Published online Jun 16, 2018. doi: 10.4253/wjge.v10.i6.121
Peer-review started: February 2, 2018
First decision: February 28, 2018
Revised: March 2, 2018
Accepted: March 20, 2018
Article in press: March 20, 2018
Published online: June 16, 2018
Processing time: 133 Days and 2.2 Hours
A 69-year-old man with advanced esophageal cancer and 2 early gastric cancers received chemoradiotherapy and was scheduled to undergo subtotal esophagectomy after gastric endoscopic submucosal dissection (ESD). However, left lower esophageal perforation induced by vomiting suddenly occurred, and he urgently underwent esophago-proximal gastrectomy and gastrostomy without reconstruction. The resected specimen showed a complete response of pretreatment for the esophageal cancer and radical resection of one gastric cancer. Radical resection of the other gastric lesion was necessary before reconstruction. The fistula of gastrostoma was gradually dilated from 6.7 to 9.3 mm in order to pass the endoscope. At nine months after emergent operation, gastric ESD was performed via only the gastrostoma. A hemoclip with thread was attached to the specimen, and the thread was pulled out of the gastrostoma. The specimen was able to be removed en bloc, resulting in radical resection. Gastric tube reconstruction through the posterior sternal route was performed at six months after the ESD. He has not developed recurrence of the esophageal or gastric cancer in the two years since the emergent operation.
Core tip: Gastric endoscopic submucosal dissection (ESD), which is a useful and minimally invasive procedure for early gastric cancer, is usually performed through the mouth. This patient’s stomach had a gastrostoma that was not connected to the mouth after surgery for esophageal perforation. The fistula of the gastrostoma was dilated in order to pass the endoscope. ESD for the early gastric cancer was performed via the gastrostoma. The specimen was able to be removed en bloc, and the residual stomach was able to be used for reconstruction. We herein report a unique gastric ESD technique using a gastrostoma.
- Citation: Sasaki T, Uesato M, Ohta T, Murakami K, Nakano A, Matsubara H. Gastric endoscopic submucosal dissection via gastrostoma before the second operation for esophageal perforation: A case report. World J Gastrointest Endosc 2018; 10(6): 121-124
- URL: https://www.wjgnet.com/1948-5190/full/v10/i6/121.htm
- DOI: https://dx.doi.org/10.4253/wjge.v10.i6.121
Endoscopic submucosal dissection (ESD) is a useful, minimally invasive procedure that is used in the management of early gastric cancer[1,2]. The insertion route of the endoscope is usually the oral route. We herein report a unique ESD technique using a gastrostoma in a patient with early gastric cancer without an esophagus.
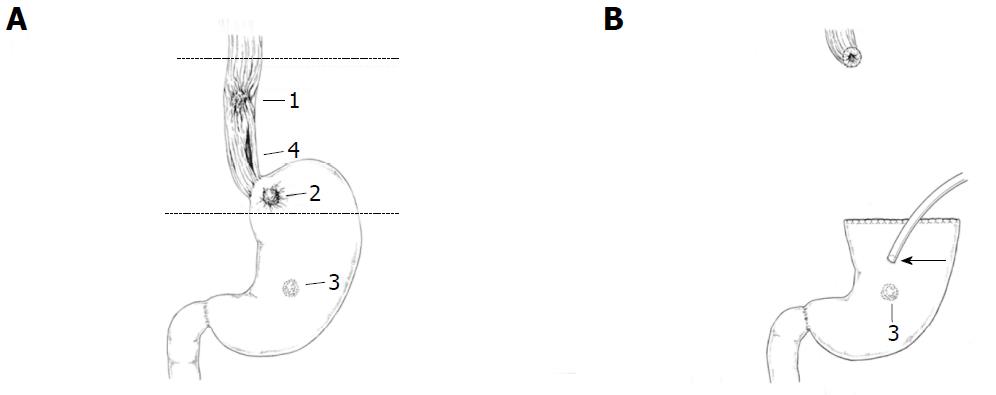
A 69-year-old man with middle thoracic esophageal cancer [T4b (trachea) N2 M0 StageIIIC] and 2 gastric cancers (T1aN0M0 StageIA, T1bN0M0 StageaIA) received chemoradiotherapy and was scheduled to undergo subtotal esophagectomy after gastric ESD. However, left lower esophageal perforation induced by vomiting suddenly occurred, and he urgently underwent esophago-proximal gastrectomy and gastrostomy without reconstruction (Figure 1). The resected specimen showed a complete response to pretreatment of the esophageal cancer and radical resection of one gastric cancer. Radical resection of the other gastric lesion was necessary before reconstruction of the gastric tube. The fistula of gastrostoma was gradually dilated from 6.7 to 9.3 mm using a urethral balloon catheter in order to pass the endoscope (GIF-Q260J; Olympus, Tokyo, Japan) after 4 wk as an outpatient.
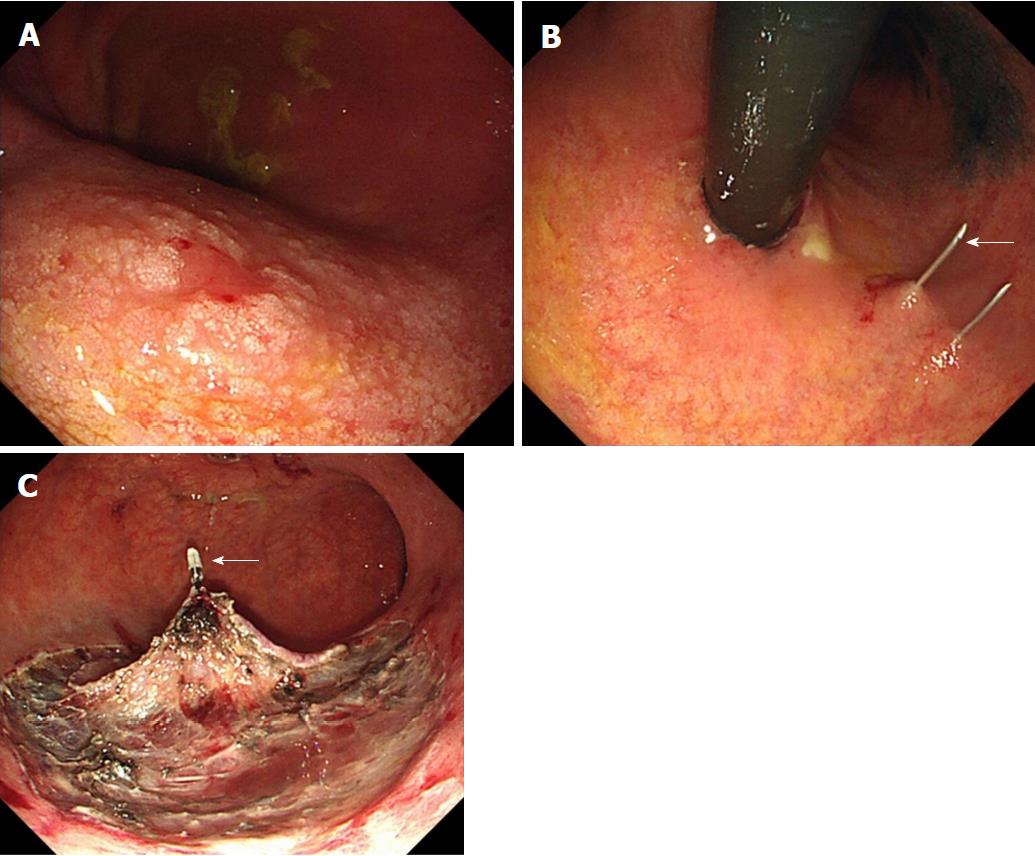
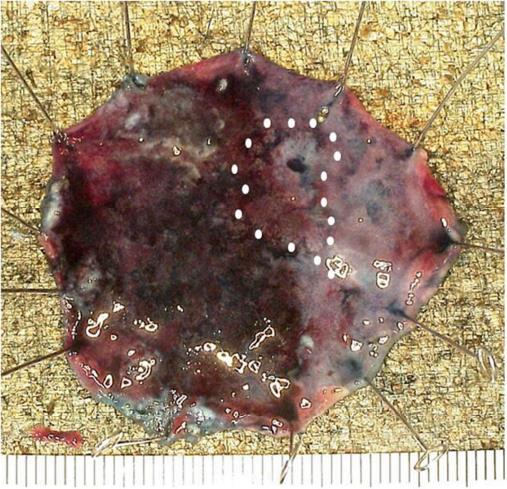
At nine months after emergent operation, gastric ESD was performed through only the gastrostoma. ESD was performed with the patient awake. The gastric lesion was located at the middle posterior wall (Figure 2A), so the patient was placed in the supine position. Just after the insertion of the scope into the stomach, Funada-type gastric wall fixation (Create Medic, Tokyo, Japan) was performed at two opposite points (Figure 2B). Marking was performed around the boundary of the lesion using a needle knife. A sufficient amount of glycerol solution was injected into the submucosal layer. After making a small incision at the anal side, we connected the incision from the anal side to the surrounding lesion using an IT Knife2 (Olympus). We felt dissection to be difficult due to the large amount of vessels and fibrosis in the submucosal layer. A hemoclip (Olympus) with thread was attached to the specimen, and the thread was pulled via the gastrostoma (Figure 2C). The specimen was able to be removed en bloc in seven hours, showing radical resection pathologically (Figure 3). Gastric tube reconstruction through the posterior sternal route was performed at six months after ESD. He has not developed recurrence of esophageal and gastric cancer in the two years since the emergent operation.
ESD is a useful, minimally invasive procedure that is used in the management of early gastric cancer[1,2]. ESD is also actively performed for cases of residual gastric cancer, since this disease is generally considered to be difficult to treat effectively. The insertion route of the endoscope is usually the oral route. However, we herein report a unique ESD technique using a gastrostoma in a patient with early residual gastric cancer without an esophagus.
Five cases of gastric ESD performed in combination via routes other than the mouth have been reported[3-7] (Table 1). Among them, two reports of animal experiments involved gastric ESD via the mouth using a percutaneous endoscopic gastrostomy (PEG) device[6,7]. All five of these reports used a gastrostoma to perform endoscopic mucosal resection or ESD more easily. When reconstruction is performed in cases of esophageal cancer, the stomach is commonly used because it has an abundant blood flow[8]. Our patient scheduled to undergo reconstruction had a stomach without a connection to the mouth. Therefore, ESD had to be performed via only the gastrostoma.
| Ref. | Asano et al[3] | Tokumo et al[4] | Nishiwaki et al[5] | Delius et al[6] | Storm et al[7] | Sasaki |
| Year | 1993 | 1997 | 2005 | 2008 | 2016 | 2018 |
| EMR/ESD | EMR | EMR | ESD | ESD | ESD | ESD |
| Subject | Human | Human | Human | Pig | Pig | Human |
| Number | 1 | 10 | 2 | 10 | 3 | 1 |
| Use of an oral endoscope | Traction | EMR | ESD | ESD | ESD | None |
| Use of a gastrostoma | EMR | Traction | Auxiliary endoscope | Traction | Traction | ESD and Traction |
| Diameter of the gastrostoma (mm) | 8 | 2.6 | 8 | 2.5 | 10, 16 | 9.3 |
| Period from PEG to EMR/ESD | 3 wk | Immediate | 3 wk | Immediate | Immediate | 7 wk1 |
| Gastropexy | Used | Used | None | None | None | Used |
As preparation, the fistula of the gastrostoma must be expanded to make it large enough for the endoscope to pass through. We previously reported a gradual tube dilation method before PEG for obstructive esophageal cancer[9]. This is a safe method, because it does not involve sudden expansion. While the method took longer than usual because our subject was an outpatient, we were able to expand to 9.3 mm without complications. The patient’s posture during ESD was supine because to ensure the stability of the endoscope. However, the lesion at the posterior wall became invisible when bleeding occurred, and without the traction of gravity, the lesion was very difficult to dissect. We were able to resolve this issue by towing the specimen with a thread clip[10]. Of particular note, the thread attached to the clip was pulled via the fistula in our case. Bleeding may be substantial during ESD of a stomach isolated from the esophagus due to poor venous return. We recommend frequent hemostasis to ensure safe ESD. Regarding the gastric wall fixation, if it is necessary to perform ESD without a PEG device, the Funada-type fixation should be performed to ensure safety.
In conclusion, we successfully performed gastric ESD via only the fistula of a gastrostoma. To ensure success, the gradual tube dilation of the fistula, traction with a hemoclip and thread through the gastrostoma and frequent hemostasis should be considered.
A 69-year-old man with advanced esophageal cancer and 2 early gastric cancers received chemoradiotherapy and he was scheduled to undergo subtotal esophagectomy after gastric endoscopic submucosal dissection. However, left lower esophageal perforation suddenly occurred, and he urgently underwent esophago-proximal gastrectomy and gastrostomy.
The patient had one early cancer in the residual stomach without a connection to the esophagus.
The only viable approach to the residual stomach was the gastrostoma.
The fistula of the gastrostoma was gradually dilated to allow the endoscope to pass through. Gastric endoscopic submucosal dissection was performed via only the gastrostoma. A hemoclip with thread was attached to the specimen, and the thread was pulled via the gastrostoma. The specimen was able to be removed en bloc. Gastric tube reconstruction was performed.
We successfully performed gastric endoscopic submucosal dissection through only the fistula of a gastrostoma. To ensure safety and success, the gradual tube dilation of fistula, traction with a hemoclip and thread through the gastrostoma and frequent hemostasis should be considered.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Hoff DAL, Lee CL, Rodrigo L S- Editor: Wang XJ L- Editor: A E- Editor: Tan WW
| 1. | Miyazaki S, Gunji Y, Aoki T, Nakajima K, Nabeya Y, Hayashi H, Shimada H, Uesato M, Hirayama N, Karube T. High en bloc resection rate achieved by endoscopic mucosal resection with IT knife for early gastric cancer. Hepatogastroenterology. 2005;52:954-958. [PubMed] |
| 2. | Uesato M, Nabeya Y, Akai T, Inoue M, Watanabe Y, Kawahira H, Mamiya T, Ohta Y, Motojima R, Kagaya A. Salivary amylase activity is useful for assessing perioperative stress in response to pain in patients undergoing endoscopic submucosal dissection of gastric tumors under deep sedation. Gastric Cancer. 2010;13:84-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Asano M, Nan Y, Ando H. A case of early gastric cancer at the cardia treated endoscopically under endoscopic gastrostomy. Gastrointest Endosc. 1993;35:2687-2692. [DOI] [Full Text] |
| 4. | Tokumo H, Komatsu H, Ishida K, Morinaka K, Ito M. Transgastrostomal endoscopic mucosal resection for the treatment of gastric mucosal lesions. Gastrointest Endosc. 1997;39:1775-1780. [DOI] [Full Text] |
| 5. | Nishiwaki S, Araki H, Niwa Y, Kubota M, Shirakami Y, Gotoh N, Iwashita M, Onogi N, Hayashi T, Maeda T. Usefulness of transgastrostomic endoscopy (TEG) in patient with post percutaneous endoscopic gastrostomy (PEG). Gastrointest Endosc. 2005;47:49-55. [DOI] [Full Text] |
| 6. | von Delius S, Karagianni A, von Weyhern CH, Feussner H, Schuster T, Schmid RM, Frimberger E. Percutaneously assisted endoscopic surgery using a new PEG-minitrocar for advanced endoscopic submucosal dissection (with videos). Gastrointest Endosc. 2008;68:365-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Storm AC, Aihara H, Thompson CC. Novel intragastric trocar placed by PEG technique permits endolumenal use of rigid instruments to simplify complex endoscopic procedures. Gastrointest Endosc. 2016;84:518-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Makuuchi H. [Reconstruction after thoracic esophagectomy]. Nihon Geka Gakkai Zasshi. 2008;109:256-260. [PubMed] |
| 9. | Uesato M, Shuto K, Kono T, Akutsu Y, Hoshino I, Murakami K, Ohta T, Shiratori T, Matsubara H. Gradual tube dilation method before percutaneous endoscopic gastrostomy for obstructive esophageal cancer. Esophagus. 2016;13:68-73. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Oyama T. Counter traction makes endoscopic submucosal dissection easier. Clin Endosc. 2012;45:375-378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 142] [Article Influence: 10.9] [Reference Citation Analysis (0)] |