Published online Dec 16, 2020. doi: 10.4253/wjge.v12.i12.542
Peer-review started: October 2, 2020
First decision: October 17, 2020
Revised: November 2, 2020
Accepted: November 17, 2020
Article in press: November 17, 2020
Published online: December 16, 2020
Processing time: 72 Days and 1.2 Hours
Medical therapy for strictures is limited and first-line treatment consists of endoscopic balloon dilatation, strictureplasty or surgical resection. Mycobacterium tuberculosis, Helicobacter pylori and Streptococcus can all cause stenosis, for which antibiotic treatment achieves stricture resolution. Mycobacterium avium ssp. paratuberculosis is a suspected causative agent in Crohn’s disease (CD). Thus, specialized antimicrobial treatment, in particular, anti-mycobacterial antibiotic therapy (AMAT) has been proposed as a potential treatment option. To our knowledge, the opening of CD strictures has not been recorded using any form of antibiotic therapy. We hypothesized that AMAT would resolve strictures in patients with CD.
To investigate the effect and outcomes of AMAT in a cohort of CD patients with an ileal stricture.
A single center, retrospective, medical record case review was conducted on an observational cohort of patients with CD who had an ileal stricture on colonoscopy and were treated with AMAT. Forty patients meeting the inclusion criteria were identified from the internal medical database. Thirty (75%) patients had follow-up colonoscopy and clinical data available. The AMAT regimen was prescribed after the initial colonoscopy for a duration of at least six months until follow-up colonoscopy with the attending gastroenterologist. Patient demographics, symptoms, colonoscopy reports, inflammatory serum markers and concurrent medications were recorded at pre-treatment and follow-up between January 1995 and June 2018.
Of the patients that returned for follow-up after > 24 mo of AMAT, twenty (67%) had complete resolution (CR) of their ileal strictures, three (10%) had partial resolution and seven (23%) had no resolution. Irrespective of stricture outcome, 21 patients (70%) demonstrated clinical response to AMAT and there was a statistically significant reduction in inflammatory serum markers C-reactive protein (P < 0.0001) and erythrocyte sedimentation rate (P = 0.04) from pre-treatment to follow-up. It was observed that 11 (37%) patients experienced side effects, but no serious adverse effects were attributable to AMAT. At follow-up there were 26 (87%) patients on concomitant medication for CD and a statistically significant association between CR and AMAT with a concomitant immunomodulator (P = 0.02).
This study demonstrated a high rate of stricture resolution (67%) similar to that seen in tuberculosis strictures (70%), suggesting a shared mycobacterial origin of strictures, and perhaps disease.
Core Tip: Crohn’s disease (CD) is a chronic, incurable inflammatory bowel disease rising in incidence and prevalence. Strictures are a significant complication of CD with current first-line treatments including dilatation and/or surgery. Mycobacterium avium ssp. paratuberculosis is a suspected causative agent in CD and the proposed treatment is anti-mycobacterial antibiotic therapy (AMAT). Preliminary results indicate AMAT for atypical Mycobacteria opens CD strictures efficaciously and reliably. Our study provides an alternative to endoscopic and surgical intervention to treat strictures in CD. Our findings should be validated by further randomized controlled trials.
- Citation: Collyer R, Clancy A, Agrawal G, Borody TJ. Crohn’s strictures open with anti-mycobacterial antibiotic therapy: A retrospective review. World J Gastrointest Endosc 2020; 12(12): 542-554
- URL: https://www.wjgnet.com/1948-5190/full/v12/i12/542.htm
- DOI: https://dx.doi.org/10.4253/wjge.v12.i12.542
Crohn’s disease (CD) is a chronic, incurable inflammatory bowel disease located at any point from the mouth through to anus. The incidence of CD in North America is 6.3-23.8 per 100000 person-years and prevalence is 96.3-318.5 per 100000 with rates increasing worldwide[1]. On presentation, patients with CD can exhibit a variety of symptoms including generalized abdominal pain, diarrhea and the passage of blood or mucus[2]. Additional symptoms may develop with disease progression such as abdominal distension, vomiting and weight loss due to obstruction, secondary to stricturing[3]. In general, the behavior of CD can be categorized into three phenotypes including inflammatory (non-stricturing, non-penetrating), stricturing and penetrating (fistulizing) disease[4]. Strictures consist of inflammatory, fibrotic or mixed processes leading to acute abdominal pain or obstruction and are a risk factor for developing internal fistulae and cancer[5,6]. At diagnosis, only a tenth of CD patients will present with a stricture however, up to one third of patients will ultimately develop stricturing within ten years of diagnosis, predominantly in the ileum[7]. Current first-line treatment for strictures includes endoscopic balloon dilatation (EBD), strictureplasty or surgical resection, and this may result in postoperative complications or recurrence of the stricture[8]. It is reported that 75% of patients diagnosed with stricturing will require at least one surgical procedure during their lifetime[9]. Since strictures remain a significant complication of CD, it is crucial to determine the most effective treatment while limiting disease burden.
Added biological therapies and exclusive enteral nutrition (EEN) have shown promise in treating CD strictures, but alone are not effective in resolving them[10-14]. In other stenosing conditions such as bowel tuberculosis (TB), Helicobacter pylori (H. pylori) duodenal ulcer disease and β-hemolytic Streptococcus oesophagitis, antimicrobial treatment can achieve complete resolution of strictures. In one prospective study, 16/23 (70%) patients with TB strictures treated with an anti-TB regimen attained stricture resolution as shown by radiography[15]. Similarly, clinical observations in a prospective study revealed patients with a peptic stenosis treated with anti-H. pylori therapy achieved a 91% endoscopic stenosis resolution[16,17]. In an isolated incident of ulcerative oesophagitis in an immunocompetent patient due to β-hemolytic Streptococcus, antibiotic treatment led to a resolution of the oesophageal stricture[18]. Hence, there appears to be a link between targeting the infection causing the stricture with specialized antimicrobial treatment and stricture resolution. To our knowledge, the opening of CD strictures has not been recorded using any form of antibiotic therapy.
Mycobacterium avium ssp. paratuberculosis (MAP) is the causative agent of Johne’s disease which shares a remarkably similar profile with CD, but occurs in ruminants such as cattle[19]. MAP, or one of its variants, may also be a causative agent of CD[20]. However, due to the difficulty in reliably detecting or culturing MAP in humans, its relationship with CD remains controversial. It is suggested that CD emerges from a chronic MAP infection, alongside a dysfunctional mucosal barrier, disrupted gut flora and a dysregulated immune response in genetically susceptible individuals[21-23]. Recently, treating CD with anti-mycobacterial antibiotic therapy (AMAT) has been shown to be highly effective as validated in a recent phase three, multicenter, randomized controlled trial (RCT) by inducing remission significantly better than placebo[24]. Additionally, even within a pediatric cohort, AMAT appears to confer a benefit yielding a robust clinical and endoscopic remission rate[25]. In a subset of CD patients given AMAT the disease can completely disappear for more than three years without further therapy, as has previously been demonstrated with duodenal ulcer disease[26,27]. It should be noted that in an up to date systematic review of AMAT, the authors conclude it may provide a benefit over placebo, but higher quality evidence is needed before it can be indicated for CD[28]. To our knowledge, the effects of antibiotic therapy on strictures has not yet been investigated nor a cohort study reported. This review reports the outcomes of AMAT in a cohort of CD patients with an ileal stricture. We hypothesized that AMAT would resolve strictures in patients with CD.
This is a single center, retrospective, medical record case review of a cohort of patients with CD and ileal strictures on colonoscopy who were treated with AMAT. All patients gave their informed consent for the off-label use of antibiotics. AMAT was prescribed after the initial colonoscopy for a duration of at least six months until follow-up colonoscopy with the attending gastroenterologist. The AMAT regimen comprised a combination of clarithromycin, rifabutin and clofazimine, some patients requiring a fourth antibiotic including ethambutol, ciprofloxacin, metronidazole or tinidazole depending on allergies. Patients were identified from the internal medical database between January 1995 and June 2018.
Inclusion criteria entailed: (1) Diagnosis of CD; (2) Stricture in the ileocecal valve (ICV) and/or terminal ileum (TI); and (3) Treated with AMAT. Exclusion criteria included: (1) No established diagnosis of CD on colonoscopy; (2) Volonic or anastomotic strictures; (3) Prior surgical intervention affecting the ICV or TI; (4) Follow-up data spanning a period of less than six months; and (5) Non-compliance with AMAT.
The primary outcome of this study was resolution of CD ileal strictures categorized as complete resolution (CR), partial resolution (PR) or no resolution (NR). The secondary outcomes pertained to clinical response and a reduction of inflammatory serum markers including both the C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR). Determinant clinical information such as diagnosis, course of treatment, symptoms and stricture outcome were obtained from the reports of the gastroenterologist.
Demographics, symptoms, colonoscopy reports, inflammatory serum markers and concurrent medications were recorded between June 2018 and June 2019 at pre-treatment and 6, 12, 18, 24, and > 24 mo on AMAT. Patient comorbidities, hospitalizations, surgeries and medical history were also documented. The data was checked by two independent reviewers to minimize bias.
Statistical analyses were performed using GraphPad Prism v8.1.1 (San Diego, CA, United States). Categorical variables were presented as percentages. Quantitative variables were presented as medians with interquartile range. Tests for association between groups and categorical variables were performed using Fisher’s exact test. Tests for comparison between groups and quantitative variables were performed using Mann-Whitney’s U test. Test results were reported as P values and considered significant if P < 0.05.
Forty patients (22 M) with a median age of 27 years (24-39 years) met the inclusion and exclusion criteria (Table 1). At pre-treatment, 15 patients had stricturing of the ICV, 24 of the TI and one with stricturing in both the TI and ICV. In total six patients had multiple ileal strictures. At presentation 39 (98%) patients were symptomatic with 10 (25%) previously requiring hospitalization for small bowel obstruction. The most common symptoms were abdominal pain and diarrhea, in 31 (78%) and 21 (53%) patients respectively. Patients had CD for a median duration of 3 years (1-8 years) pre-treatment while the median time between diagnosis and stricture found at colonoscopy was 3 years (0-6 years). Prior to patients commencing AMAT, 31 (78%) were on either biologic, steroid, immunomodulator (IMD) or 5-aminosalicylate medications.
| Pre-treatment cohort (n = 40) | |
| Demographics | |
| Age at AMAT [yr (IQR)] | 27 (24-39) |
| Sex [M (%)] | 22 (55%) |
| Duration of disease [yr (IQR)] | 3 (1-8) |
| Time between diagnosis and stricture [yr (IQR)] | 2.5 (0-6) |
| Number of strictures | |
| Single strictures, n (%) | 34 (85) |
| Multiple strictures, n (%) | 6 (15) |
| Symptoms | |
| Y, n (%) | 39 (98) |
| Abdominal pain, n (%) | 31 (78) |
| Diarrhoea, n (%) | 21 (53) |
| Fatigue, n (%) | 6 (15) |
| Weight loss, n (%) | 5 (13) |
| Vomiting, n (%) | 4 (10) |
| Constipation, n (%) | 2 (5) |
| Pathology | |
| CRP [mg/L (IQR)] | 19 (11-92) |
| ESR [mm/h (IQR)] | 12 (5-27) |
| Area of involvement | |
| ICV, n (%) | 19 (48) |
| TI, n (%) | 20 (50) |
| ICV + TI, n (%) | 1 (3) |
| Concomitant medication | |
| Y, n (%) | 31 (78) |
| N, n (%) | 9 (23) |
Thirty (75%) patients (15 M) with a median age of 26 years (24-35 years) had follow-up colonoscopy and clinical data available. Two required surgery for complications relating to fistulae; resolved by an ileocolic resection and right hemicolectomy. Both patients had successful operations but were not included in the final analysis due to the inability to reassess their stricture. The remaining eight patients were lost to follow-up.
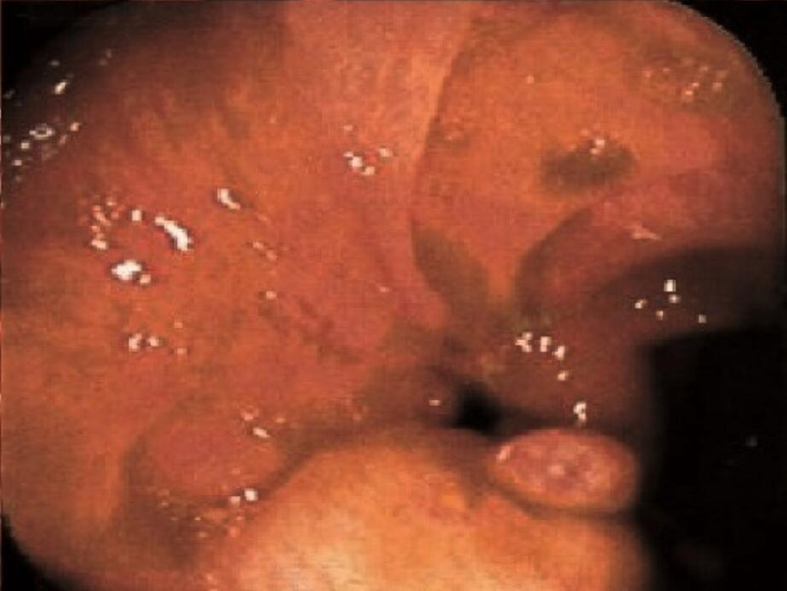
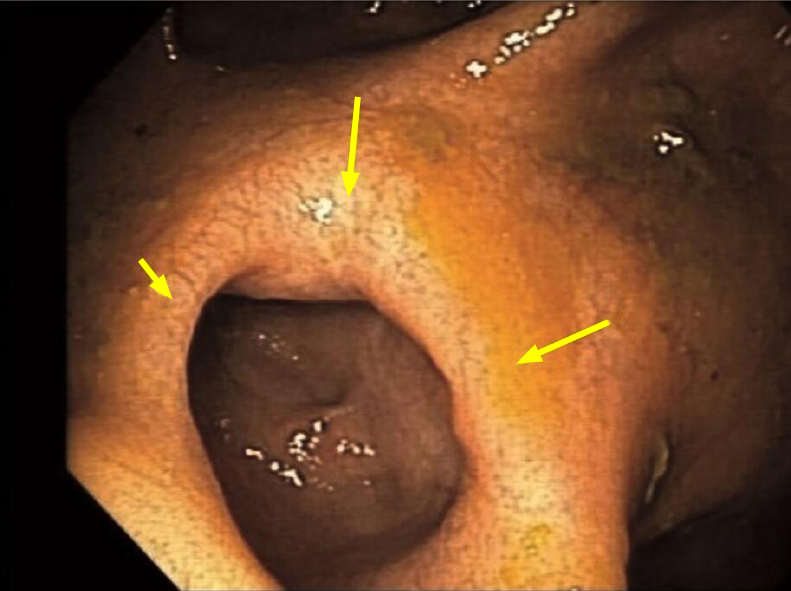
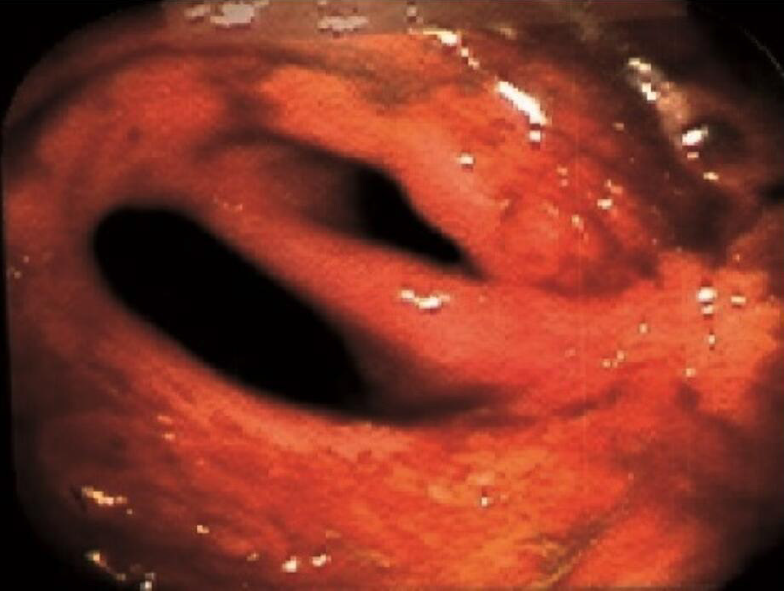
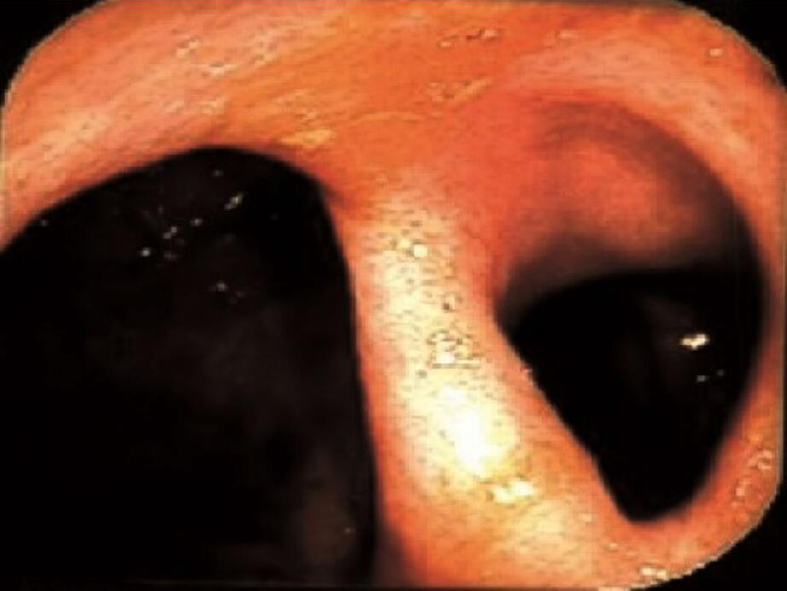
Of the patients who had follow-up colonoscopy after > 24 mo of AMAT, twenty (67%) patients had CR, three (10%) had PR and seven (23%) had NR of their strictures (Table 2). For the patients that achieved CR of their strictures (Figures 1, 2, 3, and 4), eight occurred in the ICV and 12 in the TI. Of the four patients with multiple strictures, AMAT induced one CR and one PR of both strictures which were located in the TI while the other two patients had NR of their strictures. Duration of treatment with AMAT did not seem to impact the resolution of strictures with nine patients attaining CR at 12 mo, five at > 24 mo, four at 18 mo, and one at both 6 mo and 24 mo. Interestingly, after resolution of their stricture on AMAT one patient ceased treatment. At 36 mo follow-up, the patient’s ICV stricture returned and further treatment with AMAT resolved the stricture.
| CR (n = 20) | PR + NR (n = 10) | P value | |
| Demographics | |||
| Age at AMAT [yr (IQR)] | 28 (24-32) | 26 (25-47) | 0.66 |
| Sex (M) | 11 | 4 | 0.70 |
| Duration of disease [yr (IQR)] | 4 (1-8) | 2 (1-4) | 0.45 |
| Time between diagnosis and stricture [yr (IQR)] | 3 (0-6) | 1.5 (0-3) | 0.54 |
| Stricture outcome | |||
| Single stricture, n (%) | 19 (95) | 7 (70) | 0.10 |
| Multiple strictures, n (%) | 1 (5) | 3 (30) | |
| Clinical outcome | |||
| Clinical response, n (%) | 16 (80) | 5 (50) | 0.12 |
| No clinical response, n (%) | 4 (20) | 5 (50) | |
| Pathology | |||
| CRP [mg/L (IQR)] | 4 (3-10) | 6 (4-8) | 0.71 |
| ESR [mm/h (IQR)] | 5 (2-9) | 6 (4-11) | 0.86 |
| Area of involvement | |||
| ICV, n (%) | 8 (40) | 4 (40) | |
| TI, n (%) | 12 (60) | 5 (50) | |
| ICV + TI, n (%) | 0 (0) | 1 (10) | |
| Duration of AMAT for stricture outcome | |||
| ≤ 12 mo, n (%) | 10 (50) | 3 (30) | 0.44 |
| > 12 mo, n (%) | 10 (50) | 7 (70) |
Within the cumulative follow-up period, 21 (70%) patients demonstrated clinical response to AMAT with eight becoming asymptomatic and 13 experiencing an improvement in symptoms. In contrast, nine patients exhibited no clinical response to treatment with eight having no change in symptoms and a single patient that worsened symptomatically. Additionally, measures of inflammatory serum markers demonstrated a statistically significant reduction between pre-treatment and follow-up. Median CRP decreased fourfold from 23 mg/L (13-45 mg/L) to 5 mg/L (4-9 mg/L) (P < 0.0001). Median ESR decreased threefold, falling from 15 mm/h (5-28 mm/h) at baseline to 5 mm/h (2-11 mm/h) (P = 0.04).
The 20 patients with CR of their stricture in comparison to those that had PR or NR were more likely to be older, male, with a longer duration of CD and a longer time between diagnosis and stricture found at colonoscopy. However, none of these results reached statistical significance. There was a negligible interdependence between patients with CR of their strictures conferring a greater clinical response in comparison to those with a PR or NR. Similarly, analysis of the CRP and ESR inflammatory serum markers did not reveal any discernible difference in those patients who achieved CR.
Overall, 11 (37%) patients experienced side effects, but there were no serious adverse effects (SAEs) attributable to AMAT. Concomitant medications at pre-treatment were utilized by 22 (74%) patients but this number increased to 26 (87%) at follow-up, however, these did not significantly coincide with the CR of a stricture (Table 3). The only exception to this rule was AMAT when supplemented with a concurrent IMD (P = 0.02).
| CR (n = 20) | PR + NR (n = 10) | P value | |
| Concomitant biologic medication | |||
| Y, n (%) | 3 (15) | 3 (30) | 0.37 |
| N, n (%) | 17 (85) | 7 (70) | |
| Concomitant steroid medication | |||
| Y, n (%) | 13 (65) | 3 (30) | 0.12 |
| N, n (%) | 7 (35) | 7 (70) | |
| Concomitant IMD medication | |||
| Y, n (%) | 12 (60) | 1 (10) | 0.02 |
| N, n (%) | 8 (40) | 9 (90) | |
| Concomitant 5-ASA medication | |||
| Y, n (%) | 13 (65) | 4 (40) | 0.26 |
| N, n (%) | 7 (35) | 6 (60) |
We retrospectively examined a cohort of 40 patients with CD who had colonoscopic evidence of an ileal stricture and were treated with AMAT. Overall, a positive response to AMAT was observed in just over three quarters of follow-up patients with stricturing, resulting in CR or PR without any SAEs attributable to AMAT.
At presentation, patient strictures could not be traversed by colonoscope. At follow-up, two thirds of patients achieved CR of their stricture, defined as either an adult or pediatric instrument intubating the TI and/or ICV where the previous strictures had been located. The remaining patients did not respond to the same degree and had continued evidence of stenosis. After six months or more, one tenth of patients attained PR while seven had NR of their stricture. In addition, patients on AMAT regardless of stricture outcome, conveyed a positive clinical response and a statistically significant net reduction in inflammatory serum markers, suggesting it can generally improve the symptoms and inflammation that accompanies CD. Interestingly, an association between the combination of AMAT with an IMD and the CR of a stricture was identified, albeit with small numbers. This may in part be explained by the reported anti-MAP activity of cytostatic agents, but this has not previously been reported in cases of stricturing CD[29,30]. Notably this correlation was not found with biologic therapy, which has been described to decrease both the immunogenicity and intracellular survival of MAP, and alleviate strictures[10-12,31]. In our study, patient demographics, area of involvement, number of strictures, duration of treatment and clinical response were not significantly related to stricture outcome. Lack of statistical significance in our study could be attributed to the small sample size, missing data and significant outliers. Future prospective studies should assess the effect of AMAT on stricture opening to inspect which variables are implicated in stricture outcomes.
All patients tolerated AMAT well despite the potential for side effects on a combination consisting of clarithromycin, rifabutin and clofazimine. Nine patients reported minor side effects including discoloration of the urine and skin, and arthralgia. In a single patient, there was a transient rise in their liver function tests and in another patient, a temporary inflammation of the eye not observed to be uveitis. Both cases resolved spontaneously. The minor side effects consisting of arthralgia and an orange-brown discoloration of the urine and skin, are attributed to rifabutin and clofazimine respectively[32,33]. Serious side effects comprising of hepatotoxicity and uveitis are highly unusual, and both are known to occur due to rapidly-rising dosing of clarithromycin, rifabutin or clofazimine[32-34]. Our approach is to escalate doses slowly, over 8-10 wk, circumventing most adverse effects including hepatotoxicity and uveitis. However, stricturing remains a long-term complication of CD that patients may develop years after diagnosis[7,9]. From our experience, strictures can take > 24 mo to completely open and adherence to the AMAT protocol must be stringent. Although the long-term safety profile of AMAT is being ascertained, one patient from this study was on full-dose medication for almost five years suggesting AMAT is safe within this period. Nonetheless larger cohorts need to be studied during long-term use. Upon attaining remission from CD and opening a stricture, reduced dose maintenance therapy may continue to sustain long-term remission.
EBD, strictureplasty and surgical resection are endoscopic and surgical interventions employed when medical therapy fails to adequately halt the progression of CD. If diagnosed with stricturing CD, there is a 75% likelihood patients will require at least one surgical procedure within their lifetime[9]. A systematic review on the surgical management of CD has specified that medical therapy is crucial because there is no surgical cure, however when nonresponsive to these therapeutic options surgery may be indicated[35]. As outlined by a recent meta-analysis, EBD remains a critical, non-invasive component for delaying surgery but not avoiding it, yielding a 29% surgical intervention rate for primary strictures and in total, finding 4% of patients experienced a major adverse event[36]. Strictureplasty is another conservative surgical technique that preserves bowel length and in the most comprehensive systematic review to date, has demonstrated a 30% reoperation rate and 13% complication rate for jejunoileal strictureplasty procedures, with 59% of the patients having surprisingly undergone previous bowel surgery[37]. The most invasive method to remove a CD induced refractory obstruction is surgical resection, with two separate reviews identifying a 29% risk of a second intestinal resection and a 28% early complication rate[38,39]. Evidently, surgery may result in an elevated risk of postoperative complications and short bowel syndrome, potentially leading to increased morbidity, mortality and a decreased quality of life[8,40]. Despite the risks of endoscopic and surgical practices, they are generally not intended to be preventative and with up to 67% of patients failing therapeutics for stricturing CD, new and innovative medical therapies are essential for future applications[41].
In our review, AMAT provided a high rate of stricture resolution (67%) compared with other therapies in the limited available literature. Recently, the Stricture Therapy and Research Consortium identified only ten studies involving stricture therapy, none of which included antibiotic therapy, and concede there is a need for RCTs into systemic medical therapies for CD strictures[10]. To date, the largest multicenter, prospective study (CREOLE), found adalimumab displayed efficacy in stricture success in 62/97 (64%) patients[11]. Intriguingly, there was no reference to the opening or resolution of a stricture and their respective measurement regarding stricture success was based upon an unvalidated prognostic score. In another single center, prospective study, 6/10 patients (60%) had resolution of ileal strictures after treatment with infliximab or adalimumab in the context of assessing bowel damage using the Lémann index[12]. Concerning EEN, an observational, prospective study achieved radiologic remission in 35/65 (54%) patients with the luminal cross-sectional area in the bowel increasing by 100% upon concluding treatment, but without colonoscopic data[13]. Reassuringly, EBD and surgical intervention may not need to be first-line treatment for CD strictures if simple medical therapy options such as AMAT are already opening the majority of strictures. AMAT allows physicians to apply a non-surgical treatment to correct the inflammation and fibrosis to progressively open a stricture. Our results indicate that AMAT, a targeted antimicrobial treatment, can effectively open a high percentage of CD strictures. The impact on recurrence of strictures and whether different locations and sizes affect endoscopic outcomes will require further investigative trials.
Other stenosing conditions such as bowel TB, H. pylori duodenal ulcer disease and β-haemolytic Streptococcus oesophagitis all have a proven infective agent. Our study is the first series where specific anti-atypical TB antibiotic therapy successfully opened CD strictures (67%) with results comparable with those of specific anti-typical TB therapy (70%)[15]. This points to CD being a transmural, mycobacterial-driven infection and inflammation, a concept already supported by other evidence[42-44]. Given that AMAT induces remission in CD significantly better than placebo, independently of other treatment, across multiple age ranges, and has produced prolonged disappearance of disease, our review supports the parallel between the majority of TB and CD strictures; in that both are mycobacterial infections which improve using antibiotics[24-26,45,46]. However, definitive conclusions of this relationship should be tempered until AMAT has demonstrated its benefit in CD with further supporting evidence[28]. Accordingly, the present study emphasizes the urgent need for an accurate and reliable diagnostic test for MAP in CD to solidify its causative role. Until a definitive MAP infective diagnosis can be provided the aetiology of CD will remain controversial.
There are limitations in this study, despite describing the positive, long-term, clinical and colonoscopic outcomes of CD patients with an ileal stricture undergoing AMAT. Limitations include the retrospective nature which resulted in missing data and loss to follow-up. For this reason, fecal calprotectin, radiologic medical imaging, smoking data and objective markers for both disease activity and endoscopic healing were not used. Secondly, being an observational cohort there were no case-controls to minimize confounding variables and the clinical nature of the study meant there was non-uniformity in AMAT treatments. Tolerance, dosing, side effects, clinical manifestations and effectiveness were factors implicated in this variation thereby preventing the use of identical antibiotic protocols and concomitant treatment. Thirdly, all patients were from a single center which may have permitted colonoscopic and selection bias. Consequently, it is possible that diagnostic outcomes and rate of resolution of strictures could be higher or lower in a broader population of CD patients treated with AMAT. Finally, the majority of strictures in CD are ileal, and this study exclusively focused on changes to both ICV and TI strictures[7]. As a result, patients with colonic strictures who may have had an altered outcome from AMAT were not included. Given the substantial improvement of ileal strictures with AMAT, future research should be conducted on colonic strictures using AMAT alongside IMDs to delineate their synergistic effect.
We have achieved an unexpectedly high resolution of strictures in CD by treating with AMAT, reflecting the highest rate of CR reported in the literature. In this retrospective review AMAT has displayed the capacity to provide both stricture resolution and improved clinical response in a cohort of patients with CD. Our findings should be confirmed by further prospective studies of both ileal and colonic strictures, both with and without concomitant immunotherapy to determine the most successful combination.
Crohn’s disease (CD) is a chronic, incurable inflammatory bowel disease located at any point from the mouth through to anus. Mycobacterium avium ssp. paratuberculosis is a suspected causative agent in CD and recent evidence has shown anti-mycobacterial antibiotic therapy (AMAT) to be highly effective in treating this condition. Due to the natural progression of CD, patients will often develop complications such as strictures which are inflammatory, fibrotic or mixed processes causing obstruction, for which endoscopic balloon dilatation, strictureplasty or surgical resection is currently first-line treatment.
Mycobacterium tuberculosis, Helicobacter pylori and Streptococcus can all cause stenosis and resolution can be achieved by specialized antimicrobial treatment. AMAT has proved to be an effective treatment in CD but its efficacy in opening strictures has not yet been investigated.
This study aimed to investigate the effect and outcomes of AMAT in a cohort of CD patients with an ileal stricture.
A single center, retrospective, medical record case review was conducted on an observational cohort of patients with CD who had an ileal stricture on colonoscopy and were treated with AMAT. The AMAT regimen was prescribed after the initial colonoscopy for a duration of at least six months until follow-up colonoscopy with the attending gastroenterologist. Patient demographics, symptoms, colonoscopy reports, inflammatory serum markers and concurrent medications were recorded at pre-treatment and follow-up between January 1995 and June 2018. The primary outcome was the complete resolution (CR) of CD strictures.
The majority of our cohort (67%) had CR of their ileal strictures in response to AMAT. Improvement was observed through symptomatic clinical response and a reduction in inflammatory serum markers within the cohort. There were minimal side effects attributable to AMAT that were reported in the study.
An unexpectedly high resolution of strictures in CD was observed following treatment with AMAT, reflecting the highest rate of CR reported in the literature. This rate is similar to that seen in tuberculosis strictures (70%), suggesting a shared mycobacterial origin of strictures, and perhaps disease.
The findings of this study should be confirmed by further prospective studies of both ileal and colonic strictures, both with and without concomitant immunotherapy to determine the most successful combination in opening a stricture.
The authors would like to acknowledge Professor Heller G for her statistical review and advice.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Australia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Skok P S-Editor: Chen XF L-Editor: A P-Editor: Liu JH
| 1. | Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, Panaccione R, Ghosh S, Wu JCY, Chan FKL, Sung JJY, Kaplan GG. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2018;390:2769-2778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2677] [Cited by in RCA: 4068] [Article Influence: 508.5] [Reference Citation Analysis (110)] |
| 2. | Feuerstein JD, Cheifetz AS. Crohn Disease: Epidemiology, Diagnosis, and Management. Mayo Clin Proc. 2017;92:1088-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 310] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 3. | Bessissow T, Reinglas J, Aruljothy A, Lakatos PL, Van Assche G. Endoscopic management of Crohn's strictures. World J Gastroenterol. 2018;24:1859-1867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 4. | Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, Caprilli R, Colombel JF, Gasche C, Geboes K, Jewell DP, Karban A, Loftus EV Jr, Peña AS, Riddell RH, Sachar DB, Schreiber S, Steinhart AH, Targan SR, Vermeire S, Warren BF. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19 Suppl A:5A-36A. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2148] [Cited by in RCA: 2363] [Article Influence: 214.8] [Reference Citation Analysis (0)] |
| 5. | Zeitz J, Fournier N, Labenz C, Biedermann L, Frei P, Misselwitz B, Scharl S, Vavricka SR, Sulz MC, Fried M, Rogler G, Scharl M. Risk Factors for the Development of Fistulae and Stenoses in Crohn Disease Patients in the Swiss Inflammatory Bowel Disease Cohort. Inflamm Intest Dis. 2017;1:172-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Lakatos PL, David G, Pandur T, Erdelyi Z, Mester G, Balogh M, Szipocs I, Molnar C, Komaromi E, Kiss LS, Lakatos L. Risk of colorectal cancer and small bowel adenocarcinoma in Crohn's disease: a population-based study from western Hungary 1977-2008. J Crohns Colitis. 2011;5:122-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Louis E, Collard A, Oger AF, Degroote E, Aboul Nasr El Yafi FA, Belaiche J. Behaviour of Crohn's disease according to the Vienna classification: changing pattern over the course of the disease. Gut. 2001;49:777-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 684] [Cited by in RCA: 704] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 8. | Rieder F, Zimmermann EM, Remzi FH, Sandborn WJ. Crohn's disease complicated by strictures: a systematic review. Gut. 2013;62:1072-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 385] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 9. | Cosnes J, Cattan S, Blain A, Beaugerie L, Carbonnel F, Parc R, Gendre JP. Long-term evolution of disease behavior of Crohn's disease. Inflamm Bowel Dis. 2002;8:244-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 981] [Cited by in RCA: 975] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 10. | Lu C, Baraty B, Lee Robertson H, Filyk A, Shen H, Fung T, Novak K, Ma C, Panaccione R, Achkar JP, El Ouali S, Bruining D, Jairath V, Feagan B, Rieder F; Stenosis Therapy and Research (STAR) Consortium. Systematic review: medical therapy for fibrostenosing Crohn's disease. Aliment Pharmacol Ther. 2020;51:1233-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 11. | Bouhnik Y, Carbonnel F, Laharie D, Stefanescu C, Hébuterne X, Abitbol V, Nachury M, Brixi H, Bourreille A, Picon L, Bourrier A, Allez M, Peyrin-Biroulet L, Moreau J, Savoye G, Fumery M, Nancey S, Roblin X, Altwegg R, Bouguen G, Bommelaer G, Danese S, Louis E, Zappa M, Mary JY; GETAID CREOLE Study Group. Efficacy of adalimumab in patients with Crohn's disease and symptomatic small bowel stricture: a multicentre, prospective, observational cohort (CREOLE) study. Gut. 2018;67:53-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 227] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 12. | Fiorino G, Bonifacio C, Allocca M, Repici A, Balzarini L, Malesci A, Peyrin-Biroulet L, Danese S. Bowel Damage as Assessed by the Lémann Index is Reversible on Anti-TNF Therapy for Crohn's Disease. J Crohns Colitis. 2015;9:633-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 13. | Hu D, Ren J, Wang G, Li G, Liu S, Yan D, Gu G, Zhou B, Wu X, Chen J, Ding C, Wu Y, Wu Q, Liu N, Li J. Exclusive enteral nutritional therapy can relieve inflammatory bowel stricture in Crohn's disease. J Clin Gastroenterol. 2014;48:790-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 14. | Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE. ACG Clinical Guideline: Management of Crohn's Disease in Adults. Am J Gastroenterol. 2018;113:481-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 924] [Article Influence: 132.0] [Reference Citation Analysis (0)] |
| 15. | Anand BS, Nanda R, Sachdev GK. Response of tuberculous stricture to antituberculous treatment. Gut. 1988;29:62-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Borody TJ, Bampton P, Moont M, Pearce L, Saxon J, Shortis N. Re: W. de Boer: Gastric outlet obstruction and Helicobacter pylori. Am J Gastroenterol. 1997;92:1576-1577. [PubMed] |
| 17. | Brandimarte G, Tursi A, di Cesare L, Gasbarrini G. Antimicrobial treatment for peptic stenosis: a prospective study. Eur J Gastroenterol Hepatol. 1999;11:731-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Shin JA, Lee YB, Yoon IC, Jeong HJ, Kwon T, Lee HS. Bacterial Ulcerative Esophagitis in an Immunocompetent Patient. Case Rep Gastroenterol. 2017;11:162-167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Naser SA, Sagramsingh SR, Naser AS, Thanigachalam S. Mycobacterium avium subspecies paratuberculosis causes Crohn's disease in some inflammatory bowel disease patients. World J Gastroenterol. 2014;20:7403-7415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 94] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 20. | Feller M, Huwiler K, Stephan R, Altpeter E, Shang A, Furrer H, Pfyffer GE, Jemmi T, Baumgartner A, Egger M. Mycobacterium avium subspecies paratuberculosis and Crohn's disease: a systematic review and meta-analysis. Lancet Infect Dis. 2007;7:607-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 375] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 21. | Baumgart DC, Sandborn WJ. Crohn's disease. Lancet. 2012;380:1590-1605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1347] [Cited by in RCA: 1522] [Article Influence: 117.1] [Reference Citation Analysis (0)] |
| 22. | Bach H. What Role Does Mycobacterium avium subsp. paratuberculosis Play in Crohn's Disease? Curr Infect Dis Rep. 2015;17:463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Hansen R, Thomson JM, El-Omar EM, Hold GL. The role of infection in the aetiology of inflammatory bowel disease. J Gastroenterol. 2010;45:266-276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 24. | Graham DY, Hardi R, Welton T, Krause R, Levenson S, Sarles H, Sheikh A, Epstein M, Duvall G, Freedland C, Hebzda Z, Arlukowicz T, Kopon A, Rydzewska G, Zdravkovic N, Svorcan P, Zittan E, Dugalic P, Israeli E, Anderson P, Fehrmann C, Bibliowicz A, McLean P, Fathi R, Kalfus I. Late breaking abstracts: Phase III randomized, double blind, placebo-controlled, multicenter, parallel group study to assess the efficacy and safety of add-on fixed-dose anti-mycobacterial therapy (RHB-104) in moderately to severely active Crohn’s disease (map US). United Eur Gastroenterol J. 2018;6:1586-1597. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Agrawal G, Hamblin H, Clancy A, Borody T. Anti-Mycobacterial Antibiotic Therapy Induces Remission in Active Paediatric Crohn's Disease. Microorganisms. 2020;8:1112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Agrawal G, Clancy A, Huynh R, Borody T. Profound remission in Crohn's disease requiring no further treatment for 3-23 years: a case series. Gut Pathog. 2020;12:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | George LL, Borody TJ, Andrews P, Devine M, Moore-Jones D, Walton M, Brandl S. Cure of duodenal ulcer after eradication of Helicobacter pylori. Med J Aust. 1990;153:145-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 142] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 28. | Patton PH, Parker CE, MacDonald JK, Chande N. Anti-tuberculous therapy for maintenance of remission in Crohn's disease. Cochrane Database Syst Rev. 2016;7:CD000299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Greenstein RJ, Su L, Haroutunian V, Shahidi A, Brown ST. On the action of methotrexate and 6-mercaptopurine on M. avium subspecies paratuberculosis. PLoS One. 2007;2:e161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 30. | Krishnan MY, Manning EJ, Collins MT. Effects of interactions of antibacterial drugs with each other and with 6-mercaptopurine on in vitro growth of Mycobacterium avium subspecies paratuberculosis. J Antimicrob Chemother. 2009;64:1018-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Bach H, Rosenfeld G, Bressler B. Treatment of Crohn's disease patients with infliximab is detrimental for the survival of Mycobacterium avium ssp. paratuberculosis within macrophages and shows a remarkable decrease in the immunogenicity of mycobacterial proteins. J Crohns Colitis. 2012;6:628-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Crabol Y, Catherinot E, Veziris N, Jullien V, Lortholary O. Rifabutin: where do we stand in 2016? J Antimicrob Chemother. 2016;71:1759-1771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 33. | Cholo MC, Steel HC, Fourie PB, Germishuizen WA, Anderson R. Clofazimine: current status and future prospects. J Antimicrob Chemother. 2012;67:290-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 239] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 34. | Van der Paardt AL, Akkerman OW, Gualano G, Palmieri F, Davies Forsman L, Aleksa A, Tiberi S, de Lange WC, Bolhuis MS, Skrahina A, van Soolingen D, Kosterink JG, Migliori GB, van der Werf TS, Alffenaar JC. Safety and tolerability of clarithromycin in the treatment of multidrug-resistant tuberculosis. Eur Respir J. 2017;49:1601612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Schlussel AT, Steele SR, Alavi K. Current challenges in the surgical management of Crohn's disease: a systematic review. Am J Surg. 2016;212:345-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 36. | Navaneethan U, Lourdusamy V, Njei B, Shen B. Endoscopic balloon dilation in the management of strictures in Crohn's disease: a systematic review and meta-analysis of non-randomized trials. Surg Endosc. 2016;30:5434-5443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 37. | Yamamoto T, Fazio VW, Tekkis PP. Safety and efficacy of strictureplasty for Crohn's disease: a systematic review and meta-analysis. Dis Colon Rectum. 2007;50:1968-1986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 194] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 38. | Frolkis AD, Lipton DS, Fiest KM, Negrón ME, Dykeman J, deBruyn J, Jette N, Frolkis T, Rezaie A, Seow CH, Panaccione R, Ghosh S, Kaplan GG. Cumulative incidence of second intestinal resection in Crohn's disease: a systematic review and meta-analysis of population-based studies. Am J Gastroenterol. 2014;109:1739-1748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 177] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 39. | Gutiérrez A, Rivero M, Martín-Arranz MD, García Sánchez V, Castro M, Barrio J, de Francisco R, Barreiro-de Acosta M, Juliá B, Cea-Calvo L, Romero C, Borruel Sainz N, Domènech E. Perioperative management and early complications after intestinal resection with ileocolonic anastomosis in Crohn's disease: analysis from the PRACTICROHN study. Gastroenterol Rep (Oxf). 2019;7:168-175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 40. | Chan WPW, Mourad F, Leong RW. Crohn's disease associated strictures. J Gastroenterol Hepatol. 2018;33:998-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 41. | Campos C, Perrey A, Lambert C, Pereira B, Goutte M, Dubois A, Goutorbe F, Dapoigny M, Bommelaer G, Hordonneau C, Buisson A. Medical Therapies for Stricturing Crohn's Disease: Efficacy and Cross-Sectional Imaging Predictors of Therapeutic Failure. Dig Dis Sci. 2017;62:1628-1636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 42. | Van Kruiningen HJ, Chiodini RJ, Thayer WR, Coutu JA, Merkal RS, Runnels PL. Experimental disease in infant goats induced by a Mycobacterium isolated from a patient with Crohn's disease. A preliminary report. Dig Dis Sci. 1986;31:1351-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 70] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 43. | Chamberlin W, Borody T, Naser S. MAP-associated Crohn's disease MAP, Koch's postulates, causality and Crohn's disease. Dig Liver Dis. 2007;39:792-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 44. | Greenstein RJ. Is Crohn's disease caused by a mycobacterium? Lancet Infect Dis. 2003;3:507-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 218] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 45. | Behr MA, Hanley J. Antimycobacterial therapy for Crohn's disease: a reanalysis. Lancet Infect Dis. 2008;8:344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 46. | Agrawal G, Clancy A, Sharma R, Huynh R, Ramrakha S, Borody T. Targeted Combination Antibiotic Therapy Induces Remission in Treatment-Naïve Crohn's Disease: A Case Series. Microorganisms. 2020;8:371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |