Published online Jan 16, 2018. doi: 10.4253/wjge.v10.i1.37
Peer-review started: July 28, 2017
First decision: September 11, 2017
Revised: November 4, 2017
Accepted: November 19, 2017
Article in press: November 20, 2017
Published online: January 16, 2018
Processing time: 172 Days and 10.7 Hours
To investigate the impact of endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) and positron emission tomography-computed tomography (PET-CT) in the nodal staging of upper gastrointestinal (GI) cancer in a tertiary referral centre.
We performed a retrospective review of prospectively recorded data held on all patients with a diagnosis of upper GI cancer made between January 2009 and December 2015. Only those patients who had both a PET-CT and EUS with FNA sampling of a mediastinal node distant from the primary tumour were included. Using a positive EUS-FNA result as the gold standard for lymph node involvement, the sensitivity, specificity, positive and negative predictive values (PPV and NPV) and accuracy of PET-CT in the staging of mediastinal lymph nodes were calculated. The impact on therapeutic strategy of adding EUS-FNA to PET-CT was assessed.
One hundred and twenty one patients were included. Sixty nine patients had a diagnosis of oesophageal adenocarcinoma (Thirty one of whom were junctional), forty eight had oesophageal squamous cell carcinoma and four had gastric adenocarcinoma. The FNA results were inadequate in eleven cases and the PET-CT findings were indeterminate in two cases, therefore thirteen patients (10.7%) were excluded from further analysis. There was concordance between PET-CT and EUS-FNA findings in seventy one of the remaining one hundred and eight patients (65.7%). The sensitivity, specificity, PPV and NPV values of PET-CT were 92.5%, 50%, 52.1% and 91.9% respectively. There was discordance between PET-CT and EUS-FNA findings in thirty seven out of one hundred and eight patients (34.3%). MDT discussion led to a radical treatment pathway in twenty seven of these cases, after the final tumour stage was altered as a direct consequence of the EUS-FNA findings. Of these patients, fourteen (51.9%) experienced clinical remission of a median of nine months (range three to forty two months).
EUS-FNA leads to altered staging of upper GI cancer, resulting in more patients receiving radical treatment that would have been the case using PET-CT staging alone.
Core tip: We have found that positron emission tomography-computed tomography (PET-CT) in the setting of upper gastrointestinal cancer has a high sensitivity and negative predictive value, but has poor specificity and positive predictive value for the detection of malignant mediastinal lymph nodes. This could lead to many patients being over-staged by PET-CT alone. The use of endoscopic ultrasound-guided fine-needle aspiration of mediastinal nodes results in more patients being offered radical therapy.
- Citation: Harrington C, Smith L, Bisland J, López González E, Jamieson N, Paterson S, Stanley AJ. Mediastinal node staging by positron emission tomography-computed tomography and selective endoscopic ultrasound with fine needle aspiration for patients with upper gastrointestinal cancer: Results from a regional centre. World J Gastrointest Endosc 2018; 10(1): 37-44
- URL: https://www.wjgnet.com/1948-5190/full/v10/i1/37.htm
- DOI: https://dx.doi.org/10.4253/wjge.v10.i1.37
The optimal management of oesophageal or oesophago-gastric junctional cancer relies on accurate staging to ensure that patients are directed towards the most appropriate treatment pathway for their stage of disease. Surgical resection for patients with localised disease offers the best outcomes with five year survival rates of 17%-47%[1-3]. It is particularly important to ensure that the nodal staging is as accurate as possible in these patients so that patients with incurable disease avoid radical surgical or oncological therapy but are offered a palliative approach. It is equally important that potentially curable patients are not incorrectly thought to have incurable disease.
Several imaging modalities are available and when used in combination, provide the most accurate staging in upper gastrointestinal (GI) cancer. The 2011 United Kingdom joint medical, surgical and oncology guideline advised that positron emission tomography-computed tomography (PET-CT) imaging should be used in combination with standard computed tomography (CT) and upper GI endoscopic ultrasound (EUS) in the assessment and staging of oesophageal and oesophago-gastric junctional cancer[4]. However in the era of relatively widespread use of PET-CT in this setting, the exact role of EUS remains unclear[5].
EUS has proven accuracy in both the assessment of tumour depth (T staging) and the extent of local nodal involvement (N stage) for patients with oesophageal and oesophago-gastric junctional cancer[6-8]. Standard EUS nodal imaging criteria suggestive of malignant lymphadenopathy include node size, border, shape and echogenicity. However, in practice, malignant lymph nodes rarely exhibit all of these characteristics and even with all four characteristics suggestive of malignancy, accuracy is sub-optimal[9-11]. To address this issue, other imaging techniques including tissue elastography and strain ratio have been used to help differentiate between benign and malignant mediastinal lymph nodes in upper GI cancer[12-15]. However tissue acquisition by EUS-FNA remains the optimal way to assess a (non-peritumoural) node for malignant involvement.
PET-CT imaging has been shown to be more accurate than PET alone in loco-regional nodal staging of oesophageal cancer[16]. PET-CT is also superior to both PET and CT alone in the detection of distant metastases[17,18]. It also has the potential to alter the staging and management of 12%-18% of patients[19,20]. However, it is well recognised that non-malignant processes such as inflammation can result in false positive findings which will affect the specificity of PET-CT in this setting. The false positive rate of PET-CT has been quoted as between 1.5% and 7.5% in upper GI cancer[21-24]. It has also been suggested that this may be an underestimate as positive findings are not always evaluated further[25]. However some studies have reported excellent specificity figures for PET-CT in this setting[26-32].
The aim of this study was to analyse the results and concordance of PET-CT and EUS-FNA in the staging of mediastinal lymph nodes in one tertiary referral centre, and to assess the impact of EUS-FNA on deciding the final therapeutic pathway.
This was a retrospective single centre study. Glasgow Royal Infirmary is a regional tertiary referral centre for EUS staging of upper GI cancer. Using a prospectively collected database, we reviewed the electronically held case records of all patients with a diagnosis of oesophago-gastric cancer who underwent PET-CT and EUS-FNA of at least one mediastinal lymph node between the 1st January 2009 and 31st December 2015. For each identified patient, we reviewed the PET-CT radiology report, the EUS-FNA procedure report and cytology report in addition to the final agreed therapeutic pathway after the conclusive multi-disciplinary team meeting.
Cases were described as PET-CT positive if mediastinal lymph node(s) demonstrated mild, moderate or high FDG uptake on imaging as described in the radiology report. PET-CT negative cases were those cases that demonstrated no uptake in any mediastinal lymph nodes. PET-CT indeterminate cases were those who demonstrated minimal FDG uptake and were excluded from further analysis.
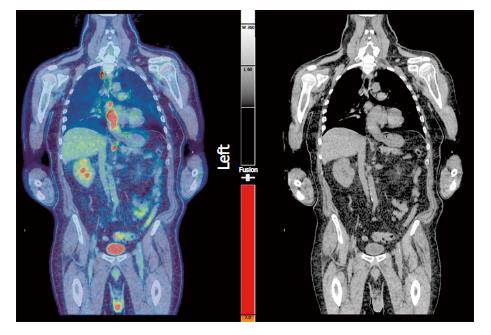
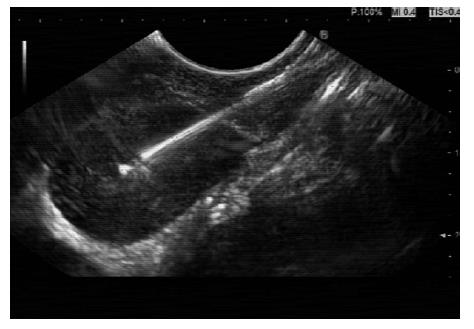
Following PET-CT imaging, all of our patients proceeded to have EUS-FNA within (a maximum of) 4 wk, but within 10-14 d for the vast majority. After MDT discussion, mediastinal nodes of concern distant from the primary tumour were targeted for FNA sampling (Figures 1 and 2).
EUS-FNA positive cases were defined as those whose cytology reports confirmed the presence of malignant cells in the sampled lymph node consistent with origin from their primary upper GI cancer. EUS-FNA negative cases were defined as those reported by the cytologist to show no evidence of malignant cells, together with benign lymphocytes consistent with lymph node sampling indicating an adequate specimen. Samples that did not meet either of these criteria were deemed to be insufficient for diagnosis and were excluded from further analysis.
Using a positive EUS-FNA result as the gold standard for lymph node involvement, we calculated the sensitivity, specificity, positive and negative predictive values (PPV and NPV) and accuracy of PET-CT in the staging of mediastinal lymph nodes. We also reviewed the final tumour stage and patient outcomes to determine the influence that EUS-FNA had in the cases where there was discordance between the PET-CT and EUS-FNA findings.
Staging EUS was undertaken by one of three experienced endosonographers (SP, NJ, AJS) using a Pentax linear ± radial echoendoscope, attached to a Hitachi EUB-8500 ultrasound processor. Standard EUS grey-scale images of suspicious lymph nodes were obtained and conventional characteristics of nodal size, shape, distinction of border and density were recorded.
EUS-FNA was performed using a Cook™ 22 gauge needle (Figure 2). A minimum of three samples were obtained by standard technique, stored in cytolite then sent to the laboratory for later cytological analysis by specialist pathologists.
A cytological report describing evidence or absence of malignancy in a sample consistent with lymph node sampling was used as the gold standard for analysis. We were then able to calculate the concordance of results between EUS-FNA and PET-CT. We also calculated the sensitivity, specificity, PPV and NPV of PET-CT in the identification of malignant mediastinal lymph nodes in patients with upper GI cancer.
One hundred and twenty one patients were identified in the study period (Table 1). Ninety one (75.2%) were male and thirty (24.8%) were female. The FNA sample was described as inadequate for analysis by the cytologist in eleven cases (8.9%) and the PET-CT findings were indeterminate in two cases (1.7%). These thirteen cases were excluded from further analysis. For the remaining one hundred and eight patients, sixty two had a histological diagnosis of adenocarcinoma (Thirty had oesophageal, twenty eight had junctional and four had gastric adenocarcinoma) and forty six had oesophageal squamous cell carcinoma. Of all these patients, thirty seven were positive on both PET-CT and EUS-FNA and thirty four were negative on both PET-CT and EUS-FNA, giving an overall concordance of 65.7%. The sensitivity, specificity, PPV and NPV results of PET-CT were 92.5%, 50%, 52.1% and 91.9% respectively.
| n = 121 | |
| Gender, n (%) | |
| Male | 91 (75.2) |
| Female | 30 (24.8) |
| Primary diagnosis, n (%) | |
| Oesophageal adenocarcinoma | 38 (31.4) |
| Oesophago-gastric junctional adenocarcinoma | 31 (25.6) |
| Oesophageal squamous cell carcinoma | 48 (39.7) |
| Gastric adenocarcinoma | 4 (3.3) |
| Excluded patients | 13 |
| EUS-FNA inadequate | 11 |
| PET-CT indeterminate | 2 |
Thirty four (31.5%) patients had positive PET-CT findings but negative EUS-FNA cytology and three (2.8%) patients had negative PET-CT findings and positive EUS-FNA cytology (Table 2). There were therefore thirty seven patients with discordant findings. The final treatment decision was unknown in five patients due to the majority of their management being undertaken at another health board, having been referred to our unit for EUS. For the remaining thirty two patients with discordant results, MDT discussion led to a radical treatment pathway in twenty seven, after the final tumour stage was altered as a consequence of the EUS-FNA findings. In all but one case this was due to downgrading of tumour stage as a result of a negative EUS-FNA in the setting of a positive PET-CT, however in one case the final tumour stage was upgraded due to a positive EUS-FNA but negative PET-CT result. Five patients were directed to a palliative management strategy (Table 3).
| PET-CT positive | PET-CT negative | |
| EUS-FNA positive | 37 (34.3%) | 3 (2.8%) |
| EUS-FNA negative | 34 (31.5%) | 34 (31.5%) |
| n = 37 | |
| Radical treatment | 27 |
| Palliative care | 5 |
| Unknown | 5 |
When all one hundred and eight cases were taken into consideration, EUS-FNA led directly to an alteration in clinical stage and subsequent clinical management in twenty seven (25%) patients.
In the group of twenty seven patients with discordant results who received radical treatment, six (22.2%) had progression of their disease whilst receiving treatment. Eleven developed progressive disease after completion of treatment at a median of nine months (range three to forty two months). Four patients remained in clinical remission post completion of radical treatment, although one of these patients died from urinary sepsis two years after completion of therapy. The median duration of clinical remission for the fifteen patients (55.6%) who experienced this was nine months (range three to forty two months).
One patient initially accepted radical treatment but refused further treatment after one cycle of neo-adjuvant chemotherapy. One other patient was not fit to have surgical resection after completing neo-adjuvant chemotherapy due to deterioration of other medical comorbidities rather than disease progression. The follow-up records after radical treatment were not available in four patients (Table 4).
| Radical treatment | n = 27 |
| Disease progression after completion of treatment | 11 |
| Disease progression whilst receiving treatment | 6 |
| Clinical remission after completion of treatment | 3 |
| Death from other cause whilst in remission | 1 |
| Consent for radical treatment withdrawn | 1 |
| Had neo-adjuvant chemo but not fit for surgery | 1 |
| Unknown | 4 |
We also analysed the data on the basis of histological subtype. For the forty six cases with oesophageal squamous cell carcinoma, nineteen were positive on both PET-CT and EUS-FNA and fourteen were negative on both investigations, resulting in a concordance of 71.7%. In the sixty two cases with adenocarcinoma (which includes oesophageal, junctional and gastric adenocarcinoma), eighteen were positive on both PET-CT and EUS-FNA and twenty were negative on both investigations, resulting in a concordance of 61.3%.
Upper GI cancer is a significant public health issue, accounting for 4% of cancers diagnosed in the United Kingdom. The most recent Cancer Research United Kingdom statistics from 2014 report an age standardised incidence of oesophageal cancer of 15.2 per 100000. The corresponding figure for gastric cancer was 11.4 per 100000 population, giving an overall incidence of upper GI cancer of 26.6 per 100000 population[33,34]. In recent years, there has been an increase in the use of PET-CT for clinical staging[5]. Its role in this setting however is controversial[21-25]. We devised this study to assess the impact of EUS-FNA in conjunction with PET-CT in the staging of patients with upper GI cancer.
We have found that PET-CT has 92.5% sensitivity for the detection of metastatic mediastinal lymphadenopathy in the setting of upper GI cancer. However, this is offset by poor specificity at 50%, leading to false-positive mediastinal nodes and the danger of over-staging upper GI cancer with PET-CT. Therefore EUS-FNA appears to have a critical role in confirming whether suspicious nodes identified on PET-CT have malignant involvement, in order to optimise staging of this disease. We feel that this is the most significant and clinically relevant finding of this study. The addition of EUS-FNA to PET-CT appears to lead to more accurate staging with the result of more patients being offered potentially curative treatment. After MDT discussion, EUS-FNA led to altered tumour stage and subsequent clinical management in 25% patients.
Our findings contrast with several previous studies which reported lower sensitivity but higher specificity rates for the detection of malignant mediastinal lymph nodes by PET-CT[26-32]. The interpretation of a positive mediastinal lymph node on PET-CT imaging in these studies seems to have been the same as our interpretation in that any FDG uptake beyond background level was considered significant. The reasons for our different findings remain unclear and require further study.
We looked in detail at the subgroup of 34 patients who had PET-CT positive, EUS-FNA negative nodes. Perhaps unexpectedly, we found that the majority (n = 22) of these patients demonstrated moderate or high (rather than just mild) uptake. The reasons for this finding are unclear, but do not suggest over-interpretation of low PET avidity.
Perhaps unexpectedly, we found three cases that had PET-CT negative but EUS-FNA positive nodes. All of these cases had adenocarcinoma; two were junctional and one case had oesophageal adenocarcinoma. Interestingly, we found that one of these cases displayed conventional EUS appearances of malignancy despite negative PET-CT appearances.
Upon analysis of our findings specifically in the context of histological subtype, we found that the concordance rate between PET-CT and EUS-FNA was 71.7% in those with oesophageal squamous cell carcinoma compared to 61.3% in those with adenocarcinoma. A recent paper which evaluated the extent of FDG uptake by malignant lymph nodes in the context of lung cancer found no significant difference on the basis of histological subtype (Which included adenocarcinoma and squamous cell carcinoma)[35]. We could not find any similar study which addresses this issue in the context of upper GI cancer. This is an area that requires further study.
Our study has several limitations. Firstly, this was a study which required us to access notes and electronic data retrospectively, albeit from a prospectively collected database. For some patients, all of the clinical information was not available because they received their follow-up care outside our tertiary referral centre, where the central staging investigations, including EUS and PET-CT, were performed. Secondly, the interpretation of mediastinal nodal involvement and designation of patients as either PET-CT positive or negative was a subjective judgement based on the radiological report rather than the maximum standardised uptake valves (SUVmax), which was only available in a minority of these reports. We agree that such data would be useful for future studies. Thirdly, the duration of follow-up was variable for each patient, although the minimum follow-up for all patients was 6 mo. This relatively short period of follow-up for some patients means that it is difficult to compare longer term survival outcomes with those reported in other studies. Finally, we accept that PET-CT and EUS-FNA are indirect ways of assessing for malignant involvement of mediastinal lymph nodes in the setting of upper GI cancer and that the most certain way to do this is by surgical resection. Unfortunately however, only a minority of our cases proceeded to surgical resection whereas they all had PET-CT followed by targeted mediastinal node sampling by EUS-FNA. The lack of surgical findings is a weakness of our study but it is reflective of our experience within our tertiary referral centre within the study period.
In conclusion and in the context of widespread use of PET-CT, we suggest that EUS-FNA remains an important diagnostic tool to optimise mediastinal nodal staging in upper GI cancer. Use of this modality ensures that patients are not potentially overstaged by PET-CT, and allows them to be directed to the appropriate therapeutic pathway after MDT discussion.
Upper GI cancer accounts for 4% of cancers diagnosed in the United Kingdom and as such is a significant public health issue. Surgical resection of the primary tumour and any involved lymph nodes results in the best outcomes. For this to be possible however, the surgical team must be confident that the disease is localised. Accurate pre-operative tumour staging is therefore paramount before any decisions regarding treatment are undertaken. In keeping with other organ systems, tumour staging of the upper digestive tract follows the TNM (Tumour, Node, Metastasis) system. The nodal staging of upper GI cancer has been an area of controversy. The 2011 United Kingdom joint medical, surgical and oncology guideline advised that positron emission tomography-computed tomography (PET-CT) imaging should be used in combination with standard computed tomography (CT) and upper GI endoscopic ultrasound (EUS) in the assessment and staging of oesophageal and oesophago-gastric junctional cancer. However in the era of relatively widespread use of PET-CT in this setting, the exact role of EUS remains unclear.
Several studies have assessed the role of PET-CT in the nodal staging of upper GI cancer. Most studies agree that PET-CT has high levels of sensitivity in the detection of malignant mediastinal lymph nodes. However, it is well documented that non-malignant processes such as inflammation can result in false positive findings which will adversely affect the specificity of PET-CT in this setting. The false positive rate of PET-CT has been quoted as between 1.5% and 7.5% in upper GI cancer. It has also been suggested that this may be an underestimate as positive findings are not always evaluated further. We performed this study to evaluate the performance of PET-CT in this setting within our centre and to compare this with the findings from other centres.
The first objective of this project was to evaluate the sensitivity, specificity, positive predictive value and negative predictive value of PET-CT in the detection of malignant mediastinal lymph nodes in the setting of upper GI cancer within the authors’ tertiary referral centre. The second objective was to evaluate the impact on subsequent therapeutic strategy that the addition of EUS-FNA had in these patients.
The authors performed a retrospective review of prospectively recorded data held on all patients with a diagnosis of upper gastrointestinal (GI) cancer made between January 2009 and December 2015. Only those patients who had both a PET-CT and EUS with FNA sampling of a mediastinal node distant from the primary tumour were included.
The authors found that EUS-FNA leads to altered staging of upper GI cancer, resulting in more patients receiving radical treatment that would have been the case using PET-CT staging alone. The authors found that EUS-FNA resulted in altered tumour staging and subsequent management in 25% of cases included in this study. The authors were also interested to find that the rate of concordance of PET-CT and EUS-FNA findings was dependent on the tumour histological subtype. There was a 71.7% rate of concordance in cases with squamous cell carcinoma compared with 61.3% concordance in cases with adenocarcinoma. The reasons for this are unclear and this is therefore an area that requires further study.
The authors suggest that EUS-FNA remains an important diagnostic tool to optimise mediastinal nodal staging in upper GI cancer. Use of this modality ensures that patients are not potentially overstaged by PET-CT, and allows them to be directed to the appropriate therapeutic pathway after MDT discussion. Therefore EUS-FNA appears to have a critical role in confirming whether suspicious nodes identified on PET-CT have malignant involvement, in order to optimise staging of this disease. The authors feel that this is the most significant and clinically relevant finding of this study.
The authors’ findings contrast with several previous studies which reported lower sensitivity but higher specificity rates for the detection of malignant mediastinal lymph nodes by PET-CT. The interpretation of a positive mediastinal lymph node on PET-CT imaging in these studies seems to have been the same as our interpretation in that any FDG uptake beyond background level was considered significant. The reasons for our different findings remain unclear and require further study. The authors also found that the rate of concordance between PET-CT and EUS-FNA findings was greater in patients with squamous cell carcinoma than in those with adenocarcinoma (71.7% and 61.3% respectively). The authors could not find any study which addresses this area in the context of upper GI cancer specifically. This is therefore an area that requires further study.
We would like to thank Dr David Colville from Glasgow Royal Infirmary, who kindly provided and reviewed the PET-CT image for this publication.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Arigami T, Shiryajev YN S- Editor: Kong JX L- Editor: A E- Editor: Li D
| 1. | Allum WH, Stenning SP, Bancewicz J, Clark PI, Langley RE. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol. 2009;27:5062-5067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 662] [Cited by in RCA: 754] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 2. | Hulscher JB, van Sandick JW, de Boer AG, Wijnhoven BP, Tijssen JG, Fockens P, Stalmeier PF, ten Kate FJ, van Dekken H, Obertop H. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med. 2002;347:1662-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1232] [Cited by in RCA: 1144] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 3. | Shapiro J, van Lanschot JJB, Hulshof MCCM, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, van Laarhoven HWM, Nieuwenhuijzen GAP, Hospers GAP, Bonenkamp JJ. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1292] [Cited by in RCA: 1824] [Article Influence: 182.4] [Reference Citation Analysis (0)] |
| 4. | Allum WH, Blazeby JM, Griffin SM, Cunningham D, Jankowski JA, Wong R; Association of Upper Gastrointestinal Surgeons of Great Britain and Ireland, the British Society of Gastroenterology and the British Association of Surgical Oncology. Guidelines for the management of oesophageal and gastric cancer. Gut. 2011;60:1449-1472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 415] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 5. | National Oesophago-Gastric Cancer Audit. 2013, Annual Report. Available from: http://www.hscic.gov.uk. |
| 6. | Smith BR, Chang KJ, Lee JG, Nguyen NT. Staging accuracy of endoscopic ultrasound based on pathologic analysis after minimally invasive esophagectomy. Am Surg. 2010;76:1228-1231. [PubMed] |
| 7. | Puli SR, Reddy JB, Bechtold ML, Antillon D, Ibdah JA, Antillon MR. Staging accuracy of esophageal cancer by endoscopic ultrasound: a meta-analysis and systematic review. World J Gastroenterol. 2008;14:1479-1490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 243] [Cited by in RCA: 246] [Article Influence: 14.5] [Reference Citation Analysis (2)] |
| 8. | van Vliet EP, Heijenbrok-Kal MH, Hunink MG, Kuipers EJ, Siersema PD. Staging investigations for oesophageal cancer: a meta-analysis. Br J Cancer. 2008;98:547-557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 336] [Cited by in RCA: 344] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 9. | Catalano MF, Alcocer E, Chak A, Nguyen CC, Raijman I, Geenen JE, Lahoti S, Sivak MV Jr. Evaluation of metastatic celiac axis lymph nodes in patients with esophageal carcinoma: accuracy of EUS. Gastrointest Endosc. 1999;50:352-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 77] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Bhutani MS, Hawes RH, Hoffman BJ. A comparison of the accuracy of echo features during endoscopic ultrasound (EUS) and EUS-guided fine-needle aspiration for diagnosis of malignant lymph node invasion. Gastrointest Endosc. 1997;45:474-479. [PubMed] |
| 11. | Chen VK, Eloubeidi MA. Endoscopic ultrasound-guided fine needle aspiration is superior to lymph node echofeatures: a prospective evaluation of mediastinal and peri-intestinal lymphadenopathy. Am J Gastroenterol. 2004;99:628-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 104] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | Saftoiu A, Vilman P. Endoscopic ultrasound elastography-- a new imaging technique for the visualization of tissue elasticity distribution. J Gastrointestin Liver Dis. 2006;15:161-165. [PubMed] |
| 13. | Janssen J, Dietrich CF, Will U, Greiner L. Endosonographic elastography in the diagnosis of mediastinal lymph nodes. Endoscopy. 2007;39:952-957. [PubMed] |
| 14. | Faige DO. EUS in patients with benign and malignant lymphadenopathy. Gastrointest Endosc. 2001;53:593-598. [PubMed] |
| 15. | Paterson S, Duthie F, Stanley AJ. Endoscopic ultrasound-guided elastography in the nodal staging of oesophageal cancer. World J Gastroenterol. 2012;18:889-895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Yuan S, Yu Y, Chao KS, Fu Z, Yin Y, Liu T, Chen S, Yang X, Yang G, Guo H. Additional value of PET/CT over PET in assessment of locoregional lymph nodes in thoracic esophageal squamous cell cancer. J Nucl Med. 2006;47:1255-1259. [PubMed] |
| 17. | Choi J, Kim SG, Kim JS, Jung HC, Song IS. Comparison of endoscopic ultrasonography (EUS), positron emission tomography (PET), and computed tomography (CT) in the preoperative locoregional staging of resectable esophageal cancer. Surg Endosc. 2010;24:1380-1386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 117] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 18. | Salahudeen HM, Balan A, Naik K, Mirsadraee S, Scarsbrook AF. Impact of the introduction of integrated PET-CT into the preoperative staging pathway of patients with potentially operable oesophageal carcinoma. Clin Radiol. 2008;63:765-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Williams RN, Ubhi SS, Sutton CD, Thomas AL, Entwisle JJ, Bowrey DJ. The early use of PET-CT alters the management of patients with esophageal cancer. J Gastrointest Surg. 2009;13:868-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Noble F, Nolan L, Bateman AC, Byrne JP, Kelly JJ, Bailey IS, Sharland DM, Rees CN, Iveson TJ, Underwood TJ. Refining pathological evaluation of neoadjuvant therapy for adenocarcinoma of the esophagus. World J Gastroenterol. 2013;19:9282-9293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Noble F, Bailey D; SWCIS Upper Gastrointestinal Tumour Panel, Tung K, Byrne JP. Impact of integrated PET/CT in the staging of oesophageal cancer: a UK population-based cohort study. Clin Radiol. 2009;64:699-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | van Westreenen HL, Westerterp M, Sloof GW, Groen H, Bossuyt PM, Jager PL, Comans EF, van Dullemen HM, Fockens P, Stoker J. Limited additional value of positron emission tomography in staging oesophageal cancer. Br J Surg. 2007;94:1515-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Torrance AD, Almond LM, Fry J, Wadley MS, Lyburn ID. Has integrated 18F FDG PET/CT improved staging, reduced early recurrence or increased survival in oesophageal cancer? Surgeon. 2015;13:19-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Han D, Yu J, Zhong X, Fu Z, Mu D, Zhang B, Xu G, Yang W, Zhao S. Comparison of the diagnostic value of 3-deoxy-3-18F-fluorothymidine and 18F-fluorodeoxyglucose positron emission tomography/computed tomography in the assessment of regional lymph node in thoracic esophageal squamous cell carcinoma: a pilot study. Dis Esophagus. 2012;25:416-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Blencowe NS, Whistance RN, Strong S, Hotton EJ, Ganesh S, Roach H, Callaway M, Blazeby JM. Evaluating the role of fluorodeoxyglucose positron emission tomography-computed tomography in multi-disciplinary team recommendations for oesophago-gastric cancer. Br J Cancer. 2013;109:1445-1450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Karashima R, Watanabe M, Imamura Y, Ida S, Baba Y, Iwagami S, Miyamoto Y, Sakamoto Y, Yoshida N, Baba H. Advantages of FDG-PET/CT over CT alone in the preoperative assessment of lymph node metastasis in patients with esophageal cancer. Surg Today. 2015;45:471-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Yamada H, Hosokawa M, Itoh K, Takenouchi T, Kinoshita Y, Kikkawa T, Sakashita K, Uemura S, Nishida Y, Kusumi T. Diagnostic value of 18F-FDG PET/CT for lymph node metastasis of esophageal squamous cell carcinoma. Surg Today. 2014;44:1258-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Redondo-Cerezo E, Martínez-Cara JG, Esquivias J, de la Torre-Rubio P, González-Artacho C, García-Marín Mdel C, de Teresa-Galván J. Endoscopic ultrasonography-fine needle aspiration versus PET-CT in undiagnosed mediastinal and upper abdominal lymphadenopathy: a comparative clinical study. Eur J Gastroenterol Hepatol. 2015;27:455-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Yoon YC, Lee KS, Shim YM, Kim BT, Kim K, Kim TS. Metastasis to regional lymph nodes in patients with esophageal squamous cell carcinoma: CT versus FDG PET for presurgical detection prospective study. Radiology. 2003;227:764-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 161] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 30. | Kneist W, Schreckenberger M, Bartenstein P, Grünwald F, Oberholzer K, Junginger T. Positron emission tomography for staging esophageal cancer: does it lead to a different therapeutic approach? World J Surg. 2003;27:1105-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Okada M, Murakami T, Kumano S, Kuwabara M, Shimono T, Hosono M, Shiozaki H. Integrated FDG-PET/CT compared with intravenous contrast-enhanced CT for evaluation of metastatic regional lymph nodes in patients with resectable early stage esophageal cancer. Ann Nucl Med. 2009;23:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 32. | Kato H, Kimura H, Nakajima M, Sakai M, Sano A, Tanaka N, Inose T, Faried A, Saito K, Ieta K. The additional value of integrated PET/CT over PET in initial lymph node staging of esophageal cancer. Oncol Rep. 2008;20:857-862. [PubMed] |
| 33. | Oesophageal cancer incidence statistics. Available from: http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/oesophageal-cancer/incidence#heading-Zero. |
| 34. | Stomach cancer incidence statistics. Available from: http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/stomach-cancer/incidence#heading-Zero. |
| 35. | Flechsig P, Frank P, Kratochwil C, Antoch G, Rath D, Moltz J, Rieser M, Warth A, Kauczor HU, Schwartz LH. Radiomic Analysis using Density Threshold for FDG-PET/CT-Based N-Staging in Lung Cancer Patients. Mol Imaging Biol. 2017;19:315-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |