Published online Mar 16, 2019. doi: 10.4253/wjge.v11.i3.219
Peer-review started: January 7, 2019
First decision: January 30, 2019
Revised: February 21, 2019
Accepted: March 11, 2019
Article in press: March 11, 2019
Published online: March 16, 2019
Processing time: 70 Days and 13.3 Hours
The role of endoscopic retrograde cholangiopancreatography (ERCP) has dramatically changed in the last years, mainly into that of a therapeutic procedure. The treatment of benign biliary disease, like “difficult” choledocolithiasis, with endoscopic papillary large balloon dilation combined with endoscopic sphinterotomy has proven an effective and safe technique. Moreover, safety in ERCP has improved as well, with the prevention of post-ERCP pancreatitis and patient-to-patient transmission of infections. The advent of self-expandable metal stenting has radically changed the management of biliopancreatic malignant strictures, while the role for therapy of benign strictures is still controversial. In addition, cholangioscopy (though the direct visualization of the biliopancreatic ductal system) has allowed for characterization of indeterminate biliary strictures and facilitated rescue therapy of large biliary stones deemed removable. Encouraging data from tissue ablation techniques, such as photodynamic therapy and radiofrequency ablation, need to be confirmed by large sample size clinical controlled trials. On the other hand, we have no drug-coated stents yet available to implant and evidence for the use of biodegradable stents is still weak. The competency and privileging of ERCP and endoscopic ultrasonography have been analyzed longer but the switch between the two procedures, at the same time, is becoming ordinary; as such, the endoscopist interested in this field should undergo parallel edification through training plans. Finally, the American Society for Gastrointestinal Endoscopy’s statement on non-anesthesiologist administration of propofol for gastrointestinal endoscopy is not actually endorsed by the European Society of Anaesthesiology, having many medical-legal implications in some European countries.
Core tip: Endoscopic retrograde cholangiopancreatography (ERCP) has seen radical changes within the last three decades. The development of endoscopic ultrasonography and other imaging technologies has changed the role of ERCP from a diagnostic tool to a unique therapeutic and imaging platform. New technological developments in ERCP for diagnosis and treatment have been slow to progress, thus increasing the necessity of interest in diagnostic and therapeutic fields.
- Citation: Salerno R, Mezzina N, Ardizzone S. Endoscopic retrograde cholangiopancreatography, lights and shadows: Handle with care. World J Gastrointest Endosc 2019; 11(3): 219-230
- URL: https://www.wjgnet.com/1948-5190/full/v11/i3/219.htm
- DOI: https://dx.doi.org/10.4253/wjge.v11.i3.219
Endoscopic retrograde cholangiopancreatography (ERCP) has seen radical changes within the last three decades. The development of endoscopic ultrasonography (EUS) and other imaging technologies has changed the role of ERCP from a diagnostic tool to a unique therapeutic and imaging platform. New technological developments in ERCP for diagnosis and treatment have been slow to progress, thus increasing the necessity of interest in diagnostic and therapeutic fields.
Some critical dilemmas, like the management of "difficult" choledocolithiasis or the decrease of ERCP-related complications like pancreatitis, have been partially solved, while some others remain. For instance, the direct visualization into the biliary tree via cholangioscopy (CS) allows for targeted tissue sampling or stone management. The self-expandable metal stent (SEMS) has also dramatically changed the management of biliopancreatic malignant strictures while the role of treatment for benign strictures is still controversial. In addition, emerging alerts from the Food and Drug Administration (commonly referred to as the ‘FDA’) to a potential association between multidrug-resistant bacteria and duodenoscopes have opened new scenarios to endoscope reprocessing procedures and stimulated the Industry to improve the research in this field as well.
Initially, EUS was introduced as a purely diagnostic procedure. Along with ERCP, technological development has gradually changed the role of EUS to therapeutic application as well. EUS and ERCP share many clinical indications, including equipment and devices, at the same time for the same patient; thus, in a manner greater than their competitors, they are truly complementary, with remarkable ability for mutual aid. This “shared approach” is changing our minds more and more in terms of training and the learning curve. The newly developed ablation therapy, tissue sampling, and endoscopic ultrasound-guided ERCP are leading us into a new dimension, wherein the future biliopancreatic endoscopy might match with genomic research to develop “personalized therapy” for our patients.
This review will critically analyze the big and small steps which have been made since ERCP has been introduced, with a look into the future.
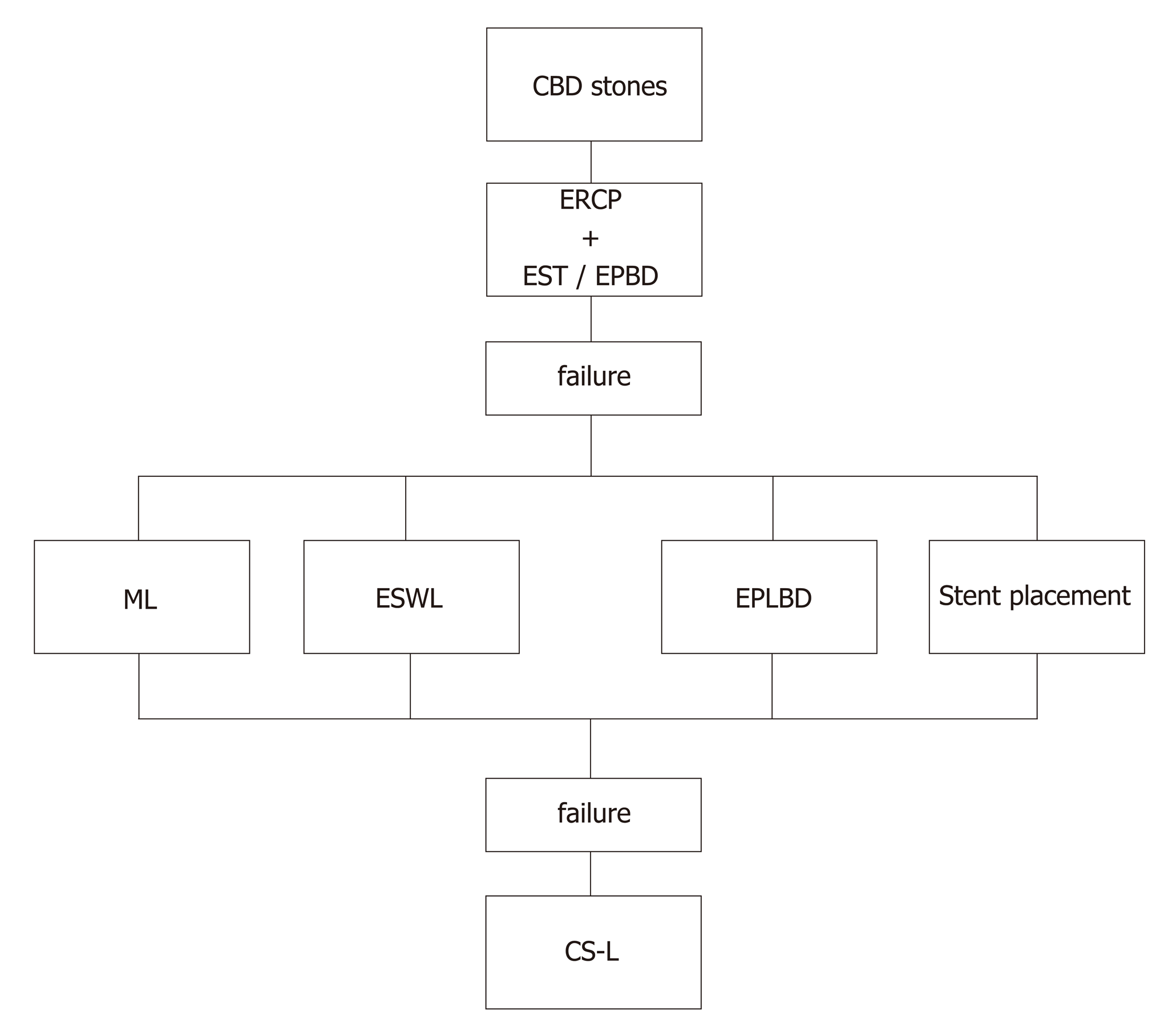
The so-called “difficult stones” are characterized as biliary stones that cannot be extracted easily with a basket or balloon after endoscopic sphincterotomy (EST) or endoscopic papillary balloon dilatation (commonly known as EPBD), mainly due to stone size (diameter > 15 mm), consistency or anatomical variations (i.e. postsurgical anatomy, diverticula, duodenal strictures). In these cases, temporary stent insertion or additional endoscopic procedures, like mechanical lithotripsy or extracorporeal shock wave lithotripsy, are performed with the need of multiple ERCP procedures and eliciting several complications. For these reasons, alternative approaches have been suggested. One of the most frequently used among these is the endoscopic papillary large balloon dilation (EPLBD) combined with EST, as described for the first time by Ersoz et al[1] and having a high success rate (90%) in extracting large stones in a single session and low complication rate (16%).
These findings have improved the endoscopist’s clinical practice since some features, like large bile duct stones on cholangiography or cross-sectional imaging and distal bile duct strictures, can easily guide our choice on when to perform EPLBD or not. This technique is relatively safe, however careful evaluation of radiological imaging is mandatory since the diameter of the balloon should not exceed the diameter of the distal bile duct and the EPLBD should not be performed when the distal bile duct is not dilated to avoid the risk of perforation[2]. A recent meta-analysis on EST plus EPLBD versus EST alone for choledocholithiasis showed fewer overall complications (OR = 0.53, 95%CI: 0.33-0.85, P = 0.008) and decreased use of mechanical lithotripsy (OR = 0.26, 95%CI: 0.08-0.82, P = 0.02) in the EST plus EPLBD group, with no significant differences regarding adverse events and stone clearance[3]. More recently, Hakuta et al[4] evaluated short- and long-term outcomes of EPLBD without EST and EPBD for large stones; in a propensity-matched analysis involving 44 patients, EPLBD without EST was significantly more effective for removal of large stones but showed worse long-term outcomes compared to EPBD. EPLBD in patients with periampullary diverticula was found to be safe in a multicentric case series involving four Italian ERCP high-volume centers with complications reported in 8/80 patients and, among these, only 1 severe (duodenal perforation)[5].
CS allows direct visualization of the biliopancreatic ductal system. Initially born as an adjunct procedure performed during surgery or percutaneous transhepatic cholangiography, today CS is mainly performed perorally during ERCP[6]. Different types of CS are possible; the single-operator CS (SOC) with “mother-daughter” cholangioscope (SpyglassTM system, Boston Scientific, Natick, MA, United States) is the most widely used technique and is gradually replacing the first-introduced dual-operator CS. More recently, ultrathin endoscopes have been introduced, permitting a direct peroral CS[7]. The main CS clinical indications include both diagnostic and therapeutic procedures.
The most intriguing of the emerging applications is the characterization of indeterminate biliary strictures. In fact, although ERCP has a high specificity (> 90%) in detecting malignancy, it is burdened by a low sensitivity (about 40%), even when brushing or biopsy is performed[8,9]; in this setting, CS allows a higher - but still not satisfying - diagnostic yield by both the direct endoscopic visualization and bioptic sampling, which is possible during the same procedure. In a recent systematic review including 456 patients among 10 studies, the pooled sensitivity and specificity of SOC-guided biopsies in the diagnosis of malignant strictures were 60.1% and 98%, respectively[10]. Interestingly, the endoscopic visual appearance seems to be more sensitive for malignancy than the targeted biopsies (but at the price of a lower specificity), as determined in the 2011 study by Chen et al[11] and subsequently confirmed by the cited review.
The visual impression at CS has emerged as a relevant aid, especially in cases of non-diagnostic brushing or biopsy performed with ERCP (pooled sensitivity and specificity 74.7% and 93.3%, respectively), suggesting a possible role in the diagnostic algorithm. However, there are some major issues to consider. At present, there are no validated imaging criteria for CS, as reflected by the sub-optimal inter-observer agreement[12]; furthermore, some concerns have been raised about the reliability of a diagnosis of malignancy based purely on visual appearance.
The main clinical application currently is the management of difficult biliary stones. CS can be successfully performed after failure of bile duct clearance during ERCP, guiding electrohydraulic or laser lithotripsy; this approach has been shown to be effective and safe, with a success rate ranging from 77% to 96% for dual-operator CS13-16] and 90% to 100% for SOC[11,17,18]. This evidence has made CS-guided lithotripsy a suitable alternative to EPLBD, as recently evaluated in a randomized controlled trial (RCT) which showed no differences between the two techniques[19]. However, since CS is significantly more expensive than EPLBD, a more reasonable approach could be to limit it to cases of EPLBD failures. Based on the current evidence, we propose an algorithm of endoscopic treatment for common bile duct stones (Figure 1).
ERCP is associated with several possible complications, including post-procedural bleeding, perforations, pancreatitis and cholangitis; their incidence largely depends on the complexity of the procedure, which can be assessed by various scoring classifications (i.e. the American Society for Gastrointestinal Endoscopy (ASGE) grading system)[20,21].
Post-ERCP pancreatitis (PEP) is the most common adverse event in ERCP, with incidence ranging from 3% up to 10%[22]. Since PEP is associated with a significant mortality rate (0.7%) as well as an extended hospitalization rate, strategies to prevent its occurrence have been largely investigated. Among the many possible drugs studied for prophylaxis of PEP, the rectal administration of 100 mg of diclofenac or indomethacin-proposed for the first time by Elmunzer et al[23] in 2012 - has been endorsed by European guidelines based on the evidence obtained from four RCTs and three meta-analysis[24].
After this first report, additional evidence about the protective role of nonsteroidal anti-inflammatory drugs (commonly known as NSAIDs) in PEP has been produced but with conflicting results[25-27]. However, two recent meta-analyses confirmed the protective role of NSAIDs for PEP, both carried out in high-risk and average-risk patients[28,29]. In light of these data, both the latest European and American guidelines[22,24] recommend the universal administration of rectal indomethacin in patients undergoing ERCP. To note, there are still variables which need further evaluation, like the best administration route (oral vs rectal), timing (before or after the procedure) and patients’ selection (high-risk vs everyone)[30].
Another prophylactic measure against PEP is an aggressive intravenous hydration. In a 2014 study, Buxbaum et al[31] found a significant reduction in PEP incidence among patients receiving hydration with lactated Ringer’s solution (3 mL/kg per hr during procedure, 20 mL/kg bolus immediately after, and then 3 mL/kg per hr for 8 hr) versus standard hydration (0% vs 17%, P = 0.016). After this pilot study, more data about the role of lactated Ringer’s in this setting have been accumulating. Two recent meta-analyses showed the effectiveness and safety of this strategy[32,33], confirming the promising role of an aggressive hydration protocol for the prevention of PEP.
Apart from pharmacologic prophylaxis, there are other factors which can impact PEP occurrence. A careful patient selection is fundamental to reducing PEP, whereby ERCP is strictly reserved for patients with high probability of therapeutic intervention[22]. In addition, the cannulation technique seems to play an important role. Wire-guided cannulation, in particular, has been found to significantly reduce PEP compared to the contrast-assisted technique (RR = 0.51; 95%CI: 0.32-0.82)[34]. The use of pancreatic duct stent was evaluated in numerous RCTs and a meta-analysis, which have demonstrated a significant reduction in PEP[35]. Thus, pancreatic duct stent placement is recommended in high-risk patients (repeated inadvertent pancreatic duct cannulation) by international guidelines[22,24].
In 2013, the Centers for Disease Control and Prevention sent an alert to the FDA about a potential association between multidrug resistant bacteria and duodenoscopes. Accurate examinations proved that these cases of infection were occurring despite confirmation that the users were following proper manufacturer cleansing and disinfection or sterilization instructions. For this reason, the FDA implemented a continuous monitoring program on the three manufacturers (Fujifilm Medical Systems USA, Inc, Olympus Medical Systems Corporation, Pentax of America) to warrant appropriate corrective actions.
Duodenoscopes are complex instruments to clean because of the many small working parts, like the elevator channel. If not adequately cleansed and disinfected, tissue or fluid from patients can lead to patient-to-patient transmission of infection. Recently, the Quality Assurance in Endoscopy Committee of the ASGE published a guideline for infection control during gastrointestinal endoscopy with new indications for reprocessing duodenoscopes, including use of double reprocessing cycles and uniform or intermittent surveillance programs with the use of a “culture and hold” policy[36,37]. In a recent systematic review, Olafsdottir et al[38] evaluated the correlation between concomitantly sampled adenosine triphosphate and bacterial contamination obtained from the instrument channel and/or elevator mechanism of the duodenoscope, as an alternative method to bacterial culture for evaluating the quality of reprocessing. The authors concluded that current data do not support the direct substitution of adenosine triphosphate for bacterial culture surveillance of duodenoscopes.
Furthermore, corrective actions from manufacturers have been implemented, such as the introduction on the market of new duodenoscopes with a single-use disposable elevator. In the era of single-use devices, like biopsy forceps, snares, sphincterotomes and now disposable elevators, we contemplate the use of "single-patient full equipment kits," so that in the near future we could hope for a single-use duodenoscope too!
ERCP has a crucial role in the diagnosis and management of cholangiocarcinoma. Tissue sampling from brushing and biopsy has a low sensitivity, ranging from 18% to 60%[39,40]. Hopefully, CS could play a role in the early diagnosis of strictures of uncertain nature, without any evidence of metastasis. If the stricture involves the carrefour or above (Bismuth II-IV), it can be potentially harmful to inject contrast[41] to enhance the intrahepatic tree or try a bilateral drainage though multiple stents placement because of the increased risk of cholangitis[42,43].
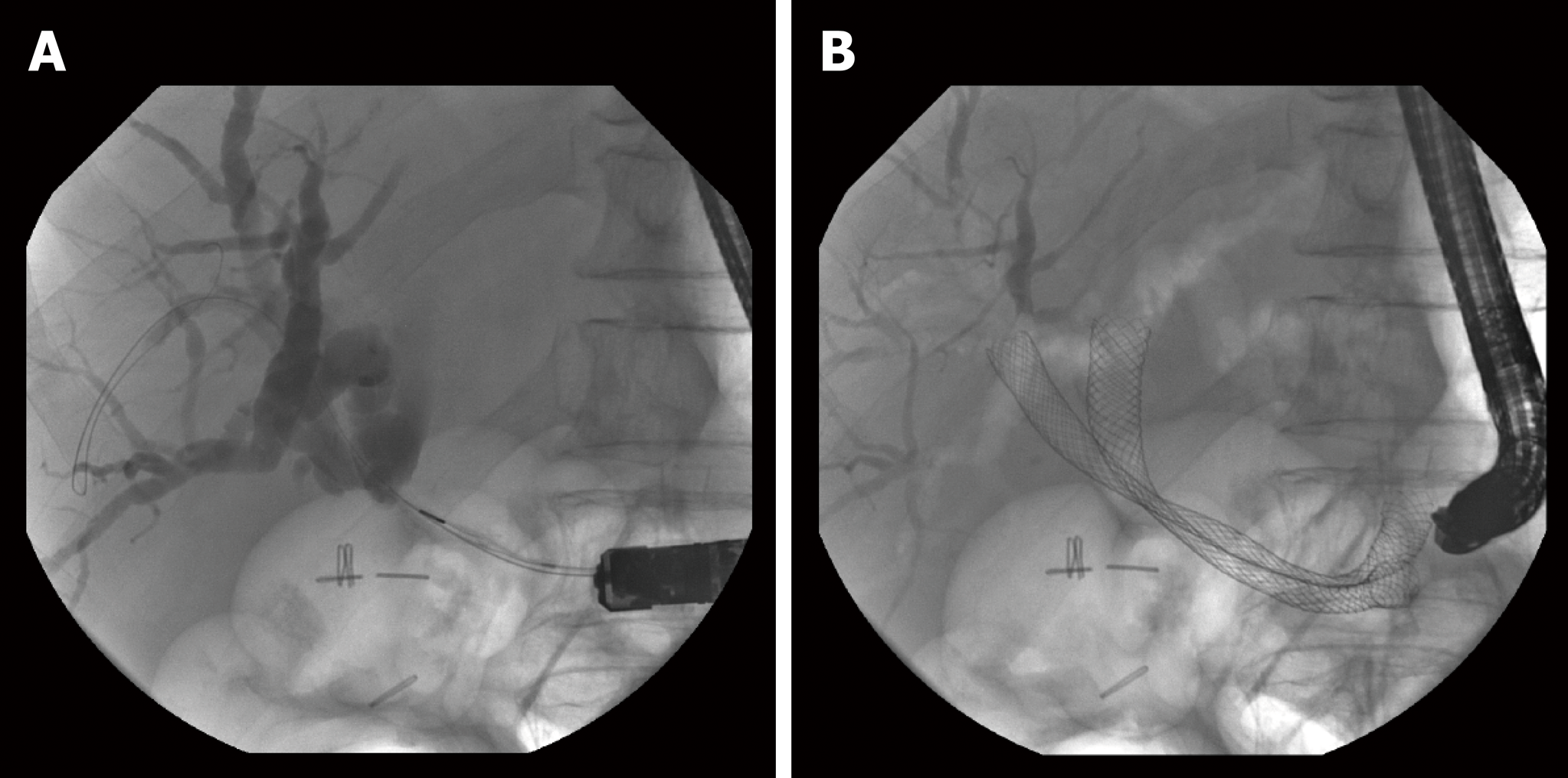
Three RCTs have evaluated the outcomes of unilateral and bilateral drainage[43-45]. On one hand, unilateral stenting has higher rates of technical success because it is easier than bilateral stenting and has a significantly lower rate of early complications[43]. On the other hand, recent data suggest that bilateral drainage has a higher clinical success rate, lower re-intervention rate and equivalent technical success rate compared with unilateral drainage[45], due as well to development in SEMS devices and technical improvement. Advanced hilar strictures are challenging to treat for the most of us, and the common feeling is that bilateral drainage fits better with physiological function of the biliary tree. From this prospective, we could might want to reconsider the term of "bilateral and unilateral" as "complete or incomplete" biliary drainage instead (Figure 2). Plastic stents are recommended when a patient’s expected survival is < 3 mo, however uncovered SEMSs are cost-effective according to one RCT[44].
Long patency and removability make fully covered (fc)SEMS appealing for therapy of benign biliary stricture (BBS) too, but the high rate of migration dramatically reduces the odds of stricture resolution[46]. For this reason, the Industry have developed a new "anti-migration" designed SEMS. In a recent meta-analysis evaluating the clinical outcome of endoscopic covered metal stenting for the resolution of benign biliary stricture, Zheng et al[47] found that the stricture recurrence in a 4-year follow-up was 11% (95%CI: 8%-14%) with the median stents dwelling time of 4.4 mo.
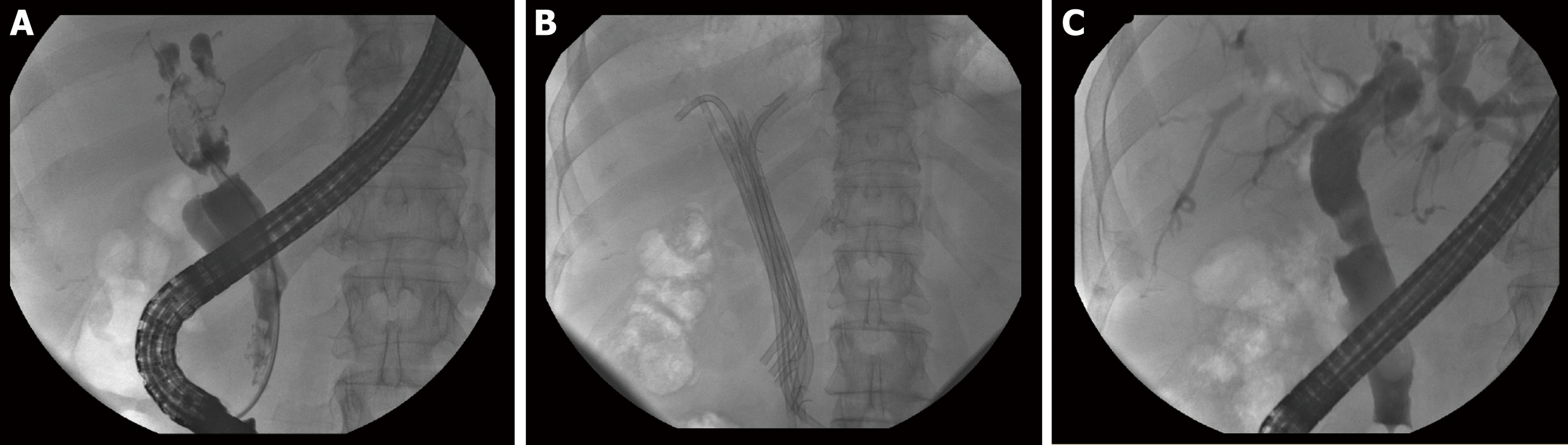
The “multi-stenting” treatment (multiple plastic stenting, insertion of the maximum number of stents, possible every 3-4 mo, for a total duration of 12 mo; Figure 3) still represents the therapy of choice for BBS[48], especially when related to liver transplantation and post-cholecystectomy injury, while the recommendation to use fcSEMS is still weak. In 2016, Coté et al[49] published the results of an RCT regarding the non-inferiority of fcSEMS to plastic stents with respect to stricture resolution. Exclusion criteria were bile duct diameter less than 6 mm and intact gallbladder in whom the cystic duct could be overlapped by a fcSEMS.
Compared with multiple plastic stents (41/48, 85.4%), the resolution rate was 92.6% (50/54) for fcSEMS and the number of ERCPs was significantly lower in the group of fcSEMS versus multiple plastic stents (mean, 2.14 vs 3.24; mean difference, 1.10; 95%CI: 0.74-1.46; P < 0.001), thus indicating that fcSEMS was not inferior to multiple plastic stents after 12 mo in achieving stricture resolution. These data have been recently confirmed in an RCT for anastomotic biliary strictures after liver transplantation[50].
Tringali et al[51] recently evaluated fcSEMS removability, stricture resolution rate, and adverse events in 15 patients with chronic pancreatitis and symptomatic main pancreatic duct stricture located in the head. Stent removability from the main pancreatic duct was feasible in all the cases, and 90% of the patients were asymptomatic after 3 yr. The main adverse event was the “de novo” stricture that fcSEMS induced in 4 patients (27%), while complete distal migration occurred in 46% of cases. The high clinical efficacy and removability are encouraging results but, on the other side, the high migration rate and the occurrence of fcSEMS–induced strictures suggest further evaluation with RCT to assess the role in this setting.
SEMS occlusion by tissue in-growth frequently occurs with uncovered SEMS inserted to treat hilar cholangiocarcinoma; this may, therefore, require more frequent procedures. The endoscopic goal is for adequate biliary drainage to palliate jaundice, but it also aims to reduce the number of reinterventions. New endoscopic techniques may extend stent patency and patient survival.
Photodynamic therapy (PDT) offers the possibility of tumor mass reduction[52] through the necrosis of the neoplastic tissue due to activation at a specific wavelength of a photosensitizing agent, given intravenously, which accumulates in malignant cells. Many studies have compared outcomes with PDT and biliary stenting versus biliary stenting only in palliation of nonresectable cholangiocarcinoma. Recently Moloe and colleagues[53] reported positive effects on biliary drainage, survival and quality of life in patients with advanced cholangiocarcinoma from a systematic review and meta-analysis comparing PDT with biliary stenting versus stenting alone. The most common adverse events were cholangitis and phototoxicity. Since cholangitis occurred in all patients with biliary stenting too, it is inappropriate to relate this effect to PDT. These data endorse the promising role of PDT even though limitations exist due to several biases, like small sample size and the heterogeneous methods used for PDT (percutaneous/endoscopic) and biliary stenting (plastic/SEMS). Well-designed prospective randomized studies are still needed.
The use of SOC for PDT makes the procedure technically more feasible, with shorter fluoroscopy time and longer median survival compared with PDT alone, as reported by Talreja et al[54].
Radiofrequency ablation (RFA) has been used historically longer as a heat delivery system for the destruction of primary and secondary hepatic tumors via localized coagulative necrosis. Recently, a new probe fit for endoscopic use has become available. A retrospective analysis of patients who have undergone RFA for malignant biliary obstruction has suggested that RFA may prolong survival in patients with advanced cholangiocarcinoma, but these findings need to be confirmed by controlled studies. Moreover, adverse events like pain, cholecystitis, hemobilia and injury to adjacent vascular structures may occur, and this suggests that caution must be taken for the endoscopic-guided use of this technique.
Competency in advanced procedures such as ERCP and EUS have been long analyzed. For ERCP, selective cannulation in at least 90% of procedures, accurately interpreting endoscopic and radiologic images, and successful sphincterotomy and stent placement are mandatory for the achievement of competency[55,56]. We know that this goal is hard to accomplish for young endoscopists and it takes a long period (according to the latest evidence, 3-yr fellowship or 1 yr of advanced endoscopy training are required[57,58]) and a large amount of procedures (> 200 ERCP under supervision of a tutor, along with 80 sphinterotomies and 60 stent insertions[59]). The EUS learning curve is not easier than that of the ERCP one and includes at least 225 hands-on cases under supervision[60].
In the past few years, simulators have been introduced with the aim of approximating the human anatomy and recreating the difficulties encountered during real-life situations in human patients. Competency-based fellowship programs have spread, to validate trainee assessment as well. In 2018, Wani and colleagues[61] evaluated, in a prospective multicenter cohort study, quality indicator adherence during the first year of independent practice among physicians who completed endoscopic training with a systematic assessment of competence. They used TEESAT (a procedure-specific competence assessment tool with strong validity evidence endorsed by the ASGE) to assess EUS and ERCP skills in a continuous fashion throughout training. At the end of training, overall technical (EUS, 91.7% and ERCP, 73.9%) and clinical (EUS, 91.7% and ERCP, 94.1%) competence were achieved by most of the trainees, thus confirming the effectiveness of training programs.
EUS and ERCP have both evolved from being a diagnostic procedure to a therapeutic procedure. Always more often in our endoscopy rooms, switches from EUS to ERCP or vice versa happen. Single-session EUS and ERCP have been shown to be accurate and effective, with minimal complication rates[62].
There are no clear data as to whether a single operator performing both procedures has better outcomes compared to those achieved by two different operators. EUS-guided biliary drainage has emerged as an alternative procedure after failed ERCP and ERCP, but expertise is needed to perform some steps, such as stent insertion. Yet, how many endoscopists can shift from ERCP to EUS is unknown. Maybe this is the time not to consider separate programs of education for learning ERCP and EUS but to instead consider one just for those who are really interested in interventional endoscopy. Endoscopists with experience in both techniques will be increasingly important, suggesting a parallel formation in training plans for all future endoscopists with an interest in the area.
Therapy with plastic or metallic stent for benign disease requires repeated endoscopy for stent removal. To avoid this, self-expanding biodegradable biliary stents (BDBSs) have recently become available for ERCP. In the past, hyperplasia or restricturing secondary to biodegradable stents encountered in the gastrointestinal stenting for benign diseases limited further use. Several studies on animal and human models to investigate the use of BDBSs with polylactide or polydioxanone in bile ducts showed good biocompatibility of BDBS, with a negligible histologic foreign body reaction and low risk of restricturing[63,64]. Siiki et al[63] evaluated the effectiveness and safety of a novel BDBS in 13 patients with iatrogenic cystic duct leaks (n = 7) and BBS (n = 6). Complete bile leak resolution was achieved in all patients and the clinical success rate in BBS was 83% in the median follow-up period of 21 mo (range: 14-25 mo). Repeated MRI during the first year demonstrated the gradual degradation pattern.
These data seem promising, but the small number of cases and the absence of control groups suggest careful evaluation and further controlled studies on long-term clinical results.
Metal stenting for malignant biliary strictures may fail because of tumor ingrowth or overgrowth of excessive epithelial or malignant cells. Drug-coated stents have been used for a long time in coronary artery disease to reduce the incidence of stent malfunction. Only paclitaxel has been trialed in humans with malignant obstruction[65,66] and provided encouraging results. Suk and colleagues[65] found overall patency rates at 3, 6 and 12 mo of 100%, 71% and 36%, respectively, in 21 patients with unresectable malignant biliary strictures treated with metallic stent coated with a paclitaxel-incorporated membrane.
The biggest limit to research in this field is that there are no cheap reproducible models to develop an ideal drug-eluting stent able to inhibit malignant cells growing with reasonable histologic tolerance to the biliary epithelium too.
ERCP may result in a prolonged procedure requiring adequate sedation.
According to the ASGE statement about non-anesthesiologist administration of propofol for gastrointestinal endoscopy[67], the administration of propofol and standard sedation by non-anesthesiologists is equivalent in terms of efficacy and safety when done in a setting of properly trained staff and accurate patient selection. Moreover, the use of anesthesiologist-administered propofol for selected patients with no risk factors for sedation-related complications is very costly and does not improve safety or procedural outcomes. The long-standing argument among anesthesiologists about the use of propofol by non-anesthesiologists is supported by the absence of an antidote and by the rapid transition from a level of moderate sedation to deep sedation or even general anesthesia, making it therefore unmanageable for a non-anesthesiologist. Moreover, the label indications report that it ‘‘should be administered only by persons trained in the administration of general anesthesia’’ and in 2012, the European Society of Anaesthesiology retracted its endorsement to the guideline on non-anesthesiologist administration of propofol for gastrointestinal endoscopy, published together with the European Society of Gastrointestinal Endoscopy and the European Society of Gastroenterology and Endoscopy Nurses and Associates[68]. These issues have deep medico-legal implications, above all in some countries in Europe, that do not make us all have sweet dreams.
In the past 30 yr, the role of ERCP has changed deeply.
Radical developments have increased our performance in the diagnostic field, such as with CS, and in the therapeutic field, such as with the advent of SEMS or with the management of "difficult biliary stone” removal. Safety has improved too with the prevention of ERCP-related complications and infections (Table 1).
| Topic | Current evidence |
| Management of " difficult" choledocolithiasis | EST plus EPLBD |
| Cholangioscopy | Electrohydraulic or laser lithotripsy/tissue sampling |
| Safety in ERCP: complications and infections | |
| PEP | Rectal administration of 100 mg of diclofenac or indomethacin and pancreatic duct stenting in high-risk and average-risk patients/aggressive intravenous hydration/wire-guided cannulation |
| Multi-drug resistant bacteria and duodenoscopes | Single-use disposable elevator |
| Management of malignant and benign biliary strictures | Bilateral drainage for hilar strictures with uSEMS /“multi-stenting” treatment for benign biliary strictures |
| Tissue ablation techniques | PDT with biliary stenting in advanced cholangiocarcinoma (more studies are needed) |
| RFA for advanced cholangiocarcinoma (more studies are needed) | |
| Training in biliopancreatic endoscopy | ERCP: at least 200 procedures under supervision of a tutor with 80 sphincterotomies and 60 stent insertions |
| EUS: at least 225 hands-on cases under supervision | |
| Biodegradable and drug-coated stents | BDBSs with polylactide or polydioxanone showed good biocompatibility (more studies are needed) |
| Only paclitaxel has been trialed in humans with malignant obstruction (more studies are needed) | |
| Sedation in ERCP | Propofol and standard sedation by non-anesthesiologists is equivalent in terms of efficacy and safety in a setting of properly trained staff and accurate patient selection (ASGE): ESA retracted its endorsement to ESGE and ESGENA |
On the other hand, there are still wide grey areas. We are still far from using biodegradable stents and this means repeating ERCP in patients with benign biliary strictures. Unlike percutaneous therapy of acute myocardial infarction, we have not yet applied drug-coated stent implantation. Even if non-anesthesiologist administration of propofol has been found to be safe in an evidence-based assessment, there is no endorsement from the Anesthesiologists and this can represent a big limitation in some countries.
RFA and PDT are promising tissue ablation techniques; the effect seems to not only be localized but it may prolong the survival of patients with advanced cholangiocar-cinoma; however, a large sample size from a controlled study is still needed.
ERCP has given us the chance to directly access the biliary tree and pancreatic duct and this has been a precious achievement, but we have focused our attention to find the best way to treat biliopancreatic disease under the "one size fits all" motto. In the near future, direct visualization and tissue sampling might lead us to understand better the genomic alterations in every single patient, thus allowing for "personalized" targeted molecular therapy.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Abd-Elsalam S, Lazăr DC, Sitkin S, Watanabe T S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Zhang YL
| 1. | Ersoz G, Tekesin O, Ozutemiz AO, Gunsar F. Biliary sphincterotomy plus dilation with a large balloon for bile duct stones that are difficult to extract. Gastrointest Endosc. 2003;57:156-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 256] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 2. | Kim TH, Kim JH, Seo DW, Lee DK, Reddy ND, Rerknimitr R, Ratanachu-Ek T, Khor CJ, Itoi T, Yasuda I, Isayama H, Lau JY, Wang HP, Chan HH, Hu B, Kozarek RA, Baron TH. International consensus guidelines for endoscopic papillary large-balloon dilation. Gastrointest Endosc. 2016;83:37-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 3. | de Clemente Junior CC, Bernardo WM, Franzini TP, Luz GO, Dos Santos MEL, Cohen JM, de Moura DTH, Marinho FRT, Coronel M, Sakai P, de Moura EGH. Comparison between endoscopic sphincterotomy vs endoscopic sphincterotomy associated with balloon dilation for removal of bile duct stones: A systematic review and meta-analysis based on randomized controlled trials. World J Gastrointest Endosc. 2018;10:130-144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Hakuta R, Kawahata S, Kogure H, Nakai Y, Saito K, Saito T, Hamada T, Takahara N, Uchino R, Mizuno S, Tsujino T, Tada M, Sakamoto N, Isayama H, Koike K. Endoscopic papillary large balloon dilation and endoscopic papillary balloon dilation both without sphincterotomy for removal of large bile duct stones: A propensity-matched analysis. Dig Endosc. 2019;31:59-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Zulli C, Grande G, Tontini GE, Labianca O, Geraci G, Sciumè C, Antypas P, Fiocca F, Manes G, Devani M, Manta R, Maurano A. Endoscopic papillary large balloon dilation in patients with large biliary stones and periampullary diverticula: Results of a multicentric series. Dig Liver Dis. 2018;50:828-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | ASGE Technology Committee; Komanduri S, Thosani N, Aslanian HR, Enestvedt BK, Manfredi M, Maple JT, Navaneethan U, Pannala R, Parsi MA, Smith ZL, Sullivan SA, Banerjee S. Cholangiopancreatoscopy. Gastrointest Endosc. 2016;84:209-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 7. | Tringali A, Lemmers A, Meves V, Terheggen G, Pohl J, Manfredi G, Häfner M, Costamagna G, Devière J, Neuhaus H, Caillol F, Giovannini M, Hassan C, Dumonceau JM. Intraductal biliopancreatic imaging: European Society of Gastrointestinal Endoscopy (ESGE) technology review. Endoscopy. 2015;47:739-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 8. | Burnett AS, Calvert TJ, Chokshi RJ. Sensitivity of endoscopic retrograde cholangiopancreatography standard cytology: 10-y review of the literature. J Surg Res. 2013;184:304-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 9. | Navaneethan U, Njei B, Lourdusamy V, Konjeti R, Vargo JJ, Parsi MA. Comparative effectiveness of biliary brush cytology and intraductal biopsy for detection of malignant biliary strictures: a systematic review and meta-analysis. Gastrointest Endosc. 2015;81:168-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 340] [Article Influence: 34.0] [Reference Citation Analysis (1)] |
| 10. | Navaneethan U, Hasan MK, Lourdusamy V, Njei B, Varadarajulu S, Hawes RH. Single-operator cholangioscopy and targeted biopsies in the diagnosis of indeterminate biliary strictures: a systematic review. Gastrointest Endosc. 2015;82:608-14.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 200] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 11. | Chen YK, Parsi MA, Binmoeller KF, Hawes RH, Pleskow DK, Slivka A, Haluszka O, Petersen BT, Sherman S, Devière J, Meisner S, Stevens PD, Costamagna G, Ponchon T, Peetermans JA, Neuhaus H. Single-operator cholangioscopy in patients requiring evaluation of bile duct disease or therapy of biliary stones (with videos). Gastrointest Endosc. 2011;74:805-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 204] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 12. | Sethi A, Widmer J, Shah NL, Pleskow DK, Edmundowicz SA, Sejpal DV, Gress FG, Pop GH, Gaidhane M, Sauer BG, Stevens PD, Kahaleh M. Interobserver agreement for evaluation of imaging with single operator choledochoscopy: what are we looking at? Dig Liver Dis. 2014;46:518-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Arya N, Nelles SE, Haber GB, Kim YI, Kortan PK. Electrohydraulic lithotripsy in 111 patients: a safe and effective therapy for difficult bile duct stones. Am J Gastroenterol. 2004;99:2330-2334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 105] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Piraka C, Shah RJ, Awadallah NS, Langer DA, Chen YK. Transpapillary cholangioscopy-directed lithotripsy in patients with difficult bile duct stones. Clin Gastroenterol Hepatol. 2007;5:1333-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Swahn F, Edlund G, Enochsson L, Svensson C, Lindberg B, Arnelo U. Ten years of Swedish experience with intraductal electrohydraulic lithotripsy and laser lithotripsy for the treatment of difficult bile duct stones: an effective and safe option for octogenarians. Surg Endosc. 2010;24:1011-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Tsuyuguchi T, Sakai Y, Sugiyama H, Ishihara T, Yokosuka O. Long-term follow-up after peroral cholangioscopy-directed lithotripsy in patients with difficult bile duct stones, including Mirizzi syndrome: an analysis of risk factors predicting stone recurrence. Surg Endosc. 2011;25:2179-2185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Chen YK, Pleskow DK. SpyGlass single-operator peroral cholangiopancreatoscopy system for the diagnosis and therapy of bile-duct disorders: a clinical feasibility study (with video). Gastrointest Endosc. 2007;65:832-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 285] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 18. | Maydeo A, Kwek BE, Bhandari S, Bapat M, Dhir V. Single-operator cholangioscopy-guided laser lithotripsy in patients with difficult biliary and pancreatic ductal stones (with videos). Gastrointest Endosc. 2011;74:1308-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 19. | Franzini T, Moura RN, Bonifácio P, Luz GO, de Souza TF, Dos Santos MEL, Rodela GL, Ide E, Herman P, Montagnini AL, D'Albuquerque LAC, Sakai P, de Moura EGH. Complex biliary stones management: cholangioscopy versus papillary large balloon dilation - a randomized controlled trial. Endosc Int Open. 2018;6:E131-E138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 20. | Cotton PB, Eisen G, Romagnuolo J, Vargo J, Baron T, Tarnasky P, Schutz S, Jacobson B, Bott C, Petersen B. Grading the complexity of endoscopic procedures: results of an ASGE working party. Gastrointest Endosc. 2011;73:868-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 192] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 21. | Sahar N, La Selva D, Gluck M, Gan SI, Irani S, Larsen M, Ross AS, Kozarek RA. The ASGE grading system for ERCP can predict success and complication rates in a tertiary referral hospital. Surg Endosc. 2019;33:448-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | ASGE Standards of Practice Committee; Chandrasekhara V, Khashab MA, Muthusamy VR, Acosta RD, Agrawal D, Bruining DH, Eloubeidi MA, Fanelli RD, Faulx AL, Gurudu SR, Kothari S, Lightdale JR, Qumseya BJ, Shaukat A, Wang A, Wani SB, Yang J, DeWitt JM. Adverse events associated with ERCP. Gastrointest Endosc. 2017;85:32-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 533] [Article Influence: 66.6] [Reference Citation Analysis (0)] |
| 23. | Elmunzer BJ, Scheiman JM, Lehman GA, Chak A, Mosler P, Higgins PD, Hayward RA, Romagnuolo J, Elta GH, Sherman S, Waljee AK, Repaka A, Atkinson MR, Cote GA, Kwon RS, McHenry L, Piraka CR, Wamsteker EJ, Watkins JL, Korsnes SJ, Schmidt SE, Turner SM, Nicholson S, Fogel EL; U. S. Cooperative for Outcomes Research in Endoscopy (USCORE). A randomized trial of rectal indomethacin to prevent post-ERCP pancreatitis. N Engl J Med. 2012;366:1414-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 503] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 24. | Dumonceau JM, Andriulli A, Deviere J, Mariani A, Rigaux J, Baron TH, Testoni PA; European Society of Gastrointestinal Endoscopy. European Society of Gastrointestinal Endoscopy (ESGE) Guideline: prophylaxis of post-ERCP pancreatitis. Endoscopy. 2010;42:503-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 185] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 25. | Levenick JM, Gordon SR, Fadden LL, Levy LC, Rockacy MJ, Hyder SM, Lacy BE, Bensen SP, Parr DD, Gardner TB. Rectal Indomethacin Does Not Prevent Post-ERCP Pancreatitis in Consecutive Patients. Gastroenterology. 2016;150:911-7; quiz e19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 148] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 26. | Thiruvengadam NR, Forde KA, Ma GK, Ahmad N, Chandrasekhara V, Ginsberg GG, Ho IK, Jaffe D, Panganamamula KV, Kochman ML. Rectal Indomethacin Reduces Pancreatitis in High- and Low-Risk Patients Undergoing Endoscopic Retrograde Cholangiopancreatography. Gastroenterology. 2016;151:288-297.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 27. | Luo H, Zhao L, Leung J, Zhang R, Liu Z, Wang X, Wang B, Nie Z, Lei T, Li X, Zhou W, Zhang L, Wang Q, Li M, Zhou Y, Liu Q, Sun H, Wang Z, Liang S, Guo X, Tao Q, Wu K, Pan Y, Guo X, Fan D. Routine pre-procedural rectal indometacin versus selective post-procedural rectal indometacin to prevent pancreatitis in patients undergoing endoscopic retrograde cholangiopancreatography: a multicentre, single-blinded, randomised controlled trial. Lancet. 2016;387:2293-2301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 156] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 28. | Lyu Y, Cheng Y, Wang B, Xu Y, Du W. What is impact of nonsteroidal anti-inflammatory drugs in the prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis: a meta-analysis of randomized controlled trials. BMC Gastroenterol. 2018;18:106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 29. | He X, Zheng W, Ding Y, Tang X, Si J, Sun LM. Rectal Indomethacin Is Protective against Pancreatitis after Endoscopic Retrograde Cholangiopancreatography: Systematic Review and Meta-Analysis. Gastroenterol Res Pract. 2018;2018:9784841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | George J, Saluja AK, Barkin JS. Indomethacin to Post-Endoscopic Retrograde Cholangiopancreatography Pancreatitis: When and How? Gastroenterology. 2017;152:306-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 31. | Buxbaum J, Yan A, Yeh K, Lane C, Nguyen N, Laine L. Aggressive hydration with lactated Ringer's solution reduces pancreatitis after endoscopic retrograde cholangiopancreatography. Clin Gastroenterol Hepatol. 2014;12:303-7.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 140] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 32. | Zhang ZF, Duan ZJ, Wang LX, Zhao G, Deng WG. Aggressive Hydration With Lactated Ringer Solution in Prevention of Postendoscopic Retrograde Cholangiopancreatography Pancreatitis: A Meta-analysis of Randomized Controlled Trials. J Clin Gastroenterol. 2017;51:e17-e26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 33. | Wu D, Wan J, Xia L, Chen J, Zhu Y, Lu N. The Efficiency of Aggressive Hydration With Lactated Ringer Solution for the Prevention of Post-ERCP Pancreatitis: A Systematic Review and Meta-analysis. J Clin Gastroenterol. 2017;51:e68-e76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 34. | Tse F, Yuan Y, Moayyedi P, Leontiadis GI. Guide wire-assisted cannulation for the prevention of post-ERCP pancreatitis: a systematic review and meta-analysis. Endoscopy. 2013;45:605-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 35. | Mazaki T, Mado K, Masuda H, Shiono M. Prophylactic pancreatic stent placement and post-ERCP pancreatitis: an updated meta-analysis. J Gastroenterol. 2014;49:343-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 142] [Article Influence: 12.9] [Reference Citation Analysis (1)] |
| 36. | ASGE Quality Assurance in Endoscopy Committee; Calderwood AH, Day LW, Muthusamy VR, Collins J, Hambrick RD 3rd, Brock AS, Guda NM, Buscaglia JM, Petersen BT, Buttar NS, Khanna LG, Kushnir VM, Repaka A, Villa NA, Eisen GM. ASGE guideline for infection control during GI endoscopy. Gastrointest Endosc. 2018;87:1167-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 135] [Article Influence: 19.3] [Reference Citation Analysis (1)] |
| 37. | U.S. Food and Drug Administration. Infections Associated with Reprocessed Duodenoscopes. Accessed February 4, 2018. Available from: https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ReprocessingofReusableMedicalDevices/ucm454630.htm. |
| 38. | Olafsdottir LB, Whelan J, Snyder GM. A systematic review of adenosine triphosphate as a surrogate for bacterial contamination of duodenoscopes used for endoscopic retrograde cholangiopancreatography. Am J Infect Control. 2018;46:697-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 39. | De Bellis M, Sherman S, Fogel EL, Cramer H, Chappo J, McHenry L, Watkins JL, Lehman GA. Tissue sampling at ERCP in suspected malignant biliary strictures (Part 1). Gastrointest Endosc. 2002;56:552-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 43] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 40. | Fogel EL, deBellis M, McHenry L, Watkins JL, Chappo J, Cramer H, Schmidt S, Lazzell-Pannell L, Sherman S, Lehman GA. Effectiveness of a new long cytology brush in the evaluation of malignant biliary obstruction: a prospective study. Gastrointest Endosc. 2006;63:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 119] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 41. | Chang WH, Kortan P, Haber GB. Outcome in patients with bifurcation tumors who undergo unilateral versus bilateral hepatic duct drainage. Gastrointest Endosc. 1998;47:354-362. [PubMed] |
| 42. | Hintze RE, Abou-Rebyeh H, Adler A, Veltzke-Schlieker W, Felix R, Wiedenmann B. Magnetic resonance cholangiopancreatography-guided unilateral endoscopic stent placement for Klatskin tumors. Gastrointest Endosc. 2001;53:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 113] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 43. | De Palma GD, Galloro G, Siciliano S, Iovino P, Catanzano C. Unilateral versus bilateral endoscopic hepatic duct drainage in patients with malignant hilar biliary obstruction: results of a prospective, randomized, and controlled study. Gastrointest Endosc. 2001;53:547-553. [PubMed] |
| 44. | Mukai T, Yasuda I, Nakashima M, Doi S, Iwashita T, Iwata K, Kato T, Tomita E, Moriwaki H. Metallic stents are more efficacious than plastic stents in unresectable malignant hilar biliary strictures: a randomized controlled trial. J Hepatobiliary Pancreat Sci. 2013;20:214-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 166] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 45. | Lee TH, Kim TH, Moon JH, Lee SH, Choi HJ, Hwangbo Y, Hyun JJ, Choi JH, Jeong S, Kim JH, Park DH, Han JH, Park SH. Bilateral versus unilateral placement of metal stents for inoperable high-grade malignant hilar biliary strictures: a multicenter, prospective, randomized study (with video). Gastrointest Endosc. 2017;86:817-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 146] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 46. | Devière J, Nageshwar Reddy D, Püspök A, Ponchon T, Bruno MJ, Bourke MJ, Neuhaus H, Roy A, González-Huix Lladó F, Barkun AN, Kortan PP, Navarrete C, Peetermans J, Blero D, Lakhtakia S, Dolak W, Lepilliez V, Poley JW, Tringali A, Costamagna G; Benign Biliary Stenoses Working Group. Successful management of benign biliary strictures with fully covered self-expanding metal stents. Gastroenterology. 2014;147:385-95; quiz e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 170] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 47. | Zheng X, Wu J, Sun B, Wu YC, Bo ZY, Wan W, Gao DJ, Hu B. Clinical outcome of endoscopic covered metal stenting for resolution of benign biliary stricture: Systematic review and meta-analysis. Dig Endosc. 2017;29:198-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 48. | Costamagna G, Pandolfi M, Mutignani M, Spada C, Perri V. Long-term results of endoscopic management of postoperative bile duct strictures with increasing numbers of stents. Gastrointest Endosc. 2001;54:162-168. [PubMed] |
| 49. | Coté GA, Slivka A, Tarnasky P, Mullady DK, Elmunzer BJ, Elta G, Fogel E, Lehman G, McHenry L, Romagnuolo J, Menon S, Siddiqui UD, Watkins J, Lynch S, Denski C, Xu H, Sherman S. Effect of Covered Metallic Stents Compared With Plastic Stents on Benign Biliary Stricture Resolution: A Randomized Clinical Trial. JAMA. 2016;315:1250-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 168] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 50. | Martins FP, De Paulo GA, Contini MLC, Ferrari AP. Metal versus plastic stents for anastomotic biliary strictures after liver transplantation: a randomized controlled trial. Gastrointest Endosc. 2018;87:131.e1-131.e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 51. | Tringali A, Vadalà di Prampero SF, Landi R, Bove V, Familiari P, Hamanaka J, Attili F, Costamagna G. Fully covered self-expandable metal stents to dilate persistent pancreatic strictures in chronic pancreatitis: long-term follow-up from a prospective study. Gastrointest Endosc. 2018;88:939-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 52. | Wiedmann M, Caca K, Berr F, Schiefke I, Tannapfel A, Wittekind C, Mössner J, Hauss J, Witzigmann H. Neoadjuvant photodynamic therapy as a new approach to treating hilar cholangiocarcinoma: a phase II pilot study. Cancer. 2003;97:2783-2790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 117] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 53. | Moole H, Tathireddy H, Dharmapuri S, Moole V, Boddireddy R, Yedama P, Dharmapuri S, Uppu A, Bondalapati N, Duvvuri A. Success of photodynamic therapy in palliating patients with nonresectable cholangiocarcinoma: A systematic review and meta-analysis. World J Gastroenterol. 2017;23:1278-1288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 80] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 54. | Talreja JP, DeGaetani M, Sauer BG, Kahaleh M. Photodynamic therapy for unresectable cholangiocarcinoma: contribution of single operator cholangioscopy for targeted treatment. Photochem Photobiol Sci. 2011;10:1233-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 55. | Coté GA, Imler TD, Xu H, Teal E, French DD, Imperiale TF, Rosenman MB, Wilson J, Hui SL, Sherman S. Lower provider volume is associated with higher failure rates for endoscopic retrograde cholangiopancreatography. Med Care. 2013;51:1040-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 56. | Cotton PB, Hawes RH, Barkun A, Ginsberg GG, Amman S, Cohen J, Ponsky J, Rex DK, Schembre D, Wilcox CM. Excellence in endoscopy: toward practical metrics. Gastrointest Endosc. 2006;63:286-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 57. | Shahidi N, Ou G, Telford J, Enns R. When trainees reach competency in performing ERCP: a systematic review. Gastrointest Endosc. 2015;81:1337-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (1)] |
| 58. | Wani S, Hall M, Wang AY, DiMaio CJ, Muthusamy VR, Keswani RN, Brauer BC, Easler JJ, Yen RD, El Hajj I, Fukami N, Ghassemi KF, Gonzalez S, Hosford L, Hollander TG, Wilson R, Kushnir VM, Ahmad J, Murad F, Prabhu A, Watson RR, Strand DS, Amateau SK, Attwell A, Shah RJ, Early D, Edmundowicz SA, Mullady D. Variation in learning curves and competence for ERCP among advanced endoscopy trainees by using cumulative sum analysis. Gastrointest Endosc. 2016;83:711-9.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 59. | Cotton PB. ERCP (Ensuring Really Competent Practice): enough words-action please! Gastrointest Endosc. 2015;81:1343-1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 60. | Wani S, Coté GA, Keswani R, Mullady D, Azar R, Murad F, Edmundowicz S, Komanduri S, McHenry L, Al-Haddad MA, Hall M, Hovis CE, Hollander TG, Early D. Learning curves for EUS by using cumulative sum analysis: implications for American Society for Gastrointestinal Endoscopy recommendations for training. Gastrointest Endosc. 2013;77:558-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 61. | Wani S, Keswani RN, Han S, Aagaard EM, Hall M, Simon V, Abidi WM, Banerjee S, Baron TH, Bartel M, Bowman E, Brauer BC, Buscaglia JM, Carlin L, Chak A, Chatrath H, Choudhary A, Confer B, Coté GA, Das KK, DiMaio CJ, Dries AM, Edmundowicz SA, El Chafic AH, El Hajj I, Ellert S, Ferreira J, Gamboa A, Gan IS, Gangarosa LM, Gannavarapu B, Gordon SR, Guda NM, Hammad HT, Harris C, Jalaj S, Jowell PS, Kenshil S, Klapman J, Kochman ML, Komanduri S, Lang G, Lee LS, Loren DE, Lukens FJ, Mullady D, Muthusamy VR, Nett AS, Olyaee MS, Pakseresht K, Perera P, Pfau P, Piraka C, Poneros JM, Rastogi A, Razzak A, Riff B, Saligram S, Scheiman JM, Schuster I, Shah RJ, Sharma R, Spaete JP, Singh A, Sohail M, Sreenarasimhaiah J, Stevens T, Tabibian JH, Tzimas D, Uppal DS, Urayama S, Vitterbo D, Wang AY, Wassef W, Yachimski P, Zepeda-Gomez S, Zuchelli T, Early D. Competence in Endoscopic Ultrasound and Endoscopic Retrograde Cholangiopancreatography, From Training Through Independent Practice. Gastroenterology. 2018;155:1483-1494.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 62. | Kawakubo K, Kawakami H, Kuwatani M, Haba S, Kudo T, Abe Y, Kawahata S, Onodera M, Ehira N, Yamato H, Eto K, Sakamoto N. Safety and utility of single-session endoscopic ultrasonography and endoscopic retrograde cholangiopancreatography for the evaluation of pancreatobiliary diseases. Gut Liver. 2014;8:329-332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 63. | Siiki A, Rinta-Kiikka I, Sand J, Laukkarinen J. A pilot study of endoscopically inserted biodegradable biliary stents in the treatment of benign biliary strictures and cystic duct leaks. Gastrointest Endosc. 2018;87:1132-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 64. | Grolich T, Crha M, Novotný L, Kala Z, Hep A, Nečas A, Hlavsa J, Mitáš L, Misík J. Self-expandable biodegradable biliary stents in porcine model. J Surg Res. 2015;193:606-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 65. | Suk KT, Kim JW, Kim HS, Baik SK, Oh SJ, Lee SJ, Kim HG, Lee DH, Won YH, Lee DK. Human application of a metallic stent covered with a paclitaxel-incorporated membrane for malignant biliary obstruction: multicenter pilot study. Gastrointest Endosc. 2007;66:798-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 66. | Jang SI, Kim JH, You JW, Rhee K, Lee SJ, Kim HG, Han J, Shin IH, Park SH, Lee DK. Efficacy of a metallic stent covered with a paclitaxel-incorporated membrane versus a covered metal stent for malignant biliary obstruction: a prospective comparative study. Dig Dis Sci. 2013;58:865-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 67. | Vargo JJ, Cohen LB, Rex DK, Kwo PY. Position statement: nonanesthesiologist administration of propofol for GI endoscopy. Gastrointest Endosc. 2009;70:1053-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 68. | Pelosi P. Retraction of endorsement: European Society of Gastrointestinal Endoscopy, European Society of Gastroenterology and Endoscopy Nurses and Associates, and the European Society of Anaesthesiology Guideline: Non-anesthesiologist administration of propofol for GI endoscopy. Endoscopy. 2012;44:302; author reply 302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |