Published online Jun 16, 2019. doi: 10.4253/wjge.v11.i6.413
Peer-review started: April 8, 2019
First decision: May 16, 2019
Revised: June 1, 2019
Accepted: June 10, 2019
Article in press: June 10, 2019
Published online: June 16, 2019
Processing time: 71 Days and 6.4 Hours
The presence of small air bubbles and foam are an impediment to a successful colonoscopy. They impair an endoscopist’s view and diminish the diagnostic accuracy of the study. This has been particularly noted to be of concern with the switch to lower volume polyethylene glycol (PEG) and bisacodyl combination preparation.
To evaluate the effect of oral simethicone addition to bowel preparation on intraluminal bubbles reduction during colonoscopy.
Described is a prospective, randomized, multi-center, double-blinded, placebo-controlled study to evaluate the use of premixed simethicone formulation with split-regimen, low-volume PEG-bisacodyl combination bowel preparation for 168 outpatients undergoing screening, surveillance, and diagnostic colonoscopies. Primary outcome includes evaluation of bubbles during colonoscopy graded using the Intraluminal Bubbles Scale. Secondary outcomes include evaluation of the Boston Bowel Preparation Scale (BBPS), total number of polyps, polyp size differentiation, polyp laterality, adenoma detection, mass detection, cecal insertion time, withdrawal time, and patient-reported adverse events.
Higher Intraluminal Bubbles grades III and IV (less than 75% of the mucosa cleared of bubbles/foam requiring intervention with simethicone infused wash) were detected in the placebo group [Simethicone n = 4/84 vs Placebo n = 20/84 (P = 0.007)]. BBPS total score was 7.42 [standard deviation (SD) = ± 1.51] in the simethicone group and 7.28 (SD = ± 1.44) in the placebo group (P = 0.542) from a total of 9. Significantly higher number of adenomas were detected in the simethicone group (P = 0.001).
The addition of simethicone to bowel preparation is well advised for its anti-foaming properties. The results of this study suggest that addition of oral simethicone can improve bowel wall visibility.
Core tip: We conducted a randomized, controlled, prospective study to analyze the efficacy of oral simethicone addition to 2-liter polyethylene glycol bowel preparation for mucosal visibility improvement during colonoscopy. Significant reduction of bubbles/foam, improvement in total polyp detection, and total adenoma detection was seen with simethicone addition. Oral simethicone prior to colonoscopy helps clear intraluminal bubbles and alleviates the need to use intraprocedural simethicone flushes as recently advised by endoscope manufacturers.
- Citation: Rishi M, Kaur J, Ulanja M, Manasewitsch N, Svendsen M, Abdalla A, Vemala S, Kewanyama J, Singh K, Singh N, Gullapalli N, Osgard E. Randomized, double-blinded, placebo-controlled trial evaluating simethicone pretreatment with bowel preparation during colonoscopy. World J Gastrointest Endosc 2019; 11(6): 413-423
- URL: https://www.wjgnet.com/1948-5190/full/v11/i6/413.htm
- DOI: https://dx.doi.org/10.4253/wjge.v11.i6.413
An adequate bowel preparation has been well established to lead to a successful colonoscopy[1,2]. Research has consistently associated inadequate bowel preparation with lower adenoma detection rates (ADR)[2,3]. Over the years, endoscopy centers have changed the contents of bowel preparation in light of new research[3]. In 2006, three medical organizations recommended the use of polyethylene glycol (PEG) solution for bowel preparation[1]. Initially, a 4-liter PEG solution was commonly used using a split-dose regimen for bowel prep. However, many patients found that this large volume gave them side effects including bloating and cramping[4]. Studies showed that a low volume PEG solution with bisacodyl tablets fared equally as far as the adequacy of bowel preparation goes[5]. Some showed that lower volumes of the preparations were better tolerated by patients[1,6]. This improved patient tolerability, clinical outcomes, and improved patient satisfaction as noted by gastroenterologists. However, with the use of lower volume preparations an increase in intraluminal bubbles have been noted in our practice. The current standard of practice includes irrigation, lavage, and suctioning using a simethicone infused saline wash through the irrigation channels during the colonoscopy. Simethicone’s ability to reduce surface tension to help dissolve bubbles and clear the field-of-view is vital during the procedure[7]. Furthermore, it does not absorb into the blood stream and is thereby considered safe[8]. However, manufacturers including Olympus (Olympus America, Center Valley, PA) have recommended against routine simethicone use due to concern about its retention inside the non-brushable irrigation channels after reprocessing[9]. Simethicone can still be mixed with water and manually flushed through the main working channel. However, this takes additional time and effort to flush and suction up this excess fluid. Therefore, the presence of simethicone in premixed bowel preparation can eliminate the need for potentially contaminating non-brushable colonoscope channels. This can improve procedural times in a busy clinical setting and theoretically allow more time to search for and remove adenomas. (RE: Use of simethicone and other non-water-soluble additives with Olympus flexible
endoscopes. 2018 cited; Available from: http://medical.olympusamerica.com /sites/us/files/pdf/Customer-Letter---Use-of-simethicone-and-lubricants.pdf)
The presence of simethicone in the colon lumen can prevent the development of bubbles and foam during the procedure[10,11]. When administered with the bowel preparation, simethicone can be present at the time of introduction of the colonoscope thereby reducing the need to use simethicone irrigation for the purpose of clearing bubbles[8,10-11]. Previous studies have shown mixing simethicone in different bowel preparations including sodium phosphate and various PEG solutions effectively cleared bubbles during the procedure[8,10-11].
The primary endpoint of this study is to evaluate the reduction of bubbles during colonoscopy by premixing simethicone in low volume PEG-bisacodyl combination preparation as compared to placebo. The adequacy of the bowel preparation, total number of polyps, polyp size differentiation, polyp laterality, adenoma detection, mass detection, cecal insertion time, withdrawal time, and patient-reported adverse events are the secondary outcomes measured in this study.
This is a prospective, parallel-group, randomized, double-blinded and placebo-controlled study conducted at two gastroenterology community-based outpatient endoscopy centers. This study was approved by University of Nevada, Reno Human Subjects Research and Institutional Review Board on December 21, 2017. Participants were recruited after study approval and until June, 2018. This clinical trial is registered with clinicaltrials.gov, NCT03410524.
Adult participants eligible for outpatient colonoscopy were given an oral syringe containing simethicone or placebo to be added to a 2-liter split bowel preparation. All participants received the same bowel preparation through the Colon Prep Center (Colon Prep Center, Olathe, KS) commonly referred to as “Gatorade Prep”. De-pending on assignment, the covered oral syringe contained either 200 mg of simethicone or placebo water. A day before the colonoscopy, participants were instructed to combine powdered PEG and oral syringe contents to 2 liters of water. Participants were instructed through Colon Prep Center to consume 1 liter of the bowel preparation with 10-mg oral bisacodyl on the afternoon prior to the planned procedure and the remaining 1 liter was to be consumed early morning on the day of the procedure. Colonoscopies and patient assessments were performed by gastroenterologists on the day of the procedure.
Inclusion criteria included adult participants eligible for outpatient elective colonoscopies. Exclusion criteria included general contraindications for colonoscopies, need for urgent or emergent colonoscopy, and a history of allergy to simethicone (Table 1). If a particular study subject was recruited and allocated but was later deemed ineligible to participate due to non-compliance or voluntary withdrawal, the cause of dropout along with participation ID was documented for the duration of the study.
| Inclusion | Exclusion |
| Male and female patients between 18-80 yr old | Patients under 18 or over 80 yr old |
| Outpatient, elective colonoscopies | Severe constipation |
| Patients using Gatorade-Polyethylene Glycol 3350-Bisacodyl bowel prep | Suspected bowel perforation or obstruction |
| Uncontrolled hypertension | |
| Urgent/emergent colonoscopies | |
| History of bowel resection | |
| Allergy to simethicone |
Patients were recruited and enrolled by medical students, residents, and gastroenterologists. Patients, endoscopists, and enrolling staff were blinded in the study during recruitment and evaluation. An online computer-generated randomization sequencer (http://randomization.com) was used to randomize sequential packets from 1 to 250 to either simethicone arm or placebo arm. All recruiters were blinded during participant recruitment and patient’s endoscopists were not involved with the randomization process. As patients were recruited, they were provided with a packet containing a copy of their signed consent form, Intraluminal Bubbles Scale form to be completed by gastroenterologists during colonoscopy, HIPAA form, and the appropriate oral syringe containing either liquid simethicone or placebo water. The oral syringes were wrapped with a label that discretely covered the contents of the syringe. Participants were assigned packets in the order in which they were recruited i.e., first recruited patient received packet number 1 and twenty-fifth patient recruited received packet number 25, etc. To ensure adherence, patients were contacted by members of the research staff a day prior to the procedure. Compliance with study drug self-administration was monitored during the day of the procedure by nursing staff upon interview on the day of colonoscopy. The following was documented during the day of the procedure: Intraluminal Bubbles Scale, Boston Bowel Preparation Scale (BBPS), total number of polyps, polyp size, polyp laterality, mass detection, cecal insertion time, withdrawal time, and patient-reported adverse events. Data was collected throughout the study duration and participant charts were reviewed by those members of the team blinded to document demographics, procedure reports, and pathologies.
The primary outcome measure was bubble reduction during the withdrawal phase starting from the cecum. Since no standardized or validated bubble scales exist to evaluate effects on colonoscopy outcomes, Intraluminal Bubbles Scale was adapted from studies performed by Yoo et al[10] and Matro et al [11]. To limit inter-observer variability and to objectify the amount of bubbles noted, gastroenterologists were familiarized with the scale and subjective scores of “minimal”, “moderate”, and “severe” levels of bubbles were replaced by 4 grades. A grade of 1-4 was assigned which correlated with the percent circumference of colonic mucosa clear of all bubbles/foam. Grade 1 was equivocal to > 90% mucosa clear of bubbles not requiring irrigation; Grade 2 was 75%-89% mucosa clear of bubbles not requiring irrigation; Grade 3 was 50%-74% mucosa clear of bubbles and required irrigation; Grade 4 was < 50% mucosa clear of bubbles and required irrigation. This scale was not divided between segments of the colon; instead the worst score noted during the entire colonoscopy. If a procedure required simethicone flushes, higher Intraluminal Bubbles Grades 3 or 4 were assigned.
Adequacy of bowel preparation was assessed through the validated BBPS[12]. Each segment of the colon (ascending, transverse, descending) was given a score out of three for a total of 9 points. Cecal insertion time was monitored from time of insertion of scope into the anus until reaching the cecum. Withdrawal time was recorded as withdrawal from cecum until anus. Withdrawal time was calculated on all procedures regardless of whether a biopsy or polypectomy was performed or not. Histologic evaluation was performed on all suspected neoplastic lesions including tubular adenomas, tubulo-villous adenomas, and sessile serrated adenomas. Patients were monitored for adverse events related to the bowel preparation and simethicone use. These were asked by nursing staff during preparation for colonoscopy. Adverse events were divided into two categories. Mild events were classified as abdominal pain/cramping, nausea, and vomiting. Serious adverse events were classified as rash, itching/swelling, dizziness, and difficulty breathing.
Sample: All statistical analysis was performed by a biomedical statistician. To achieve a power of at least 80%, mean Intraluminal Bubbles grade of 1.0 in simethicone group and 2.2 in placebo group[10,13-15], at least 129 participants were needed accounting for an expected dropout rate of about 30%[16,17]. This was accepting an alpha error of 0.05. Two hundred and fifty participants were recruited for the study and 168 of the 250 were included in the final analysis. The baseline characteristics and group differences were compared using Pearson’s Chi square (χ2) test for proportions and Mann-Whitney U test. Continuous variables including BBPS, Intraluminal Bubbles Scale, age, cecal insertion time and withdrawal time were analyzed with the student t-test. A P-value < 0.05 was deemed statistically significant in this study. The adverse events were small in this study and the raw frequencies are reported in Table 2. Participants who withdrew from the study, did not schedule colonoscopy, or failed to comply with oral syringe addition to bowel preparation were excluded.
| Simethicone, n = 84 | Placebo, n = 84 | |
| Abdominal pain/Cramping | 10 (8) | 12 (14) |
| Nausea | 13 (15) | 20 (24) |
| Vomiting | 4 (5) | 6 (7) |
| Serious adverse events | ||
| Rash | 1 (1) | 1 (1) |
| Itching | 1 (1) | None |
Observer variance: 8 gastroenterologists were given picture images of various grades for training prior to start of the study. To measure inter-observer variability after training, they were asked to grade the bubbles on pictures of 9 colonoscopies 2 weeks after training. The variation in response was measured using Cohen’s Kappa[18]. Agreement of 0 would be observed by chance and 1 when there is perfect agreement. Intermediate values fall between 0 and 1 and interpreted as follows: 0.0 Poor agreement, 0.01-0.20 Slight agreement, 0.21-0.40 Fair agreement, 0.41-0.60 Moderate agreement, 0.61-0.80 Substantial agreement, 0.81-1.00 almost perfect agreement. (Table 3)
| Intraluminal bubbles grade | Kappa value | P-value |
| 1 | 0.5687 | < 0.001 |
| 2 | 0.2605 | < 0.001 |
| 3 | 0.3481 | < 0.001 |
| 4 | 0.5440 | < 0.001 |
| Combined | 0.4024 | < 0.001 |
All statistical analyses were performed using Stata version 14.2 (StataCorp, College Station, Texas, United States)
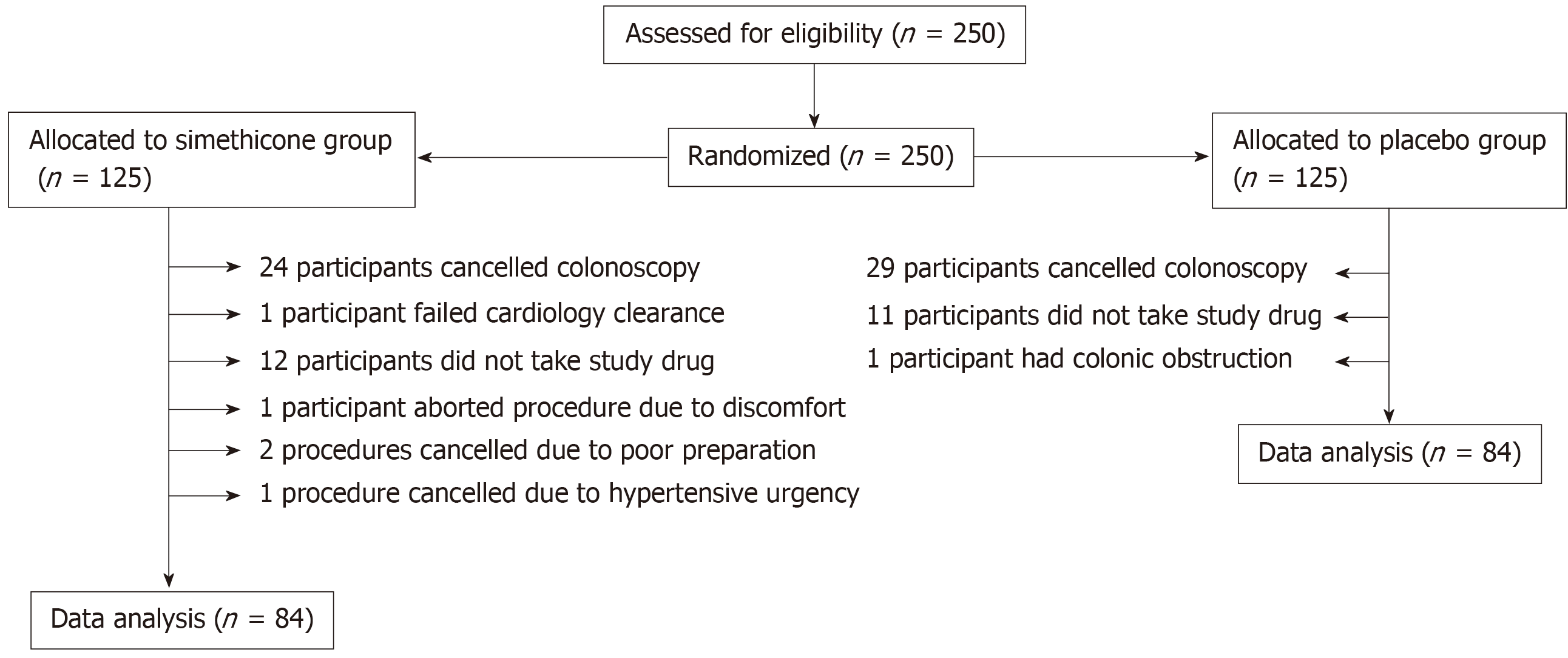
Two hundred and fifty patients were eligible and consented for elective colonoscopy over a period of 1 year. One hundred and twenty-five patients were assigned to placebo group. Of those patients, 29 patients cancelled their appointment, 11 patients failed to mix study drug with bowel preparation, and one patient’s procedure was aborted due to presence of colonic obstruction. Ultimately, data of 84 patients in the placebo group was analyzed.
Of the 125 patients assigned to the simethicone group, 24 patients cancelled their appointment, 12 failed to mix oral syringe with bowel preparation, one patient failed cardiology clearance, one patient’s procedure was aborted due to patient discomfort, two procedures cancelled due to poor preparation, and one procedure cancelled due to hypertensive urgency. Ultimately, data of 84 patients in the experimental group was analyzed. (Figure 1)
In total, 168 subjects underwent colonoscopy [female and male (56.3% vs 43.7%; P = 0.477)], with no significant gender difference between simethicone and placebo groups (P = 0.436) (Table 4). The mean age was 59.6 years (SD = ± 14.0) and 54.0 years (SD = ± 16.4) in simethicone and placebo groups respectively (P = 0.019). The mean Intraluminal Bubbles grade between the simethicone vs placebo (1.20 ± 0.60 vs 1.77 ± 1.00; P < 0.001) was significantly different. Inter-observer variability for Intraluminal Bubbles Scale was measured and noted to have moderate agreement for Grades 1 (κ = 0.569) and 4 (κ = 0.544). Grades 2 (κ = 0.260) and 3 (κ = 0.348) had fair agreement among the raters.
| Simethicone | Placebo | P-value | |
| Baseline characteristics | |||
| Gender | 0.436 | ||
| Female | 50 (59.5) | 45 (53.6) | |
| Male | 34 (40.5) | 39 (46.4) | |
| mean age (yr ± SD) | 59.6 ± 14.0 | 54.0 ± 16.4 | 0.019 |
| Median age (yr) | 63 (IQR; 51-70) | 57 (IQR; 43-68) | 0.033 |
| Outcomes | |||
| Intraluminal Bubbles Scale | < 0.001 | ||
| 1 | 73 (86.9) | 46 (54.8) | |
| 2 | 7 (8.3) | 18 (21.4) | |
| 3 | 2 (2.4) | 13 (15.5) | |
| 4 | 2 (2.4) | 7 (8.3) | |
| Boston Bowel Prep Scale | 0.832 | ||
| 3 | 2 (2.4) | 1 (1.2) | |
| 4 | 0 (0.0) | 1 (1.2) | |
| 5 | 2 (2.4) | 1 (1.2) | |
| 6 | 26 (31.0) | 31 (36.9) | |
| 7 | 13 (15.5) | 13 (15.5) | |
| 8 | 9 (10.7) | 9 (10.7) | |
| 9 | 32 (38.1) | 27 (32.1) | |
| Missing | 0 (0.0) | 1 (1.2) | |
Amongst all of the patients with high Intraluminal Bubbles grade (grade III/IV), 16.7% were from the simethicone group and 83.3% were from the placebo group (P = 0.007). The mean BBPS was 7.42 ± 1.51 with simethicone and 7.28 ± 1.44 with placebo group (P = 0.542). The total number of polyps detected in simethicone group was significantly higher vs placebo [127 (56.7%) vs 97 (43.3%); P = 0.047]. There was no significant difference in mean small polyp detection (1.56 ± 1.92 vs 1.00 ± 1.14; P = 0.093) or large polyp detection (1.13 ± 1.71 vs 1.16 ± 1.27; P = 0.937). Total number of adenomas were noted to be significantly higher in the simethicone group [86 (65.6%) vs 45 (34.4%); P = 0.001].
The mean cecal insertion time between the simethicone group vs placebo was not significantly different (6.06 ± 3.55 vs 5.48 ± 2.82; P = 0.252). The mean cecal withdrawal time between the simethicone group versus placebo was not significantly different (11.73 ± 5.52 vs 11.23 ± 3.99; P = 0.500) (Table 5).
| Simethicone, n = 84 | Placebo, n = 84 | P-value | |
| Intraluminal Bubbles Scale | |||
| mean (± SD) | 1.20 ± 0.60 | 1.77 ± 1.00 | < 0.001 |
| Low grade (I+II) | 80 (55.6) | 64 (44.4) | 0.184 |
| High grade (III + IV) | 4 (16.7) | 20 (83.3) | 0.007 |
| Boston Bowel Preparation Score (BBPS) | |||
| mean (± SD) | 7.42 ± 1.51 | 7.28 ± 1.44 | 0.542 |
| Mean cecal insertion time (± SD) | 6.06 ± 3.55 | 5.48 ± 2.82 | 0.252 |
| Mean withdrawal time (± SD) | 11.73 ± 5.52 | 11.23 ± 3.99 | 0.500 |
| No polyps | 37 (49.3) | 38 (50.7) | 0.907 |
| Total polyps | 127 (56.7) | 97 (43.3) | 0.047 |
| Adenomas | 86 (65.6) | 45 (34.4) | 0.001 |
| Sessile serrated polyps | 4 (3.2) | 7 (7.2) | 0.163 |
| Large polyps (> 5 mm), (mean ± SD) | 1.13 ± 1.71 | 1.16 ± 1.27 | 0.937 |
| Small polyps (1 mm - 4 mm), (mean ± SD) | 1.56 ± 1.92 | 1.00 ± 1.14 | 0.093 |
| Right sided polyps (mean ± SD) | 1.23 ± 1.95 | 0.98 ± 0.96 | 0.429 |
| Left sided polyps | 1.53 ± 1.56 | 1.18 ± 1.19 | 0.228 |
Two masses were found in the entire study, both of which were noted to be in the simethicone test group. One was an adenocarcinoma and one was anal squamous cell carcinoma. Adverse events (simethicone vs placebo) such as abdominal pain/ cramping (11.9% vs 14.3%), nausea (15.5% vs 23.8%), and vomiting (4.8% vs 7.1%) were more prevalent in placebo group, however this did not reach statistical significance. A total of 3 serious adverse events were reported, one event of rash and one event of itching was reported in simethicone group; one event of rash was reported in placebo group (Table 2).
Colonoscopy is the current gold standard for colon cancer screening, prevention, and evaluation of colonic mucosal disease. Various bowel preparations have been devised throughout the years to accommodate a desire for cleaner bowel preparation and patient satisfaction[1-3]. Mucosal visibility is a key factor in the diagnostic accuracy of the procedure19. Low volume PEG has improved patient tolerability and satisfaction with equally effective bowel cleansing capability[1,4-6]. However, many gastro-enterologists have noted an increase in bubbles and foam with the switch to lower volume PEG-bisacodyl combination preparation. Validated and reliable scales to assess the adequacy of bowel preparation exist to measure colonoscopy outcomes, however, the impact of bubbles remains debatable in the literature[12]. We believe that the presence of intraluminal bubbles impedes the efficacy and outcome of the endoscopic exam.
Colonic bubbles are universally found with various bowel preparations during colonoscopy and various methods exist to reduce bubbles during colonoscopy including water and simethicone flushing. Multiple other studies have found the addition of simethicone to bowel preparation useful to clear the visual field of bubbles[11,14,20-23]. The adapted Intraluminal Bubbles Scale was created to utilize stringent requirements for irrigation and mucosal circumference visibility. If more than 25% of the visual field was obscured or if irrigation was required, the procedure was considered to have high-grade bubbles. This scale was designed to incorporate irrigation, lavage, and suctioning as impactful factors for procedures. The strict mucosal visibility criteria and the addition of irrigation as a requirement for grading shows that approximately 30% of routine colonoscopies have significant bubbles. With the addition of liquid simethicone to bowel preparation, this number reduced drastically. Complete mucosal visibility was achieved prior to withdrawing colonoscope even if extra irrigation was required.
Simethicone is a silicone-based polymer which reduces surfaces tension and helps with bubble breakdown[7]. Most commonly, it is infused with water irrigated through the irrigation/water channel during colonoscopy. However, recent studies have shown that simethicone is detectable in non-brushable endoscope irrigation channels despite reprocessing steps of pre-cleaning, manual cleaning, and high-level disinfection[9]. Simethicone may contribute to the microbial growth and biofilm development even with low concentration use according to Barakat et al[24] in non-brushable endoscope channels. Recent endoscope manufacturer recommendations against adding simethicone to irrigation channels has caused endoscopists to utilize working channels to manually inject simethicone flushes during the procedure. Notably, this can increase procedure times or possibly lead to less colon mucosa inspection time in a busy community-based endoscopy center setting. In spite of the lack of significance between the two groups, oral simethicone may have a meaningful impact on procedural times in a lively community-based outpatient practice. Furthermore, artificial intelligence use in colonoscopy for automatic colon polyp detection has been developing to target adenoma miss rate ranging from 6%-27% in routine colonoscopies[25]. Presence of bubbles among other distractors has been implicated with higher false positives[26]. Specificity of neoplastic lesion detection in the new era of computer aided detection tools remains an important marker. Given the findings of our study, adding simethicone to the bowel preparation can eliminate the need for contaminating irrigation channels and improve visualization for artificial intelligence use.
Our study showed that more adenomas were found in the simethicone arm compared to the placebo arm. It is unclear if this would translate to an increase in ADR since this term is a quality measurement applied to individual gastro-enterologists performing colorectal cancer screening colonoscopies and is the proportion of screening colonoscopy patients who are found to have atleast one adenoma. Furthermore, the measurement of ADR does not account an endoscopist’s ability to identify multiple adenomatous polyps in a patient. We recruited par-ticipants who underwent screening, surveillance, and diagnostic colonoscopies instead of screening colonoscopies alone. Additionally, a wide range of participants were recruited including those younger than the screening age. In theory, with improved bowel wall visualization there should be an observed increased in adenoma detection. Our data correlates with other studies evaluating simethicone premix in various PEG preparations including Bai et al[15] and Zhang et al[20] which show increased adenoma detection. In fact this benefit may be underestimated because irrigation in both groups utilized simethicone flushes and sole water flushes were not performed in the placebo arm as we believe irrigation with water alone can paradoxically increase bubbles[11]. Upon literature review, no prior studies evaluate the effect of simethicone addition on adenoma detection without flushing with supplemental simethicone in all study arms. To our knowledge, this is the first trial evaluating the effect of premixing simethicone in 2-liter PEG and bisacodyl combination preparation.
Similarly, other studies have evaluated the effect of simethicone on adenoma detection with varying bowel preparations. A meta-analysis by Yeh et al[27] evaluated 12 randomized controlled trials with a total number of 6003 patients and concluded no significant difference in ADR when simethicone was used, although sub group analysis revealed a difference in populations with low baseline ADRs. On the contrary, meta-analysis performed by Pan et al[28] showed supplemental simethicone improved ADR for colonoscopy. Discordant findings in the literature are likely attributed to limiting factors including varying bowel preparations and inconsistent blinding amongst the studies analyzed.
Use of simethicone flushes does not significantly reduce insertion time or the cecal withdrawal time although its addition can certainly reduce the number of lavage and aspiration of bubbles as noted in studies[11,13]. This may increase colonic mucosal inspection time in a busy outpatient community-based endoscopy center. Ad-ditionally, our study showed that simethicone addition did not demonstrate any significant change in adequacy of bowel cleansing through a higher BBPS. These findings are consistent with trials involving simethicone addition to varying bowel preparations including de Leone et al[23] however in contrast to trials reported by Yoo et al[10], Bai et al[15], and Zhang et al[20]. Simethicone is not a colonic purgative and in theory, should not improve bowel cleansing.
Our subjects were adequately randomized without significant differences between the two groups. 30% of the subjects who were initially enrolled were not included in data analysis but there was an equal dropout rate between the two groups. Noncompliance with administration of the test drug and failure to schedule colonoscopy were the most common causes for exclusion (Figure 1). We attempted to limit inter-observer variability by familiarizing the gastroenterologists with the Intraluminal Bubbles Scale and provided a reference sheet to utilize during procedure grading. Results show moderate agreement when comparing images on the extremes of the Intraluminal Bubbles Scale. Agreement decreased to “fair” when comparing images for bubbles scale 2 and 3. Reasons for the discrepancy include strict mucosal visibility criteria and subjective difference between gastroenterologists on the amount of visible bubbles requiring irrigation.
There are several limitations to our study. This study is not powered to detect secondary outcomes. Also, all intra procedural irrigation was performed with simethicone infused water in both groups. Further studies need to analyze outcomes without the use of supplemental simethicone flushing in the placebo arm although this may be challenging. In addition, several gastroenterologists performed colonoscopies and despite education on Intraluminal Bubbles Scale significant subjective variability was present. Medical supervision of simethicone and placebo administration was not performed although patients were asked of its use. Indeed, significant dropout was noted due to noncompliance that could have been circumvented with premixed bowel preparation. This study was not designed to calculate ADR and adenoma localization was not recorded. Gastroenterologists were not randomized between the two study arms. Despite these limitations, this study provides valuable analysis of colonoscopy outcomes with simethicone addition to low volume bowel preparations.
In summary, the addition of simethicone reduces intraluminal bubbles, improves mucosal visibility, and improves adenoma detection during colonoscopy. Investigators find its addition to bowel preparation clinically useful in practice although colonoscopy outcomes including adequacy of bowel preparation, cecal insertion, and withdrawal time were not shown to be affected. Although larger studies need to be carried out to evaluate these outcomes, simethicone supple-mentation with low volume PEG and bisacodyl bowel preparation shows promise for clinical use since many endoscopy centers and hospitals are avoiding simethicone in irrigation fluid due to new concerns for channel contamination and increased potential for infection.
The presence of small air bubbles and foam are an impediment to a successful colonoscopy. They impair an endoscopist’s view and diminish the diagnostic accuracy of the study. This has been particularly noted to be of concern with the switch to lower volume polyethylene glycol (PEG) and bisacodyl combination preparation.
Simethicone is commonly added to water for irrigation during colonoscopy to clear bubbles/ foam and to improve mucosal visibility. However, recent endoscope manufacturer guidelines recommend against intraprocedural irrigation with simethicone infused water due to concerns for its retention in irrigation channels despite reprocessing. Our initial motivation to perform this study was to evaluate whether the addition of liquid simethicone to low volume PEG bowel preparation would improve intraluminal visibility during colonoscopy and thereby eliminate the need for intra procedural irrigation of simethicone.
The primary outcome measurement was the comparison of bubbles and foam utilizing the Intraluminal Bubbles Scale. Secondary outcomes measured include Boston Bowel Preparation Scale measurement, total number of polyps, polyp size differentiation, polyp laterality, total adenoma detection, cecal insertion time, withdrawal time, and adverse events.
This is a prospective, parallel-group, randomized, double-blinded and placebo-controlled study conducted at two gastroenterology community-based outpatient endoscopy centers. Adult participants were instructed to add liquid simethicone to 2-liter split bowel preparation with bisacodyl. Patients, endoscopists, and enrolling staff were blinded during patient enrollment. Endoscopists were also blinded during conduction of the procedure. Intraluminal Bubbles Scale was recorded during the procedure and a grade of 1-4 was allocated correlating with the percent circumference of colonic mucosa clear of all bubbles/foam. Grade 1 was equivocal to > 90% mucosa clear of bubbles not requiring irrigation; Grade 2 was 75%-89% mucosa clear of bubbles not requiring irrigation; Grade 3 was 50%-74% mucosa clear of bubbles and required irrigation; Grade 4 was < 50% mucosa clear of bubbles and required irrigation.
Higher Intraluminal Bubbles grades III and IV (less than 75% of the mucosa cleared of bubbles/foam requiring intervention with simethicone infused wash) were detected in the placebo group [Simethicone n = 4/84 vs Placebo n = 20/84 (P = 0.007)]. BBPS total score was 7.42 (SD = ± 1.51) in the simethicone group and 7.28 (SD = ± 1.44) in the placebo group (P = 0.542) from a total of 9. Significantly higher number of adenomas were detected in the simethicone group (P = 0.001).
The addition of simethicone to bowel preparation is well advised for its anti-foaming properties during colonoscopy. The results of this study suggest that addition of oral simethicone can improve bowel wall visibility and reduce the need for intraprocedural irrigation.
Larger research studies with screening colonoscopy patients should be carried out to evaluate the effect of simethicone on adenoma detection rate.
We are thankful for Ting-Hui Hsieh MD, Clark Harrison MD, Darren Hill MSN APRN, Timothy Halterman MD, James Nachiondo MD, and James Doyle MD for making contributions and support throughout the entire study process.
Manuscript Source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Madalinski M, Liu YH S-Editor: Dou Y L-Editor: A E-Editor: Wu YXJ
| 1. | Wexner SD, Beck DE, Baron TH, Fanelli RD, Hyman N, Shen B, Wasco KE; American Society of Colon and Rectal Surgeons; American Society for Gastrointestinal Endoscopy; Society of American Gastrointestinal and Endoscopic Surgeons. A consensus document on bowel preparation before colonoscopy: prepared by a task force from the American Society of Colon and Rectal Surgeons (ASCRS), the American Society for Gastrointestinal Endoscopy (ASGE), and the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES). Gastrointest Endosc. 2006;63:894-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 211] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 2. | Harrison NM, Hjelkrem MC. Bowel cleansing before colonoscopy: Balancing efficacy, safety, cost and patient tolerance. World J Gastrointest Endosc. 2016;8:4-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 3. | Toledo TK, DiPalma JA. Review article: colon cleansing preparation for gastrointestinal procedures. Aliment Pharmacol Ther. 2001;15:605-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | da Silva MM, Briars GL, Patrick MK, Cleghorn GJ, Shepherd RW. Colonoscopy preparation in children: safety, efficacy, and tolerance of high- versus low-volume cleansing methods. J Pediatr Gastroenterol Nutr. 1997;24:33-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Adams WJ, Meagher AP, Lubowski DZ, King DW. Bisacodyl reduces the volume of polyethylene glycol solution required for bowel preparation. Dis Colon Rectum. 1994;37:229-33; discussion 233-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 121] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 6. | Kojecky V, Matous J, Keil R, Dastych M, Kroupa R, Zadorova Z, Varga M, Dolina J, Kment M, Hep A. A head-to-head comparison of 4-L polyethylene glycol and low-volume solutions before colonoscopy: which is the best? A multicentre, randomized trial. Int J Colorectal Dis. 2017;32:1763-1766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Brecević L, Bosan-Kilibarda I, Strajnar F. Mechanism of antifoaming action of simethicone. J Appl Toxicol. 1994;14:207-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Tongprasert S, Sobhonslidsuk A, Rattanasiri S. Improving quality of colonoscopy by adding simethicone to sodium phosphate bowel preparation. World J Gastroenterol. 2009;15:3032-3037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 9. | Ofstead CL, Wetzler HP, Johnson EA, Heymann OL, Maust TJ, Shaw MJ. Simethicone residue remains inside gastrointestinal endoscopes despite reprocessing. Am J Infect Control. 2016;44:1237-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (1)] |
| 10. | Yoo IK, Jeen YT, Kang SH, Lee JH, Kim SH, Lee JM, Choi HS, Kim ES, Keum B, Chun HJ, Lee HS, Kim CD. Improving of bowel cleansing effect for polyethylene glycol with ascorbic acid using simethicone: A randomized controlled trial. Medicine (Baltimore). 2016;95:e4163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 11. | Matro R, Tupchong K, Daskalakis C, Gordon V, Katz L, Kastenberg D. The effect on colon visualization during colonoscopy of the addition of simethicone to polyethylene glycol-electrolyte solution: a randomized single-blind study. Clin Transl Gastroenterol. 2012;3:e26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Lai EJ, Calderwood AH, Doros G, Fix OK, Jacobson BC. The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc. 2009;69:620-625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 930] [Cited by in RCA: 924] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 13. | Parente F, Vailati C, Bargiggia S, Manes G, Fontana P, Masci E, Arena M, Spinzi G, Baccarin A, Mazzoleni G, Testoni PA. 2-Litre polyethylene glycol-citrate-simethicone plus bisacodyl versus 4-litre polyethylene glycol as preparation for colonoscopy in chronic constipation. Dig Liver Dis. 2015;47:857-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Repici A, Cestari R, Annese V, Biscaglia G, Vitetta E, Minelli L, Trallori G, Orselli S, Andriulli A, Hassan C. Randomised clinical trial: low-volume bowel preparation for colonoscopy - a comparison between two different PEG-based formulations. Aliment Pharmacol Ther. 2012;36:717-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Bai Y, Fang J, Zhao SB, Wang D, Li YQ, Shi RH, Sun ZQ, Sun MJ, Ji F, Si JM, Li ZS. Impact of preprocedure simethicone on adenoma detection rate during colonoscopy: a multicenter, endoscopist-blinded randomized controlled trial. Endoscopy. 2018;50:128-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Alexander W. The uphill path to successful clinical trials: keeping patients enrolled. P T. 2013;38:225-227. [PubMed] |
| 17. | Sakpal TV. Sample size estimation in clinical trial. Perspect Clin Res. 2010;1:67-69. [PubMed] |
| 18. | Cohen J. A Coefficient of Agreement for Nominal Scales. Educ Psychol Meas. 1960;20:37-46. [DOI] [Full Text] |
| 19. | Rex DK, Petrini JL, Baron TH, Chak A, Cohen J, Deal SE, Hoffman B, Jacobson BC, Mergener K, Petersen BT, Safdi MA, Faigel DO, Pike IM; ASGE/ACG Taskforce on Quality in Endoscopy. Quality indicators for colonoscopy. Am J Gastroenterol. 2006;101:873-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 532] [Cited by in RCA: 561] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 20. | Zhang S, Zheng D, Wang J, Wu J, Lei P, Luo Q, Wang L, Zhang B, Wang H, Cui Y, Chen M. Simethicone improves bowel cleansing with low-volume polyethylene glycol: a multicenter randomized trial. Endoscopy. 2018;50:412-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 21. | Pontone S, Angelini R, Standoli M, Patrizi G, Culasso F, Pontone P, Redler A. Low-volume plus ascorbic acid vs high-volume plus simethicone bowel preparation before colonoscopy. World J Gastroenterol. 2011;17:4689-4695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 56] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 22. | Kump P, Hassan C, Spada C, Brownstone E, Datz C, Haefner M, Renner F, Schoefl R, Schreiber F. Efficacy and safety of a new low-volume PEG with citrate and simethicone bowel preparation for colonoscopy (Clensia): a multicenter randomized observer-blind clinical trial vs. a low-volume PEG with ascorbic acid (PEG-ASC). Endosc Int Open. 2018;6:E907-E913. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | de Leone A, Tamayo D, Fiori G, Ravizza D, Trovato C, De Roberto G, Fazzini L, Dal Fante M, Crosta C. Same-day 2-L PEG-citrate-simethicone plus bisacodyl vs split 4-L PEG: Bowel cleansing for late-morning colonoscopy. World J Gastrointest Endosc. 2013;5:433-439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Barakat MT, Huang RJ, Banerjee S. Simethicone is retained in endoscopes despite reprocessing: impact of its use on working channel fluid retention and adenosine triphosphate bioluminescence values (with video). Gastrointest Endosc. 2019;89:115-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 25. | Alagappan M, Brown JRG, Mori Y, Berzin TM. Artificial intelligence in gastrointestinal endoscopy: The future is almost here. World J Gastrointest Endosc. 2018;10:239-249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 106] [Cited by in RCA: 103] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 26. | Wang Y, Tavanapong W, Wong J, Oh JH, de Groen PC. Polyp-Alert: near real-time feedback during colonoscopy. Comput Methods Programs Biomed. 2015;120:164-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 27. | Yeh JH, Hsu MH, Tseng CM, Chen TH, Huang RY, Lee CT, Lin CW, Wang WL. The benefit of adding oral simethicone in bowel preparation regimen for the detection of colon adenoma: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2019;34:830-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 28. | Pan P, Zhao SB, Li BH, Meng QQ, Yao J, Wang D, Li ZS, Bai Y. Effect of supplemental simethicone for bowel preparation on adenoma detection during colonoscopy: A meta-analysis of randomized controlled trials. J Gastroenterol Hepatol. 2019;34:314-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |