Published online Jan 16, 2020. doi: 10.4253/wjge.v12.i1.23
Peer-review started: June 29, 2019
First decision: August 2, 2019
Revised: August 13, 2019
Accepted: November 18, 2019
Article in press: November 18, 2019
Published online: January 16, 2020
Processing time: 172 Days and 18 Hours
Accurate detection of gastric infection by Helicobacter pylori (H. pylori) and premalignant lesions are important for effective provision of treatment, preventing the development of gastric neoplasia. Optical enhancement systems with optical magnification improved the identification of mucosal superficial and vascular patterns in patients with dyspepsia.
To evaluate an optical enhancement system with high-definition magnification, for diagnosis of normal gastric mucosa, H. pylori-associated gastritis, and gastric atrophy.
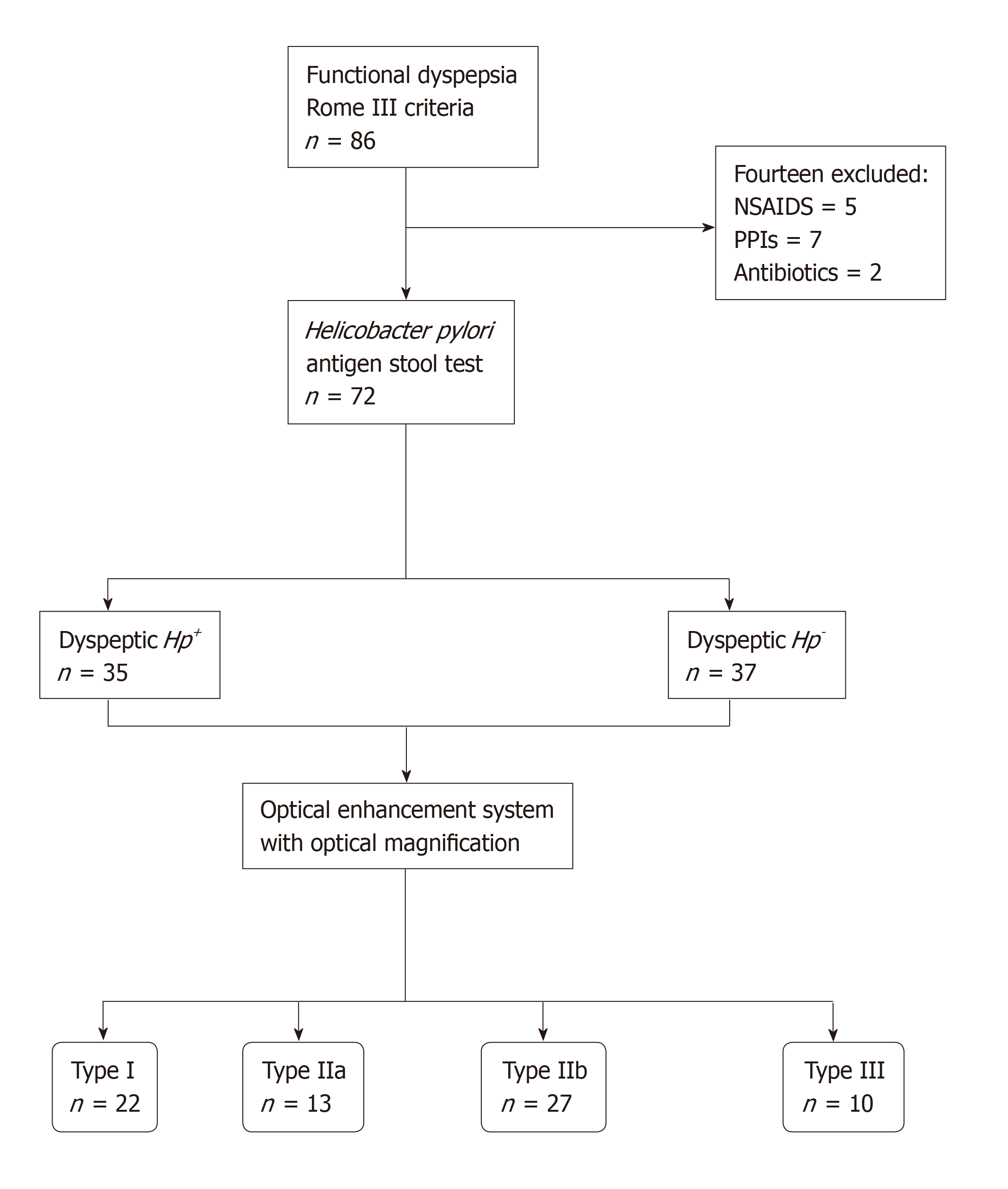
A cross-sectional, nonrandomized study from November 2015 to April 2016 performed in a single-tertiary academic center from Ecuador. Seventy-two consecutive patients with functional dyspepsia according to the Rome III criteria, were tested for H. pylori using a stool antigen test and were assigned to an Hp+ group or an Hp− control group. Esophagogastroduodenoscopy with high-definition optical magnification and digital chromoendoscopy was performed, and patients were classified into 4 groups, in accordance to the microvascular-architecture pattern of the mucosa. Interobserver and intraobserver agreement among operators were calculated.
Of the 72 participants, 35 were Hp+ and 37 were Hp−. Among 10 patients with normal mucosal histology in biopsy samples, 90% had a Type I pattern of microvascular architecture by endoscopy. Among participants with type IIa and type IIb patterns, significantly more were Hp+ than Hp− (32 vs 8), and most (31 out of 40) had histological diagnoses of chronic active gastritis. Two of the three participants with a histological diagnosis of atrophy had a type III microvascular pattern. The type I pattern predicted normal mucosa, type IIa–IIb predicted H. pylori infection, and type III predicted atrophy with sensitivities of 90.0%, 91.4%, and 66.7%, respectively. The intraobserver and interobserver agreements had kappa values of 0.91 and 0.89, respectively.
High-definition optical magnification with digital chromoendoscopy is useful for diagnosis of normal gastric mucosa and H. pylori-associated gastritis with high accuracy, but further studies are needed to determine whether endoscopic diagnosis of gastric atrophy is feasible.
Core tip: The accurate and reliable detection of Helicobacter pylori-associated gastritis and gastric atrophy is imperative for appropriate therapy, preventing the development of gastric neoplasia. Digital chromoendoscopy with optical magnification improved the identification of mucosal superficial and vascular patterns in the gastric mucosa of dyspeptic patients. We described a high sensitivity and accuracy for predicting normal gastric mucosa and Helicobacter pylori-associated gastritis, with a high interobserver agreement. The accurate detection of gastric infection by Helicobacter pylori and the presence of premalignant lesion at an early stage are important for the effective provision of treatment.
- Citation: Robles-Medranda C, Valero M, Puga-Tejada M, Oleas R, Baquerizo-Burgos J, Soria-Alcívar M, Alvarado-Escobar H, Pitanga-Lukashok H. High-definition optical magnification with digital chromoendoscopy detects gastric mucosal changes in dyspeptic-patients. World J Gastrointest Endosc 2020; 12(1): 23-32
- URL: https://www.wjgnet.com/1948-5190/full/v12/i1/23.htm
- DOI: https://dx.doi.org/10.4253/wjge.v12.i1.23
Gastric cancer is the second most common cause of cancer-related death worldwide, and upper endoscopy is essential to enable its identification at an early stage[1]. Helicobacter pylori (H. pylori) is involved in the pathogenesis of gastric diseases, such as peptic ulcers, gastric lymphoma, and gastric cancer[2-6].Thus, it is important to detect H. pylori infection and premalignant gastric lesions at an early stage so that the best therapeutic approaches can be offered[7]. Diagnosis of H. pylori-associated gastritis and gastric atrophy on the basis of endoscopic findings can be difficult, so histology is considered to be the diagnostic gold standard for these conditions[8,9]. H. pylori infection and the resultant gastric atrophy are known premalignant lesions linked to gastric carcinogenesis[10]. However, the reliability of detection of H. pylori infection or gastric atrophy by histology depends on the location, number, and size of the endoscopy-guided gastric-biopsy specimens. Alternatively, obtaining samples for histological assessment by endoscopic gastric biopsy can result in significant sampling errors.
Pentax Medical (HOYA, Tokyo, Japan) developed the Optical Enhancement™ (OE) System, which combines bandwidth-limited light with an endoscopy video system. This technology combines digital signal processing (similar to I-SCAN™) with optical filters that limit the spectral characteristics of the illuminating light, enhancing visualization of the mucosal surface and microvessels[11]. In addition, MagniView™ endoscopes have been developed, and can combine high-definition imaging with optical magnification.
The present study was designed to enable evaluation of the OE System with optical magnification for diagnosis of normal gastric mucosa, H. pylori-associated gastritis, and gastric atrophy. Additionally, interobserver and intraobserver reproducibility in the assessment of endoscopic patterns detected was assessed.
The investigation involved a cross-sectional, nonrandomized, double-blind study that was performed at the Instituto Ecuatoriano de Enfermedades Digestivas, Academic Tertiary Center, Ecuador, between November 2015 and April 2016. The study protocol and consent form were approved by the Institutional Review Board, registered at ClinicalTrials.gov (ID: NCT02597517), and the study was conducted according to the guidelines of the Declaration of Helsinki. All patients provided written informed consent. All authors had access to the study data and had reviewed and approved the final manuscript.
The required sample size was estimated with a 95% confidence interval (CI) and a 7.5% margin of error, on the basis of the results of Tongtawee et al[12]. All consecutive participants had functional dyspepsia according to the Rome III criteria, and were ≥ 18 years old. Participants had an epigastric pain syndrome (defined as localized pain or burning pain in the upper abdomen at least once a week, which was intermittent, nongeneralized, not relieved by defecation, and did not meet the criteria for pathology of the gallbladder or sphincter of Oddi) and/or a postprandial distress syndrome (defined as the presence of a nagging feeling of postprandial fullness after normal-volume meals, and/or early satiety that prevented the completion of a regular meal several times a week). The criteria had to be present within the three months prior to enrolment, and to have started ≥ 6 mo prior to diagnosis of dyspepsia[13]. Patients with severe uncontrolled coagulopathy, prior history of gastric surgery, or ongoing pregnancy, as well as patients who had received nonsteroidal anti-inflammatory drugs (NSAIDs), proton-pump inhibitors (PPIs) or antibiotics in the preceding three weeks were excluded.
Participants were tested for H. pylori infection with the H. pylori stool antigen test (Wondfo®, Wondfo Biotech Co., Guangzhou, China), prior to allocation into two groups: Hp+ and Hp− (control group) (Figure 1). Finally, upper endoscopy was performed with the OE System and optical magnification. Endoscopists and participants were blinded to the group allocation.
Complete endoscopic procedures were performed, with evaluation of the entire stomach with conventional white light, to exclude obvious lesions. Participants were also evaluated by upper endoscopy with the OE System (including OPTIVISTA EPK-i7010 HD Video Processor; Pentax Medical, Hoya Corp., Japan) and MagniView™ EG-2990Zi Video Gastroscope (Pentax Medical, Hoya Corp., Japan) under intravenous sedation in a standardized manner. This technique involved the use of a distal black rubber hood (OE-A58; Pentax) at the tip of the endoscope, to fix the distance between the tip of the endoscope and the gastric mucosa at 2 mm. The OE System was initially used in mode 1 and mode 2 without optical magnification, to obtain an overview of the gastric body and identify any gross changes in the mucosa, then optical magnification was implemented. At maximum magnification, the hood was brought into contact with the gastric mucosa, and water was instilled. Any residue in the stomach was removed with a water-ejection pump prior to the procedure. Each endoscopy was performed by one of three endoscopists (Robles-Medranda C, Valero M, and Soria-Alcívar M), who were assigned by randomized allocation, blinded to the group selection, and trained in the use of the OE system with optical magnification.
OE System: The OE System combines digital signal processing with optical filters that limit the spectral characteristics of the illuminating light, connecting the peaks of the hemoglobin absorption spectrum (415 nm, 540 nm, and 570 nm) to create a continuous wavelength spectrum. The OE System has two modes that use different filters to optimize visualization of specific features. Here, only mode 1 was used for the high-magnification studies, because of its ability to enhance microvessel visualization.
MagniView endoscope: The MagniView™ combines a high-definition endoscope with optical magnification, to produce detailed images with magnification of up to 136×. This imaging facilitates the evaluation of the superficial vascular aspects of the mucosa, enabling identification of early signs of inflammation or lesions not previously seen with conventional endoscopy.
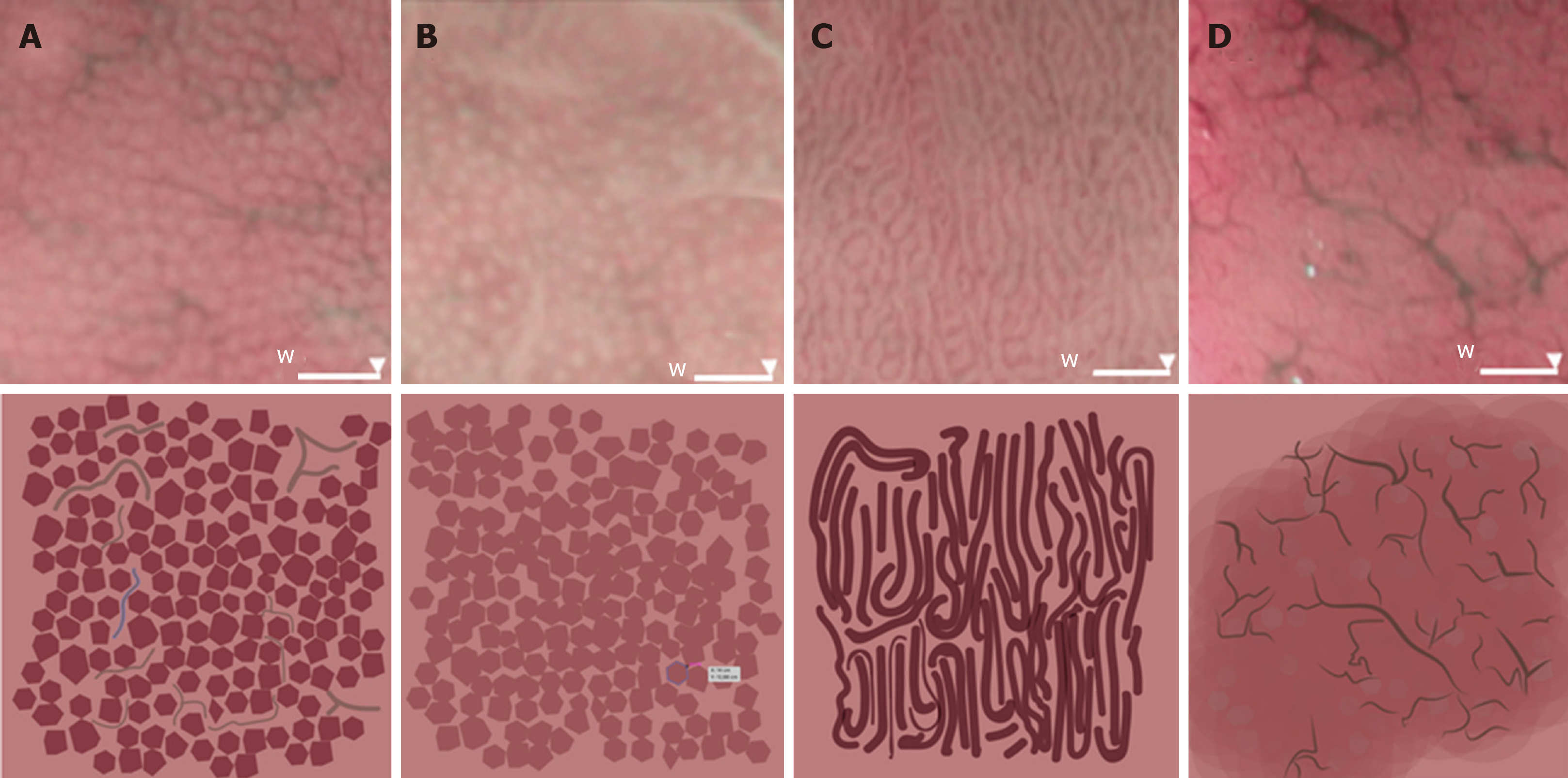
The gastric body was chosen instead of the antrum for evaluation of the mucosa. The microvascular architecture of the normal stomach shows two distinct patterns depending on the region of the stomach. The gastric body has a honeycomb-like subepithelial capillary network (SECN) pattern with collecting venules, whereas the gastric antrum has a coil-shaped SECN pattern, where collecting venules lie in deeper layers and cannot be seen[14,15].
Endoscopic evaluation enabled classification of participants according to four patterns of microvascular architecture, on the basis of the combination of the SECN, collecting venules, and round pits[16]. The type I pattern consisted of a honeycomb-type SECN with a regular arrangement of collecting venules (RAC) and regular round pits; type IIa involved a honeycomb-type SECN with regular round pits, but with loss of collecting venules; the type IIb pattern consisted of enlarged white pits surrounded by erythema with loss of normal SECN and collecting venules; and the type III pattern involved loss of normal SECN and round pits, with irregular arrangements of collecting venules (Figure 2).
Endoscopic images were recorded, and biopsy samples were obtained to correlate the images with histological assessments. The biopsies were taken with regular biopsy forceps at random locations following the Sydney protocol (two from the gastric body, two from the antrum and one from the incisura angularis) in cases of type I pattern, with additional targeted biopsies from areas with type IIa, IIb, or III patterns[17]. The specimens were fixed immediately in 10% formalin solution, stained with hematoxylin–eosin for histopathological assessment, and stained with the Giemsa stain for H. pylori detection. Detection of H. pylori infection was performed by histology and by the H. pylori stool antigen test. A positive result with either of these tests was considered to indicate H. pylori infection. The pathologist was blinded to the endoscopic diagnosis.
A dataset containing 60 photographs taking during the study of the gastric body was presented in a blinded manner to four endoscopists who were individually asked to classify the photographs according to the four microvascular patterns at three time points, each 1 wk apart. At each evaluation, the same photographs were shown to the endoscopists in a different order. The four endoscopists were trained to evaluate the four patterns. Interobserver agreement was assessed by comparison of the photographic analyses by each endoscopist (Alvarado-Escobar H, Puga-Tejada M, Oleas R, and Baquerizo-Burgos J), and intraobserver agreement was assessed by comparison of the photographic analysis by each endoscopist at each time point.
The statistical review of the study was performed by a biomedical statistician. The baseline characteristics of Hp+ and Hp− patients were compared by Pearson’s chi-square or Fisher’s exact tests for categorical variables and the Mann-Whitney U-test for continuous variables. Continuous variables are expressed as the mean (standard deviation) or median (interquartile range), according to their statistical distribution. Categorical variables are presented as percentages. The sensitivity, specificity, predictive values, and accuracy of the endoscopic findings for normal gastric mucosa, H. pylori infection, and gastric atrophy were calculated with 95% CIs. For interobserver and intraobserver agreement, kappa values were calculated[18]. Kappa coefficients < 0.4 indicated “poor agreement”, values of 0.4-0.8 represented “moderate-to-good agreement”, and values > 0.8 indicated “excellent agreement”. A P value < 0.05 was considered statistically significant. Data analysis was performed using R v3.4.3 (R Foundation for Statistical Computing, Vienna, Austria).
The sample-size calculation indicated that the study required at least 68 patients. Of the 72 participants who were enrolled, 35 (48.6%) were dyspeptic Hp+ and 37 (51.4%) were dyspeptic Hp−. Histopathology and the H. pylori stool antigen test showed 100% agreement for diagnosis of H. pylori infection. There were no significant differences between Hp+ and Hp− groups in terms of age (mean 46.3 ± 13.7 years), sex (69.4% female), or primary symptom (58.3% epigastric pain) (Table 1).
| Total (n = 72) | Hp+ (n = 35) | Hp− (n = 37) | P value | |
| Sex (female), n (%) | 50 (69.4) | 27 (77.1) | 23 (62.1) | 0.1681 |
| Age (yr), mean ± SD | 46.3 ± 13.7 | 43.3 ± 13.5 | 49.1 ± 13.5 | 0.0752 |
| Symptoms, n (%) | 0.5121 | |||
| Epigastric pain syndrome | 42 (58.3) | 21 (60.0) | 21 (56.8) | |
| Postprandial distress syndrome | 14 (19.4) | 5 (14.3) | 9 (24.3) | |
| Both | 16 (22.2) | 9 (25.7) | 7 (18.9) | |
| Endoscopic classification, n (%) | < 0.0011 | |||
| Type I | 22 (30.6) | 3 (8.6) | 19 (51.4) | |
| Type IIa and IIb | 40 (55.6) | 32 (91.4) | 8 (21.6) | |
| Type III | 10 (13.9) | 0 | 10 (27.0) |
Endoscopic images were analyzed and patients were classified following agreement among endoscopists (Robles-Medranda C, Valero M, and Soria-Alcívar M), into type I (30.6%), type II 55.6%, and type III (13.9%). We compared distribution of patients according to the microvascular types with distribution according to histopathology and H. pylori-infection results. Among the 10 individuals with normal mucosal histology, 90% were type I (Table 2). The type I pattern was also present in three individuals with chronic active gastritis and 10 with chronic inactive gastritis. Most individuals with type IIa and type IIb patterns (10 out of 13 and 21 out of 27, respectively) had chronic active gastritis, and similar numbers were Hp+. Notably, 32 of the 35 Hp+ individuals (91.5%) were Type IIa–IIb. Only three individuals had gastric atrophy identified by histology; one with type IIb microvascular pattern and two (66.7%) with type III pattern. Eight of the 10 individuals with a type III pattern had chronic inactive gastritis, and two exhibited gastric atrophy, but none of them were Hp+.
Predictive performance of the microvascular patterns was calculated (Table 3). The type I pattern was predictive of normal mucosa, with sensitivity of 90.0% and accuracy of 80.5%. The presence of a type IIa or type IIb pattern was predictive of H. pylori infection, with sensitivity of 91.4% and accuracy of 84.7%. The type III pattern predicted gastric atrophy with a sensitivity 66.7% and accuracy of 87.5%. For assessment of the three endoscopic patterns, interobserver agreement had a kappa value of 0.89 (95%CI: 0.84-0.93) and intraobserver agreement had a kappa value of 0.91 (95%CI: 0.85-0.96).
| Sensitivity, % (95%CI) | Specificity, % (95%CI) | PPV, % (95%CI) | NPV, % (95%CI) | Accuracy, % | |
| Type I1 | 90.0 (55.5-99.8) | 79.0 (66.8-88.3) | 40.9 (20.7-63.7) | 90.0 (89.4-99.9) | 80.5 |
| Type IIa–IIb2 | 91.4 (76.9-98.2) | 78.4 (61.8-90.2) | 80.0 (64.4-90.9) | 90.6 (74.9-98.0) | 84.7 |
| Type III3 | 66.7 (9.4-99.2) | 88.4 (78.4-94.9) | 20.0 (2.5-55.6) | 98.4 (91.3-99.9) | 87.5 |
In the present study of individuals with dyspepsia, 90% of those with histologically normal gastric mucosa had the type I microvascular pattern on endoscopy and were Hp−, 91.5% of Hp+ participants had the type IIa or type IIb pattern, and of the three individuals with gastric atrophy on histology, two had the type III pattern on endoscopy. Identification of these microvascular patterns with the OE System with optical magnification enabled prediction of normal gastric mucosa or H. pylori-associated gastritis with high sensitivity and accuracy, with excellent interobserver and intraobserver agreement.
In recent decades, the role of upper endoscopy in the real-time identification of H. pylori infection of the stomach and gastric atrophy has been evaluated. Initially, studies that were designed to determine whether there was a relationship between endoscopic features and H. pylori-induced gastritis used white-light endoscopy[8,19-21]. The results of these studies showed that some endoscopic features related to H. pylori infection were difficult to distinguish from non-H. pylori gastritis and were not specific[8,21]. Additionally, prediction of gastric atrophy through endoscopic signs such as the absence of gastric folds and the presence of visible vessels had low sensitivity (67%) and specificity (48%)[22]. These results suggested that H. pylori infection and gastric atrophy can be suspected, but not confirmed, by endoscopy alone, and that histology is needed for a definitive diagnosis[8,9].
The reliability of detection of H. pylori infection or gastric atrophy by histology depends on the location, number, and size of the gastric biopsies, but considerable sampling errors can occur during endoscopic biopsy sampling. Advances in magnification technology have made it possible to determine endoscopically whether patients have H. pylori infection. The blood-vessel network in the surface layer of the gastric body has been shown endoscopically to be associated with previous histopathology findings[23]. Normal gastric-body mucosal microvascular architecture consists of a honeycomb-type SECN and collecting venules in a regular arrangement[24,25]. The presence and regular distribution of numerous red spots (collecting venules) in the gastric body have been shown to indicate a stomach with no H. pylori infection[14,26-29]. The RAC as an endoscopic feature has been shown to have sensitivity, specificity, and accuracy for identification of an Hp− stomach of 93.8%, 96.2%, and 95.5%, respectively, with RAC-negative findings having an accuracy of 95% for identification of H. pylori infection[30]. In the current study, RAC positivity (a type I microvascular pattern) had a sensitivity, specificity, and accuracy to predict Hp− of 90%, 79%, and 80.5%, respectively, and RAC negativity (a type IIa or type IIb pattern) had a sensitivity, specificity, and accuracy to predict Hp+ of 91.4%, 78.3%, and 84.7%, respectively.
Nakagawa et al[31] evaluated the association of patterns of collecting venules (regular, irregular, or obscured) with H. pylori infection and histopathological gastritis. The presence of a regular pattern of collecting venules in the gastric mucosa was shown to indicate an absence of H. pylori infection, which suggests that a biopsy is unnecessary. Conversely, observation of an obscure or irregular pattern was shown to indicate H. pylori infection, with the irregular pattern suggesting the presence of severe gastric mucosal atrophy[31].
The endoscopic classification that we used was based on a previous evaluation of the gastric body by magnified white-light endoscopy[16]. Here, the use of the OE System with optical magnification improved the identification of mucosal superficial and vascular patterns and made it easier to classify images, thereby improving interobserver agreement (0.89 vs 0.73). In the previous study, type I and type II/III sensitivities for prediction of normal mucosa and H. pylori-related gastritis (92.7% and 100%) were similar to our findings (90% and 91.4% for type IIa and IIb, respectively). However, the type IV pattern sensitivity for prediction of gastric atrophy was 90% with white-light endoscopy compared with 66% (type III) with the OE System, although this difference could be explained by the greater number of individuals with the type IV pattern in the previous study compared with the current study (21 vs 10)[16].
The present investigation had some limitations. First, the use of the magnified OE System with optical magnification for these indications has not been studied systematically, and there is a learning curve in obtaining and interpreting the images. This was a small, single-center study, and further investigations with multiple endoscopists are needed. Neither the severity of gastritis nor the degree of endoscopic gastric mucosal atrophy was evaluated. The sample size of patients with gastric atrophy was small. Finally, intake of NSAIDs and PPIs in the three weeks preceding the study was considered an exclusion criterion in our study, to avoid bias and an incorrect interpretation.
Our results demonstrate the potential of the OE System with optical magnification to enable the identification of histopathological changes in the gastric mucosa. In this study, the reproducibility of the classification was excellent. High-definition optical magnification and digital chromoendoscopy exhibited high accuracy for the diagnosis of normal gastric mucosa and H. pylori-associated gastritis, with lower accuracy for the evaluation of gastric atrophy. Clinical practice experience needs to be further tested in order to determine the applicability of these techniques in defining chronic active gastritis and H. pylori infection. In order to validate our results, the optical enhancement with magnification technologies should be evaluated in randomized, controlled trials. In addition, further studies are needed to determine whether endoscopic diagnosis of gastric atrophy is feasible.
Helicobacter pylori (H. pylori) infection and atrophic gastritis are linked to gastric carcinogenesis. The endoscopic diagnosis of H. pylori infection and gastric atrophy is challenging, requiring histological confirmation; however, sampling errors might occur.
Digital chromoendoscopy with optical magnification improved the identification of mucosal superficial and vascular patterns in the gastric mucosa and might provide a more accurate endoscopic visual impression of the gastric mucosa.
The main objective of the study was to evaluate digital chromoendoscopy with optical magnification for the diagnosis of normal gastric mucosa, H. pylori associated gastritis and atrophic gastritis.
This was a cross-sectional, nonrandomized, single-center study in which consecutive patients with functional dyspepsia were evaluated via esophagogastroduodenoscopy using a high definition endoscope with digital chromoendoscopy and optical magnification for the endoscopic diagnosis of H. pylori associated gastritis and atrophic gastritis. The endoscopic visual impression was compared to H. pylori stool antigen test and histological analysis.
We described a high sensitivity and accuracy for predicting normal gastric mucosa and H. pylori associated gastritis, with a high inter and intraobserver agreement. Atrophic gastritis was detected with a low sensitivity, and further studies are required to determine if endoscopic diagnosis of atrophic gastritis is feasible.
In our study, esophagogastroduodenoscopy with digital chromoendoscopy and optical magnification enable the identification of histological changes in the gastric mucosa of consecutive patients with functional dyspepsia.
We encourage a randomized multicenter trial evaluating high definition white light endoscopy versus digital chromoendoscopy with optical magnification for the evaluation of the gastric mucosa of dyspeptic patients, in order to determine the clinical practice applicability of these techniques. Further studies are needed to determine if endoscopic diagnosis of atrophic gastritis is feasible.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Ecuador
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Altonbary A S-Editor: Yan JP L-Editor: A E-Editor: Ma YJ
| 1. | Huang JQ, Sridhar S, Chen Y, Hunt RH. Meta-analysis of the relationship between Helicobacter pylori seropositivity and gastric cancer. Gastroenterology. 1998;114:1169-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 625] [Cited by in RCA: 618] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 2. | NIH Consensus Conference. Helicobacter pylori in peptic ulcer disease. NIH Consensus Development Panel on Helicobacter pylori in Peptic Ulcer Disease. JAMA. 1994;272:65-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 483] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 3. | Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2805] [Cited by in RCA: 2739] [Article Influence: 80.6] [Reference Citation Analysis (0)] |
| 4. | Forman D, Newell DG, Fullerton F, Yarnell JW, Stacey AR, Wald N, Sitas F. Association between infection with Helicobacter pylori and risk of gastric cancer: evidence from a prospective investigation. BMJ. 1991;302:1302-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 941] [Cited by in RCA: 925] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 5. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3126] [Cited by in RCA: 3187] [Article Influence: 132.8] [Reference Citation Analysis (0)] |
| 6. | Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735-6740. [PubMed] |
| 7. | Whiting JL, Sigurdsson A, Rowlands DC, Hallissey MT, Fielding JW. The long term results of endoscopic surveillance of premalignant gastric lesions. Gut. 2002;50:378-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 193] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 8. | Bah A, Saraga E, Armstrong D, Vouillamoz D, Dorta G, Duroux P, Weber B, Froehlich F, Blum AL, Schnegg JF. Endoscopic features of Helicobacter pylori-related gastritis. Endoscopy. 1995;27:593-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Calabrese C, Di Febo G, Brandi G, Morselli-Labate AM, Areni A, Scialpi C, Biasco G, Miglioli M. Correlation between endoscopic features of gastric antrum, histology and Helicobacter pylori infection in adults. Ital J Gastroenterol Hepatol. 1999;31:359-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Spence AD, Cardwell CR, McMenamin ÚC, Hicks BM, Johnston BT, Murray LJ, Coleman HG. Adenocarcinoma risk in gastric atrophy and intestinal metaplasia: A systematic review. BMC Gastroenterol. 2017;17:157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 11. | Neumann H, Fujishiro M, Wilcox CM, Mönkemüller K. Present and future perspectives of virtual chromoendoscopy with i-scan and optical enhancement technology. Dig Endosc. 2014;26 Suppl 1:43-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Tongtawee T, Kaewpitoon S, Kaewpitoon N, Dechsukhum C, Loyd RA, Matrakool L. Correlation between Gastric Mucosal Morphologic Patterns and Histopathological Severity of Helicobacter pylori Associated Gastritis Using Conventional Narrow Band Imaging Gastroscopy. Biomed Res Int. 2015;2015:808505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology. 2006;130:1377-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1467] [Cited by in RCA: 1479] [Article Influence: 77.8] [Reference Citation Analysis (0)] |
| 14. | Yagi K, Nakamura A, Sekine A, Goto T. Endoscopic features of the normal gastric mucosa without Helicobacter pylori infection. Gastroenterol Endosc. 2000;42:1977-1987. |
| 15. | Yao K. Gastric microvascular architecture as visualized by magnifying endoscopy: body and antral mucosa without pathologic change demonstrate two different patterns of microvascular architecture. Gastrointest Endosc. 2004;59:596-7; author reply 597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Anagnostopoulos GK, Yao K, Kaye P, Fogden E, Fortun P, Shonde A, Foley S, Sunil S, Atherton JJ, Hawkey C, Ragunath K. High-resolution magnification endoscopy can reliably identify normal gastric mucosa, Helicobacter pylori-associated gastritis, and gastric atrophy. Endoscopy. 2007;39:202-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 107] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 17. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3221] [Cited by in RCA: 3558] [Article Influence: 122.7] [Reference Citation Analysis (3)] |
| 18. | Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43944] [Cited by in RCA: 41949] [Article Influence: 873.9] [Reference Citation Analysis (0)] |
| 19. | Laine L, Cohen H, Sloane R, Marin-Sorensen M, Weinstein WM. Interobserver agreement and predictive value of endoscopic findings for H. pylori and gastritis in normal volunteers. Gastrointest Endosc. 1995;42:420-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 76] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Mond DJ, Pochaczevsky R, Vernace F, Bank S, Chow KW. Can the radiologist recognize Helicobacter pylori gastritis? J Clin Gastroenterol. 1995;20:199-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Yela MC, Manzano ML, Rodríguez-Muñoz S, Sánchez F, Pérez-Carreras M, Sánchez-Pobre P, Garfia P, Castellano G. Assessment of the usefulness of endoscopic signs in Helicobacter pylori associated gastritis. Rev Esp Enferm Dig. 1997;89:3-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 22. | Redéen S, Petersson F, Jönsson KA, Borch K. Relationship of gastroscopic features to histological findings in gastritis and Helicobacter pylori infection in a general population sample. Endoscopy. 2003;35:946-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 113] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 23. | Tsuchihashi Y, Tani T, Maruyama K, Yorioka S, Okada K, Sudo H, Ashihara T, Fujita S, Kawai K, Manabe H, Zweifach BW, Messmer K. Structural alterations of mucosal microvascular system in human chronic gastritis. Microcirculation in circulatory disorders. Manabe H, Zweifach BW, Messmer K. Tokyo: Springer 1988; 161-169. |
| 24. | Yao K, Oishi T, Matsui T, Yao T, Iwashita A. Novel magnified endoscopic findings of microvascular architecture in intramucosal gastric cancer. Gastrointest Endosc. 2002;56:279-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 162] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 25. | Yao K, Tatsuhiro O. Microgastroscopic findings of mucosal microvascular architecture as visualized by magnifying endoscopy. Novel magnified endoscopic findings of microvascular architecture in intramucosal gastric cancer. Dig Endosc. 2001;13:27-33. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Yagi K, Nakamura A, Sekine A. Characteristic endoscopic and magnified endoscopic findings in the normal stomach without Helicobacter pylori infection. J Gastroenterol Hepatol. 2002;17:39-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 130] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 27. | Yagi K, Nakamura A, Sekine A. Comparison between magnifying endoscopy and histological, culture and urease test findings from the gastric mucosa of the corpus. Endoscopy. 2002;34:376-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 28. | Yagi K, Honda H, Yang JM, Nakagawa S. Magnifying endoscopy in gastritis of the corpus. Endoscopy. 2005;37:660-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Yagi K, Nakamura A, Sekine A. Magnifying endoscopy of the gastric body: a comparison of the findings before and after Helicobacter pylori eradication. Dig Endosc. 2002;14:76-82. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Yagi K, Aruga Y, Nakamura A, Sekine A. Regular arrangement of collecting venules (RAC): a characteristic endoscopic feature of Helicobacter pylori-negative normal stomach and its relationship with esophago-gastric adenocarcinoma. J Gastroenterol. 2005;40:443-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Nakagawa S, Kato M, Shimizu Y, Nakagawa M, Yamamoto J, Luis PA, Kodaira J, Kawarasaki M, Takeda H, Sugiyama T, Asaka M. Relationship between histopathologic gastritis and mucosal microvascularity: observations with magnifying endoscopy. Gastrointest Endosc. 2003;58:71-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |