Published online Jan 16, 2018. doi: 10.4253/wjge.v10.i1.51
Peer-review started: July 18, 2017
First decision: September 7, 2017
Revised: October 22, 2017
Accepted: November 10, 2017
Article in press: November 10, 2017
Published online: January 16, 2018
Processing time: 182 Days and 4 Hours
To correlate the length of endoscope hang time and number of bacteria cultured prior to use.
Prospectively, we cultured specimens from 19 gastroscopes, 24 colonoscopes and 5 side viewing duodenoscopes during the period of 2011 to 2015. A total of 164 results had complete data denoting date of cleansing, number of days stored and culture results. All scopes underwent initial cleaning in the endoscopy suite utilizing tap water, and then manually cleaned and flushed. High level disinfection was achieved with a Medivator© DSD (Medivator Inc., United States) automated endoscope reprocessor following manufacturer instructions, with Glutacide® (Pharmax Limited, Canada), a 2% glutaraldehyde solution. After disinfection, all scopes were stored in dust free, unfiltered commercial cabinets for up to 7 d. Prior to use, all scopes were sampled and plated on sheep blood agar for 48 h; the colony count was obtained from each plate. The length of endoscope hang time and bacterial load was analyzed utilizing unpaired t-tests. The overall percentage of positive and negative cultures for each type of endoscope was also calculated.
All culture results were within the acceptable range (less than 200 cfu/mL). One colonoscope cultured 80 cfu/mL after hanging for 1 d, which was the highest count. ERCP scopes cultured at most 10 cfu, this occurred after 2 and 7 d, and gastroscopes cultured 50 cfu/mL at most, at 1 d. Most cultures were negative for growth, irrespective of the length of hang time. Furthermore, all scopes, with the exception of one colonoscope which had two positive cultures (each of 10 cfu/mL), had at most one positive culture. There was no significant difference in the number of bacteria cultured after 1 d compared to 7 d when all scopes were combined (day 2: P = 0.515; day 3: P = identical; day 4: P = 0.071; day 5: P = 0.470; day 6: P = 0.584; day 7: P = 0.575). There was also no significant difference in the number of bacteria cultured after 1 day compared to 7 d for gastroscopes (day 2: P = 0.895; day 3: P = identical; day 4: P = identical; day 5: P = 0.893; day 6: P = identical; day 7: P = 0.756), colonoscopes (day 2: P = 0.489; day 4: P = 0.493; day 5: P = 0.324; day 6: P = 0.526; day 7: P = identical), or ERCP scopes (day 2: P = identical; day 7: P = 0.685).
There is no correlation between hang time and bacterial load. Endoscopes do not need to be reprocessed if reused within a period of 7 d.
Core tip: Several cases of transmission of antibiotic resistant microbes have recently been reported, most notably carbapenem-resistant Enterobacteriaceae. However, according to our research, there does not appear to be a correlation between the number of days that an endoscope has been hanging and the bacterial load. Therefore, reprocessing of endoscopes is unnecessary prior to use, if they undergo cleaning according to guidelines, maintained in a ventilated, dust-free cabinet between use and the period of hang time does not exceed 7 d.
- Citation: Mallette KI, Pieroni P, Dhalla SS. Bacterial presence on flexible endoscopes vs time since disinfection. World J Gastrointest Endosc 2018; 10(1): 51-55
- URL: https://www.wjgnet.com/1948-5190/full/v10/i1/51.htm
- DOI: https://dx.doi.org/10.4253/wjge.v10.i1.51
The use of flexible endoscopes is instrumental in the diagnosis and management of gastrointestinal and hepatobiliary disease. Due to the invasive nature of these procedures they carry a risk of infection, either by bacteria within the individuals’ gastrointestinal tract or through bacteria contaminating the endoscope[1,2]. Endoscopes are defined as “semi critical” devices as per the Spaulding classification of medical devices; in order to minimize the risk of inoculating patients with microbes from a previous patient, they must undergo high level disinfection between patients[1].
Previous guidelines established by several international societies, including the European Society of Gastrointestinal Endoscopy, suggested that in addition to high level disinfection after use, endoscopes should be reprocessed the day of procedure prior to use[3,4]. However, these guidelines were based on very limited research and data[5]. This extra reprocessing of endoscopes is extremely expensive for facilities and leads to extra wear and damage to the equipment (both processing machines and endoscopes)[6]. A study conducted at our institution examined the necessity of the aforementioned guidelines, and established that endoscopes could be stored up to 7 d prior to use without the need for reprocessing when maintained in a ventilated, dust free cabinet[7]. Thus, our institution has been following these guidelines for the past few years. Similarly, a limited study conducted in Czechoslovakia identified that colonoscopes and duodenoscopes, if properly disinfected and stored, did not require reprocessing for up to 5 d[8].
Several cases of transmission of antibiotic resistant microbes have been documented recently, most notably carbapenem-resistant Enterobacteriaceae, in the United States via endoscopy[9]. One of the most concerning aspects of these recent cases is that no breaches in reprocessing of the endoscopes was identified[9]. The aim of this study was to verify a previous study conducted at our institution, correlating endoscope hang time and bacterial load prior to use, as well as to evaluate our procedures in light of the recent cases of transmission of bacteria between patients.
During the period of 2011 to 2015, we prospectively sampled specimens from nineteen gastroscopes, twenty-four colonoscopes, and five side viewing duodenoscopes, available in our institution. Each week during that time frame, two scopes were sampled on a rotating basis, accounting for a total of 327 samples. Only 164 results could be obtained which had complete data including date of cleansing, number of days stored and culture results.
Prior to removal from the endoscopy suite, all scopes are flushed with tap water and then the outer surface is wiped clean with tap water. Endoscopes were initially manually brushed to remove debris from the ports. For the duodenoscopes and the colonoscopes, the suction cylinder (to distal end and suction connector end) and instrument channel ports were manually brushed a total of three times and then flushed with at least 500 cc while submersed in detergent. With respect to ERCP scopes, the elevator recess, in both the up and down position, suction cylinder and instrument channel port were manually flushed three times each. In addition, for the ERCP scopes, the elevator wire and forceps elevator (in the up and down position) were manually cleaned three times. The elevator recess was flushed with a 30 mL water/detergent mixture in the up and down position. Using an automated flushing pump all scopes were flushed with a water/detergent mixture for 1 min and 15 s and then with air for 30 s; during flushing of ERCP scopes, the elevator mechanism was moved up and down.
The endoscopes then underwent high level disinfection using a Medivator© DSD (Medivator Inc., United States) automated endoscope reprocessor (AER). High level disinfection was achieved utilizing Glutacide® (Pharmax Limited, Canada), a 2% glutaraldehyde solution that can be utilized for 30 d. The AER cycle consists of a 1-min flush with reverse osmosis water, followed by a 5-min detergent disinfection and a 20-min detergent soak. Next, the scopes undergo two rinses with reverse osmosis water (4:10 min and 3 min each), then a 1-min rinse with 70% alcohol. Finally, they undergo a 5-min air dry and a 5-min manual air dry (utilizing filtered medical, non-heated air), of the suction channel, air/water channel and dials. All endoscopes are then stored in dust free, unfiltered, roll top commercial cabinets manufactured by Olympus. The cabinets were wiped clean by staff health care aides monthly, as well as, on an as needed basis.
Samples for culture were obtained using a protocol, developed at our institution, in accordance with those developed by the Endoscopy Working Group as part of the Manitoba Advisory Committee on Infectious Diseases[10]. The endoscopes were all sampled after a period of hang time, as described below. Sampling of the endoscopes was undertaken outside the reprocessing room, within the health care aide room, within a designated area. The distal end of the endoscope is held inside a sterile specimen container, 10 mL of sterile water is drawn up, and 5 mL is flushed through the biopsy channel. An endoscopy brush is then dipped into sterile water and passed through the biopsy channel until it emerges out the distal end, it’s then pulled back up the channel and pushed through once more until it emerges 2 cm into the sterile container. Scissors are then cleaned with an alcohol pad and used to cut off 2 cm of the brush into the sterile container. Finally, the remaining 5 mL of sterile water are passed through the biopsy channel and collected in the sterile container. Prior to plating, the water containing the cleaning brush was vortexed, to ensure a representative sample was obtained. A 100 μL aliquot of the samples were placed on a sheep blood agar plate, spread with a glass rod until absorbed by the media. Plates were then incubated at 35 °C for 48 h. The colony count was obtained after 48hours and was then equated to colony forming units per milliliter. It should be noted that ERCP scopes were cultured with the elevator in the down position.
Hang time was determined by calculating the total number of days between cleaning and microbiological sampling. Guidelines at our facility dictate that any samples greater than 200 cfu/mL (cut-off for acceptable microbial levels for potable water) are deemed as an unacceptable level of bioburden and the scope would be removed from use to be reprocessed[10]. The data was evaluated using an unpaired t-test with Minitab statistical software©, comparing the number of colony forming units cultured on each type of endoscope after 1 d of hang time compared to subsequent days (up to day 7). Overall, the percentage of negative cultures (i.e., no growth) and positive cultures, for each type of endoscope was also calculated. The statistical methods in the manuscript were reviewed and approved by all authors with the help of the quality improvement specialist affiliated with the Brandon Reginal Health Centre.
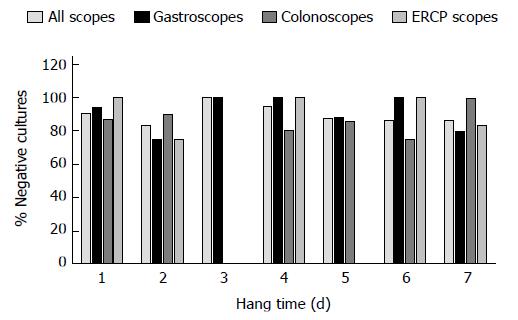
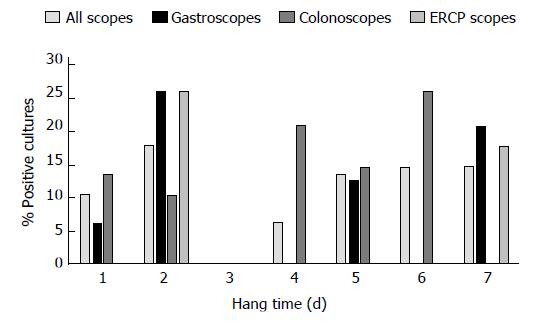
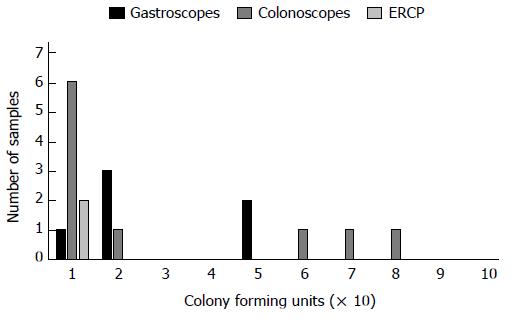
All positive culture results were less than 200 cfu/mL, and thus no endoscopes required additional reprocessing or quarantine. It should be noted that samples which were excluded from our study, due to missing data and inability to calculate hang time, all had culture results within the acceptable limit. A colonoscope cultured the highest bacterial load at 80 cfu/mL, with a hang time of 1 d. The highest bacterial load for ERCP scopes was 10 cfu/mL, this occurred at hang times 2 and 7 d. The highest count for gastroscopes was 50 cfu/mL after a hang time of 1 d. Most cultures, regardless of hang time, were negative for growth (Figures 1-3). Only one endoscope had more than one positive culture, one colonoscope had two positive cultures (of 4 obtained), each of 10 cfu/mL.
There was no significant difference at the 95% confidence interval, in the number of bacteria cultured after 1 d compared to 7 d when grouping all scopes (Table 1). At the 95%CI no statistical differences were observed, in culture results after 1 d of hang time compared to subsequent days for each scope type (Table 1).
| Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | |
| All Scopes (Day 1: n = 82) | P = 0.515 (n = 18) | P = identical (n = 3) | P = 0.071 (n = 18) | P = 0.470 (n = 15) | P = 0.584 (n = 7) | P = 0.575 (n = 21) |
| Gastroscopes (Day 1: n = 34) | P = 0.895 (n = 4) | P = identical (n = 3) | P = identical (n = 12) | P = 0.893 (n = 8) | P = identical (n = 2) | P = 0.756 (n = 10) |
| Colonoscopes (Day 1: n = 46) | P = 0.489 (n = 10) | No data (n = 0) | P = 0.493 (n = 5) | P = 0.324 (n = 7) | P = 0.526 (n = 4) | P = identical (n = 5) |
| ERCP Scopes (Day 1: n = 2) | P = identical (n = 4) | No data (n = 0) | Insufficient data (n = 1) | No data (n = 0) | No data (n = 0) | P = 0.685 (n = 6) |
The percentage of negative cultures is similar for both day 1 and day 7 of storage for each type of endoscope, suggesting that storage of endoscopes for 7 d is safe, and that the risk of patient transmission is relatively low. This correlates with the previous findings of the study conducted at our institution[7].
Furthermore, all culture results were less than 200 cfu/mL, the acceptable limit for potable water, and thus were within the guidelines for use in endoscopy[10]. It is also of note that the highest bacterial load was cultured from a colonoscope, and the lowest was from an ERCP scope. This is despite the fact that ERCP scopes have a large number of moving parts, which are more likely to harbour bacteria[11]. Overall, it appears that proper disinfection and storage of endoscopes makes reprocessing prior to use unnecessary within a period of 7 d. Interim guidelines produced by the Centers for Disease Control and Prevention have suggested that cultures obtained after processing should possess less than 10 cfu[12]. All samples obtained in our study were less than this new limit, however our centre should adjust our guidelines to fit these new suggestions.
One limitation to this study is the relatively small sample size, especially with regards to ERCP scopes, as a statistical difference may not have been detected utilizing the t-test even if it existed. Furthermore, the type of bacteria cultured was not assessed in this study and therefore in future studies, it would be important to assess which bacteria are able to withstand the disinfection process. It has been suggested that sterilization of endoscopes may be required for prevention of transmission of certain species of bacteria rather than disinfection[13]. Lastly, not all bacteria are amenable to culture using the medium employed in this study. Moving forward, our institution will be assessing the use of different culture media in comparison to the commonly used sheep blood agar, including reasoner’s 2A agar which may identify water stressed or damaged organisms[14]. For future studies, it may be valuable to initially plate a 0.5 mL sample onto MacConkey media to allow for rapid screening for organisms which may lead to patient harms[15].
In conclusion, there is no clear correlation between the duration of hang time of an endoscope and bacterial load. This further supports the previous study conducted at our institution indicating that there is not a need to reprocess endoscopes prior to use if they are properly disinfected, and properly stored for up to 7 d[7]. It is important to stress that proper cleansing of endoscopes be carried out immediately after use, according to manufacturer suggestions. Further work in this area should focus on assessing the type of bacteria cultured in order to determine the true risk to the patient, as well as determining methods to further decrease the risk of transmission of antibiotic resistant organisms. Lastly, new research should assess whether a limit of 200 cfu/mL is appropriate or if transmission of virulent organisms can occur below this limit.
Due to the nature of endoscopy, all endoscopes must undergo high level disinfection after use. Previously, guidelines suggested that endoscopes be reprocessed prior to use, regardless of the hang time. These guidelines led to excessive wear on the instruments, and were quite costly for institutions. A previous study conducted at our institution suggested that endoscopes could be stored for up to 7 d prior to requiring reprocessing. The aim of this study was to determine if there was a correlation between the hang time and bacterial load on endoscopes.
There have recently been several documented cases of transmission of antibiotic resistant organisms, specifically carbapenem-resistant Enterobacteriaceae via endoscopy. This has led to increased interest in the bacterial contamination on endoscopes after thorough disinfection.
The study demonstrates that endoscopes can be stored for a period of up to 7 d without significant levels of bacterial contamination, there does not appear to be a correlation between hang time and bacterial load. There does not appear to be a need for reprocessing of endoscopes prior to use if disinfected and stored properly. This is contrary to previous society guidelines which suggested disinfection prior to use.
Endoscopes if disinfected and stored properly can be stored for up to 7 d without requiring reprocessing prior to use.
Hang time refers to the number of days an endoscope was stored, from disinfection to microbiological evaluation.
The authors thank members of the endoscopy department at Brandon Regional Health Centre, the Office of Rural and Northern Health, and Prairie Mountain Health.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Canada
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Lakatos PL, Lee CL, Toshniwal JJ S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | ASGE Quality Assurance In Endoscopy Committee. Petersen BT, Chennat J, Cohen J, Cotton PB, Greenwald DA, Kowalski TE, Krinsky ML, Park WG, Pike IM, Romagnuolo J; Society for Healthcare Epidemiology of America, Rutala WA. Multisociety guideline on reprocessing flexible gastrointestinal endoscopes: 2011. Gastrointest Endosc. 2011;73:1075-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 123] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 2. | ASGE Standards of Practice Committee. Banerjee S, Shen B, Nelson DB, Lichtenstein DR, Baron TH, Anderson MA, Dominitz JA, Gan SI, Harrison ME, Ikenberry SO, Jagannath SB, Fanelli RD, Lee K, van Guilder T, Stewart LE. Infection control during GI endoscopy. Gastrointest Endosc. 2008;67:781-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 3. | Cleaning and disinfection of equipment for gastrointestinal endoscopy. Report of a Working Party of the British Society of Gastroenterology Endoscopy Committee. Gut. 1998;42:585-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 85] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Association of periOperative Registered Nurses. Recommended practices for cleaning and processing endoscopes and endoscope accessories. AORN J. 2003;77:434-438, 441-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Nelson DB. Recent advances in epidemiology and prevention of gastrointestinal endoscopy related infections. Curr Opin Infect Dis. 2005;18:326-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Burdick JS, Hambrick D. Endoscope reprocessing and repair costs. Gastrointest Endosc Clin N Am. 2004;14:717-724, ix-ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Vergis AS, Thomson D, Pieroni P, Dhalla S. Reprocessing flexible gastrointestinal endoscopes after a period of disuse: is it necessary? Endoscopy. 2007;39:737-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Rejchrt S, Cermák P, Pavlatová L, McKová E, Bures J. Bacteriologic testing of endoscopes after high-level disinfection. Gastrointest Endosc. 2004;60:76-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Epstein L, Hunter JC, Arwady MA, Tsai V, Stein L, Gribogiannis M, Frias M, Guh AY, Laufer AS, Black S. New Delhi metallo-β-lactamase-producing carbapenem-resistant Escherichia coli associated with exposure to duodenoscopes. JAMA. 2014;312:1447-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 319] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 10. | Manitoba Health, Manitoba Advisory Committee on Infectious Disease, Infection Control Subcommittee EWG. Guidelines for Infection Prevention and Control in Endoscopy. Infect Control. 2000;1-12. |
| 11. | Higa JT, Gluck M, Ross AS. Duodenoscope-Associated Bacterial Infections: A Review and Update. Curr Treat Options Gastroenterol. 2016;14:185-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Centers for Disease Control and Prevention. Interim Protocol for Healthcare Facilities Regarding Surveillance for Bacterial Contamination of Duodenoscopes after Reprocessing [Internet]. 2015;Mar 11]. [cited 2017 May 02] Available from: https://www.cdc.gov/hai/organisms/cre/cre-duodenoscope-surveillance-protocol.html. |
| 13. | Rutala WA, Weber DJ. Gastrointestinal endoscopes: a need to shift from disinfection to sterilization? JAMA. 2014;312:1405-1406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 14. | Reasoner DJ, Geldreich EE. A new medium for the enumeration and subculture of bacteria from potable water. Appl Environ Microbiol. 1985;49:1-7. [PubMed] |
| 15. | Centers for Disease Control and Prevention. Interim culture method for the duodenoscope - distal end and instrument channel [Internet]. 2015;Aug 19]. [cited 2017 May 02] Available from: https://www.cdc.gov/hai/settings/lab/lab-duodenoscope-culture-method.html. |