Published online Oct 16, 2018. doi: 10.4253/wjge.v10.i10.250
Peer-review started: May 29, 2018
First decision: June 6, 2018
Revised: June 24, 2018
Accepted: June 28, 2018
Article in press: June 29, 2018
Published online: October 16, 2018
Processing time: 140 Days and 17 Hours
Patients with long-standing ulcerative colitis (UC) and extensive Crohn’s colitis (CC) are at increased risk for dysplasia and colorectal cancer (CRC). Several studies have shown that UC extending proximal to the rectum, CC involving at least 1/3 of the colon, co-existence of primary sclerosing cholangitis, undetermined or unclassified colitis, family history of CRC and young age at diagnosis appear to be independent risk factors for inflammatory bowel disease (IBD) - related CRC. Therefore, screening and surveillance for CRC in IBD patients is highly recommended by international and national guidelines, whilst colonoscopy remains the unequivocal tool in order to detect potentially resectable dysplastic lesions or CRC at an early stage. Although the importance of screening and surveillance is widely proven, there is a controversy regarding the time of the first colonoscopy and the criteria of who should undergo surveillance. In addition, there are different recommendations among scientific societies concerning which endoscopic method is more efficient to detect dysplasia early, as well as the terminology for reporting visible lesions and the management of those lesions. This article concisely presents the main endoscopic methods and techniques performed for detecting dysplasia and CRC surveillance in patients with IBD focusing on their evidence-based accuracy and efficiency, as well as their cost-effectiveness. Finally, newer methods are mentioned, highlighting their applicability in daily endoscopic practice.
Core tip: There is an established association between inflammatory bowel disease (IBD) and colorectal cancer (CRC). Therefore, surveillance of these patients for CRC is crucial and recommended by international guidelines. In this review we present the main endoscopic methods and techniques performed for detecting dysplasia and CRC surveillance in patients with IBD, highlighting chromoendoscopy with targeted biopsies as the gold standard method. Finally, newer methods are mentioned, examining their applicability in daily endoscopic practice.
- Citation: Galanopoulos M, Tsoukali E, Gkeros F, Vraka M, Karampekos G, Matzaris GJ. Screening and surveillance methods for dysplasia in inflammatory bowel disease patients: Where do we stand? World J Gastrointest Endosc 2018; 10(10): 250-258
- URL: https://www.wjgnet.com/1948-5190/full/v10/i10/250.htm
- DOI: https://dx.doi.org/10.4253/wjge.v10.i10.250
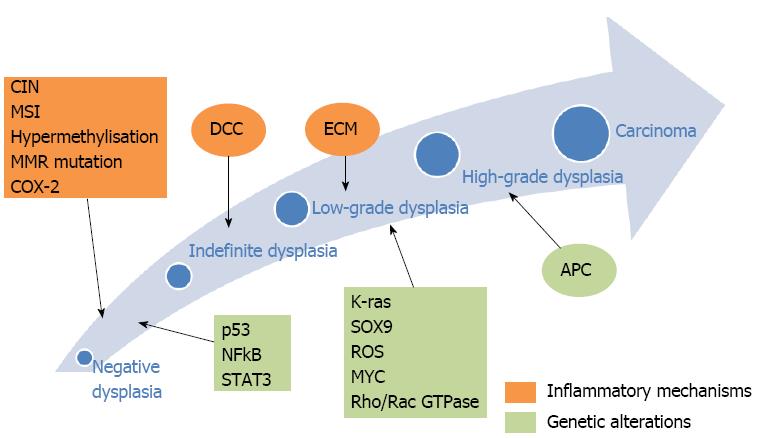
Patients with inflammatory bowel disease (IBD) have a higher incidence of colorectal cancer (CRC) compared to the general population, even though only 1% of all CRC cases are attributed to IBD[1]. The incidence rates reported by Eaden et al[2,3], as well as the St. Mark’s group in the United Kingdom, showed comparable cumulative probabilities of CRC and dysplasia, approximately 8% and 18% by 20 and 30 years of ongoing disease, respectively. According to Bernstein et al[4], both Crohn’s disease (CD) and ulcerative colitis (UC) patients face an increased risk for colon cancer [relative risk (RR) 2.64 and 2.75, respectively]. Factors linked to an increased incidence of CRC include: prolonged duration of colitis, extensive colonic involvement, presence of primary sclerosing cholangitis (PSC), positive family history for CRC and, according to some studies, earlier onset and severity of inflammation[1,5-9] (Table 1). Oncogenesis in IBD has been well described as a result of chronic inflammation, leading via low- and high-grade dysplasia, finally, to CRC[1,10-24] (Figure 1). Dysplasia is divided into two categories: (1) Endoscopically visible dysplastic lesion, e.g., polyps, which are detected by targeted biopsies or resection of endoluminal masses; and (2) Endoscopically invisible dysplasia which is detected by blinded random biopsies on endoscopically normal lumen and is characterized as the most dependable marker for increased CRC risk in IBD patients[1,25,26]. The resection of visible dysplasia, in combination with a rigorous follow-up program has been shown to be a safe alternative to colectomy for select patients[27,28]. On the other hand, a study by Picco et al[29] showed that the detection rate for dysplasia with the use of white light endoscopy (WLE) was 9.3%, compared to 21.3% when using both WLE and dye-spray chromoendoscopy (DCE). This demonstrates the need for the implementation of a surveillance strategy in IBD patients based on better techniques and technologies, aiming at reducing the prevalence of metachronous lesions during follow-up. However, uncertainties exist regarding the soundness of this approach on preventing CRC. In a recent systematic review, people undergoing periodic surveillance for CRC were not found to have lower mortality when compared to those under no surveillance (RR 0.81, 95%CI: 0.17 to 3.83)[30,31].
| High risk factors |
| Annual surveillance |
| Extensive colonic involvement (pancolitis, CD with > 50% colonic involvement) |
| Moderate-severe endoscopic or histological active inflammation sustained over time |
| PSC |
| Disease commencing at age < 15 yr |
| Family history of sporadic CRC in a first-degree relative < 50 yr |
| Presence of a stricture or dysplasia detected during the previous 5 yr |
| High risk factors in case of pouch existence |
| Dysplasia |
| Previous CRC |
| Type C mucosa |
| Intermediate risk |
| Every three years surveillance |
| Mild or moderate endoscopic/histological inflammation sustained over time |
| Family history of sporadic CRC in a first-degree relative older than 50 yr |
| Presence of inflammatory polyps |
| Low risk factors |
| Every five years surveillance |
| Pancolitis without inflammation |
| Left-sided UC or CD with < 50% colonic involvement |
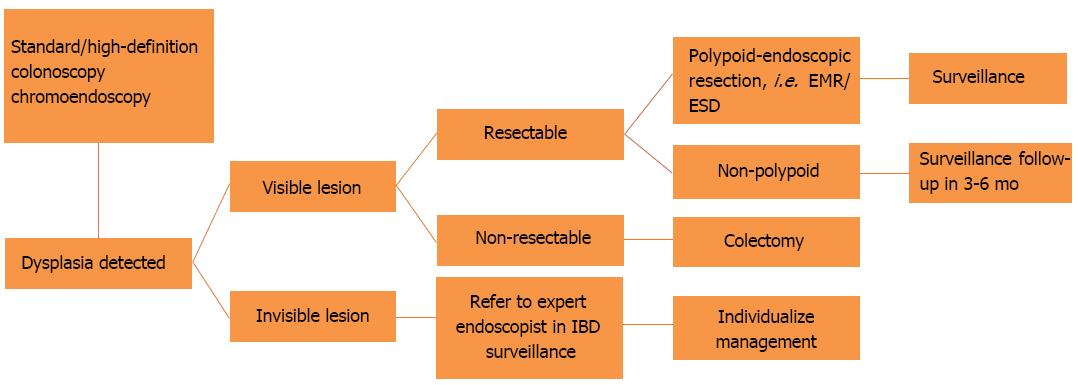
Nevertheless, the current recommendations favor DCE with targeted biopsies of any identified lesions[1,26,32,33] (Figure 2). Whenever DCE is not available, WLE with random, four quadrant biopsies every 10 cm should be performed with additional targeted biopsies from visible lesions. Other endoscopic modalities, like narrow band imaging (NBI), i-SCAN and autofluorescence imaging, did not achieve superior dysplasia detection rates when compared to standard (SD)- or high-definition (HD) WLE in randomized controlled trials[34-39].
Taking all these into consideration, the aim of our review is the brief and up-to-date description of the basic screening endoscopic modalities, as well as their efficacy and accuracy for CRC surveillance in IBD patients.
The standard method in CRC surveillance has until recently been SD colonoscopy, with the use of targeted as well as random quadrant biopsies every 10 cm, which amounts to at least 33 biopsies to achieve 90% confidence of detecting dysplasia. However, this technique ultimately inspects less than 1% of the mucosal surface of the colon[40]. According to a Dutch study examining long-standing UC, the overall rate of dysplasia detection with SD colonoscopy was 0.19[36]. With the advent of HD endoscopes and monitors, the endoscopist is able to better identify dysplastic lesions. A study by Subramanian et al[41] comparing SD to HD colonoscopy for dysplasia screening in UC, reported a three-fold increase in the yield of the HD endoscope combined with targeted, as well as random biopsies, especially in the right colon. Based on the aforementioned study, the SCENIC consensus statement by American Society for Gastrointestinal Endoscopy (ASGE) favors HD- over SD-WLE when implementing a surveillance program, even though the HD cost remains a limitation[33]. This improvement in detection of dysplastic lesions by HD-WLE and targeted-biopsy sampling changed the therapeutic considerations regarding colectomy, favoring more conservative approaches[41]. Furthermore, it was pointed out that the increased turnout with HD colonoscopy is probably a true reflection of the increased yield of this technique[41]. Nevertheless, based on the same study, neither significant change in the detection of lesions with high grade dysplasia nor early carcinoma or flat lesions were observed.
On the contrary, the study by van den Broek et al[36] showed no substantial difference in clinical outcomes for patients, in whom low grade dysplasia was revealed using random biopsies, thus advocating the use of improved visualization through advanced techniques[36,41].
Concluding, even though the most widespread technique for dysplasia surveillance in IBD until recently has been the WLE with random biopsies, it is arduous and protracted[40]. Furthermore, the diagnostic reliability of WLE is challenged in a recent review, which found a sensitivity of 76%[42]. Therefore, this method’s practicability has been clearly questioned and the research for the development of diagnostic modalities is supported[43].
Four quadrant biopsies every 10 cm throughout the colon has been the gold standard of IBD surveillance for more than 30 years. This approach originates from the theory of “flat dysplasia”, which suggests that dysplasia is difficult to visualize in colitis-affected mucosa[40,44]. Random biopsy only samples less than 1% of the luminal mucosa; has a subpar detection rate (< 2 per 1000 biopsies taken) and when used in conjunction with advanced endoscopic techniques, it does not affect clinical decisions[44]. A large retrospective analysis by van den Broek et al[36] reviewing 1010 colonoscopies during 10 years of surveillance stated that the result of random biopsy surveillance was poor, and neoplasia was detected only in four patients with random biopsies. Additionally, neoplasia was macroscopically visible in 94% of colonoscopies[43,44]. Current guidelines by British Society of Gastroenterology (BSG) and ASGE advocate the use of DCE without the need for random biopsies; however, it is suggested that random biopsies be acquired during HD colonoscopy, if DCE is not available or technically feasible[26]. Random biopsies remain a reasonable alternative if there are conditions that lower the diagnostic yield, such as inflammation, pseudo-polyposis, poor preparation or a poorly visualised mucosa[26,45].
Several studies have proven the efficacy of DCE in the detection of dysplasia in patients with IBD. DCE may reduce the need for random biopsies and may allow prolonged surveillance-interval, leading to cost reduction, as well as an increase the detection sensitivity of dysplastic lesions per examination[46].
This technique helps to augment dysplasia detection by topical application of dye on the colonic mucosa during colonoscopy. Areas that are macroscopically elevated or depressed, friable, obscure in vasculature, and with a villous or nodular pattern, can be detected more easily and biopsies can be taken. The most common dyes that in use are methylene blue and indigo carmine[47]. Dye solution can be sprayed by catheter, or flushing pumps, or administered as controlled release tablets, taken with bowel preparation[48]. When performing DCE, it is important to avoid active disease and to have adequate bowel preparation. Paris classification seems to be the standard method to describe any visible lesion, and targeted biopsies should be taken from any suspected area. If the lesion is well-defined, en-bloc endoscopic resection should be performed and biopsies should be taken from the adjacent mucosa. In case the lesion is unresectable, the endoscopist should take biopsies and tattoo the area.
Kiesslich et al[49] were the pioneers conducting a large randomized study with 263 individuals with long-standing UC. In the DCE-group, there was a statistically important correlation between the endoscopic estimation of the level and extent of inflammation of the colon (P = 0.0002) and the histology report, when compared to WLE (P = 0.0002) (89% vs 52% P < 0.0001). Additionally, more targeted biopsies were possible and these biopsies detected significantly more intraepithelial neoplasia (INs) when performing DCE (32 vs 10 P = 0.003). In a well-designed prospective study, Hurlstone et al[50] examined 350 patients with long-standing UC undergoing colonoscopy surveillance with high-magnification chromoscopic colonoscopy (HMCC) comparing the data with matched controls who had undergone WLE. The HMCC-group found significantly more intraepithelial neoplasias compared to controls (69 vs 24 P < 0.0001), and only 0.16% of the random biopsies have shown INs vs 8% from the targeted biopsies. Furthermore, Marion et al[51] studied 102 patients with IBD who underwent in a single examination, initially a WLE with random biopsies, then a targeted biopsy protocol and finally, DCE with targeted biopsies. They reported that biopsies obtained by the latter method detected significantly more dysplastic lesions than random biopsies with WLE (P = 0.001), as well as more than WLE with targeted biopsies (P = 0.057).
According to Subramanian et al[52] meta-analysis study including a large number of patients, the overall difference between the DCE and WLE in the detection of dysplasia was approximately 7% (95%Cl: 3.2-11.3), with the former showing a better rate of dysplastic lesions detected by targeted biopsies, as well as a higher rate of detection for flat lesions at 27% (95%CI: 11.2-41.9). On the other hand, the omission of random biopsies during chromoendoscopy will result in missing endoscopically invisible dysplasia. According to another meta-analysis, Wu et al[47] reported that DCE offers median to good sensitivity and a very good accuracy for revealing lesions with dysplasia in UC after analyzing six randomized controlled trials with 1.528 patients. The pooled sensitivity and specificity for DCE with targeted biopsies were 83.3% (95%CI: 35.9%-99.6%) and 91.3% (95%CI: 43.8%-100%) respectively, with conventional colonoscopy demonstrating lower rates. Soetikno et al[53] in a well-designed meta-analysis with 665 patients with IBD, demonstrated that the pooled positive percentage of DCE over WLE for the discernment of dysplasia of any grade per patient was 7% (95%CI: 3.3%-10.3%), as well as the possibility to miss dysplasia was 93% lower by performing chromoendoscopy with targeted biopsies (the pooled OR was 0.07; 95%CI: 0.03-0.21). Interestingly, according to a prospective study, Marion et al[54] showed that apart from the superiority of DCE when compared to WLE, a DCE examination without any findings was considered as the most probable indicator for a patient without any level of dysplasia, whereas an exam with any sort of findings was positively correlated with earlier referral for colectomy(hazard ratio, 12.1; 95%CI: 3.2-46.2).
Nevertheless, lately, the advantages of DCE over WLE have come into question, as well as the practicability of applying DCE in a real world setting of hectic endoscopy units. Trying to highlight this problem, a large retrospective non-randomized trial with different types of endoscopes used over time showed that the performance of DCE for IBD surveillance did not increase detection of dysplasia compared with WLE with targeted and random biopsies (11% vs 10%, P = 0.80)[55]. The number of lesions with neoplasia was also comparable between the DCE and WLE groups (P = 0.30).
As a final point, an interesting cohort analysis regarding cost-effectiveness was conducted by Konijeti et al[56], that compared DCE with targeted biopsies to WLE with random biopsies at various surveillance intervals and no surveillance at all. Chromoendoscopy was more efficient in the detection of dysplasia and cost more effective when compared with WLE. DCE exhibited cost-effectiveness relative to patients not undergoing any surveillance when performed at intervals bigger than 7 years.
Technological progression has enabled newer modalities based on older technologies for mucosal assessment. Given the success rate of chromoendoscopy in assessing colonic mucosa, the newest endoscopic devices have filters and algorithms that enable the mimicry of chromoendoscopy by filtering some light wavelengths to better underline abnormal tissues, while foregoing the limitating factors of chromoendoscopy. Dye-less or virtual chromoendoscopy has been developed by three major manufacturers for their respective endoscopic platforms. NBI filters out red and green light bands while contributing more to blue light bands at the 415 nm wavelength. This modality allows for visualization of the vasculature of the upper mucosa and different patterns correlating to different degrees of mucosal inflammation and predicts disease relapse. In the same vein, the i-Scan system provides detailed analysis, which is based on principles similar to NBI, with parameters allowing the processing of light through specific algorithms. This process provides detailed analysis based on vessel, mucosal pattern or surface architecture (i-Scan v, i-Scan p and i-Scan SE, respectively), with each analysis being readily available during endoscopy[57].
It has been reported that the yield of surveillance can be improved by the use of autofluorescence with NBI[36]. According to a study by Dekker et al[34], 52 suspicious lesions were detected in 17 patients using NBI, in comparison to 28 lesions in 13 patients detected with WLE. The pathology of the targeted biopsies revealed neoplasia in 11 patients; neoplasia was detected in 4 patients with both those modalities, in another 4 neoplasia was detected only by use of NBI, and in 3 patients neoplasia was discovered only by WLE, demonstrating non-statistical significance (P = 0.705) for those three modalities. In addition to targeted biopsies, 1522 random biopsies were taken in the context of surveillance. The pathology of these biopsies added only 1 patient with dysplasia that remained undetected by both NBI and WLE[34]. A prospective multicenter study by Leifeld et al[35] concluded that the two techniques did not differ in the statistical probability of lesion detection, but NBI required less withdrawal time (23 min vs 13 min, respectively P < 0.001) and biopsy samples (11.9 vs 38.6 biopsy specimens, respectively P < 0.001), when compared to WLE. These results are backed by a randomized study by Ignjatovic et al[38], which revealed no difference between the two modalities, regarding the detection of dysplasia. Overall, NBI does not seem to achieve a significantly higher probability of dysplasia detection, compared to conventional HD colonoscopy.
In the same vein Pellisé et al[58] conducted a prospective, randomized, controlled trial comparing NBI to DCE in 60 patients with long-standing inactive colonic IBD. The authors reported that NBI was less time-consuming (P < 0.01), equally effective in detecting dysplastic lesions and had a lower rate of false-positive biopsies (P = 0.001). However, NBI missed suspicious lesions with a non-significant miss rate difference of 30.7% (95%CI: -64.2% to 2.8%). As a result, the study surmised that NBI should not be standard modality for surveillance.
In general, NBI did not substantially differ from DCE, a claim that needs to be verified by more robust data pooling. A possible explanation is that NBI can more readily identify non-neoplastic inflammatory lesions than WLE, which were not pooled in the meta-analysis comparing those techniques[37]. Furthermore, the iterations of NBI are different in those studies, with older generation systems producing suboptimal, darker images[37,42]. Based on the current level of evidence, DCE remains the standard technique for the surveillance in IBD patients.
A large randomized prospective study comparing HD-iScan and HD-WLE to standard DCE did not prove inferiority for those two techniques, with the question of whether i-Scan and HD-WLE will benefit an expert endoscopist remaining unanswered[39]. The authors conclude that they need more multiple-operator studies to assess the helpful potential of these new techniques.
One of the newest tools in the arsenal of mucosal assessment for dysplasia is the confocal laser endomicroscopy (CLE) that allows in vivo microscopic inspection and evaluations of a targeted lesion in the gastrointestinal tract. This new and evolving method is used in conjunction with HD-WLE and DCE to further define suspicious lesions and assess their histology, by performing real time analysis of the cellular and subcellular characteristics at high resolution. The technique is based on fluorescence, which requires the addition of fluorescein intravenously or topically, but results in high quality images, comparable to traditional histology.
Kiesslich et al[59] first used the endoscope-based integrated system in 2007 to demonstrate that neoplastic changes in patients with UC can be identified with very good accuracy (94.7% sensitivity, 98.3% specificity, 97.8% accuracy), compared with standard surveillance endoscopy. Overall, 4.75-fold more neoplastic areas could be identified than with a WLE (P = 0.005), while requiring only half the number of biopsy samples (median 21.2 in the CLE group vs 42.2 undergoing surveillance endoscopy), despite the fact that CLE prolonged colonoscopy by an additional 10 min on average (P > 0.05). A recent study by Wanders et al[60], on the application of integrated CLE for surveillance in CD, which was terminated early due to critical equipment failure at 4 of the 5 participating centers, came up with a much lower diagnostic yield, with sensitivity of 42.9%, specificity of 92.4% and accuracy of 86.7%. The authors concluded that the technique probably will not be used in the daily practice of screening for CRC in patients with colitis.
A recent study of the probe-based CLE (pCLE) comes from Sweden where it was used for the surveillance of dysplasia in patients with PSC-IBD, a population with 6-fold increase in the incidence of CRC compared with the average risk for CRC population[61]. The study showed good diagnostic accuracy, with the estimated accuracy at 96%, sensitivity at 89% and specificity at 96%, with a low PPV at 41%, but with a very high NPV at 99% for the pCLE. The authors noted that the yield for accuracy fell when assessing areas with mucosal inflammation being misinterpreted as dysplasia. This study challenges the earliest attempts at pCLE systems for CRC surveillance in IBD patients by van den Broek et al[62], where the authors reported much lower diagnostic yield.
Despite the fact that DCE with targeted biopsies is the gold standard technique for IBD surveillance, it has some limitations. The need for adequate bowel preparation, the long procedure time, and its operator dependence are some of them. Moreover, the presence of active mucosal inflammation or post-inflammatory polyps may affect the images of chromoendoscopy and, in these cases random biopsies are still justified. There are no sufficient data about the effectiveness of the different dyes in detecting dysplasia and there are some concerns about methylene blue inducing DNA damage but have not yet been validated. Two recent editorials have questioned the SCENIC consensus, because chromoendoscopy and targeted biopsies have not been shown to improve CRC mortality[63,64]. Even when accounting for those limitations, chromoendoscopy remains a validated technique that becomes more and more recommended for CRC surveillance in IBD patients, whilst white light endoscopy with random biopsies should only be performed when the skill or the equipment for chromoendoscopy is unavailable.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Greece
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Gkekas I, Lorenzo-Zúñiga V, Muguruma N, Osawa S S- Editor: Ji FF L- Editor: A E- Editor: Wu YXJ
| 1. | Farraye FA, Odze RD, Eaden J, Itzkowitz SH. AGA technical review on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010;138:746-774, 774.e1-4; quiz e12-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 341] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 2. | Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1985] [Cited by in RCA: 2074] [Article Influence: 86.4] [Reference Citation Analysis (1)] |
| 3. | Rutter MD, Saunders BP, Wilkinson KH, Rumbles S, Schofield G, Kamm MA, Williams CB, Price AB, Talbot IC, Forbes A. Thirty-year analysis of a colonoscopic surveillance program for neoplasia in ulcerative colitis. Gastroenterology. 2006;130:1030-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 450] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 4. | Bernstein CN, Blanchard JF, Kliewer E, Wajda A. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer. 2001;91:854-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 5. | Itzkowitz SH, Harpaz N. Diagnosis and management of dysplasia in patients with inflammatory bowel diseases. Gastroenterology. 2004;126:1634-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 311] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 6. | Centre for Clinical Practice at NICE (UK). Diagnosis and management of dysplasia in patients with inflammatory bowel diseases. . [PubMed] |
| 7. | Annese V, Daperno M, Rutter MD, Amiot A, Bossuyt P, East J, Ferrante M, Götz M, Katsanos KH, Kießlich R. European evidence based consensus for endoscopy in inflammatory bowel disease. J Crohns Colitis. 2013;7:982-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 584] [Article Influence: 48.7] [Reference Citation Analysis (1)] |
| 8. | Cairns SR, Scholefield JH, Steele RJ, Dunlop MG, Thomas HJ, Evans GD, Eaden JA, Rutter MD, Atkin WP, Saunders BP. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut. 2010;59:666-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 897] [Cited by in RCA: 807] [Article Influence: 53.8] [Reference Citation Analysis (2)] |
| 9. | Itzkowitz SH, Present DH; Crohn’s and Colitis Foundation of America Colon Cancer in IBD Study Group. Consensus conference: Colorectal cancer screening and surveillance in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:314-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 395] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 10. | Rutter M, Saunders B, Wilkinson K, Rumbles S, Schofield G, Kamm M, Williams C, Price A, Talbot I, Forbes A. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. 2004;126:451-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 895] [Cited by in RCA: 881] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 11. | Gupta RB, Harpaz N, Itzkowitz S, Hossain S, Matula S, Kornbluth A, Bodian C, Ullman T. Histologic inflammation is a risk factor for progression to colorectal neoplasia in ulcerative colitis: a cohort study. Gastroenterology. 2007;133:1099-1105; quiz 1340-1341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 617] [Cited by in RCA: 570] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 12. | Loughrey MB, Shepherd NA. The pathology of bowel cancer screening. Histopathology. 2015;66:66-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology. 2011;140:1807-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 853] [Article Influence: 60.9] [Reference Citation Analysis (0)] |
| 14. | Popivanova BK, Kitamura K, Wu Y, Kondo T, Kagaya T, Kaneko S, Oshima M, Fujii C, Mukaida N. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest. 2008;118:560-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 456] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 15. | Kim S, Keku TO, Martin C, Galanko J, Woosley JT, Schroeder JC, Satia JA, Halabi S, Sandler RS. Circulating levels of inflammatory cytokines and risk of colorectal adenomas. Cancer Res. 2008;68:323-328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 234] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 16. | Chan IH, Jain R, Tessmer MS, Gorman D, Mangadu R, Sathe M, Vives F, Moon C, Penaflor E, Turner S. Interleukin-23 is sufficient to induce rapid de novo gut tumorigenesis, independent of carcinogens, through activation of innate lymphoid cells. Mucosal Immunol. 2014;7:842-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 120] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 17. | Tong Z, Yang XO, Yan H, Liu W, Niu X, Shi Y, Fang W, Xiong B, Wan Y, Dong C. A protective role by interleukin-17F in colon tumorigenesis. PLoS One. 2012;7:e34959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 18. | Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1747] [Cited by in RCA: 1767] [Article Influence: 110.4] [Reference Citation Analysis (0)] |
| 19. | Brighenti E, Calabrese C, Liguori G, Giannone FA, Trerè D, Montanaro L, Derenzini M. Interleukin 6 downregulates p53 expression and activity by stimulating ribosome biogenesis: a new pathway connecting inflammation to cancer. Oncogene. 2014;33:4396-4406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 20. | Wang S, Liu Z, Wang L, Zhang X. NF-kappaB signaling pathway, inflammation and colorectal cancer. Cell Mol Immunol. 2009;6:327-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 330] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 21. | Agoff SN, Brentnall TA, Crispin DA, Taylor SL, Raaka S, Haggitt RC, Reed MW, Afonina IA, Rabinovitch PS, Stevens AC. The role of cyclooxygenase 2 in ulcerative colitis-associated neoplasia. Am J Pathol. 2000;157:737-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 125] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 22. | De Simone V, Franzè E, Ronchetti G, Colantoni A, Fantini MC, Di Fusco D, Sica GS, Sileri P, MacDonald TT, Pallone F. Th17-type cytokines, IL-6 and TNF-α synergistically activate STAT3 and NF-kB to promote colorectal cancer cell growth. Oncogene. 2015;34:3493-3503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 448] [Cited by in RCA: 456] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 23. | Liu S, Sun X, Wang M, Hou Y, Zhan Y, Jiang Y, Liu Z, Cao X, Chen P, Liu Z. A microRNA 221- and 222-mediated feedback loop maintains constitutive activation of NFκB and STAT3 in colorectal cancer cells. Gastroenterology. 2014;147:847-859.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 144] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 24. | Shi C, Yang Y, Xia Y, Okugawa Y, Yang J, Liang Y, Chen H, Zhang P, Wang F, Han H. Novel evidence for an oncogenic role of microRNA-21 in colitis-associated colorectal cancer. Gut. 2016;65:1470-1481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 121] [Article Influence: 13.4] [Reference Citation Analysis (1)] |
| 25. | Bernstein CN, Shanahan F, Weinstein WM. Are we telling patients the truth about surveillance colonoscopy in ulcerative colitis? Lancet. 1994;343:71-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 362] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 26. | American Society for Gastrointestinal Endoscopy Standards of Practice Committee, Shergill AK, Lightdale JR, Bruining DH, Acosta RD, Chandrasekhara V, Chathadi KV, Decker GA, Early DS, Evans JA, Fanelli RD, Fisher DA, Fonkalsrud L, Foley K, Hwang JH, Jue TL, Khashab MA, Muthusamy VR, Pasha SF, Saltzman JR, Sharaf R, Cash BD, DeWitt JM. The role of endoscopy in inflammatory bowel disease. Gastrointest Endosc. 2015;81:1101-21.e1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 259] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 27. | Rubin PH, Friedman S, Harpaz N, Goldstein E, Weiser J, Schiller J, Waye JD, Present DH. Colonoscopic polypectomy in chronic colitis: conservative management after endoscopic resection of dysplastic polyps. Gastroenterology. 1999;117:1295-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 226] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 28. | Vieth M, Behrens H, Stolte M. Sporadic adenoma in ulcerative colitis: endoscopic resection is an adequate treatment. Gut. 2006;55:1151-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Picco MF, Pasha S, Leighton JA, Bruining D, Loftus EV Jr, Thomas CS, Crook JE, Krishna M, Wallace M. Procedure time and the determination of polypoid abnormalities with experience: implementation of a chromoendoscopy program for surveillance colonoscopy for ulcerative colitis. Inflamm Bowel Dis. 2013;19:1913-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Lichtenstein GR, Hanauer SB, Sandborn WJ; Practice Parameters Committee of American College of Gastroenterology. Management of Crohn’s disease in adults. Am J Gastroenterol. 2009;104:465-483; quiz 464, 484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 591] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 31. | Collins PD, Mpofu C, Watson AJ, Rhodes JM. Strategies for detecting colon cancer and/or dysplasia in patients with inflammatory bowel disease. Cochrane Database Syst Rev. 2006;CD000279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 96] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 32. | Van Assche G, Dignass A, Bokemeyer B, Danese S, Gionchetti P, Moser G, Beaugerie L, Gomollón F, Häuser W, Herrlinger K. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 3: special situations. J Crohns Colitis. 2013;7:1-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 339] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 33. | Laine L, Kaltenbach T, Barkun A, McQuaid KR, Subramanian V, Soetikno R; SCENIC Guideline Development Panel. SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastrointest Endosc. 2015;81:489-501.e26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 270] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 34. | Dekker E, van den Broek FJ, Reitsma JB, Hardwick JC, Offerhaus GJ, van Deventer SJ, Hommes DW, Fockens P. Narrow-band imaging compared with conventional colonoscopy for the detection of dysplasia in patients with longstanding ulcerative colitis. Endoscopy. 2007;39:216-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 202] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 35. | Leifeld L, Rogler G, Stallmach A, Schmidt C, Zuber-Jerger I, Hartmann F, Plauth M, Drabik A, Hofstädter F, Dienes HP. White-Light or Narrow-Band Imaging Colonoscopy in Surveillance of Ulcerative Colitis: A Prospective Multicenter Study. Clin Gastroenterol Hepatol. 2015;13:1776-1781.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 36. | van den Broek FJ, Fockens P, van Eeden S, Reitsma JB, Hardwick JC, Stokkers PC, Dekker E. Endoscopic tri-modal imaging for surveillance in ulcerative colitis: randomised comparison of high-resolution endoscopy and autofluorescence imaging for neoplasia detection; and evaluation of narrow-band imaging for classification of lesions. Gut. 2008;57:1083-1089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 190] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 37. | van den Broek FJ, Fockens P, van Eeden S, Stokkers PC, Ponsioen CY, Reitsma JB, Dekker E. Narrow-band imaging versus high-definition endoscopy for the diagnosis of neoplasia in ulcerative colitis. Endoscopy. 2011;43:108-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 38. | Ignjatovic A, East JE, Subramanian V, Suzuki N, Guenther T, Palmer N, Bassett P, Ragunath K, Saunders BP. Narrow band imaging for detection of dysplasia in colitis: a randomized controlled trial. Am J Gastroenterol. 2012;107:885-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 39. | Iacucci M, Kaplan GG, Panaccione R, Akinola O, Lethebe BC, Lowerison M, Leung Y, Novak KL, Seow CH, Urbanski S. A Randomized Trial Comparing High Definition Colonoscopy Alone With High Definition Dye Spraying and Electronic Virtual Chromoendoscopy for Detection of Colonic Neoplastic Lesions During IBD Surveillance Colonoscopy. Am J Gastroenterol. 2018;113:225-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 109] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 40. | Rubin CE, Haggitt RC, Burmer GC, Brentnall TA, Stevens AC, Levine DS, Dean PJ, Kimmey M, Perera DR, Rabinovitch PS. DNA aneuploidy in colonic biopsies predicts future development of dysplasia in ulcerative colitis. Gastroenterology. 1992;103:1611-1620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 365] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 41. | Subramanian V, Ramappa V, Telakis E, Mannath J, Jawhari AU, Hawkey CJ, Ragunath K. Comparison of high definition with standard white light endoscopy for detection of dysplastic lesions during surveillance colonoscopy in patients with colonic inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:350-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 42. | Rubin DT, Rothe JA, Hetzel JT, Cohen RD, Hanauer SB. Are dysplasia and colorectal cancer endoscopically visible in patients with ulcerative colitis? Gastrointest Endosc. 2007;65:998-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 149] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 43. | East JE. Colonoscopic Cancer Surveillance in Inflammatory Bowel Disease: What’s New Beyond Random Biopsy? Clin Endosc. 2012;45:274-277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 44. | van den Broek FJ, Stokkers PC, Reitsma JB, Boltjes RP, Ponsioen CY, Fockens P, Dekker E. Random biopsies taken during colonoscopic surveillance of patients with longstanding ulcerative colitis: low yield and absence of clinical consequences. Am J Gastroenterol. 2014;109:715-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 45. | Navaneethan U, Kochhar G, Venkatesh PG, Bennett AE, Rizk M, Shen B, Kiran RP. Random biopsies during surveillance colonoscopy increase dysplasia detection in patients with primary sclerosing cholangitis and ulcerative colitis. J Crohns Colitis. 2013;7:974-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 46. | Shah SA, Rubin DT, Farraye FA. Chromoendoscopy for colorectal cancer surveillance in patients with inflammatory bowel disease. Curr Gastroenterol Rep. 2014;16:407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 47. | Wu L, Li P, Wu J, Cao Y, Gao F. The diagnostic accuracy of chromoendoscopy for dysplasia in ulcerative colitis: meta-analysis of six randomized controlled trials. Colorectal Dis. 2012;14:416-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 48. | Repici A, Di Stefano AF, Radicioni MM, Jas V, Moro L, Danese S. Methylene blue MMX tablets for chromoendoscopy. Safety tolerability and bioavailability in healthy volunteers. Contemp Clin Trials. 2012;33:260-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 49. | Kiesslich R, Fritsch J, Holtmann M, Koehler HH, Stolte M, Kanzler S, Nafe B, Jung M, Galle PR, Neurath MF. Methylene blue-aided chromoendoscopy for the detection of intraepithelial neoplasia and colon cancer in ulcerative colitis. Gastroenterology. 2003;124:880-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 557] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 50. | Hurlstone DP, Sanders DS, Lobo AJ, McAlindon ME, Cross SS. Indigo carmine-assisted high-magnification chromoscopic colonoscopy for the detection and characterisation of intraepithelial neoplasia in ulcerative colitis: a prospective evaluation. Endoscopy. 2005;37:1186-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 185] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 51. | Marion JF, Waye JD, Present DH, Israel Y, Bodian C, Harpaz N, Chapman M, Itzkowitz S, Steinlauf AF, Abreu MT. Chromoendoscopy-targeted biopsies are superior to standard colonoscopic surveillance for detecting dysplasia in inflammatory bowel disease patients: a prospective endoscopic trial. Am J Gastroenterol. 2008;103:2342-2349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 209] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 52. | Subramanian V, Mannath J, Ragunath K, Hawkey CJ. Meta-analysis: the diagnostic yield of chromoendoscopy for detecting dysplasia in patients with colonic inflammatory bowel disease. Aliment Pharmacol Ther. 2011;33:304-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 154] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 53. | Soetikno R, Subramanian V, Kaltenbach T, Rouse RV, Sanduleanu S, Suzuki N, Tanaka S, McQuaid K. The detection of nonpolypoid (flat and depressed) colorectal neoplasms in patients with inflammatory bowel disease. Gastroenterology. 2013;144:1349-1352, 1352.e1-1352.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 54. | Marion JF, Waye JD, Israel Y, Present DH, Suprun M, Bodian C, Harpaz N, Chapman M, Itzkowitz S, Abreu MT. Chromoendoscopy Is More Effective Than Standard Colonoscopy in Detecting Dysplasia During Long-term Surveillance of Patients With Colitis. Clin Gastroenterol Hepatol. 2016;14:713-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 55. | Mooiweer E, van der Meulen-de Jong AE, Ponsioen CY, Fidder HH, Siersema PD, Dekker E, Oldenburg B. Chromoendoscopy for Surveillance in Inflammatory Bowel Disease Does Not Increase Neoplasia Detection Compared With Conventional Colonoscopy With Random Biopsies: Results From a Large Retrospective Study. Am J Gastroenterol. 2015;110:1014-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 56. | Konijeti GG, Shrime MG, Ananthakrishnan AN, Chan AT. Cost-effectiveness analysis of chromoendoscopy for colorectal cancer surveillance in patients with ulcerative colitis. Gastrointest Endosc. 2014;79:455-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 57. | Iacucci M, Panaccione R, Ghosh S. Advances in novel diagnostic endoscopic imaging techniques in inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:873-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 58. | Pellisé M, López-Cerón M, Rodríguez de Miguel C, Jimeno M, Zabalza M, Ricart E, Aceituno M, Fernández-Esparrach G, Ginès A, Sendino O. Narrow-band imaging as an alternative to chromoendoscopy for the detection of dysplasia in long-standing inflammatory bowel disease: a prospective, randomized, crossover study. Gastrointest Endosc. 2011;74:840-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 59. | Kiesslich R, Goetz M, Lammersdorf K, Schneider C, Burg J, Stolte M, Vieth M, Nafe B, Galle PR, Neurath MF. Chromoscopy-guided endomicroscopy increases the diagnostic yield of intraepithelial neoplasia in ulcerative colitis. Gastroenterology. 2007;132:874-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 376] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 60. | Wanders LK, Kuiper T, Kiesslich R, Karstensen JG, Leong RW, Dekker E, Bisschops R. Limited applicability of chromoendoscopy-guided confocal laser endomicroscopy as daily-practice surveillance strategy in Crohn’s disease. Gastrointest Endosc. 2016;83:966-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 61. | Dlugosz A, Barakat AM, Björkström NK, Öst Å, Bergquist A. Diagnostic yield of endomicroscopy for dysplasia in primary sclerosing cholangitis associated inflammatory bowel disease: a feasibility study. Endosc Int Open. 2016;4:E901-E911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 62. | van den Broek FJ, van Es JA, van Eeden S, Stokkers PC, Ponsioen CY, Reitsma JB, Fockens P, Dekker E. Pilot study of probe-based confocal laser endomicroscopy during colonoscopic surveillance of patients with longstanding ulcerative colitis. Endoscopy. 2011;43:116-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 63. | Higgins PD. Miles to Go on the SCENIC Route: Should Chromoendoscopy Become the Standard of Care in IBD Surveillance? Am J Gastroenterol. 2015;110:1035-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 64. | Marion JF, Sands BE. The SCENIC consensus statement on surveillance and management of dysplasia in inflammatory bowel disease: praise and words of caution. Gastroenterology. 2015;148:462-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |