Published online Apr 16, 2020. doi: 10.4253/wjge.v12.i4.119
Peer-review started: December 15, 2019
First decision: January 6, 2020
Revised: February 18, 2020
Accepted: March 1, 2020
Article in press: March 1, 2020
Published online: April 16, 2020
Processing time: 117 Days and 1.8 Hours
Endoscopic submucosal dissection (ESD) represents an organ-preserving alternative to surgical resection of early gastric cancer. However, even with ESD yielding en-bloc resection specimens, there are concerns regarding tumor spread such as with larger lesions, ulcerated lesions, undifferentiated pathology and submucosal invasion. Sentinel node navigational surgery (SNNS) when combined with ESD offers a minimally invasive alternative to the traditional extended gastrectomy and lymphadenectomy if lack of lymph node spread can be confirmed. This would have a clear advantage in terms of potential complications and quality of life. However, SNNS, though useful in other malignancies such as breast cancer and melanoma, may not have a sufficient sensitivity for malignancy and negative predictive value in EGC to justify this as standard practice after ESD. The results of SNNS may improve with greater standardization and more involved dissection, technological innovations and more experience and validation such that the paradigm for post-ESD resection of EGC may change and include SNNS.
Core tip: Sentinel node navigation surgery after endoscopic submucosal dissection represents a minimally invasive approach to gastric cancer. However, this approach is controversial because it is not standardized nor has it been well validated outside of few centers in Asia. We will discuss these controversies and the potential of sentinel node navigational surgery to become an accepted diagnostic modality for select early gastric cancer patients.
- Citation: Friedel D, Zhang X, Stavropoulos SN. Burgeoning study of sentinel-node analysis on management of early gastric cancer after endoscopic submucosal dissection. World J Gastrointest Endosc 2020; 12(4): 119-127
- URL: https://www.wjgnet.com/1948-5190/full/v12/i4/119.htm
- DOI: https://dx.doi.org/10.4253/wjge.v12.i4.119
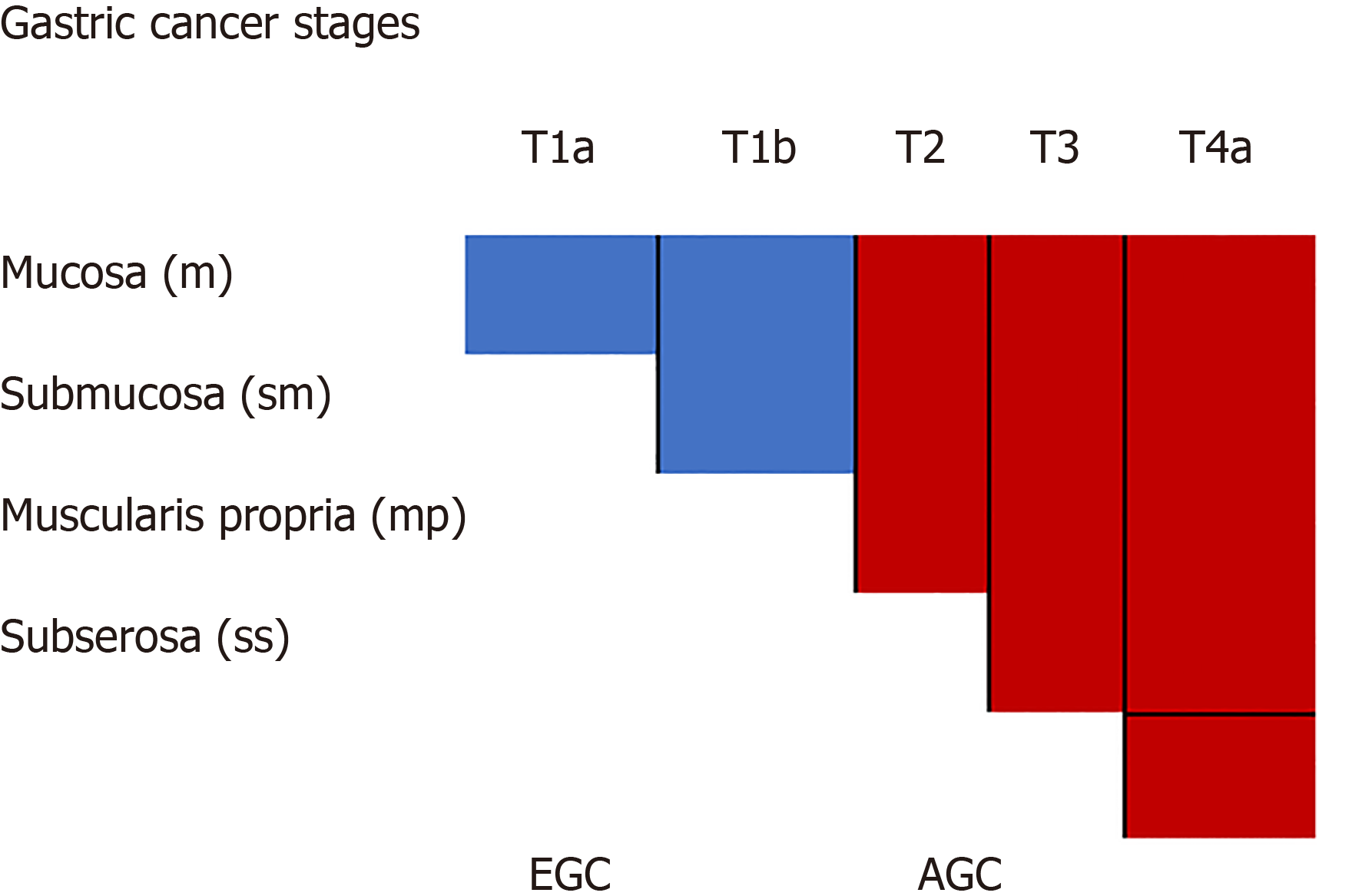
Gastric cancer (GC) is a common and lethal malignancy ranking fifth and second in global prevalence and cancer-related mortality, respectively[1]. There are mass screening programs in Asia but not the West. Mortality after gastrectomy for cancer is lower in Asia compared to the West; largely due to predominance of earlier stages representation[2]. Early GC (EGC) is defined as intramucosal cancer (T1a) or limited to mucosa and submucosa (T1b). Figure 1 Similar to other luminal GI malignancies, prognosis relates largely to stage of disease with post-surgical groups for EGC having a respective 5-year survival rate for T1a and T1b of 96% and 83%[3]. Endoscopic submucosal dissection (ESD) has become the standard mode of resection for T1a lesions with expanded criteria also considered from Japanese centers[4]. However, additional surgery is recommended for subjects undergoing ESD for these expanded indications (larger diameter, ulcerated, submucosal invasion, undifferentiated histology); especially for Western subjects[5]. Table 1 Concerns regarding oncologic cure linger even after apparent en-bloc resections.
| Histology | Depth | |||||
| Mucosal cancer | Submucosal cancer | |||||
| No ulceration | Ulcerated | SM1 | SM2 | |||
| ≤ 20 | > 20 | ≤ 30 | > 30 | ≤ 30 | Any size | |
| Differentiated | - | -- | -- | --- | -- | --- |
| Undifferentiated | ---- | --- | --- | --- | --- | --- |
A sentinel node resection is removal of draining lymph nodes that are deemed likely to first receive lymph flow from the area of the resected gastric lesion and is examined by a pathologist to determine presence of metastasis. Lack of noted metastasis can infer no likely spread of the gastric malignancy to other lymph nodes or organs. Sentinel node navigation surgery (SNNS) is combined with ESD (ESN) with the premise that this will ensure complete resection for EGC with organ preservation and assessment of pathological nodes. However, the SNNS concept was first described almost 20 years ago but has not been well validated subsequently; currently its implementation has been concentrated in a few Asian centers and there is sparse Western use. Moreover, though the minimal invasiveness and organ-preservation concept is attractive, doubts linger as to sensitivity of malignant lymph node detection and negative predictive value.
Surgical approach to EGC: Surgery for invasive EGC is involved with either total or subtotal gastrectomy depending on tumor localization. Lymphadenectomy is mandated with local (D1) or extended (D2) resection. There was a trend favoring the D1 resection in European studies in terms of lesser postoperative complications and similar outcomes[6,7], but 15-year follow-up for the Dutch group noted better survival in the D2 cohort[8]. D2 lymphadenectomy remains the standard in Japan for advanced cancer[9]. However, GC is prevalent in Japan and there has been an impetus for less drastic surgeries to improve quality of life and the concept of “function-sparing gastrectomy” including pylorus-sparing gastrectomy, local tumor resection and segmental gastrectomy in conjunction with SNNS[10]. Laparoscopic gastrectomy yields similar technical and oncological results as open gastrectomy with less invasiveness, and robotic gastrectomy has promise[11].
Sentinel Node Navigational Surgery: SNNS has been used been used extensively for staging in breast cancer and malignancy, and sporadically in a variety of other solid tumors including thyroid tumors, head/neck squamous cancer and pelvic tumors[12]. The goal of SNNS is to avoid the morbidity of extensive gastric resection with preservation of gastric function and goal of likely complete cancer resection. Sentinel node navigational surgery for EGC was described in 2001 with concerns that continue today including micrometastases, aberrant lymph drainage, accuracy of frozen section and criteria for sentinel node[13].
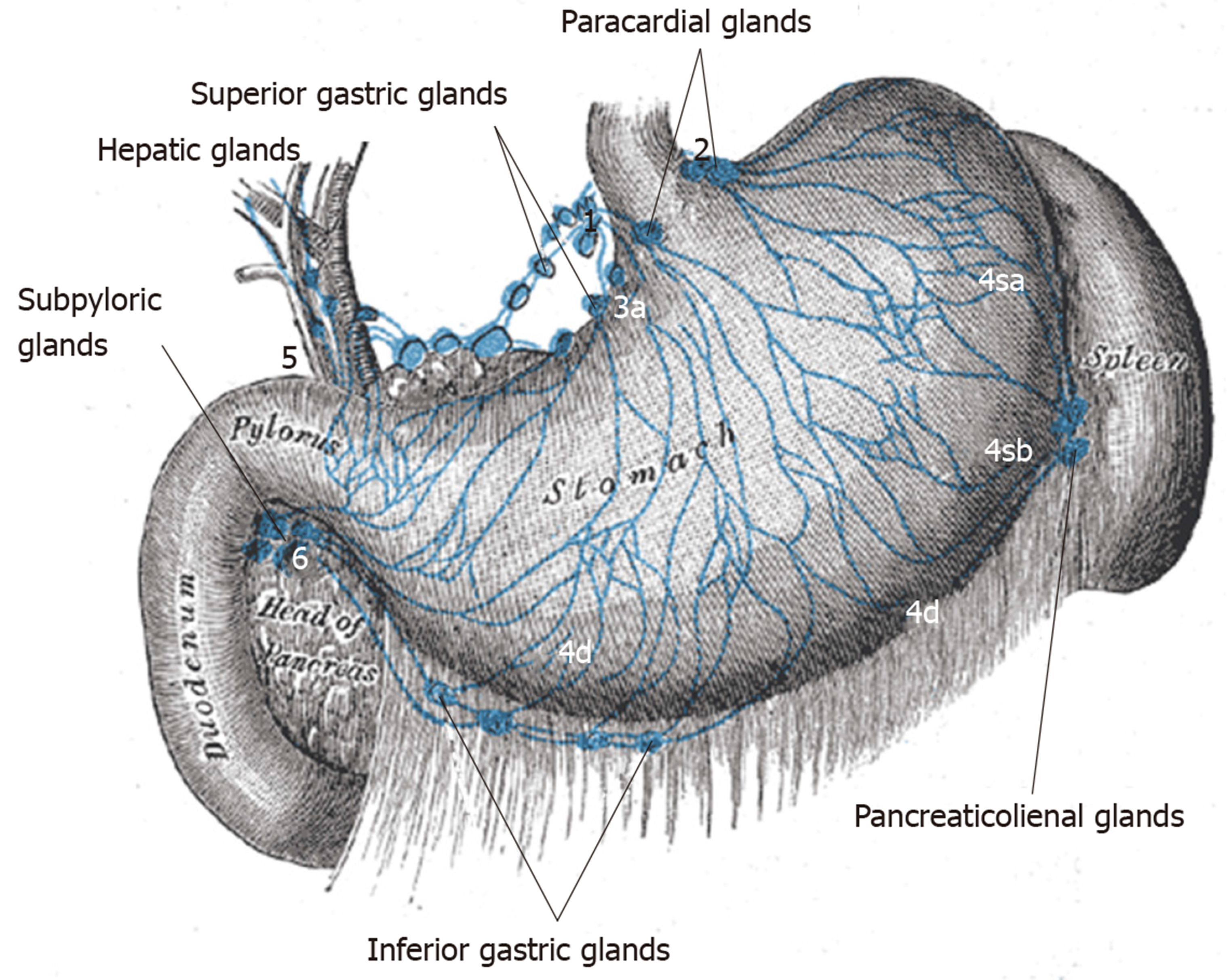
Techniques for detecting sentinel lymph nodes: The premise of SN dissection is the status of the sentinel node (i.e., tumor-free or not) determines the status of the adjacent draining nodes as well. The primary draining peri-gastric LN stations usually can be defined though there is variability and challenges for the surgeon. Larger tumors may have multidirectional lymph flow and post-ESD scarring may alter flow[14,15] (Figure 2).
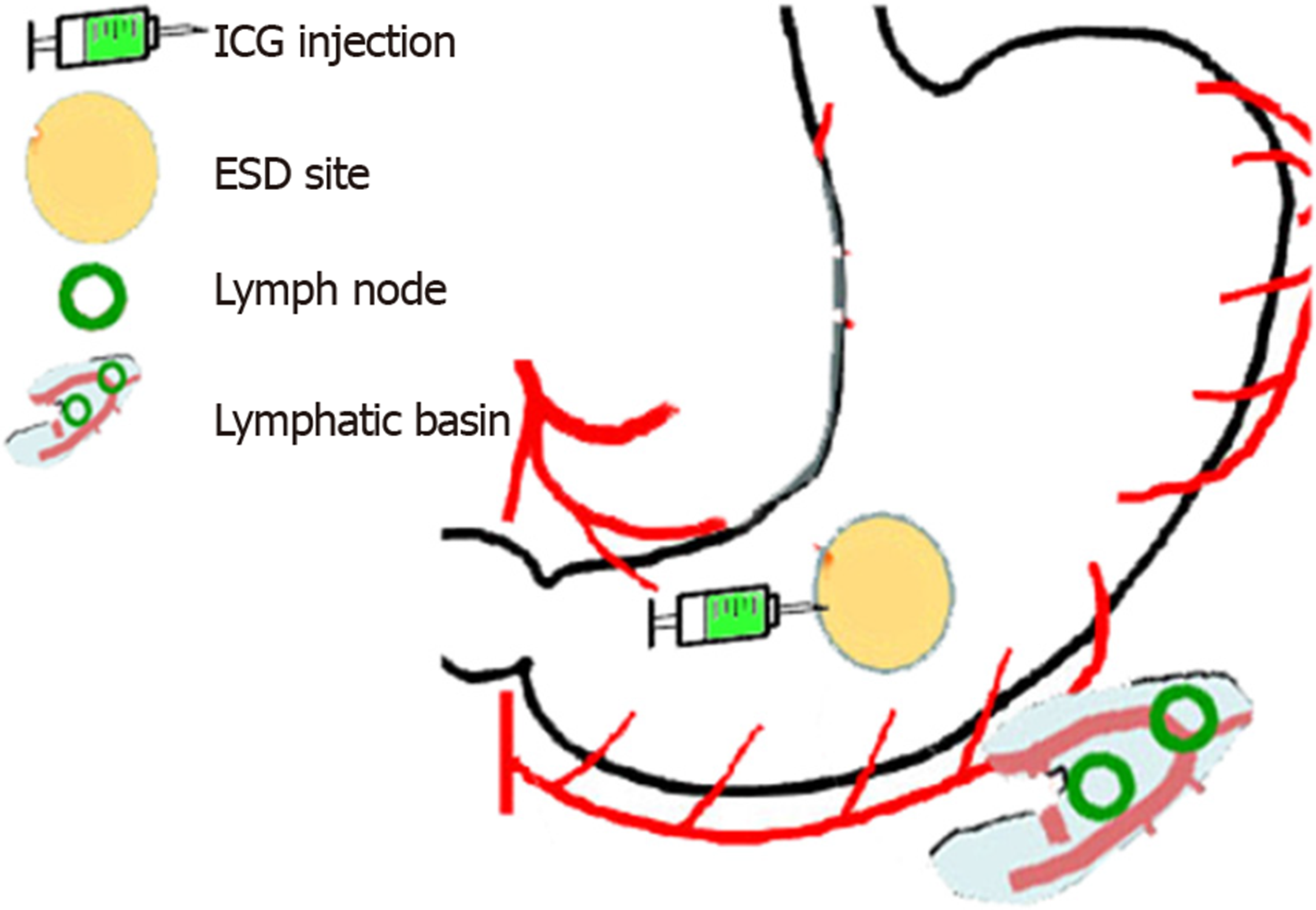
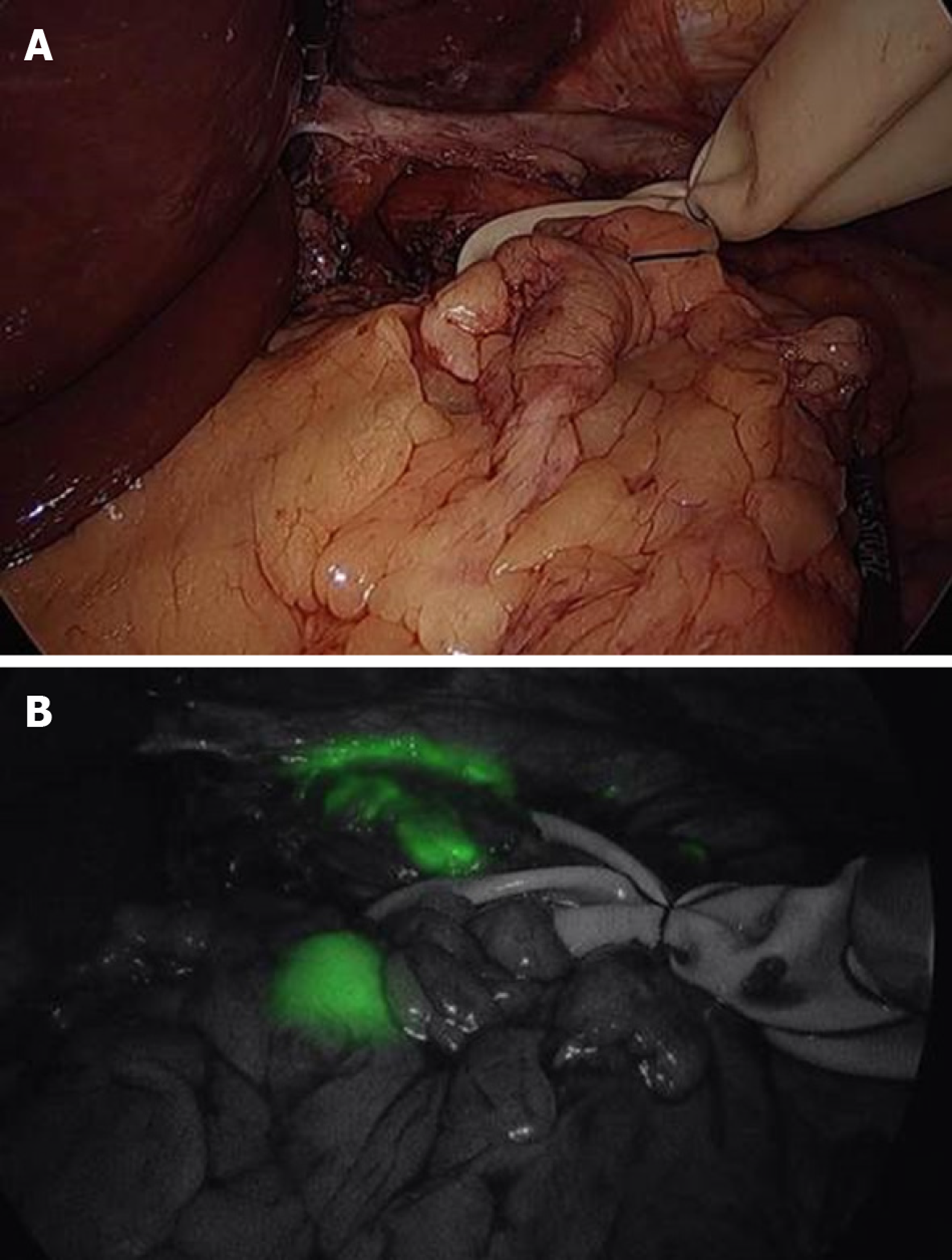
The most commonly used tracers are indocyanine green, carbon nanoparticles and blue dyes (patent, sulfan, isosulfan) which are injected into the submucosa at endoscopy done just prior to surgery or sub-serosally during surgery. Radioisotopes such as Technetium can be injected solely or in addition to the tracer. Tracers usually delineate draining LN’s well (Figure 3, Figure 4) but adiposity can be an obstacle. Injection should be done optimally intraoperatively to allow the surgeon to well delineate lymphatic drainage. Imaging is enhanced by a variety of electronic systems including some packaged into the laparoscope such as a florescence imaging system for indocyanine green (ICG) or using electronic infrared filtering where there is less concern for adiposity[16]. Probably, the greatest challenge to considering SNNS in EGC to be standard practice is the issue of how metastases in retrieved LN’s are verified[17]. Typically, this is done via frozen section using hematoxylin-eosin staining. The “lymphatic basin” concept of dissection has been advanced where LN dissection is dictated by the apparent path of the tracer during the surgery to cover the entire area of drainage[18]. This concept allows for more LN dissection than a “pick-up” approach of dissecting only obviously involved nodes but less than a gastrectomy-associated lymphadenectomy. LB dissection is superior to the “pick-up” method in terms of micrometastases detection[19].
The surgeon is tasked with potentially sampling multiple LN’s including possibly those in the second tier of gastric drainage, and the pathologist would be required to do the LN analysis (requiring multiple slices) which would be a tedious endeavor! Moreover, the accuracy of H&E staining for malignancy is suspect with a reported false-negative rate of 46% in one study which was therefore terminated[20]. This was felt to be largely due to insufficient sectioning of LN’s. One study noted that almost a quarter of ultimately positive LN’s were not identified in real time by H&E staining[21]. One experienced Japanese group noted a 10% intraoperative and 3% ultimate false negative rate using ICG alone[22]. Most other studies using dye tracer alone report lesser results[17,21,23].
Combined dye and radiotracer use is clearly superior to dye alone in terms of detection of involved LN’s and undetected pathology was associated with higher T stage and undifferentiated histology[23,24]. Micrometastases are a prime concern for SNNS in EGC; there is no accepted biomarker for GC, but there is a concerted effort to improve pathologic analysis of LN’s. This includes reverse transcriptase-PCR with CEA as mRNA rather than standard immunohistochemistry[25,26]. RT-PCR for MUC2 and CEA demonstrated good sensitivity and specificity[27]. Using real-time RT-PCR for specific cytokeratins and CEA demonstrated high sensitivity and no false negative LN’s[28]. More work in this area is awaited.
Despite its attractiveness in concept and prior scrutiny, SNNS remains relatively unvalidated for EGC with concern for patient outcome in terms of oncological cure and dubious QOL benefits with lesser resections[29]. A basic concern again is the complexity and variability of gastric drainage after ESD and in relation to the original lesion with the possibility of “skip” metastases[30]. One small Korean study noted a skip metastases rate of 17%[31]! The difficulty of accurate real-time LN analysis has been noted[21]. Nonetheless, the SNNS concept has been validated at least in some Japanese centers. This was demonstrated by a multicenter study where subgroup analysis of D2 lymphadenectomy subjects with EGC showed SNNS sensitivity and accuracy of 93% and 99%, respectively[21]. Only 4 patients (1%) had false negative SN dissection and ¾ of these were in the lymphatic basin with the fourth having a primary lesion > 4 cm[21]. The authors affirmed a LBD strategy rather than simple SND. The extrapolation of this study is limited however as these were very experienced operators with both EGC and SNNS.
An earlier meta-analysis also suggested that SNNS alone was inadequate to support limited lymphadenectomy for EGC, and a minimum of 4 LN’s should be harvested to ensure adequate sensitivity-overall sensitivity and negative predictive value was 98% and 92%, respectively[32]. Another meta-analysis had similar favorable results[24]. Limited results outside Asia showed lesser results likely reflecting less experience and issues with technique[33]. One American study noted a false-negative rate of 17%[34].
There is much less SNNS experience outside Asia and very little in the Americas[34-36]. The greatest obstacle to pursuing SNNS for EGC in the West is that GC-especially EGC- is generally less prevalent in the West and relatedly ESD is not commonplace. The potential solutions include multicenter trials to garner enough cases and to extrapolate the SNNS after ESD concept to GEJ tumors including Barrett’s esophagus. Siewert II and III GEJ tumors are probably best treated as GCs[37,38]. Surgeons beginning SNNS should consider travel to Asia for instruction or at least converse with surgeons who perform this for other entities (breast cancer). They probably should follow the typical path noted in Asian studies of performing SNNS prior to a planned gastrectomy and extended lympadenectomy to familiarize themselves before embarking on a SNNS directed strategy. The learning curve for SNNS has been suggested to be 25-30 cases[21,39].
Our experience included SNNS performed on 10 elderly patients with comorbid disease and early foregut cancers (7 Barrett’s, 3 EGC). Staging was as follow: T1a-mm(5), T1b(5) Mean lesion diameter was 4.0(2.2-8.6)cm-histology was G1(4), G2(5), G4(1). R0 resection and curative resection noted in 8 and 5 patients, respectively. SNNS was performed with a median of 9 (4-20) LN’s resected. Four had (+) SN’s with staging N1(1), N2(2), N3(1). These four received adjuvant chemotherapy; 2 with radiation. None of N0 subjects received chemotherapy. After a median follow-up of 30 months, 8 patients (including the 6 N0 patients) were in remission. Two patients with (+) SN’s died. We used endoscopic submucosal injection of ICG intra-operatively and unenhanced tracer detection. Of note, diagnostic laparoscopy with SNNS was the goal at onset and any gastric resection was to be performed at another time. Again, real time pathologic analysis is challenging and yield may increase with delayed assessment. SN analysis was useful in our multidisciplinary conference to direct management. Our experience suggests that SNNS is best reserved for those who value potential minimal resection, lesser postoperative complications and better global QOL over oncological safety. These would include the elderly including those with significant comorbid disease.
Skipped metastases: Skipped metastases refers to the discontinuous spread of malignancy with uninvolved contiguous lymph nodes interspersed among those harboring malignancy. This phenomenon runs counter to the sentinel node concept and would mandate extended lymphadenectomy if skipped metastases were common after ESD for EGC. Risk factors for LN spread with EGC surgery or ESD include tumor > 2 cm, submucosal invasion, undifferentiated histology and lymphovascular invasion and these are also risk factors for skipped metastases[40]. It is not entirely clear whether a skip metastases relates to direct spread from the resection site to second-tier LN’s or that spread to the first tier of LN’s is simply undetected[27]. This is academic and emphasizes the fragility of the SNNS concept; especially for lesions in the expanded criteria group, and also highlights that although SNNS is a multidisciplinary endeavor, the major onus is on the surgeon to adequately dissect appropriate and sufficient LN’s. The surgeon could be aided by enhanced technical aspects-dual dye and radiolabel tracer (gamma probe in abdomen and on resected LN’s on back table), IR electronic endoscopy and fluorescence imaging with improved as well as dedicated pathologic analysis (not simple H&E and one slice, but rather multiple slices and use of nuclear amplification, immunohistochemistry, imprint cytology). Lymphatic basin dissection rather than simple SND is essential[31]. A consideration is to have a dedicated SNNS independent of findings and have subsequent time for pathological analysis.
Lesion location: Lesion location has a significant impact on variability of LN drainage and possibility of missed or skipped metastases. GC anywhere can have atypical metastases but this is more likely for distal tumors and those on the lesser curvature[41]. Proximal tumors extending towards the middle of the stomach often have drainage to multiple LN basins[42]. Antral location may be a predictor of LN metastases after non-curative EGC resection[43].
Beginning a SNND program: There are many obstacles to initiating a SNND program and this includes both direct and indirect costs. Surgical faculty may need to be recruited. SNND would potentially also require more faculty, time, efforts and costs for gastroenterology, nuclear medicine and pathology. Formal cost analysis of gastric SNND has not been described but is likely a significant barrier; especially in the West where EGC is less common.
New techniques with SNNS: Endoscopist and laparoscopic surgeons can “cooperate” to effect removal of gastric lesions; this has been done widely for gastric GIST’s and described for a 6 cm lateral spreading GC[44]. Conceptually, this approach could include SNND for treating EGC[45]. A further enhancement of this cooperation is laparoscopic sero-muscular incision and suturing to evert a GC with endoscopic performance of ESD (EFTR) and preventing tumor seeding into the peritoneum; the specimen is removed orally, and SNNS is actually performed initially to assess for LN spread[46]. Finally, a NOTES (transvaginal entry) approach to EGC and SNNS has been described[47].
A Korean study analyzing SNNS with subsequent extended gastrectomy and D2 lympadenectomy noted 100% sensitivity and accuracy with dual tracer and radiolabel in detecting metastatic LN’s, but > 20% of cases were technical failures due to inability to dissect at least five SB LN’s[48]. These results together with the recent Japanese study[21] suggest that the treatment paradigm for EGC may change and incorporate SNNS use. Nonetheless, it is apparent that there will always be a chance of missed micrometastases so the sentinel node concept is imperfect and both the patient and surgeon have to realize this. The attraction of organ and function preservation has to be balanced with oncologic safety. One Japanese surgeon opined: “endoscopic and laparoscopic limited gastrectomy combined with SLN navigation surgery has the potential to become the standard minimally invasive surgery in EGC[29].” This optimism runs counter to the current swing back to extended lymphadenectomy[8] in GC surgery and number of LN’s dissected regarded as a quality measure[49]. An optimistic outcomes study of patients after SNNS for EGC noted that none of 93 subjects with (-) SNNS LN exam died of gastric cancer with follow-up of up to 15 years and a 5 year survival rate > 98%; metachronous GC developed in 6 patients with “diminished” gastrectomy emphasizing the need for continued gastric surveillance[50]. We are hopeful for similar positive outcomes regarding SNNS in future studies.
SNNS was first conceptualized almost 20 years ago but remains controversial and only recently has gained traction as a plausible option for patients with EGC. It is only performed routinely in a handful of select Japanese and Korean centers, where experience has increased confidence in the technique and as a stratifying modality. SNNS is more recently conceptualized as the surgical complement to ESD for the treatment and potential cure of EGC. Prolonged disease-free survival after successful ESD for EGC has been noted. SNNS after ESD is part of the continuum of minimal resection with organ and function preservation. However, SNNS as a technique has not been well validated outside of these centers, nor has the technique been standardized. Experience to date favors a lymphatic basin resection approach based on intraoperative determination of lymph drainage as opposed to a dedicated sentinel node dissection or “pick-up” approach. Dual use of both injected dye tracer in the ESD site and radiolabeled injection is superior to dye injection alone. The benefit of minimal resection including SNNS has to be balanced with oncological safety; specifically, likelihood of missed dissemination of malignancy and related lesser prognosis. These issues have to be explained to the patient giving informed consent. Western centers are handicapped by relative lack of EGC and ESD operators. A reasonable path to acquire SNNS experience and expertise is to perform this prior to extended gastrectomy and lymphadenectomy in order to gain experience without risking missed malignancy. It is inevitable that SNNS following ESD becomes an option in the management of EGC; especially for patients who are older, have significant comorbid disease and prefer avoidance of significant organ resection. We also expect that subsequent to more studies on the standardization and validation of sentinel node navigational surgery, the technique will be widely utilized globally.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shi J, Unger MM S-Editor: Ma YJ L-Editor: A E-Editor: Liu MY
| 1. | Khanderia E, Markar SR, Acharya A, Kim Y, Kim YW, Hanna GB. The Influence of Gastric Cancer Screening on the Stage at Diagnosis and Survival: A Meta-Analysis of Comparative Studies in the Far East. J Clin Gastroenterol. 2016;50:190-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 2. | Markar SR, Karthikesalingam A, Jackson D, Hanna GB. Long-term survival after gastrectomy for cancer in randomized, controlled oncological trials: comparison between West and East. Ann Surg Oncol. 2013;20:2328-2338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 3. | Onodera H, Tokunaga A, Yoshiyuki T, Kiyama T, Kato S, Matsukura N, Masuda G, Tajiri T. Surgical outcome of 483 patients with early gastric cancer: prognosis, postoperative morbidity and mortality, and gastric remnant cancer. Hepatogastroenterology. 2004;51:82-85. [PubMed] |
| 4. | Yamaguchi N, Isomoto H, Fukuda E, Ikeda K, Nishiyama H, Akiyama M, Ozawa E, Ohnita K, Hayashi T, Nakao K, Kohno S, Shikuwa S. Clinical outcomes of endoscopic submucosal dissection for early gastric cancer by indication criteria. Digestion. 2009;80:173-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 5. | Hatta W, Gotoda T, Koike T, Masamune A. A Recent Argument for the Use of Endoscopic Submucosal Dissection for Early Gastric Cancers. Gut Liver. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Bonenkamp JJ, Hermans J, Sasako M, van de Velde CJ, Welvaart K, Songun I, Meyer S, Plukker JT, Van Elk P, Obertop H, Gouma DJ, van Lanschot JJ, Taat CW, de Graaf PW, von Meyenfeldt MF, Tilanus H; Dutch Gastric Cancer Group. Extended lymph-node dissection for gastric cancer. N Engl J Med. 1999;340:908-914. [PubMed] |
| 7. | Degiuli M, Sasako M, Ponti A; Italian Gastric Cancer Study Group. Morbidity and mortality in the Italian Gastric Cancer Study Group randomized clinical trial of D1 versus D2 resection for gastric cancer. Br J Surg. 2010;97:643-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 223] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 8. | Hartgrink HH, van de Velde CJ, Putter H, Bonenkamp JJ, Klein Kranenbarg E, Songun I, Welvaart K, van Krieken JH, Meijer S, Plukker JT, van Elk PJ, Obertop H, Gouma DJ, van Lanschot JJ, Taat CW, de Graaf PW, von Meyenfeldt MF, Tilanus H, Sasako M. Extended lymph node dissection for gastric cancer: who may benefit? Final results of the randomized Dutch gastric cancer group trial. J Clin Oncol. 2004;22:2069-2077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 647] [Cited by in RCA: 653] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 9. | Zhang CD, Yamashita H, Seto Y. Gastric cancer surgery: historical background and perspective in Western countries versus Japan. Ann Trans Med. 2019;7(18):493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Takeuchi H, Goto O, Yahagi N, Kitagawa Y. Function-preserving gastrectomy based on the sentinel node concept in early gastric cancer. Gastric Cancer. 2017;20:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Russo A, Strong VE. Minimally invasive surgery for gastric cancer in USA: current status and future perspectives. Transl Gastroenterol Hepatol. 2017; 30(2):38. E collection.. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Gipponi M. Clinical applications of sentinel lymph-node biopsy for the staging and treatment of solid neoplasms. Minerva Chir. 2005;60:217-233. [PubMed] |
| 13. | Aikou T, Higashi H, Natsugoe S, Hokita S, Baba M, Tako S. Can sentinel node navigation surgery reduce the extent of lymph node dissection in gastric cancer? Ann Surg Oncol. 2001;8:90S-93S. [PubMed] |
| 14. | Shida A, Mitsumori N, Fujioka S, Takano Y, Fujisaki M, Hashizume R, Takahashi N, Ishibashi Y, Yanaga K. Sentinel Node Navigation Surgery for Early Gastric Cancer: Analysis of Factors Which Affect Direction of Lymphatic Drainage. World J Surg. 2018;42:766-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Nohara K, Goto O, Takeuchi H, Sasaki M, Maehata T, Yahagi N, Kitagawa Y. Gastric lymphatic flows may change before and after endoscopic submucosal dissection: in vivo porcine survival models. Gastric Cancer. 2019;22:723-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Takeuchi H, Kitagawa Y. Sentinel node navigation surgery in patients with early gastric cancer. Dig Surg. 2013;30:104-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Rino Y, Takanashi Y, Hasuo K, Kawamoto M, Ashida A, Harada H, Inagaki D, Hatori S, Ohshima T, Yamada R, Imada T. The validity of sentinel lymph node biopsy using dye technique alone in patients with gastric cancer. Hepatogastroenterology. 2007;54:1882-1886. [PubMed] |
| 18. | Kinami S, Fujimura T, Ojima E, Fushida S, Ojima T, Funaki H, Fujita H, Takamura H, Ninomiya I, Nishimura G, Kayahara M, Ohta T, Yoh Z. PTD classification: proposal for a new classification of gastric cancer location based on physiological lymphatic flow. Int J Clin Oncol. 2008;13:320-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Kelder W, Nimura H, Takahashi N, Mitsumori N, van Dam GM, Yanaga K. Sentinel node mapping with indocyanine green (ICG) and infrared ray detection in early gastric cancer: an accurate method that enables a limited lymphadenectomy. Eur J Surg Oncol. 2010;36:552-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 20. | Miyashiro I, Hiratsuka M, Sasako M, Sano T, Mizusawa J, Nakamura K, Nashimoto A, Tsuburaya A, Fukushima N; Gastric Cancer Surgical Study Group (GCSSG) in the Japan Clinical Oncology Group (JCOG). High false-negative proportion of intraoperative histological examination as a serious problem for clinical application of sentinel node biopsy for early gastric cancer: final results of the Japan Clinical Oncology Group multicenter trial JCOG0302. Gastric Cancer. 2014;17:316-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 113] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 21. | Kitagawa Y, Takeuchi H, Takagi Y, Natsugoe S, Terashima M, Murakami N, Fujimura T, Tsujimoto H, Hayashi H, Yoshimizu N, Takagane A, Mohri Y, Nabeshima K, Uenosono Y, Kinami S, Sakamoto J, Morita S, Aikou T, Miwa K, Kitajima M. Sentinel node mapping for gastric cancer: a prospective multicenter trial in Japan. J Clin Oncol. 2013;31:3704-3710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 233] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 22. | Miyashiro I, Hiratsuka M, Kishi K, Takachi K, Yano M, Takenaka A, Tomita Y, Ishiguro S. Intraoperative diagnosis using sentinel node biopsy with indocyanine green dye in gastric cancer surgery: an institutional trial by experienced surgeons. Ann Surg Oncol. 2013;20:542-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Cozzaglio L, Bottura R, Di Rocco M, Gennari L, Doci R. Sentinel lymph node biopsy in gastric cancer: possible applications and limits. Eur J Surg Oncol. 2011;37:55-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Wang Z, Dong ZY, Chen JQ, Liu JL. Diagnostic value of sentinel lymph node biopsy in gastric cancer: a meta-analysis. Ann Surg Oncol. 2012;19:1541-1550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 25. | Arigami T, Uenosono Y, Yanagita S, Nakajo A, Ishigami S, Okumura H, Kijima Y, Ueno S, Natsugoe S. Clinical significance of lymph node micrometastasis in gastric cancer. Ann Surg Oncol. 2013;20:515-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Yanagita S, Natsugoe S, Uenosono Y, Arigami T, Funasako Y, Hirata M, Kozono T, Ehi K, Arima H, Green G, Wang Y, Aikou T. The utility of rapid diagnosis of lymph node metastasis in gastric cancer using a multiplex real-time reverse transcription polymerase chain reaction assay. Oncology. 2009;77:205-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Sonoda H, Yamamoto K, Kushima R, Okabe H, Tani T. Detection of lymph node micrometastasis in gastric cancer by MUC2 RT-PCR: usefulness in pT1 cases. J Surg Oncol. 2004;88:63-70. [PubMed] |
| 28. | Shimizu Y, Takeuchi H, Sakakura Y, Saikawa Y, Nakahara T, Mukai M, Kitajima M, Kitagawa Y. Molecular detection of sentinel node micrometastases in patients with clinical N0 gastric carcinoma with real-time multiplex reverse transcription-polymerase chain reaction assay. Ann Surg Oncol. 2012;19:469-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Tani T, Sonoda H, Tani M. Sentinel lymph node navigation surgery for gastric cancer: Does it really benefit the patient? World J Gastroenterol. 2016;22:2894-2899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Lianos GD, Bali CD, Hasemaki N, Glantzounis GK, Mitsis M, Rausei S. Sentinel Node Navigation in Gastric Cancer: Where Do We Stand? J Gastrointest Cancer. 2019;50:201-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Li C, Kim S, Lai JF, Oh SJ, Hyung WJ, Choi WH, Choi SH, Noh SH. Solitary lymph node metastasis in gastric cancer. J Gastrointest Surg. 2008;12:550-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Ryu KW, Eom BW, Nam BH, Lee JH, Kook MC, Choi IJ, Kim YW. Is the sentinel node biopsy clinically applicable for limited lymphadenectomy and modified gastric resection in gastric cancer? A meta-analysis of feasibility studies. J Surg Oncol. 2011;104:578-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 33. | Symeonidis D, Tepetes K. Techniques and Current Role of Sentinel Lymph Node (SLN) Concept in Gastric Cancer Surgery. Front Surg. 2018;5:77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Becher RD, Shen P, Stewart JH, Geisinger KR, McCarthy LP, Levine EA. Sentinel lymph node mapping for gastric adenocarcinoma. Am Surg. 2009;75:710-714. [PubMed] |
| 35. | Mueller CL, Lisbona R, Sorial R, Siblini A, Ferri LE. Sentinel Lymph Node Sampling for Early Gastric Cancer-Preliminary Results of A North American Prospective Study. J Gastrointest Surg. 2019;23:1113-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 36. | Bravo Neto GP, Dos Santos EG, Victer FC, Neves MS, Pinto MF, Carvalho CE. Sentinel Lymph Node Navigation Surgery for Early Gastric Cancer: Is It a Safe Procedure in Countries with Non-Endemic Gastric Cancer Levels? A Preliminary Experience. J Gastric Cancer. 2016;16:14-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 37. | Mullen JT, Kwak EL, Hong TS. What's the Best Way to Treat GE Junction Tumors? Approach Like Gastric Cancer. Ann Surg Oncol. 2016;23:3780-3785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 38. | Azari FS, Roses RE. Management of Early Stage Gastric and Gastroesophageal Junction Malignancies. Surg Clin North Am. 2019;99:439-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 39. | Lee JH, Ryu KW, Lee SE, Cho SJ, Lee JY, Kim CG, Choi IJ, Kook MC, Kim MJ, Park SR, Lee JS, Nam BH, Kim YW. Learning curve for identification of sentinel lymph node based on a cumulative sum analysis in gastric cancer. Dig Surg. 2009;26:465-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 40. | Wang Z, Ma L, Zhang XM, Zhou ZX. Risk of lymph node metastases from early gastric cancer in relation to depth of invasion: experience in a single institution. Asian Pac J Cancer Prev. 2014;15:5371-5375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 41. | Lee JH, Lee HJ, Kong SH, Park DJ, Lee HS, Kim WH, Kim HH, Yang HK. Analysis of the lymphatic stream to predict sentinel nodes in gastric cancer patients. Ann Surg Oncol. 2014;21:1090-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 42. | Ohi M, Toiyama Y, Omura Y, Ichikawa T, Yasuda H, Okugawa Y, Fujikawa H, Okita Y, Yoshiyama S, Hiro J, Araki T, Kusunoki M. Possibility of limited gastrectomy for early gastric cancer located in the upper third of the stomach, based on the distribution of sentinel node basins. Surg Today. 2019;49:529-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 43. | Yang HJ, Kim SG, Lim JH, Choi J, Im JP, Kim JS, Kim WH, Jung HC. Predictors of lymph node metastasis in patients with non-curative endoscopic resection of early gastric cancer. Surg Endosc. 2015;29:1145-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 44. | Nunobe S, Hiki N, Gotoda T, Murao T, Haruma K, Matsumoto H, Hirai T, Tanimura S, Sano T, Yamaguchi T. Successful application of laparoscopic and endoscopic cooperative surgery (LECS) for a lateral-spreading mucosal gastric cancer. Gastric Cancer. 2012;15:338-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 45. | Aisu Y, Yasukawa D, Kimura Y, Hori T. Laparoscopic and endoscopic cooperative surgery for gastric tumors: Perspective for actual practice and oncological benefits. World J Gastrointest Oncol. 2018;10:381-397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 46. | Goto O, Takeuchi H, Kawakubo H, Sasaki M, Matsuda T, Matsuda S, Kigasawa Y, Kadota Y, Fujimoto A, Ochiai Y, Horii J, Uraoka T, Kitagawa Y, Yahagi N. First case of non-exposed endoscopic wall-inversion surgery with sentinel node basin dissection for early gastric cancer. Gastric Cancer. 2015;18:434-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 47. | Asakuma M, Cahill RA, Lee SW, Nomura E, Tanigawa N. NOTES: The question for minimal resection and sentinel node in early gastric cancer. World J Gastrointest Surg. 2010;2:203-206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 48. | An JY, Min JS, Lee YJ, Jeong SH, Hur H, Han SU, Hyung WJ, Cho GS, Jeong GA, Jeong O, Park YK, Jung MR, Park JY, Kim YW, Yoon HM, Eom BW, Ryu KW. Which Factors Are Important for Successful Sentinel Node Navigation Surgery in Gastric Cancer Patients? Analysis from the SENORITA Prospective Multicenter Feasibility Quality Control Trial. Gastroenterol Res Pract. 2017;2017:1732571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 49. | Claassen YHM, de Steur WO, Hartgrink HH, Dikken JL, van Sandick JW, van Grieken NCT, Cats A, Trip AK, Jansen EPM, Kranenbarg WMM, Braak JPBM, Putter H, van Berge Henegouwen MI, Verheij M, van de Velde CJH. Surgicopathological Quality Control and Protocol Adherence to Lymphadenectomy in the CRITICS Gastric Cancer Trial. Ann Surg. 2018;268:1008-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 50. | Isozaki H, Matsumoto S, Murakami S. Survival outcomes after sentinel node navigation surgery for early gastric cancer. Ann Gastroenterol Surg. 2019;3:552-560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |