Published online May 16, 2019. doi: 10.4253/wjge.v11.i5.345
Peer-review started: March 20, 2019
First decision: May 8, 2019
Revised: May 11, 2019
Accepted: May 13, 2019
Article in press: May 14, 2019
Published online: May 16, 2019
Processing time: 60 Days and 2.7 Hours
Endoscopic ultrasound-guided biliary drainage (EUS-BD) has been developed as an alternative means of biliary drainage for malignant biliary obstruction (MBO). Compared to percutaneous transhepatic biliary drainage, EUS-BD offers effective internal drainage in a single session in the event of failed endoscopic retrograde cholangiopancreatography and has fewer adverse events (AE). In choosing which technique to use for EUS-BD, a combination of factors appears to be important in decision-making; technical expertise, the risk of AE, and anatomy. With the advent of novel all-in-one EUS-BD specific devices enabling simpler and safer techniques, as well as the growing experience and training of endosonographers, EUS-BD may potentially become a first-line technique in biliary drainage for MBO.
Core tip: Endoscopic ultrasound-guided biliary drainage (EUS-BD) has been developed as an alternative means of biliary drainage for malignant biliary obstruction. EUS-BD must replace percutaneous transhepatic biliary drainage as the salvage procedure of choice in failed endoscopic retrograde cholangiopancreatography when endoscopic expertise is available. The advent of novel all-in-one EUS-BD specific devices, as well as the growing experience and training of endosonographers are promising for the development of EUS-BD as a first-line technique.
- Citation: Leung Ki EL, Napoleon B. Endoscopic ultrasound-guided biliary drainage: A change in paradigm? World J Gastrointest Endosc 2019; 11(5): 345-353
- URL: https://www.wjgnet.com/1948-5190/full/v11/i5/345.htm
- DOI: https://dx.doi.org/10.4253/wjge.v11.i5.345
Endoscopic retrograde cholangiopancreatography (ERCP) is the current first-line approach for drainage of malignant biliary obstruction (MBO)[1-3]. Although success rate is high, difficult cannulation or access due to surgically altered anatomy, prior duodenal obstruction or stenting, periampullary diverticulum, and large tumors account for a failure rate of 5%-10%[1-3].
Percutaneous transhepatic biliary drainage (PTBD) is the conventional salvage procedures for failed ERCP. However, it is associated with significant morbidity, discomfort, and re-interventions[1-3].
ERCP and PTBD have proven their usefulness over 40 years of experience. Since the first report by Giovannini et al[4] in 2001, endoscopic ultrasound-guided biliary drainage (EUS-BD) has been developed as an alternative means of biliary drainage. Several methods have been described. Rendez-vous technique and antegrade stenting (AGS) are alternative means to achieve trans-papillary drainage. However, choledocoduodenostomy (EUS-CBD), and hepaticogastrostomy (EUS-HGS) are newer approaches which achieve extra-papillary drainage by trans-mural stenting.
Initially, EUS-BD was considered an advanced technique performed by experts in referral centers. Significant morbidity limited its indications despite its efficacy.
The last published guidelines accept the following indications for EUS-BD drainage[1-3,5]: (A) failed ERCP performed by a referral center with high expertise; (B) altered anatomy or malignant obstruction precluding papillary access; (C) failed cannulation due to occluding tumor; and (D) contraindication to percutaneous access such as large volume ascites.
Expert consensus and guidelines agree that specialized pancreaticobiliary endoscopists should perform EUS-BD[1-3,5]. Surgical and interventional radiology back up must be available due to potential severe adverse events (AE).
With the growing experience in EUS-BD and new EUS specific tools, overall improvement in efficacy, and safety are apparent. A growing body of evidence suggests that EUS-BD may not only be feasible as salvage to failed ERCP but also as a first-line technique for biliary drainage in MBO[6]. Compared to ERCP it confers two important theoretical advantages: (1) it avoids papillary trauma and subsequent risk of pancreatitis; and (2) it does not traverse the malignant stricture hence reducing the risk of tumor ingrowth that ultimately leads to stent dysfunction and re-intervention.
Our review aims to present the evolving data on EUS-BD that could potentially change the current algorithm by making it the first-line technique for biliary drainage. As data in benign conditions remains scarce and its role is uncertain[7], we will focus on extra-papillary drainage in MBO.
EUS-BD can be performed through intra-hepatic (transgastric-transhepatic) or extra-hepatic (transenteric-transcholedochal) approaches. For the intrahepatic route, the echoendoscope is positioned in the distal esophagus, gastric cardia or lesser curvature, which enables left intra-hepatic access. For the extra-hepatic route, the echoendoscope is frequently positioned in the duodenal bulb and sometimes the pre-pyloric antrum.
Until recently, EUS-BD was performed using devices borrowed from ERCP. It was first demonstrated using a plastic stent for EUD-choledocoduodenostomy (EUS-CDS)[4]. Plastic stents present the risk of bile leak, bile peritonitis, and occlusion[1,2,5]. SEMS have largely superseded them. Partially covered (PC) and fully covered (FC) SEMS are preferred over uncovered (UC) SEMS to prevent bile leak [1-2], however, conventional designs still lack anti-migratory property.
Device-related shortcomings have led to the development of specifically designed EUS-BD stents including lumen-apposing metal stents (LAMS), hybrid metal stents (distal covered and proximal UC portions with anti-migratory properties), and one-step dedicated devices with pre-mounted hybrid stents[8].
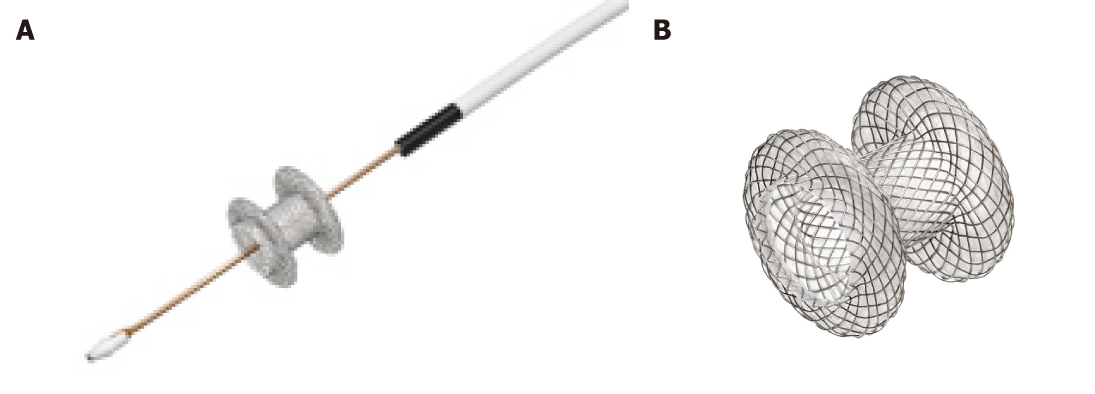
The most data and extensive experience are on LAMS. LAMS are a recently developed, revolutionary device, designed for EUS trans-luminal drainage[9]. They are short dumbbell-shaped FC metallic stents with wide flanges to allow anchoring across non-adherent structures (Figure 1). LAMS were initially designed for drainage of pancreatic fluid collections. Indications have expanded to EUS-CDS for distal MBO. The newer version of dedicated LAMS have integrated an electrocautery-enhanced delivery system (ECE-LAMS) to allow puncture and release of the stent in a single step procedure hence decreasing the number of accessory exchanges, and reducing the potential of complications[10-12]. There are several different LAMS available with different lengths and diameters. The AXIOS stent with diameters of 6 and 8 mm and saddle length of 8 mm is custom designed for EUS-CDS.
Four meta-analyses reported a technical success rate of EUS-BD of 90%-94.7%, clinical success rate of 87%-94%, and AE rate of 16%-29%[7,13-15]. MBO was the most frequent indication.
AE of EUS-BD depend on the route, the device used, type and extent of disease and operator experience. Overall AE rate for EUS-BD is 16.5%-23.3%[7,13,14]. The most frequent AE are bleeding, bile leak, pneumo-peritoneum, cholangitis, stent migration, abdominal pain, and peritonitis. Although these complications are often self-limited and can be treated conservatively or with endoscopic re-intervention, some complications such as stent migration into the peritoneal cavity may be fatal[8].
In EUS-CDS, the most frequent complications are pneumo-peritoneum and biliary leak predominantly occurring with plastic or UCSEMS[16]. In EUS-HGS, needle puncture into the peritoneal cavity increases the risk of pneumo-peritoneum and bile leak. Smaller intra-hepatic duct caliber precluding the placement of a wider metallic stent may also predispose to these complications due to incomplete sealing of the bilio-enteric fistula. Finally, the movement of the liver during respiration may lead to stent migration, resulting in biliomas and trauma to the bilio-enteric tract.
AE progressively decrease as experience grows and with the development of new stents. In a more recent prospective international multicenter study on efficacy and safety of EUS-BD, by Khashab et al[17] (n = 96), 10.5% (10/96) AE occurred: 2 pneumo-peritoneum, 1 sheared wire, 1 bleeding, 3 bile leaks, 2 cholangitis, and 1 perforation. 4 AE were graded mild, 4 were moderate, 1 was severe, and 1 was fatal due to unintended perforation. 91.3% of inserted stents were SEMS (44 FC, 26 PC, 14 UC) as oppose to plastic stents. The necessity for track dilation with the use of plastic stents or SEMS was a likely predisposing factor for bile or air leakage.
The advent of LAMS for EUS-CDS confers the theoretical advantage of decreasing migration and bile leak. ECE-LAMS also removes the need for tract dilation, and numerous guide-wire exchanges, potentially reducing complications. Data on LAMS show excellent efficiency and safety profile in short series[6]. Two larger trials have been published for the use of LAMS in distal MBO: (1) A multicenter, retrospective study by Kunda et al[10] (n = 57) showed that EUS-CDS with LAMS or ECE-LAMS, had a technical success rate of 98.2% (56/57) and clinical success rate of 94.7% (54/57). Mean procedure time was 22.4 min. Overall AE rate was 7% with 2 duodenal perforations, 1 bleed, and 1 transient cholangitis. During follow-up, 9.3% (5/54) with clinical success required re-intervention for 1 stent migration and 4 sump syndromes; (2) A recent multicenter, retrospective study by Jacques et al[11] (n = 52), showed that EUS-CDS with ECE-LAMS had a technical success of 88.5% (46/52), and clinical success rate of 100% (46/46). Mean procedure time was 10.2 min. 3.8% (2/46) patients presented short-term complications (1 bleed and 1 cholangitis due to obstructive bezoar). Long-term AE were 13.5% including 6 (11.5%), recurrent jaundice due to 4 tumor obstructions and 2 sump-syndromes. One patient experienced stent migration at 6 wk. In univariate analyses, a small common bile duct diameter and not following the recommended procedure technique were significant risk factors for technical failure. Median survival time without biliary complications was 135 d. Interestingly, expert and non-experts performed the procedure however no difference in technical or clinical success was found in the two groups. Finally, 2 patients underwent pancreaticoduodenectomy with no interference of the stent on the procedure.
Currently, there is no established consensus for the choice of EUS-BD technique, and data remains conflicting[1,7,18]. Subgroup analyses from 2 meta-analyses compared extra-hepatic and intra-hepatic routes for EUS-BD[7,13]. Technical and clinical success rates were similar although AE were less frequent with the extra-hepatic compared to the intra-hepatic approach (OR = 0.40, 95%CI: 0.18-0.87, P = 0.022)[13]. A multicenter retrospective study by Dhir et al[18] compared success and complication rates in patients undergoing EUS-BD via different methods. This study showed that success rates of different techniques were comparable, but that AE rates were higher for trans-hepatic vs trans-duodenal route (30.5% vs 9.3%, P = 0.03). A systematic review by Alvarez-Sanchez et al[16] also showed that AE rates were higher for intra-hepatic (18%) compared to the extra-hepatic (14%) approach. On the other hand, a systematic review and meta-analysis by Uemura et al[19] of 10 studies (n = 434), concluded that EUS-HGS and EUS-CDS had equal efficacy and safety.
In summary, AE in EUS-BD are non-negligible. The more recent data shows an overall lower rate of AE in EUS-BD compared to older publications, which could reflect increasing experience and the development of EUS-BD specific devices.
In choosing which technique to use for EUS-BD, a combination of factors appears to be important in decision making; technical expertise, the risk of AE, and anatomy[12]. It is also generally admitted that EUS-HGS is technically more challenging than EUS-CDS. In general, in patients with distal common bile duct obstruction and adequate duct dilatation, the trans-duodenal and trans-hepatic approaches for EUS-BD have similar efficacy, but extra-hepatic route may be a safer option. Future trials will probably rapidly confirm that LAMS specifically designed for EUS-CDS further reduce complications of this route of drainage and simplify the technique. Hence EUS-HGS will probably be reserved to patients where EUS-CDS is not possible.
PTBD has a high success rate (87%-100%). However, the external drainage catheter causes discomfort to the patient, and AE are non-negligible, reaching 30%, including pneumothorax, bleeding bile leak, and infection. PTBD is also contraindicated in the presence of ascites or multiple liver metastases[20-23]. EUS-BD offers drainage in a single session in the event of failed ERCP; provides internal drainage with less physical discomfort; allows better nutritional absorption; and avoids electrolyte loss.
The result of randomized controlled trials and meta-analyses comparing EUS-BD to PTBD after failed ERCP show comparable technical and clinical success of 90%-100% with higher complication rates in PTBD[20-23]. Sharaiha et al[23] performed a systematic review and meta-analysis of 9 studies (n = 483), which showed no difference in technical success between EUS-BD and PTBD (OR = 1.78, 95%CI: 0.69-4.59, I2 = 22%) after failed ERCP. EUS-BD was associated with better clinical success (OR = 0.45, 95%CI: 0.23-0.89, I2 = 0%), fewer post-procedure AE (OR = 0.23, 95%CI: 0.12-0.47, I2 = 57%), and lower re-intervention (OR = 0.13, 95%CI: 0.7-0.24, I2 = 0%). There was no difference in length of hospital stay with a pooled standard mean difference of -0.48 (95%CI: -1.13-0.16). EUS-BD was more cost-effective.
An interesting multicenter survey by Nam et al[24] (n = 313) examined patient perception and preference of EUS-BD and PTBD. After explaining the procedure and AE, patients were asked to choose between 2 simulated scenarios. 80.2% of patients preferred EUS-BD. EUS-BD preference declined as AE increased. The authors concluded that technical innovation and improved proficiency to reduce complications of EUS-BD would increase patient acceptability.
In summary EUS-BD must replace PTBD as the standard procedure of choice in failed ERCP in high volume centers with skilled pancreaticobiliary endoscopists.
Only 6 very recent studies have compared EUS-BD to ERCP[25-30]. These studies were performed in patients with distal MBO and used PC or FC-SEMS for EUS-BD. All 6 trials included patients treated by EUS-CDS, and of this one trial also used EUS-AGS and another EUS-HGS. We hereby discuss the available data, also summarized in Table 1.
| Authors | Yr | Study type patients (n) | Type of EUS-BD/ stent used | Technical success (%) EUS-BD/ERCP (P-value) | Functional or clinical success (%) EUS-BD/ERCP (P-value) | Procedure time (min) BD/ERCP (P-value) | AE (%) BD/ERCP (P-value); PPP (%) EUS-BD/ERCP (P-value) | Stent dysfunction (%)EUS-BD/ERCP (P-value) | Re-intervention (%) EUS-BD/ERCP (P-value) |
| Kawakubo et al[25] | 2016 | Single center, retrospective cohort study (82) | EUS-CDS/ PCSEMS | - | 96.2/98.2 (0.54) | Mean 19.7/30.2 (﹤0.01) | 26.9/35.7 (0.46); 0/16.1 (0.50) | - | 20/12.7 (0.50) |
| Dhir et al[26] | 2015 | Multicenter, retrospective (208) | EUS-CDS + EUS-HGS/ FC +UCSEMS | 93.26/94.23 (1.00) | 89.42/91.34 (0.814) | Median 35.95/30.1 (0.05) | 8.65/8.65 (1.00); 0/4.8 (0.59) | - | - |
| Nakai et al[27] | 2018 | Multicenter, prospective (34) | EUS-CDS/ PC + FCSEMS | 97 | 100 | Median 25/52 (﹤0.01) | 15/24 | 29/36 (0.78) | - |
| Park et al[28] | 2018 | Single center, prospective, RCT (30) | EUS-CDS/ PCSEMS | 92.8/100 (1.00) | 92.8/100 (1.00) | Median 43/31 (0.2) | 0/0 (1.00) | 15.4/30.8 (0.65) | - |
| Bang et al[29] | 2018 | Single center, prospective, RCT (67) | EUS-CDS/ FCSEMS | 90.9/94.1 (0.67) | 97/91.2 (0.61) | Median 25/21 (0.173) | 21.2/14.7 (0.49) | 1/1 (0.97) | 3/2.9 (0.99) |
| Paik et al[30] | 2018 | Multicenter, prospective RCT (125) | Distal MBO/ EUS-CDS, EUS-HGS/hybrid PCSEMS | 93.8/90.2 (0.003 for non-inferiority margin 10%) | 90/94.5 (0.49) | Median 5/11 (﹤0.01) | Early AE 6.3/19.7 (0.03); 0/14.8 (0.001) | - | 15.6/42.6 (0.001) (stent patency 85.1 vs 48.9, P = 0.001) |
Three trials compared a group of patients with EUS-CDS +/- EUS-AGS to a retrospective ERCP control group:
A single-center retrospective study by Kawakubo et al[25] (n = 82) comparing the clinical efficacy and safety of EUS-CDS (PCSEMS) vs ERCP (PC or FCSEMS) showed that clinical success rates were equivalent between the groups (EUS-CDS 96.2%, ERCP 98.2%; P = 0.54). Mean procedure time was significantly shorter with EUS-CDS than ERCP (19.7 vs 30.2 min; P < 0.01). Overall AE were not significantly different between the groups (EUS-CDS 26.9%, ERCP 35.7%; P = 0.46). Post-procedure pancreatitis was only seen with ERCP (0% vs 16.1%; P = 0.03). Re-intervention rate at 1 year was not significantly different (16.6% vs 13.6%, P = 0.5).
A multicenter, retrospective analysis by Dhir et al[26] (n = 208) compared the outcomes of EUS-BD vs ERCP. Patients in the EUS-BD group underwent EUS-CDS or EUS-AGS with FCSEMS or UCSEMS respectively after 1 or more failed ERCP attempts. Patients in the ERCP group underwent retrograde SEMS placement. In the ERCP and EUS-BD groups respectively; technical success was 94.23% vs 93.26%, P = 1; AE were 4.8% vs 0%, P = 0.06; and mean procedure time was 30.1 vs 35.95 min, P = 0.05.
Nakai et al[27] performed a multicenter prospective study (n = 34) evaluating EUS-CDS (PC or FC-SEMS) vs ERCP (PC or FC-SEMS). For EUS-CDS, technical success rate was 97% and functional success rate 100%, with median procedure time of 25 min. Overall AE were 15% (5/34); 2 with mild abdominal pain and 3 with moderate cholecystitis. Rate of recurrent biliary obstruction (RBO) was 29% (10/34) and non-tumor related. Migration occurred in 6, sludge or food impaction in 3, and stent impaction in duodenal wall in 1. Median time to RBO was 11.3 months. In comparison to the ERCP control group, the rate of RBO and cumulative time to RBO of EUS-CDS was comparable to ERCP, which were 36% and 9.1 months respectively. ERCP procedure time was significantly longer (median of 52 min, P < 0.01), and AE rate were comparable.
Three randomized trials compared EUS-CDS +/- HGS to ERCP.
Park et al[28] performed a prospective randomized controlled study comparing efficacy and safety of EUS-CDS (n = 15) vs ERCP (n = 15). Both arms used the same PCSEMS. 27 had unresectable pancreatic ductal adenocarcinoma, 1 had distal biliary cancer, and 2 patients had metastatic malignant lymphadenopathy. There were no significant differences for both arms in terms of technical, and clinical success rates (100% vs 93%, P = 1.00 and 93% vs 100%, P = 1.00 respectively). 4 patients (31%) had tumor ingrowth causing stent dysfunction in the ERCP group. 2 patients had food impaction and 2 patients had stent migration in the EUS-CDS group. There were no significant procedure-related AE in either group. The authors concluded that EUS-CDS and ERCP had similar safety and that EUS-CDS was not superior to ERCP in terms of relieving MBO. EUS-CDS had fewer cases of tumor ingrowth but more cases of food impaction and stent migration.
Bang et al[29] performed a single center, single-blind, randomized trial to compare EUS-CDS (n = 33) vs ERCP (n = 34) as primary treatment for distal biliary obstruction from pancreatic cancer. Both arms used the same FCSEMS. The primary endpoint was the rate of AE for EUS-CDS compared to ERCP, which was not significantly different (21.2% vs 14.7% respectively, risk ratio 0.69, 95%CI: 0.24-1.07, P = 0.49). Moderate AE in both groups were around 6%, with no severe AE or procedure-related deaths. For secondary endpoints there were no significant differences between EUS-CDS and ERCP in the rates of technical success (90.9% vs 94.1%, P = 0.67), treatment success (97% vs 91.2%, P = 0.61), or re-interventions (3.0% vs 2.9%, P = 0.99). EUS-CDS did not impede subsequent pancreaticoduodenectomy that was performed in 5/33 (15.2%) of these patients and in 5/34 (14.7%) in the ERCP group (P = 0.99). Median procedure time was similar for EUS-CDS and ERCP (25 min vs 21 min respectively, P = 0.178).
In a larger multicenter randomized non-inferiority study by Paik et al[30] (n = 125) EUS-BD (EUS-CDS, and EUS-HGS) was compared to ERCP in palliative drainage of distal MBO. In the EUS-BD group a dedicated hybrid PCSEMS pre-mounted on a one-step delivery device was used, whereas in the ERCP group either a PC or FCSEMS was used. Technical success rates were 93.8% vs 90.2% (P = 0.003), and clinical success rates 90% vs 94.5% (P = 0.49) for EUS-BD and ERCP respectively. EUS-BD had lower rates of overall AE (6.3% vs 19.7% P = 0.03) including post-procedure pancreatitis (0 vs 14.8%), and re-intervention (15.6% vs 42.6%). EUS-BD had higher rates of stent patency (85.1% vs 48.9%). There was no difference in patency between EUS-CDS and EUS-HGS. Median procedure time was significantly shorter in EUS-BD 5 min (IQR 3-12) vs ERCP 11 min (IQR 7-18), P < 0.001. EUS-BD was associated with higher quality of life (QOL) compared to ERCP at 12 wk post procedure. This study had a notably higher rate of post ERCP pancreatitis and a lower rate of EUS-BD complications compared to other studies. The authors explained these discrepancies by the high number of complex papillary access, and the specific EUS-BD delivery devices used.
In summary, recent randomized studies suggest that EUS-CDS is an effective and safe alternative to ERCP that could reduce the re-intervention rate, and risk of pancreatitis without impeding potential curative surgery. Thus EUS-CDS is a practical route of drainage that should be considered in preoperative drainage.
ERCP in non-operable hilar stenosis is more challenging than for distal MBO. Bilateral biliary drainage with placement of multiple metallic stent is often required in order to drain ≥ 50% of the liver volume[2,3,31]. The failure rate can reach 27%, with lower clinical response despite successful stent placement. EUS-HGS enables trans-luminal stenting of the left biliary tree without traversing the stricture. It can be combined with ERCP to drain both left and right hepatic ducts. When feasible, the right biliary ducts can also be accessed via EUS-HGS with bridge trans-hilar stenting[31].
Data on EUS-HGS for proximal MBO are limited[7,31], and there is no data comparing EUS-HGS to ERCP in this situation. Furthermore, except for a single-step delivery device only commercially available in Korea, most EUS-HGS specific stents still require a multi-step procedure for adequate positioning.
In summary, the development of new EUS-HGS specific tools, comparative studies between EUS-HGS and ERCP/PTBD, as well as standardization of procedures should be a future goal.
Gastro-duodenal and biliary obstruction may occur in advanced pancreatic cancer, and double stenting may be required. ERCP is challenging in the presence of prior duodenal stent placement. Yamao et al[32] performed a multicenter retrospective study (n = 39) to evaluate the outcome of EUS-BD in pancreatic patients with an indwelling gastro-duodenal stent (GDS). This study showed that when a GDS overlay the papilla, EUS-BD technical and clinical success were higher than ERCP (95.2% vs 56 % P < 0.01, and 90.5% vs 52% P = 0.01 respectively). There was no significant difference in the incidence of AE. The authors concluded that EUS-BD could be a first-line technique for biliary drainage in patients who had a GDS overlying the papilla. In a case series by Anderloni et al[33], single session EUS-CDS with LAMS and duodenal stenting was performed. Results showed 100% technical success with no early or late complications. The short length and design of LAMS did not to interfere with duodenal stenting.
Until recently, EUS-BD was reserved for cases of failed ERCP.
Current data suggests that in multi-disciplinary centers with endoscopic pancreatobiliary expertise EUS-BD is a viable alternative to ERCP and should be favored in cases of prior duodenal stenting. EUS-CDS appears to be a simpler and safer procedure than EUS-HGS, and should be favored when both techniques are possible.
Although recent randomized studies have shown that EUS-CDS is as effective as ERCP with longer stent patency, similar AE profile and reduced risk of pancreatitis precluding early surgery, they also show a higher than expected rate complications and failure of ERCP. In contrast, other studies show a meager failure rate of ERCP in expert hands. A prospective study by Holt et al[34] (n = 52) showed that ERCP had a high success rate, in particular when advanced techniques of cannulation were available; hence only 0.6% of native papilla having failed ERCP required EUS-BD. Another retrospective study by Ardengh et al[35] (n = 3538), also showed that the failure rate for ERCP was low, 0.68%. In light of the long experience and excellent results with ERCP, this technique should be difficult to replace despite the advantages of EUS-BD.
Nevertheless, the development of ECE-LAMS is a significant milestone in EUS-CDS. Growing data suggests it is an efficient and safe tool that reduces procedure time and AE. By virtue of its simple, all-in-one application, ECE-LAMS may reduce the risk of procedural complications such as biliary leakage. Selecting patients with a common bile duct dilation of at least 15 mm diameter, and distal MBO below mid common bile duct appear to be effective measures to reduce procedure-related complications[11,12]. Prospective multicenter, randomized studies are required to compare ECE-LAMS to ERCP in distal MBO. Based on current data it can be hypothesized that such studies would show a comparable efficiency of the two techniques, with reduced pancreatitis and prolonged stent patency in the ECE-LAMS group. Nonetheless, the requirement of EUS and ERCP training to perform EUS-CDS with ECE-LAMS should likely limit the applicability of this technique in a widespread manner. Data are lacking with regards to the learning curve for EUS-BD. A prospective study by Oh et al[36] (n = 129) showed that 33 procedures were required to reach a stabilization level in terms of AE and to reduce procedure time. Concerning ECE-LAMS a second follow-up study by Jacques and col[12] (n = 61) re-examined the efficacy of ECE-LAMS in distal MBO after a year of further experience. This study under abstract form showed 98.4% technical and clinical success, 1.6% procedure-related complication (1 bleed during fistulotomy which was self-limited with the expansion of the stent), 0% early complications. Thus, when experience with ECE-LAMS was acquired for EUS-CDS, this technique was effective and safe for biliary drainage.
Finally, concerning EUS-HGS as an alternative to ERCP, the development of effective all-in-one dedicated devices would reduce AE rates and make it an attractive means of drainage in particular for proximal MBO. Due to the complex nature or proximal MBO, it is likely that ERCP, and EUS-HGS will remain complementary in the future.
EUS-BD has enormous potential and has already replaced PTBD in salvage of failed ERCP in expert centers. Several challenges remain before it can fully represent a paradigm shift and replace standard biliary drainage techniques in a widespread manner. The advent of novel EUS-BD specific tools enabling simpler and safer techniques, as well as the growing experience and training of endosonographers, will undoubtedly push the frontiers of its application forward.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: France
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yakoot M S-Editor: Dou Y L-Editor: A E-Editor: Xing YX
| 1. | Teoh AYB, Dhir V, Kida M, Yasuda I, Jin ZD, Seo DW, Almadi M, Ang TL, Hara K, Hilmi I, Itoi T, Lakhtakia S, Matsuda K, Pausawasdi N, Puri R, Tang RS, Wang HP, Yang AM, Hawes R, Varadarajulu S, Yasuda K, Ho LKY. Consensus guidelines on the optimal management in interventional EUS procedures: results from the Asian EUS group RAND/UCLA expert panel. Gut. 2018;67:1209-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 154] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 2. | Dumonceau JM, Tringali A, Papanikolaou IS, Blero D, Mangiavillano B, Schmidt A, Vanbiervliet G, Costamagna G, Devière J, García-Cano J, Gyökeres T, Hassan C, Prat F, Siersema PD, van Hooft JE. Endoscopic biliary stenting: indications, choice of stents, and results: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline - Updated October 2017. Endoscopy. 2018;50:910-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 484] [Article Influence: 69.1] [Reference Citation Analysis (0)] |
| 3. | Mukai S, Itoi T, Baron TH, Takada T, Strasberg SM, Pitt HA, Ukai T, Shikata S, Teoh AYB, Kim MH, Kiriyama S, Mori Y, Miura F, Chen MF, Lau WY, Wada K, Supe AN, Giménez ME, Yoshida M, Mayumi T, Hirata K, Sumiyama Y, Inui K, Yamamoto M. Indications and techniques of biliary drainage for acute cholangitis in updated Tokyo Guidelines 2018. J Hepatobiliary Pancreat Sci. 2017;24:537-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 119] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 4. | Giovannini M, Moutardier V, Pesenti C, Bories E, Lelong B, Delpero JR. Endoscopic ultrasound-guided bilioduodenal anastomosis: a new technique for biliary drainage. Endoscopy. 2001;33:898-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 480] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 5. | Kahaleh M, Artifon EL, Perez-Miranda M, Gupta K, Itoi T, Binmoeller KF, Giovannini M. Endoscopic ultrasonography guided biliary drainage: summary of consortium meeting, May 7th, 2011, Chicago. World J Gastroenterol. 2013;19:1372-1379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Jain D, Shah M, Patel U, Sharma A, Singhal S. Endoscopic Ultrasound Guided Choledocho-Enterostomy by Using Lumen Apposing Metal Stent in Patients with Failed Endoscopic Retrograde Cholangiopancreatography: A Literature Review. Digestion. 2018;98:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Wang K, Zhu J, Xing L, Wang Y, Jin Z, Li Z. Assessment of efficacy and safety of EUS-guided biliary drainage: a systematic review. Gastrointest Endosc. 2016;83:1218-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 233] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 8. | Minaga K, Kitano M. Recent advances in endoscopic ultrasound-guided biliary drainage. Dig Endosc. 2018;30:38-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 9. | Mussetto A, Fugazza A, Fuccio L, Triossi O, Repici A, Anderloni A. Current uses and outcomes of lumen-apposing metal stents. Ann Gastroenterol. 2018;31:535-540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 10. | Kunda R, Pérez-Miranda M, Will U, Ullrich S, Brenke D, Dollhopf M, Meier M, Larghi A. EUS-guided choledochoduodenostomy for malignant distal biliary obstruction using a lumen-apposing fully covered metal stent after failed ERCP. Surg Endosc. 2016;30:5002-5008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 144] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 11. | Jacques J, Privat J, Pinard F, Fumex F, Valats JC, Chaoui A, Cholet F, Godard B, Grandval P, Legros R, Kerever S, Napoleon B. Endoscopic ultrasound-guided choledochoduodenostomy with electrocautery-enhanced lumen-apposing stents: a retrospective analysis. Endoscopy. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 12. | Jacques J, Fumex F, Privat J, Pinard F, Chaput U, Valats JC, Cholet F, Grandval P, Legros R, Jézéquel J, Lepetit H, Napoléon B. Anastomose choledoco-bulbaire sous écho-endoscopie par système Hot-AXIOS: étude multicentrique française d'évaluation de l'efficacité du système après apprentissage. Endoscopy. 2019;51:1-1. |
| 13. | Khan MA, Akbar A, Baron TH, Khan S, Kocak M, Alastal Y, Hammad T, Lee WM, Sofi A, Artifon EL, Nawras A, Ismail MK. Endoscopic Ultrasound-Guided Biliary Drainage: A Systematic Review and Meta-Analysis. Dig Dis Sci. 2016;61:684-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 141] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 14. | Moole H, Bechtold ML, Forcione D, Puli SR. A meta-analysis and systematic review: Success of endoscopic ultrasound guided biliary stenting in patients with inoperable malignant biliary strictures and a failed ERCP. Medicine (Baltimore). 2017;96:e5154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 15. | Fabbri C, Luigiano C, Lisotti A, Cennamo V, Virgilio C, Caletti G, Fusaroli P. Endoscopic ultrasound-guided treatments: are we getting evidence based--a systematic review. World J Gastroenterol. 2014;20:8424-8448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 105] [Cited by in RCA: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 16. | Alvarez-Sánchez MV, Jenssen C, Faiss S, Napoléon B. Interventional endoscopic ultrasonography: an overview of safety and complications. Surg Endosc. 2014;28:712-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Khashab MA, Van der Merwe S, Kunda R, El Zein MH, Teoh AY, Marson FP, Fabbri C, Tarantino I, Varadarajulu S, Modayil RJ, Stavropoulos SN, Peñas I, Ngamruengphong S, Kumbhari V, Romagnuolo J, Shah R, Kalloo AN, Perez-Miranda M, Artifon EL. Prospective international multicenter study on endoscopic ultrasound-guided biliary drainage for patients with malignant distal biliary obstruction after failed endoscopic retrograde cholangiopancreatography. Endosc Int Open. 2016;4:E487-E496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (1)] |
| 18. | Dhir V, Artifon EL, Gupta K, Vila JJ, Maselli R, Frazao M, Maydeo A. Multicenter study on endoscopic ultrasound-guided expandable biliary metal stent placement: choice of access route, direction of stent insertion, and drainage route. Dig Endosc. 2014;26:430-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 120] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 19. | Uemura RS, Khan MA, Otoch JP, Kahaleh M, Montero EF, Artifon ELA. EUS-guided Choledochoduodenostomy Versus Hepaticogastrostomy: A Systematic Review and Meta-analysis. J Clin Gastroenterol. 2018;52:123-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 20. | Artifon EL, Aparicio D, Paione JB, Lo SK, Bordini A, Rabello C, Otoch JP, Gupta K. Biliary drainage in patients with unresectable, malignant obstruction where ERCP fails: endoscopic ultrasonography-guided choledochoduodenostomy versus percutaneous drainage. J Clin Gastroenterol. 2012;46:768-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 177] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 21. | Lee TH, Choi JH, Park do H, Song TJ, Kim DU, Paik WH, Hwangbo Y, Lee SS, Seo DW, Lee SK, Kim MH. Similar Efficacies of Endoscopic Ultrasound-guided Transmural and Percutaneous Drainage for Malignant Distal Biliary Obstruction. Clin Gastroenterol Hepatol. 2016;14:1011-1019.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 159] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 22. | Baniya R, Upadhaya S, Madala S, Subedi SC, Shaik Mohammed T, Bachuwa G. Endoscopic ultrasound-guided biliary drainage versus percutaneous transhepatic biliary drainage after failed endoscopic retrograde cholangiopancreatography: a meta-analysis. Clin Exp Gastroenterol. 2017;10:67-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Sharaiha RZ, Khan MA, Kamal F, Tyberg A, Tombazzi CR, Ali B, Tombazzi C, Kahaleh M. Efficacy and safety of EUS-guided biliary drainage in comparison with percutaneous biliary drainage when ERCP fails: a systematic review and meta-analysis. Gastrointest Endosc. 2017;85:904-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 285] [Article Influence: 35.6] [Reference Citation Analysis (2)] |
| 24. | Nam K, Kim DU, Lee TH, Iwashita T, Nakai Y, Bolkhir A, Castro LA, Vazquez-Sequeiros E, de la Serna C, Perez-Miranda M, Lee JG, Lee SS, Seo DW, Lee SK, Kim MH, Park DH. Patient perception and preference of EUS-guided drainage over percutaneous drainage when endoscopic transpapillary biliary drainage fails: An international multicenter survey. Endosc Ultrasound. 2018;7:48-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 25. | Kawakubo K, Kawakami H, Kuwatani M, Kubota Y, Kawahata S, Kubo K, Sakamoto N. Endoscopic ultrasound-guided choledochoduodenostomy vs. transpapillary stenting for distal biliary obstruction. Endoscopy. 2016;48:164-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Dhir V, Itoi T, Khashab MA, Park DH, Yuen Bun Teoh A, Attam R, Messallam A, Varadarajulu S, Maydeo A. Multicenter comparative evaluation of endoscopic placement of expandable metal stents for malignant distal common bile duct obstruction by ERCP or EUS-guided approach. Gastrointest Endosc. 2015;81:913-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 129] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 27. | Nakai Y, Isayama H, Kawakami H, Ishiwatari H, Kitano M, Ito Y, Yasuda I, Kato H, Matsubara S, Irisawa A, Itoi T. Prospective multicenter study of primary EUS-guided choledochoduodenostomy using a covered metal stent. Endosc Ultrasound. 2019;8:111-117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 28. | Park JK, Woo YS, Noh DH, Yang JI, Bae SY, Yun HS, Lee JK, Lee KT, Lee KH. Efficacy of EUS-guided and ERCP-guided biliary drainage for malignant biliary obstruction: prospective randomized controlled study. Gastrointest Endosc. 2018;88:277-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 167] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 29. | Bang JY, Navaneethan U, Hasan M, Hawes R, Varadarajulu S. Stent placement by EUS or ERCP for primary biliary decompression in pancreatic cancer: a randomized trial (with videos). Gastrointest Endosc. 2018;88:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 189] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 30. | Paik WH, Lee TH, Park DH, Choi JH, Kim SO, Jang S, Kim DU, Shim JH, Song TJ, Lee SS, Seo DW, Lee SK, Kim MH. EUS-Guided Biliary Drainage Versus ERCP for the Primary Palliation of Malignant Biliary Obstruction: A Multicenter Randomized Clinical Trial. Am J Gastroenterol. 2018;113:987-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 252] [Article Influence: 36.0] [Reference Citation Analysis (1)] |
| 31. | Nakai Y, Kogure H, Isayama H, Koike K. Endoscopic Ultrasound-Guided Biliary Drainage for Unresectable Hilar Malignant Biliary Obstruction. Clin Endosc. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 32. | Yamao K, Kitano M, Takenaka M, Minaga K, Sakurai T, Watanabe T, Kayahara T, Yoshikawa T, Yamashita Y, Asada M, Okabe Y, Hanada K, Chiba Y, Kudo M. Outcomes of endoscopic biliary drainage in pancreatic cancer patients with an indwelling gastroduodenal stent: a multicenter cohort study in West Japan. Gastrointest Endosc. 2018;88:66-75.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (1)] |
| 33. | Anderloni A, Buda A, Carrara S, Di Leo M, Fugazza A, Maselli R, Repici A. Single-session double-stent placement in concomitant malignant biliary and duodenal obstruction with a cautery-tipped lumen apposing metal stent. Endoscopy. 2016;48:E321-E322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 34. | Holt BA, Hawes R, Hasan M, Canipe A, Tharian B, Navaneethan U, Varadarajulu S. Biliary drainage: role of EUS guidance. Gastrointest Endosc. 2016;83:160-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 35. | Ardengh JC, Lopes CV, Kemp R, Dos Santos JS. Different options of endosonography-guided biliary drainage after endoscopic retrograde cholangio-pancreatography failure. World J Gastrointest Endosc. 2018;10:99-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Oh D, Park DH, Song TJ, Lee SS, Seo DW, Lee SK, Kim MH. Optimal biliary access point and learning curve for endoscopic ultrasound-guided hepaticogastrostomy with transmural stenting. Therap Adv Gastroenterol. 2017;10:42-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |