with cirrhosis, and midazolam can trigger encephalopathy in these patients. Propofol does not need dose adjustment in patients with cirrhosis and has a faster onset of action, shorter effect, and faster recovery times[13]. Many studies have compared propofol with midazolam for sedation in cirrhosis showing variable results. Therefore, we aimed to perform a systematic review and meta-analysis of randomized controlled trials (RCTs) to compare sedation with propofol and midazolam in patients with cirrhosis undergoing elective endoscopy.
This systematic review was carried out in accordance with the Cochrane Handbook for Systematic Reviews of Interventions and Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA). The study was registered by The International Prospective Registry of Continuous Systematic Reviews of the National Institutes of Health Research (PROSPERO), under the code CRD42019137659 and was approved by the Scientific Ethics Committee of the Department of Gastroenterology of the Faculty of Medicine of the University of São Paulo.
The search was carried out using MEDLINE (Pubmed); EMBASE; Cochrane Central Register of Randomized Controlled Clinical Trials/CENTRAL; and Latin-American and Caribbean Health Sciences Literature LILACS electronic databases from their date of inception to November 2019 with no language restriction. A gray literature search was also performed. The terms used for database search were "Sedation OR Sedations OR anesthesia OR Propofol OR Midazolam OR benzodiazepine" AND "Endoscopy OR endoscopic OR panendoscopy" AND "Cirrhosis OR liver OR hepatic."
RCTs comparing propofol and midazolam for sedation during elective gastrointestinal endoscopy in patients with cirrhosis more than 18 years of age were included. Studies were excluded if they included patients without cirrhosis, patients with upper gastrointestinal bleeding, decompensated liver disease, neurological or psychiatric diseases; patients who used illicit drugs that could alter their central nervous system; patients that used drugs such as benzodiazepines, anti-depressants, antiepileptics, and patients with ASA class IV-V. Case series and studies that did not provide enough data for outcome analysis or full text were also excluded. The outcomes of our study were procedure time, recovery time, discharge time, and adverse events (bradycardia, hypotension, and hypoxemia).
All data were extracted from article texts, tables, and figures with any estimates made based on the presented data and figures. Two investigators independently reviewed each included article, and its eligibility was determined based on predetermined inclusion and exclusion criteria. Any discrepancy resolved by discussion and re-evaluation by senior authors. The following data were collected: Study model, the total number of included patients, gender, age, etiology of cirrhosis, Child-Turcotte-Pugh score, and adverse events related to the sedation.
The risk of bias in the studies was assessed using the Revised Cochrane Risk-of-Bias tool for randomized trials (RoB-2). We performed a complete analysis using RoB-2 for each of the outcomes in each selected study. In order to simplify the analysis, we assessed the overall risk of bias for each study using the same domains suggested in RoB-2.
We evaluated the randomized trials using the criteria from Bias Risk Assessment by the Cochrane Collaboration's tool - ROB2 - Risk of Bias[14]. The tool analyzes the risk of bias by classifying it in five different domains: Randomization process, deviations in the intention of the intervention, loss of data on outcomes, methods of measuring outcomes, and selection of reported results. The risk of bias for each specific domain is categorized as "low risk,” "some concerns," or "high risk" for each of the outcomes, according to the criteria described in detail in the Cochrane Handbook[14].
GRADE (quality of evidence): The quality of evidence was assessed with the objective criteria of GRADE (Grading of Recommendations Assessment, Development, and Evaluation) for each of the pre-specified results and outcomes using the software GRADEpro - Guideline Development Tool (Mc Master University, 2015; Evidence Prime, Inc., Ontario, Canada). GRADE is a tool used to assess the quality of evidence-based on criteria that involve assessing the risk of bias, inconsistency, indirect evidence, imprecision, and publication bias. The evaluation of the risk of bias and the quality of the studies was carried out under the supervision of our statistical analysis team.
Statistical analysis: The metanalysis was performed using RevMan 5 (Review Manager version 5.3.5 - Cochrane Collaboration, Oxford, United Kingdom). The risk of difference (RD) with a 95% confidence interval (CI) for dichotomous variables was calculated by using the Mantel-Haenszel Cochran method with the fixed-effects model. For continuous variables, we calculated the mean difference (MD) with 95%CI using random effect with inverse variance. The semi-quantitative values were reported as weighted mean with standard deviation determined by the number of patients in each study. All estimates were made based on an intention-to-treat analysis. Heterogeneity values were estimated according to Chi-square (χ²) and Higgins method (I²). Heterogeneity values greater than 50% were considered high. We used the fixed-effects model if the heterogeneity was < 50%. Absolute numbers, means, and standard deviations were used for data analysis. If the means and standard deviations were not reported, they were estimated using mathematical formulas (SP Hozo, B. Djulbegovic, I. Hozo). A P value of less than 0.05 was considered statistically significant.
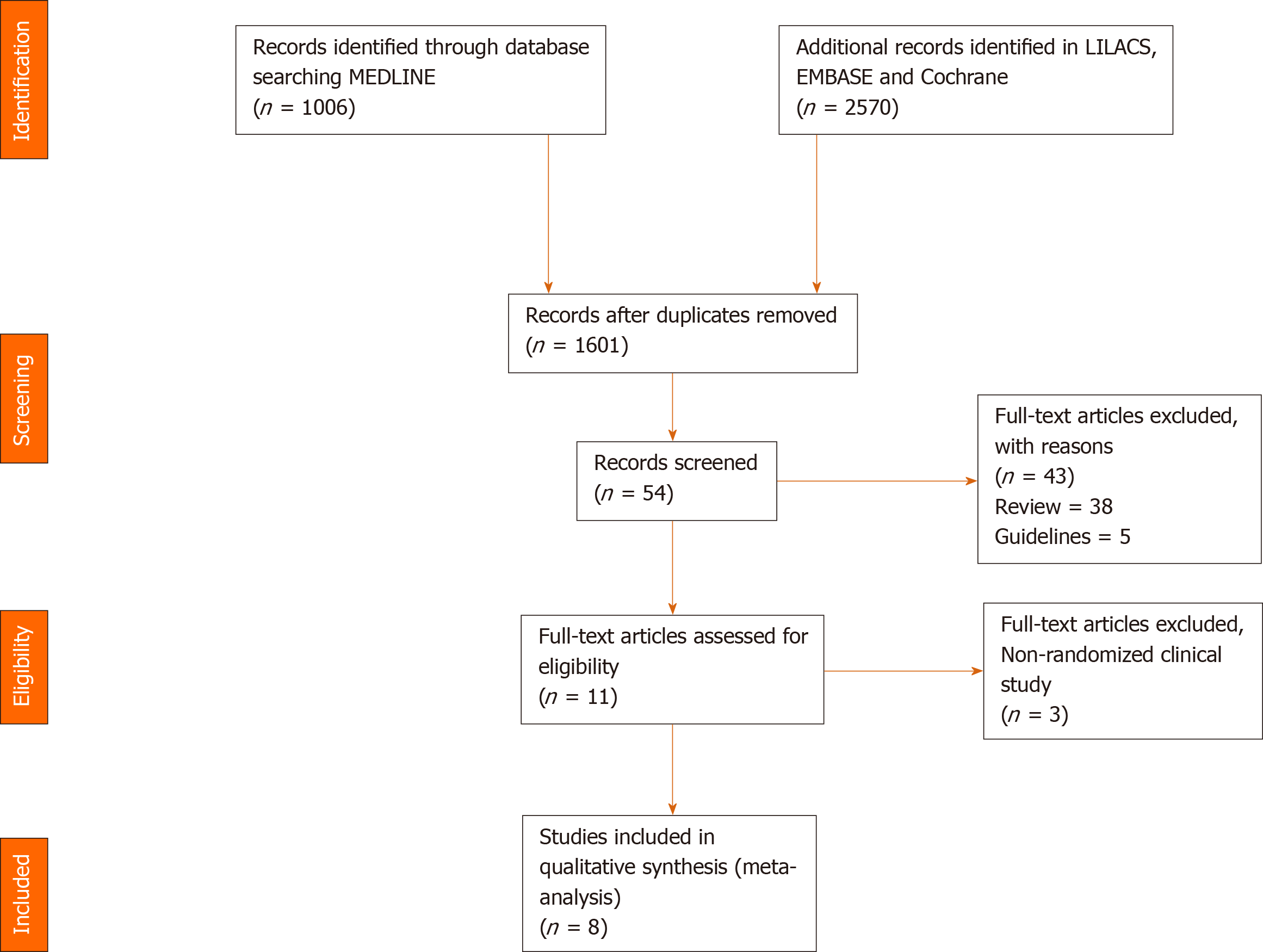
The initial search identified a total of 3576 citations. After eliminating duplicates, 1601 citations were selected for title and abstract review. Out of these, 54 studies were selected for full-text review. Eleven articles were then selected to examine for eligibility, out of which 3 were excluded because they were not RCTs. Finally, 8 studies[15-22] were included in our meta-analysis (Figure 1).
Eight RCTs with a total of 596 patients were included; 302 in the propofol group and 294 patients in the midazolam group. Individual study characteristics are summarized in Table 1. Four studies[15-18] used sedation with only propofol or midazolam. The other four studies[19-22] also used additional medications for sedation, such as opioid analgesic Pentazocine (Watanabe et al[19]), fentanyl (Ahmed et al[20] and Correia et al[21]) and Meperidine in the midazolam group (Weston et al[22]).
| Ref. | Study type | Inclusion criteria and outcomes | Medication | Intervention |
| Yoo et al[18], 2019, South Korea | RCT | Inclusion: Patients aged 19 to 75 yr for evaluation of portal hypertension; ASA I-III; Child-Turcotte-Pugh A, B, and C. Outcomes: Exacerbation of MHE, adverse events and discharge time; suggestive satisfaction measurements. | Propofol (20) | Propofol: 0.5 mg/kg in patients < 65 yr old or with body weight > 55 kg. In patients older than 65 yr and with body weight < 55 kg, the initial dose was 50% lower. |
| Midazolam (20) | Midazolam: 0.03 mg/kg or 2 mg if the patient is < 65 yr old or with a body weight > 55 kg. In patients older than 65 yr and with body weight under 55 kg, the initial dose was 20% lower. | |||
| Midazolam and propofol (20) | Midazolam: 0.03 mg/kg or 2 mg; Propofol: 20 mg. If the patient is > 65 yr old or has a body weight < 55 kg, the midazolam and propofol doses were respectively 20% and 50% lower. | |||
| Watanabe et al[19], 2018, Japan | RCT | Inclusion: Patients aged 20 to 80 yr, hepatic cirrhosis for the treatment of sclerosis, primary prophylaxis, Child- Turcotte-Pugh A and B. Outcomes: Exacerbation of MHE, patient and operator satisfaction, and adverse events. | Propofol (11) | Pentazocine 15 mg + Propofol 1% 20 mg IV followed by BIC of 3-5 mg/kg/h. In case of body movements or discomfort, 20 mg of Propofol (IV) was administered. |
| Midazolam (12) | Pentazocine 15 mg + midazolam 2.5-5 mg. In case of body movement or signs of discomfort, an IV infusion with an additional 2.5 mg of midazolam was administered. | |||
| Ahmed et al[20], 2017, Egypt | RCT | Inclusion: Patients aged 40 to 60 yr, Child-Turcotte-Pugh B or C, patients willing to be part of the study. Outcomes: Procedure duration, recovery time, discharge time, sedation scores, and adverse events. | Propofol (50) | Propofol 1 mg/kg + 0.5 mcg/kg IV until a satisfactory level of sedation is reached. An additional dose of 0.2 mg/kg of propofol was administered in case of discomfort. |
| Midazolam (50) | Midazolam 3 mg IV + fentanyl 0.5 mcg/kg until a satisfactory level of sedation is reached. A supplementary dose of 1 mg of midazolam was administered in case of an unsatisfactory level of sedation. | |||
| Agrawal et al[15], 2012, India | RCT | Inclusion: Patients aged 18 to 70 yr, hepatic cirrhosis confirmed and staged by Child-Turcotte-Pugh A and B, MELD, ASA I-III. Outcomes: Deterioration of psychometric tests before and after the examination, critical flicker frequency before and after, adverse events. | Propofol (40) | Propofol 0.5-1 mg IV, followed by an additional bolus if necessary. |
| Midazolam (42) | Midazolam 0.5 - 1 mg IV, with an increasing dosage every 1-3 min, until a satisfactory level of sedation is reached. | |||
| No-sedation (45) | ||||
| Correia et al[21], 2011, Brazil | RCT | Inclusion: Patients aged 18 to 75 yr, with hepatic cirrhosis, Child-Turcotte-Pugh A, B or C, ASA I-III. Outcomes: Procedure duration, discharge time, recovery time, and adverse events. | Propofol (100) | Midazolam 0.05 mg/kg with a dosage of 1 mg every 2 min, if necessary, up to a maximum dose of 0.1 mg/ kg or 10 mg + 50 mcg of fentanyl. |
| Midazolam (110) | Propofol 0.25 mg/kg with a dosage of 20-30 mg, if necessary, every 30-60 s up to a maximum dose of 400 mg + fentanyl 50 mcg. | |||
| Khamaysi et al[16], 2011, Israel | RCT | Inclusion: Compensated liver cirrhosis, Child-Turcotte-Pugh A and B. Outcomes: Sub-clinical hepatic encephalopathy before and after, procedure duration, induction time, recovery time, discharge time, adverse events. | Propofol (31) | Propofol: 30-50 mg followed by repeated dosages of 10-20 mg at intervals of 15 s, at the endoscopist's discretion, up to a 70-100 mg dose, considering the level of satisfactory sedation. |
| Midazolam (30) | Midazolam: (0.5-1.0 mg) administered by intravenous bolus injection, with incremental dosages at intervals of approximately 1 to 3 min until a satisfactory level of sedation for the procedure was reached (variation of 3-6 mg). | |||
| Control/No-sedation (30) | ||||
| Riphaus et al[17], 2009, Germany | RCT | Inclusion: Patients over 18 yr old diagnosed with hepatic cirrhosis, Child-Turcotte-Pugh A, B and C, without using benzodiazepine or antiepileptics, ASA I-III. Control group: Non-cirrhotic. Outcomes: Acute deterioration of minimal encephalopathy before and after sedation, procedure duration, recovery time, and adverse events. | Propofol (40) | Propofol: 40 mg of propofol 1% or 60 mg in patients weighing 70 kg; an extra dose of 10 mg was administered if necessary. |
| Midazolam (20) | Midazolam: 2.5 mg IV, with repeated doses administered to ensure satisfactory sedation within a limit of 7.5 mg total. | |||
| Control/No-sedation (20) | ||||
| Weston et al[22], 2003, United States | RCT | Inclusion: Patients over 18 yr old, confirmed hepatic cirrhosis, Child-Turcotte-Pugh A and B, ASA I-II. Outcomes: Procedure duration, recovery time, discharge time, and adverse events. | Propofol (10) | Propofol: 30-50 mg IV, followed by a 10-20 mg dosage every 15 s, at the discretion of an endoscopist or nurse, until a satisfactory level of sedation is reached. |
| Midazolam (10) | Midazolam: 0.5-1 mg + meperidine (12.5-25 mg), with an additional dosage every 1-3 min if necessary. |
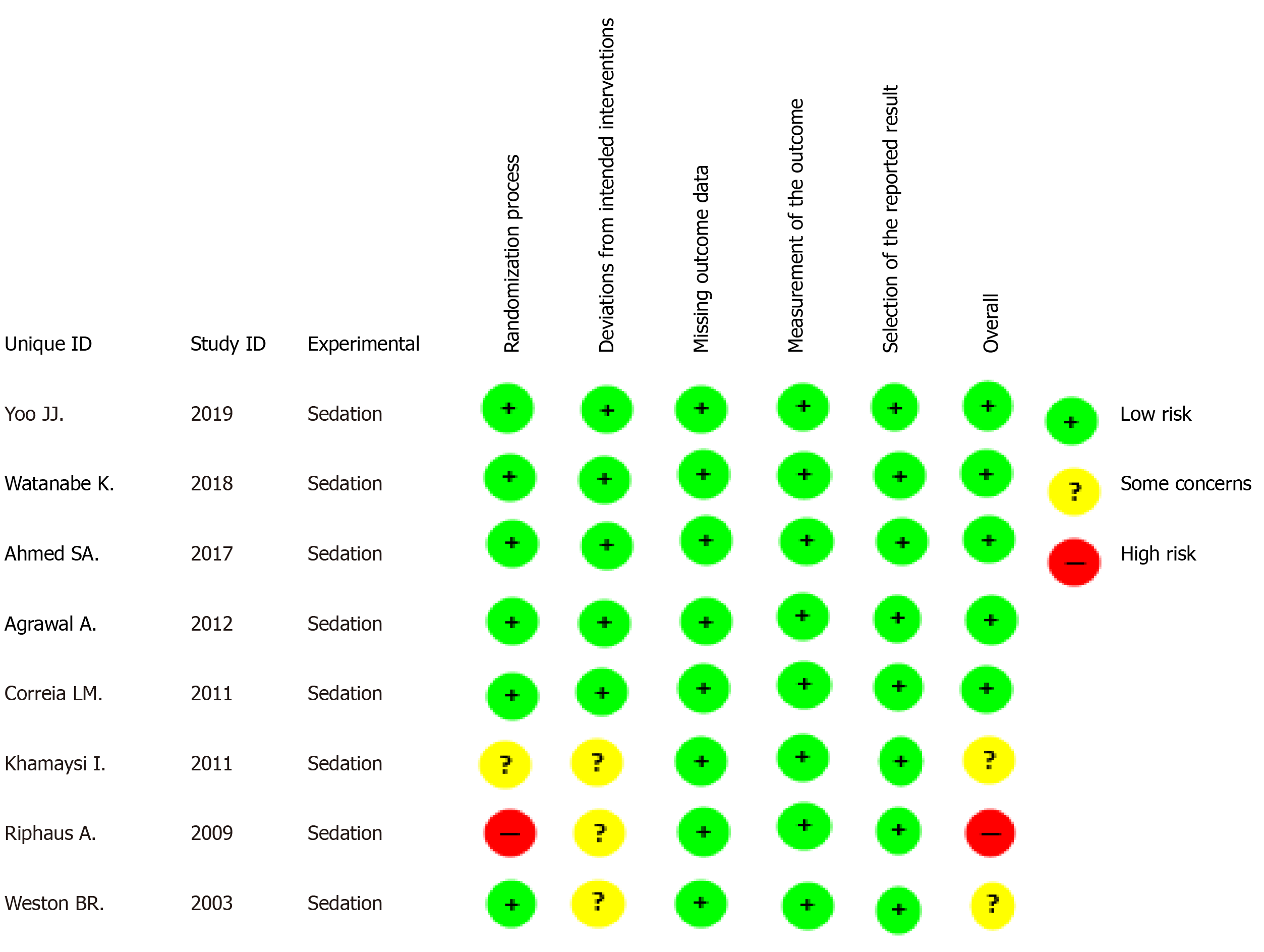
Risk of bias: Studies by Yoo et al[18], Watamabe et al[19], Ahmed et al[20], Agrawal et al[15], and Correia et al[21] were considered low risk when globally assessed per outcome, while the studies by Khamaysi et al[16] and Weston et al[22] had some concerns, and the study by Riphaus et al[17] had a high risk of bias (Figure 2).
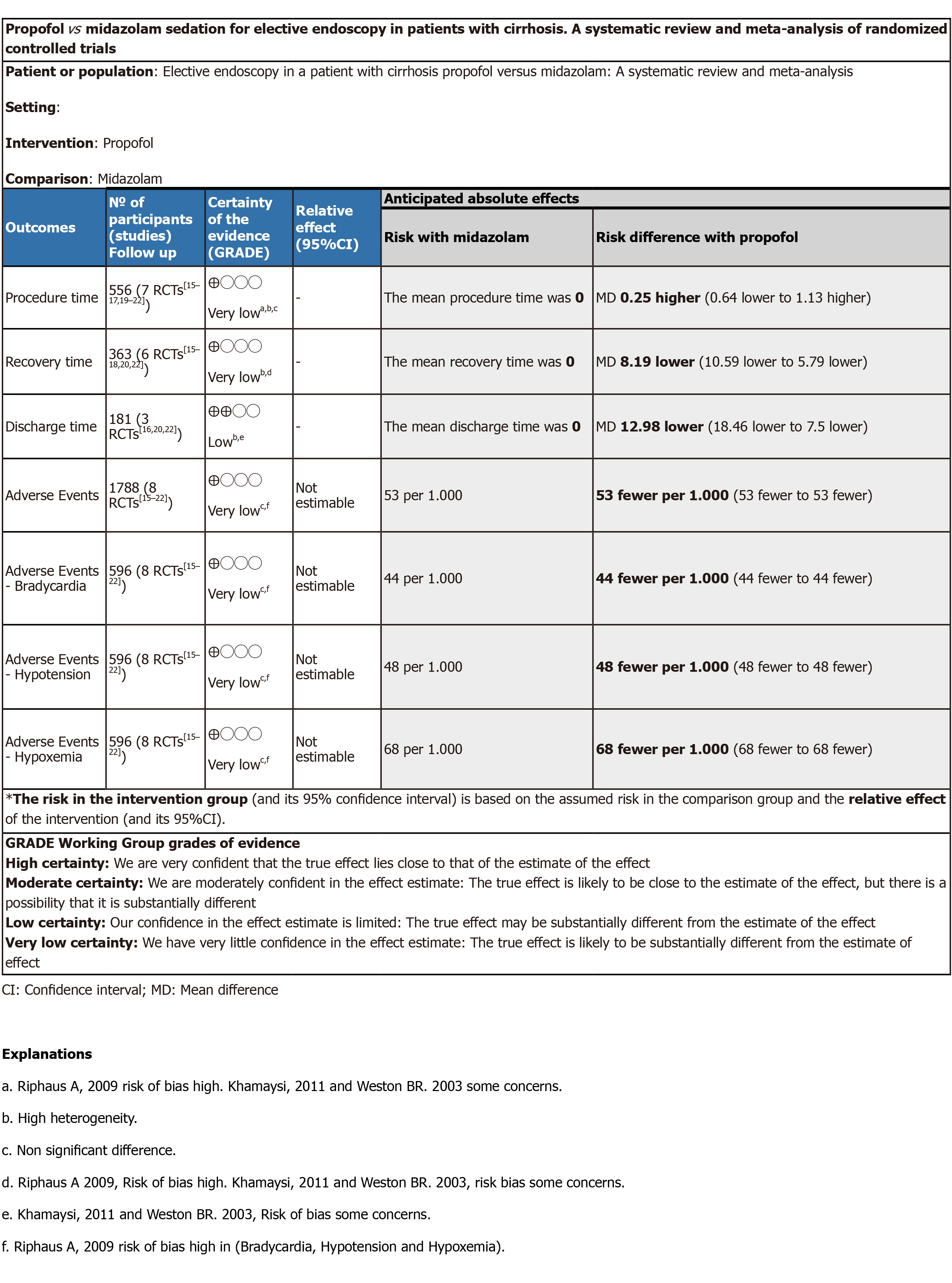
GRADEpro: The estimated outcomes of procedure time, recovery time, and adverse events showed very low quality of evidence, and discharge time showed low quality of evidence (Figure 3).
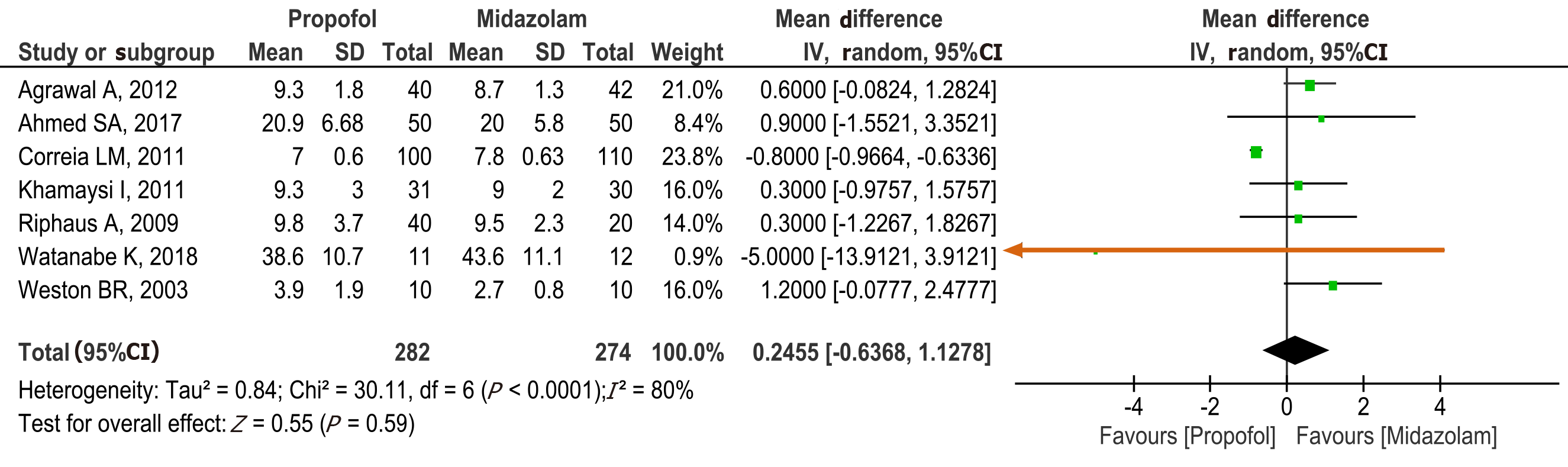
Procedure time: Seven studies[15-17,19-22] with a total of 556 patients (282 propofol group and 274 midazolam group) reported procedure time. No statistical difference was found between the propofol and midazolam groups (MD: 0.25, 95%CI: -0.64 to 1.13, P = 0.59) (Figure 4).
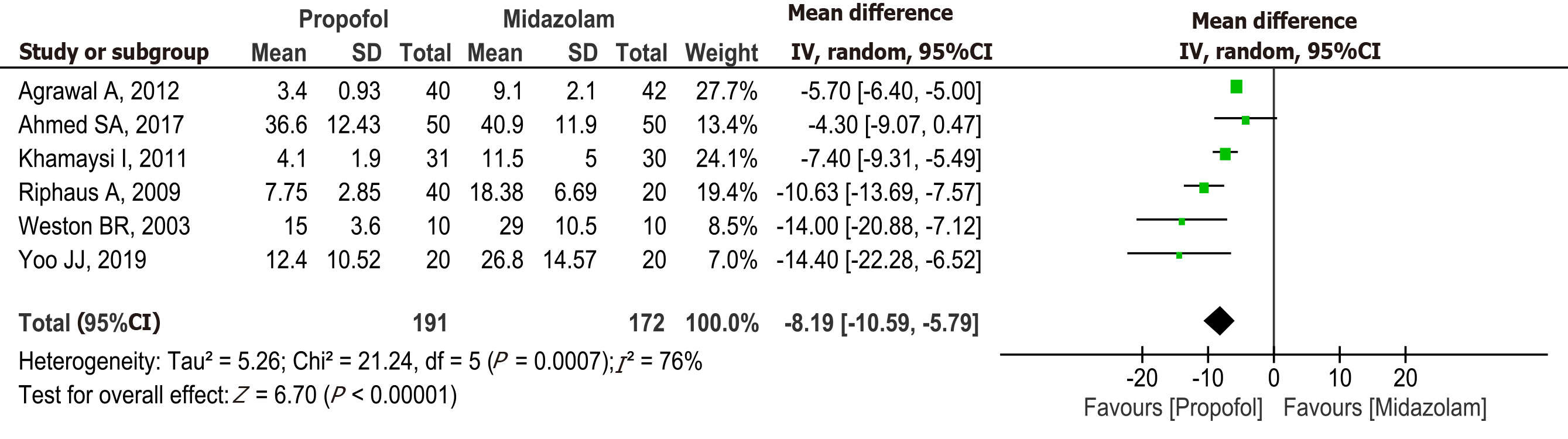
Recovery time: Six studies[15-18,20,22] with a total of 363 patients (191 in the propofol group and 172 in the midazolam group) reported recovery time after sedation. The recovery time was significantly higher in the midazolam group (MD: -8.19, 95%CI: -10.59 to -5.79, P < 0.00001) (Figure 5).
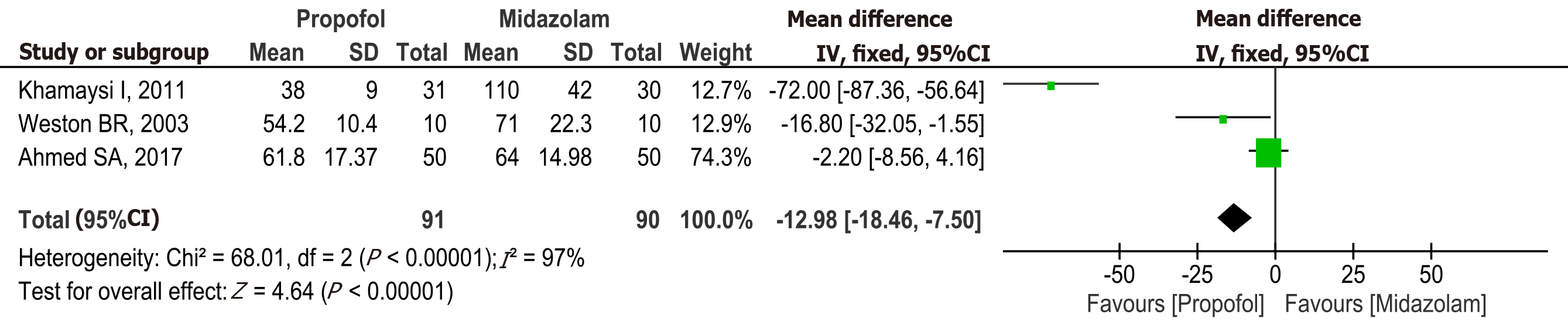
Discharge time: Three studies[16,20,22] with a total of 181 patients (91 in the propofol group and 90 in the midazolam group) reported discharge time after sedation. The discharge was significantly lower in the propofol group compared to midazolam (MD: -12.98, 95%CI: -18.46 to -7.50, P < 0.00001) (Figure 6).
Adverse events: All included studies[15-22] reported the incidence of adverse events related to sedation during upper gastrointestinal endoscopy (bradycardia, hypotension, and hypoxemia).
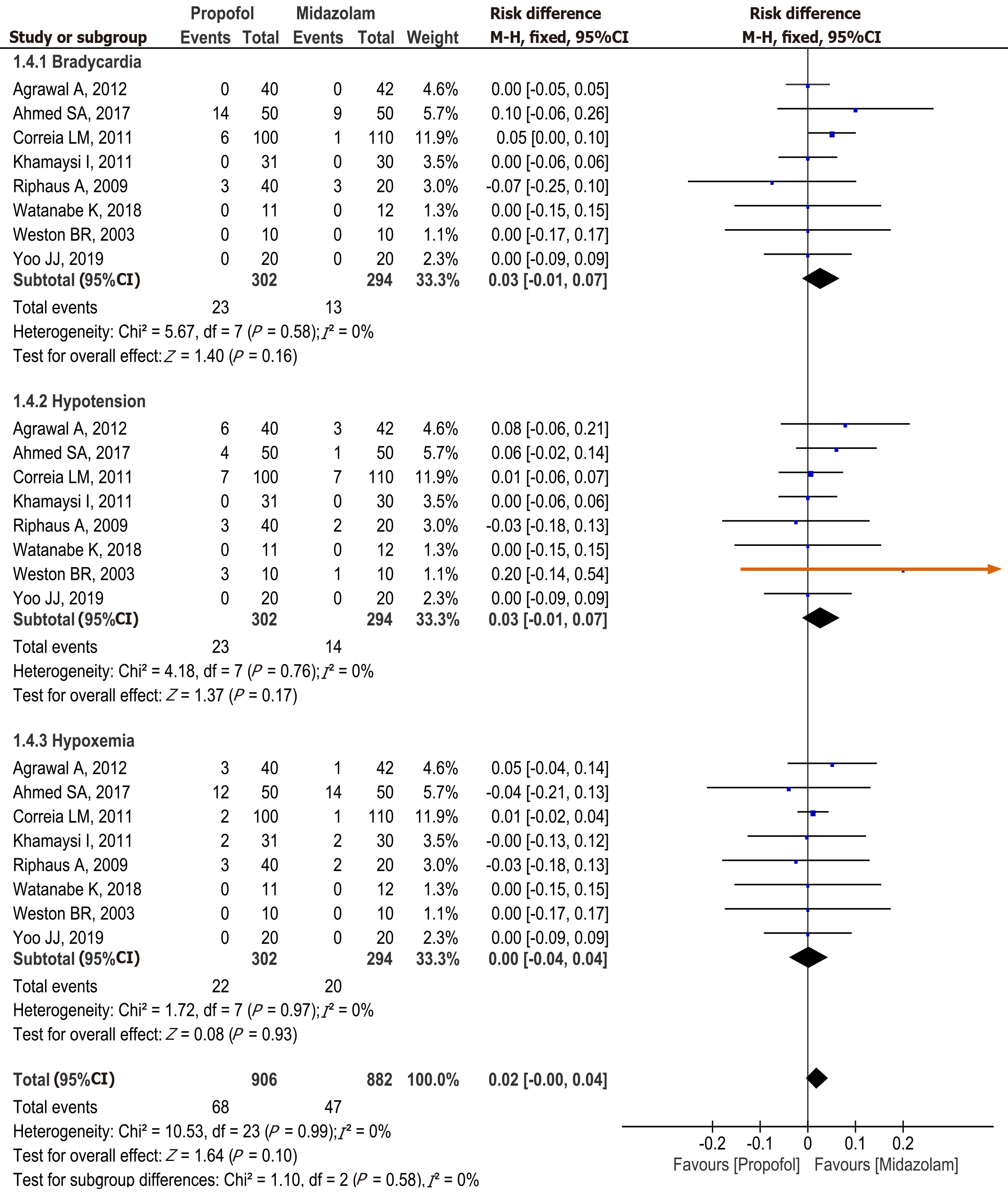
Adverse events were similar in both groups (RD: 0.02, 95%CI: 0-0.04, P = 0.58). Also, no significant difference was found when comparing each adverse event individually (Figure 7).
Bradycardia: Eight studies[15-22] with a total of 596 patients (302 in the propofol group and 294 in the midazolam group) reported the incidence of bradycardia related to sedation during upper gastrointestinal endoscopy. Increase incidence of bradycardia was seen in patients receiving midazolam for sedation; however, the difference was not statistically significant (RD: 0.03, 95%CI: -0.01 to 0.07, P = 0.16).
Hypotension: All studies[15-22] reported the incidence of hypotension related to sedation during upper gastrointestinal endoscopy. An increase in the incidence of hypotension was seen with the use of midazolam; however, it was not statistically significant (RD: 0.03, 95%CI: -0.01 to 0.07, P = 0.17).
Hypoxemia: All eight studies[15-22] reported the incidence of hypoxemia related to sedation. No statistically significant difference was found between groups (RD: 0.00, 95%CI: -0.04 to 0.04, P = 0.93).
Historically endoscopy was performed without sedation, which can be painful and uncomfortable for patients[23]. Over time, the application of topical anesthesia was introduced, and some countries still only use topical anesthesia because of low cost, patient preference, or institutional availability[24-26]. Administration of analgesics and intravenous sedation during endoscopy was a significant breakthrough worldwide, for both physicians and patients alike because of several advantages such as patient comfort, reduced discharge time, and early recovery after the procedure[27-29]. These can be used either alone or in combination for a synergetic effect to comfortably perform the procedure while maintaining an adequate level of sedation. Sedation in endoscopy is safe when we correctly select, individualize, and optimize the medicine dosage for each type of patient. One of the primary considerations is patient comorbidities, including hepatic dysfunction– which can lead to difficulty in clearance, recirculation, and increased half-life of drugs[30-36]. Sedation during endoscopy in patients with hepatic dysfunction was highlighted in 1975 when benzodiazepine use was compared in patients with and without liver abnormalities. Patients with cirrhosis can have alterations in the metabolism of benzodiazepines, which can result in impaired psychomotor function and increased recovery time; therefore, it was suggested to use benzodiazepines with caution[31,37-39]. Whereas, short-duration hypnotic agent propofol does not need dose adjustment in patients with cirrhosis and has a faster onset of action, shorter effect, and quick recovery time[13].
We studied the optimal approach for sedation during an elective upper gastrointestinal endoscopy in patients with cirrhosis. In our analysis, we included 8 RCTs[15-22] all with adequate designs, with a total of 596 patients. Our analysis showed that propofol had a faster recovery and discharge time. However, procedure time and adverse events were similar between propofol and midazolam group. Our results are consistent with the previous metanalysis by Tsai et al[40]; however, we included three more recent RCTs as well. Despite our study population only composed of patients with liver cirrhosis, our results are similar to a recent meta-analysis by Delgado et al[41] showing that propofol to be a better approach during upper GI endoscopy for all patients. The use of propofol for endoscopy in patients with cirrhosis has been increasing; however, one of the limitations for widespread use is that propofol is restricted mostly to anesthesiologists in some countries[42-47].
Seven RCTs were included[15-17,19-22] in our analysis for procedure time, and we found no statistical difference between midazolam and propofol. Five diagnostic and therapeutic procedures studies[15-17,20,22] showed shorter procedure tie for the midazolam group; however, 2 RCTs[19,21], including therapeutic procedures, showed shorter procedure time for the propofol group. The study by Agrawal et al[15] also included patients without any sedation and showed a shorter procedure time in the sedation group, likely due to a reduction in the discomfort that patients felt during endoscopy without sedation. All 6 RCTs[15-18,20,22] evaluating recovery time demonstrated a faster recovery time when using propofol compared to midazolam. Therefore, a statistically significant difference in recovery time was found in the metanalysis favoring the propofol group, although the methods to assess recovery varied slightly in studies. Three RCTs[15,16,22] used blood pressure and heart rate parameters within 20% of the baseline, oxygen saturation greater than 90% in ambient air, ability to tolerate oral fluids, and bedside support capacity without help or regaining basal function. While Yoo et al[18] and Ahmed et al[20]. evaluated patients for recovery using blood pressure, pulse oxymetry, and heart rate parameters. Different from other studies, Riphaus et al[17] used the post-anesthesia recovery score (PARS) which consists of five parameters (1) activity (inability to move the limbs, ability to move two or four limbs with or without command); (2) respiration (evidence of apnea, labored breathing, or normal breathing pattern); (3) circulation (blood pressure compared with baseline: ± 50% to baseline, ± 20% to 50% compared to baseline, ± 20% to baseline); (4) consciousness (non-arousable, arousable, or fully awake); and (5) skin color (cyanotic, pink, or normal). 0, 1 or 2 points are given for each parameter, and complete recovery is indicated by the maximum PARS of 10 points. Our metanalysis, including 3 RCTs[16,20,22], showed that propofol was associated with a faster discharge time than midazolam. Khamaysi et al[16] and Weston et al[22] showed results favoring propofol. While Ahmed et al[20] showed no difference in discharge time between propofol and midazolam.
Many adverse events in endoscopy are related to sedation. Our study found no statistical difference when comparing adverse events related to the use of propofol and midazolam. Our results were similar to a retrospective study[30] of 1667 patients with cirrhosis, which showed no difference in adverse events between midazolam plus fentanyl vs propofol sedation for endoscopy. Another recent multicenter cross-sectional study[48] that included 9007 endoscopic procedures in patients with cirrhosis reported that adverse events were infrequent and cardiovascular adverse events were related to unfit patients and those requiring general anesthesia. Cardiopulmonary adverse events in our study were mainly seen in 3 RCTs[17,20,21], which included endoscopic therapeutic procedures (varices treatment) likely because of the prolonged procedure time and the need for higher sedation dose for patient comfort. Given the significance of cardiopulmonary adverse events with sedation, we further evaluated adverse events like bradycardia, hypotension, and hypoxemia individually.
In our metanalysis, there was no difference in the incidence of bradycardia between propofol and midazolam. Bradycardia was described as a heart rate (HR) < 50 in most studies[16,17,20,22], HR < 45 by Watanabe et al[19], 25% decrease in initial HR or HR < 55 bpm by Correia et al[21] and a 20% decrease in initial HR by Agrawal et al[15] Patients in only one study (Ahmed et al[20]) were administered atropine 0.3 mg IV to control bradycardia. Hypotension with propofol is well recognized due to a reduction in systemic vascular reduction and depression of myocardial contractility. In our analysis, Agrawal et al[15] and Weston et al[22] notably used high doses of propofol, which could potentially result in the development of hypotension. However, in our analysis, we did not find any difference in the incidence of hypotension between propofol and midazolam. The included studies used various parameters to define hypotension. Agrawal et al[15] defined a blood pressure < 20% of the baseline, while Correia et al[21] considered a 20% decrease in MAP or a systolic blood pressure < 90 mmHg or a diastolic blood pressure < 50 mmHg as hypotension. Khamaysi et al[16], Riphaus et al[17], and Weston et al[22] considered a systolic blood pressure < 90 mmHg as hypotension. Ahmed et al[20] considered a decrease in mean arterial pressure (MAP) of 20 mmHg from baseline as hypotension and administrated ephedrine 10 mg and Ringer's lactate 5 mL/kg when it occurred. Watanabe et al[19] considered a systolic blood pressure < 80 mmHg as hypotension. Unlike other studies, Yoo et al[18] did not report any hypotension in both groups. Similarly, there was no difference in the incidence of hypoxemia seen in the propofol and midazolam group, although the definition of hypoxemia varied in studies. In most included studies[15-17,19-21], hypoxemia was defined as oxygen saturation of less than 90%. Weston et al[22] considered an oxygen saturation < 85% as hypoxemia and also measured hypoventilation if the respiratory rate was < 8 breaths per minute or by using a capnograph. Yoo et al[18] did not specify the values for hypoxemia, or if the patients were receiving oxygen. Seven studies[15-17,19-22] reported the use of oxygen through the nasal cannula at a rate of 2 to 5 L/min with an increase if necessary.
Hepatic encephalopathy is a multifaceted disorder in patients with cirrhosis and more evident in patients with high Child-Turcotte-Pugh and MELD scores. Benzodiazepines can particularly exacerbate hepatic encephalopathy after endoscopy in some patients[49,50], while the risk of encephalopathy reported with propofol is relatively low. Studies by Khamaysi et al[16], Riphaus et al[17] and Agrawal et al[15] included in our analysis reported that the risk of exacerbating minimal hepatic encephalopathy was less in the propofol group compared to midazolam. However, studies by Watanabe et al[19] and Yoo et al[18] did not present a statistically significant difference in minimal hepatic encephalopathy, with the latter using a software ("Stroop") for testing. In our meta-analysis, we could not quantitatively estimate the incidence of hepatic encephalopathy after sedation with propofol or midazolam since it was not uniformly reported. Five RCTs[15-19] that evaluated change in cognition used different tests to assess minimal hepatic encephalopathy prior to and after endoscopy without time standardization. Some of the tests described in the literature[16,17,19] to assess hepatic encephalopathy are Number Connection Tests (NCT), test and combination of psychometric[15], Portosystemic Encephalopathy (PSE)[17] Psychometric tests and Critical Flicker Frequency (CFF)[15], Cognitive Function Score (CFS)[16], Digital Symbol Tests (DST)[15], Line Tracing Tests (LTT)[15], Serial Dotting Tests (SDT)[15], and a test using the app "Stroop" [18] (limitation in patients of advanced age, low education level, and high MELD).
Despite our rigorous meta-analysis, including only RCTs, our study has several limitations. The quality of our systematic review and meta-analysis is inherently limited by the quality of the included studies. A high degree of statistical heterogeneity was found in some of our estimates. The included studies had patients with different Child-Turcotte-Pugh scores (A-B, B-C, and A-B-C). The doses of sedation used in studies were not consistent. Higher sedation doses of propofol and midazolam were used in the studies by Watanabe et al[19], Ahmed et al[20], Agrawal et al[15], Correia et al[21], Khamaysi et al[16], Riphaus et al[17], and Weston et al[22] as compared to the doses used in the study by Yoo et al[18]. This variance in doses was likely related to differences in BMI, height, and ethnicity of the patients included in these studies[18]. Additionally, some studies also used synthetic analgesics. We could not quantitatively estimate the minimal hepatic encephalopathy after sedation since the tests used in the included studies to assess hepatic encephalopathy were not uniform.
In conclusion, propofol has faster recovery time and a shorter patient discharge time compared with midazolam, with similar adverse events. Therefore, propofol should be the preferred agent for sedation in patients with cirrhosis undergoing upper gastrointestinal endoscopy.
Administration of analgesics and intravenous sedation during endoscopy in patients with cirrhosis has several advantages such as patient comfort, reduced discharge time, and early recovery after the procedure. However, proper selection of sedative medications is essential because of the risk of complications mainly due to underlying hepatic dysfunction– which can lead to difficulty in clearance, recirculation, and increased half-life of drugs.
Many diagnostic or therapeutic upper gastrointestinal endoscopy procedures are often performed in cirrhosis, but choosing effective and safe sedative medications can be a real challenge. Therefore, we wanted to compare commonly used sedation protocols in an attempt to understand the best approach.
To perform a systematic review and meta-analysis of Randomized Controlled Trials comparing sedation with propofol and midazolam in patients with cirrhosis undergoing elective endoscopy.
We performed a systematic review and meta-analysis using the PRISMA guidelines. Electronic searches were performed using MEDLINE, EMBASE, Central Cochrane, LILACS databases. Only randomized control trials (RCTs) were included. The outcomes studied were procedure time, recovery time, discharge time, and adverse events (bradycardia, hypotension, and hypoxemia).
Eight randomized clinical trials were included in the final analysis with a total of 596 patients, of whom 302 belonged to the propofol group and 294 to the midazolam group. Procedure time was similar between midazolam and propofol groups; however, the recovery time and discharge time were significantly less in the propofol group. Adverse events were similar in both groups, and no significant difference was found in rates of bradycardia, hypotension, and hypoxemia.
Our study showed that propofol has shorter recovery and patient discharge time as compared to midazolam with a similar rate of adverse events. These results suggest that propofol should be the preferred agent for sedation in patients with cirrhosis.
Sedation medications used during endoscopy can differ in outcomes in patients with cirrhosis. Randomized control trials comparing outcomes and adverse events of multiple sedation protocols in patients with cirrhosis should be carried out in the future.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lei JJ, Shen ZY S-Editor: Gong ZM L-Editor: A P-Editor: Wang LL
| 1. | Anthony PP, Ishak KG, Nayak NC, Poulsen HE, Scheuer PJ, Sobin LH. The morphology of cirrhosis. Recommendations on definition, nomenclature, and classification by a working group sponsored by the World Health Organization. J Clin Pathol. 1978;31:395-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 309] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 2. | American Association for the Study of Liver Diseases; European Association for the Study of the Liver. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases. J Hepatol. 2014;61:642-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 332] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 3. | Lôbo MR de A, Chaves DM, DE Moura DTH, Ribeiro IB, Ikari E, DE Moura EGH. Safety and efficacy of EUS-guided coil plus cyanoacrylate versus conventional cyanoacrylate technique in the treatment of gastric varices: a randomized controlled trial. Arq Gastroenterol. 2019;56:99-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 4. | Runyon BA; AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology. 2009;49:2087-2107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 613] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 5. | Garg H, Gupta S, Anand AC, Broor SL. Portal hypertensive gastropathy and gastric antral vascular ectasia. Indian J Gastroenterol. 2015;34:351-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | de Moura DTH, McCarty TR, Jirapinyo P, Ribeiro IB, Hathorn KE, Madruga-Neto AC, Lee LS, Thompson CC. Evaluation of endoscopic ultrasound fine-needle aspiration versus fine-needle biopsy and impact of rapid on-site evaluation for pancreatic masses. Endosc Int Open. 2020;8:E738-E747. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 7. | de Rezende DT, Brunaldi VO, Bernardo WM, Ribeiro IB, Mota RCL, Baracat FI, de Moura DTH, Baracat R, Matuguma SE, de Moura EGH. Use of hemostatic powder in treatment of upper gastrointestinal bleeding: a systematic review and meta-analysis. Endosc Int Open. 2019;7:E1704-E1713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Luz GO, Matuguma SE, Madruga Neto AC, Ribeiro IB, Dal Bello F, de Moura DTH, de Moura EGH. A novel technique in the management of refractory variceal bleeding. Endoscopy. 2020;52:310-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | de Moura DTH, do Monte Junior ES, Hathorn KE, Ribeiro IB, de Medeiros FS, Thompson CC, de Moura EGH. The use of novel modified endoscopic vacuum therapies in the management of a transmural rectal wall defect. Endoscopy. 2020;Online ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Brito HP, Ribeiro IB, de Moura DTH, Bernardo WM, Chaves DM, Kuga R, Maahs ED, Ishida RK, de Moura ETH, de Moura EGH. Video capsule endoscopy vs double-balloon enteroscopy in the diagnosis of small bowel bleeding: A systematic review and meta-analysis. World J Gastrointest Endosc. 2018;10:400-421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Ribeiro IB, Rezende DT, Madruga Neto AC, Ide E, Furuya CK, De Moura DTH, De Moura EGH. Endoscopic dual therapy for giant peptic ulcer hemorrhage. Endoscopy. 2018;50:E316-E317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Amornyotin S. Registered nurse-administered sedation for gastrointestinal endoscopic procedure. World J Gastrointest Endosc. 2015;7:769-776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Edelson JC, Rockey DC. Endoscopic Sedation of the Patient With Cirrhosis. Clin Liver Dis (Hoboken). 2018;12:165-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Cochrane. Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019) [Internet]. Cochrane; 2019. Available from: https://training.cochrane.org/handbook. |
| 15. | Agrawal A, Sharma BC, Sharma P, Uppal R, Sarin SK. Randomized controlled trial for endoscopy with propofol versus midazolam on psychometric tests and critical flicker frequency in people with cirrhosis. J Gastroenterol Hepatol. 2012;27:1726-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Khamaysi I, William N, Olga A, Alex I, Vladimir M, Kamal D, Nimer A. Sub-clinical hepatic encephalopathy in cirrhotic patients is not aggravated by sedation with propofol compared to midazolam: a randomized controlled study. J Hepatol. 2011;54:72-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Riphaus A, Lechowicz I, Frenz MB, Wehrmann T. Propofol sedation for upper gastrointestinal endoscopy in patients with liver cirrhosis as an alternative to midazolam to avoid acute deterioration of minimal encephalopathy: a randomized, controlled study. Scand J Gastroenterol. 2009;44:1244-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Yoo JJ, Goong HJ, Moon JE, Kim SG, Kim YS. Safety profile of sedative endoscopy including cognitive performance in liver cirrhosis: A double-blind randomized controlled trial. Sci Rep. 2019;9:16798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Watanabe K, Hikichi T, Takagi T, Suzuki R, Nakamura J, Sugimoto M, Kikuchi H, Konno N, Takasumi M, Sato Y, Hashimoto M, Irie H, Obara K, Ohira H. Propofol is a more effective and safer sedative agent than midazolam in endoscopic injection sclerotherapy for esophageal varices in patients with liver cirrhosis: a randomized controlled trial. Fukushima J Med Sci. 2018;64:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Ahmed SA, Selim A, Hawash N, Tawfik AK, Yousef M, Kobtan A, Badawi R, Elnawasany S, Elkhouly RA, Hanafy AS, Rizk FH, Mansour L, Abd-Elsalam S. Randomized Controlled Study Comparing Use of Propofol Plus Fentanyl versus Midazolam Plus Fentanyl as Sedation in Diagnostic Endoscopy in Patients with Advanced Liver Disease. Int J Hepatol. 2017;2017:8462756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Correia LM, Bonilha DQ, Gomes GF, Brito JR, Nakao FS, Lenz L, Rohr MR, Ferrari AP, Libera ED. Sedation during upper GI endoscopy in cirrhotic outpatients: a randomized, controlled trial comparing propofol and fentanyl with midazolam and fentanyl. Gastrointest Endosc. 2011;73:45-51, 51.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Weston BR, Chadalawada V, Chalasani N, Kwo P, Overley CA, Symms M, Strahl E, Rex DK. Nurse-administered propofol versus midazolam and meperidine for upper endoscopy in cirrhotic patients. Am J Gastroenterol. 2003;98:2440-2447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 67] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Modlin IM, Kidd M, Lye KD. From the lumen to the laparoscope. Arch Surg. 2004;139:1110-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Smith JL, Opekun A, Graham DY. Controlled comparison of topical anesthetic agents in flexible upper gastrointestinal endoscopy. Gastrointest Endosc. 1985;31:255-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Froehlich F. Topical pharyngeal anesthesia during gastroscopy. Gastrointest Endosc. 2001;54:803-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 26. | Evans LT, Saberi S, Kim HM, Elta GH, Schoenfeld P. Pharyngeal anesthesia during sedated EGDs: is "the spray" beneficial? A meta-analysis and systematic review. Gastrointest Endosc. 2006;63:761-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 27. | Hoare AM, Hawkins CF. Upper gastrointestinal endoscopy with and without sedation: patients' opinions. Br Med J. 1976;2:20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Reed WD, Hopkins BE, Joske RA, Laurence BH. A comparative study of conventional premedication (pethidine, promethazine, and atropine) and neuroleptanalgesia (droperidol and phenoperidine) for peroral endoscopy. Gut. 1971;12:736-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Findlay CW. The value of promethazine hydrochloride in preparing patients for peroral endoscopy. Am Rev Respir Dis. 1962;86:272-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 30. | Edelson J, Suarez AL, Zhang J, Rockey DC. Sedation During Endoscopy in Patients with Cirrhosis: Safety and Predictors of Adverse Events. Dig Dis Sci. 2020;65:1258-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | MacGilchrist AJ, Birnie GG, Cook A, Scobie G, Murray T, Watkinson G, Brodie MJ. Pharmacokinetics and pharmacodynamics of intravenous midazolam in patients with severe alcoholic cirrhosis. Gut. 1986;27:190-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 97] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Schilling D, Rosenbaum A, Schweizer S, Richter H, Rumstadt B. Sedation with propofol for interventional endoscopy by trained nurses in high-risk octogenarians: a prospective, randomized, controlled study. Endoscopy. 2009;41:295-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 33. | Heuss LT, Schnieper P, Drewe J, Pflimlin E, Beglinger C. Conscious sedation with propofol in elderly patients: a prospective evaluation. Aliment Pharmacol Ther. 2003;17:1493-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 71] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 34. | Razavi F, Gross S, Katz S. Endoscopy in the elderly: risks, benefits, and yield of common endoscopic procedures. Clin Geriatr Med. 2014;30:133-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 35. | Cohen LB, Delegge MH, Aisenberg J, Brill JV, Inadomi JM, Kochman ML, Piorkowski JD; AGA Institute. AGA Institute review of endoscopic sedation. Gastroenterology. 2007;133:675-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 308] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 36. | Bell GD, Spickett GP, Reeve PA, Morden A, Logan RF. Intravenous midazolam for upper gastrointestinal endoscopy: a study of 800 consecutive cases relating dose to age and sex of patient. Br J Clin Pharmacol. 1987;23:241-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 101] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 37. | Klotz U, Avant GR, Hoyumpa A, Schenker S, Wilkinson GR. The effects of age and liver disease on the disposition and elimination of diazepam in adult man. J Clin Invest. 1975;55:347-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 448] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 38. | Hamdy NA, Kennedy HJ, Nicholl J, Triger DR. Sedation for gastroscopy: a comparative study of midazolam and Diazemuls in patients with and without cirrhosis. Br J Clin Pharmacol. 1986;22:643-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 39. | Haq MM, Faisal N, Khalil A, Haqqi SA, Shaikh H, Arain N. Midazolam for sedation during diagnostic or therapeutic upper gastrointestinal endoscopy in cirrhotic patients. Eur J Gastroenterol Hepatol. 2012;24:1214-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | Tsai HC, Lin YC, Ko CL, Lou HY, Chen TL, Tam KW, Chen CY. Propofol versus midazolam for upper gastrointestinal endoscopy in cirrhotic patients: a meta-analysis of randomized controlled trials. PLoS One. 2015;10:e0117585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 41. | Delgado AAA, de Moura DTH, Ribeiro IB, Bazarbashi AN, Dos Santos MEL, Bernardo WM, de Moura EGH. Propofol vs traditional sedatives for sedation in endoscopy: A systematic review and meta-analysis. World J Gastrointest Endosc. 2019;11:573-588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 42. | Amorós A, Aparicio JR, Garmendia M, Casellas JA, Martínez J, Jover R. Deep sedation with propofol does not precipitate hepatic encephalopathy during elective upper endoscopy. Gastrointest Endosc. 2009;70:262-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 43. | Thuluvath PJ. Toward safer sedation in patients with cirrhosis: have we done enough? Gastrointest Endosc. 2009;70:269-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 44. | Sharma P, Singh S, Sharma BC, Kumar M, Garg H, Kumar A, Sarin SK. Propofol sedation during endoscopy in patients with cirrhosis, and utility of psychometric tests and critical flicker frequency in assessment of recovery from sedation. Endoscopy. 2011;43:400-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 45. | Tanaka N, Horiuchi A, Nakayama Y, Katsuyama Y, Isobe M, Aoyama T, Tanaka E, Ohmori S. Safety and effectiveness of low-dose propofol sedation during and after esophagogastroduodenoscopy in child A and B cirrhotic patients. Dig Dis Sci. 2013;58:1383-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 46. | Suh SJ, Yim HJ, Yoon EL, Lee BJ, Hyun JJ, Jung SW, Koo JS, Kim JH, Kim KJ, Choung RS, Seo YS, Yeon JE, Um SH, Byun KS, Lee SW, Choi JH, Ryu HS. Is propofol safe when administered to cirrhotic patients during sedative endoscopy? Korean J Intern Med. 2014;29:57-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 47. | Wahab EA, Hamed EF, Ahmad HS, Abdel Monem SM, Fathy T. Conscious sedation using propofol versus midazolam in cirrhotic patients during upper GI endoscopy: A comparative study. JGH Open. 2019;3:25-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 48. | Lieber SR, Heller BJ, Howard CW, Sandler RS, Crockett S, Barritt AS. Complications Associated with Anesthesia Services in Endoscopic Procedures Among Patients with Cirrhosis. Hepatology. 2020;Online ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 49. | Assy N, Rosser BG, Grahame GR, Minuk GY. Risk of sedation for upper GI endoscopy exacerbating subclinical hepatic encephalopathy in patients with cirrhosis. Gastrointest Endosc. 1999;49:690-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 57] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 50. | Vasudevan AE, Goh KL, Bulgiba AM. Impairment of psychomotor responses after conscious sedation in cirrhotic patients undergoing therapeutic upper GI endoscopy. Am J Gastroenterol. 2002;97:1717-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |