Published online Jan 16, 2018. doi: 10.4253/wjge.v10.i1.1
Peer-review started: November 17, 2017
First decision: December 6, 2017
Revised: December 11, 2017
Accepted: December 29, 2017
Article in press: December 29, 2017
Published online: January 16, 2018
Processing time: 61 Days and 10.7 Hours
Increases in the quality as well as utilization of cross-sectional imaging have led to rising diagnoses of pancreatic cystic lesions (PCL). Accurate presurgical diagnosis enables appropriate triage of PCLs. Unfortunately, current diagnostic approaches have suboptimal accuracy and may lead to unnecessary surgical resections or missed diagnoses of advanced neoplasia. Additionally, early detection represents an opportunity for intervention to prevent the progression to pancreatic adenocarcinoma. Our aim for this review is to systematically review the current literature on confocal endomicroscopy and molecular biomarkers in the evaluation of PCLs. Confocal laser endomicroscopy is a novel technology that allows for real-time in vivo microscopic imaging with multiple clinical trials identifying characteristic endomicroscopy findings of various pancreatic cystic lesions. DNA-based molecular markers have also emerged as another diagnostic modality as the pattern of genetic alternations present in cyst fluid can provide both diagnostic and prognostic data. We propose that both techniques can be utilized to improve patient outcomes.
Core tip: Current diagnostic guidelines for the evaluation of pancreatic cystic lesions have suboptimal accuracy and may lead to unnecessary surgical resections or missed diagnoses of advanced neoplasia. We propose that two new diagnostic technologies, confocal laser endomicroscopy and DNA-based molecular markers, may be used synergistically to improve diagnostic accuracy. In this review, we summarize the current literature regarding these two techniques.
- Citation: Li F, Malli A, Cruz-Monserrate Z, Conwell DL, Krishna SG. Confocal endomicroscopy and cyst fluid molecular analysis: Comprehensive evaluation of pancreatic cysts. World J Gastrointest Endosc 2018; 10(1): 1-9
- URL: https://www.wjgnet.com/1948-5190/full/v10/i1/1.htm
- DOI: https://dx.doi.org/10.4253/wjge.v10.i1.1
Increases in the quality as well as utilization of cross-sectional imaging have led to rising diagnoses of pancreatic cystic lesions (PCL) with a reported incidence ranging from 2.4%-19.6%[1-3]. Unfortunately, current diagnostic approaches have suboptimal accuracy and may lead to unnecessary surgical resections or missed diagnoses of advanced neoplasia[4]. Accurate pre-surgical diagnosis enables appropriate triage of PCLs, allowing for surveillance of lower-risk lesions and surgical resection of high-risk lesions. Additionally, early detection represents an opportunity for intervention to prevent the progression to pancreatic adenocarcinoma.
Our aim for this review is to summarize the current literature on confocal endomicroscopy and molecular biomarkers in the evaluation of PCLs. We propose that both techniques can be complementary to improve patient outcomes.
Pancreatic cysts can be divided into mucinous cysts [intraductal papillary mucinous neoplasm (IPMN), mucinous cystic neoplasm (MCN)], non-mucinous cystic neoplasms [serous cystadenoma (SCA), pseudocysts], cystic neuroendocrine tumors (cystic-NETs), and solid pseudopapillary neoplasm (SPN)[5]. Each of these lesions have unique characteristics and malignancy potential requiring different management strategies.
The current standard of care in the evaluation of PCLs utilizes a multimodality approach, including clinical and radiographic assessment, Endoscopic ultrasound (EUS)-guided fine needle aspiration (EUS-FNA), cyst fluid analysis (i.e., tumor markers such as CEA), and cytology. Despite these techniques, the pre-surgical differentiation of PCLs remains challenging with continued need for improved diagnostic accuracy. A landmark prospective study comparing cyst fluid CEA, cytology, and EUS showed that that cyst fluid CEA > 192 ng/mL had a diagnostic accuracy of 79.2%, cytology had a diagnostic accuracy of 58.7%, and EUS morphology had a diagnostic accuracy of 50.9%[6]. However, a more recent, larger multicenter retrospective study showed that a CEA cutoff of 192 ng/mL for the diagnosis of mucinous cysts resulted in a sensitivity of only 61%[7].
In an effort to improve diagnostic accuracy, multiple guidelines have been developed over the past decade to assist in the management of PCLs, including the International Consensus Guidelines (Sendai 2006, Fukuoka 2012, and 2017 revision of the Fukuoka guidelines) and the American Gastroenterological Association (AGA) 2015 guidelines[8-10]. The 2006 Sendai guidelines recommended surgical resection of any suspected MCN, main duct IPMN, or mixed duct IPMN. Additional criteria for surgical resection included: clinical symptoms, dilated pancreatic duct (≥ 6 mm), intracystic mural nodules, or positive cytology[8]. While the Sendai guidelines have a sensitivity approaching 100%, specificity is limited, ranging from 23%-31%[11,12]. In 2012, stricter surgical criteria were developed for the revised Fukuoka guidelines for IPMN and MCN including: pancreatic duct ≥ 10 mm, presence of an enhancing solid component, obstructive jaundice with a pancreatic cyst[9]. Although the Fukuoka guidelines were more specific compared to the Sendai guidelines, sensitivity was decreased. In a retrospective analysis, the updated Fukuoka (2012) guidelines were not superior to the Sendai guidelines for detection of invasive carcinoma or high-grade dysplasia[13].
Given these limitations, the AGA introduced guidelines in 2015 for the management of all asymptomatic neoplastic pancreatic cysts, whereas neither the Sendai nor the Fukuoka guidelines address the management of non-mucinous cysts. Compared to the Fukuoka guidelines, the AGA guidelines have a higher threshold for both endoscopic evaluation and surgical resection. EUS-FNA was recommended if 2 high-risk features were present, including size ≥ 3 cm, a dilated main pancreatic duct, or associated solid component. Surgical resection was recommended if a cyst had both a solid component and a dilated pancreatic duct and/or concerning features on EUS-FNA[10]. In a retrospective study of 225 patients who underwent EUS-FNA for pancreatic cysts, applying the AGA criteria detected advanced neoplasia with 62% sensitivity, 79% specificity, 57% positive predictive value, and 82% negative predictive value. Unfortunately, 45% of IPMNs with adenocarcinoma or high-grade dysplasia were missed[14].
In 2017, the International Consensus Group released updated guidelines regarding the prediction of invasive carcinoma and high-grade dysplasia, as well as the surveillance and post-operative follow-up of IPMNs. In the revised guidelines, increased serum CA19-9 and cyst growth rate greater than 5 mm in diameter over 2 years were added as “worrisome features” for BD-IPMN. These limitations show that current guidelines are suboptimal to accurately diagnose PCLs and additional imaging and molecular biomarkers are necessary to improve diagnostic accuracy of these increasingly prevalent lesions. EUS-guided needle-based confocal laser endomicroscopy (nCLE) and pancreatic cyst fluid molecular markers are promising new diagnostic modalities to aid in diagnosis and management of PCLs.
CLE is a novel technology that allows for real-time in vivo microscopic imaging. The CLE probe can be inserted through a 19-gauge FNA needle for real-time microscopic examination of the pancreatic cyst epithelium during EUS.
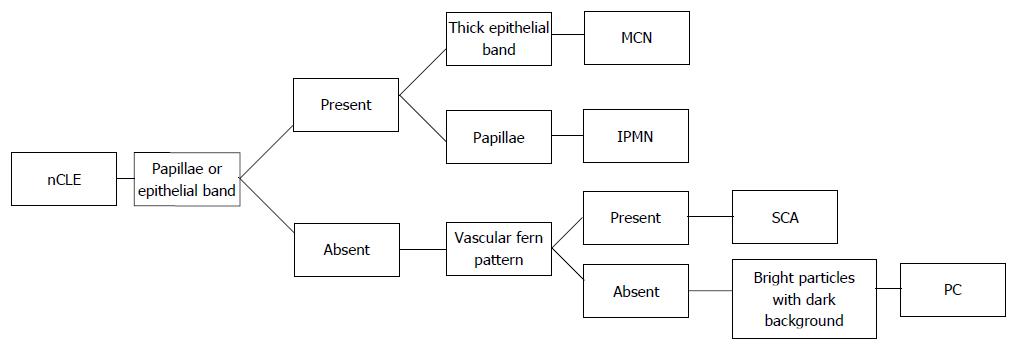
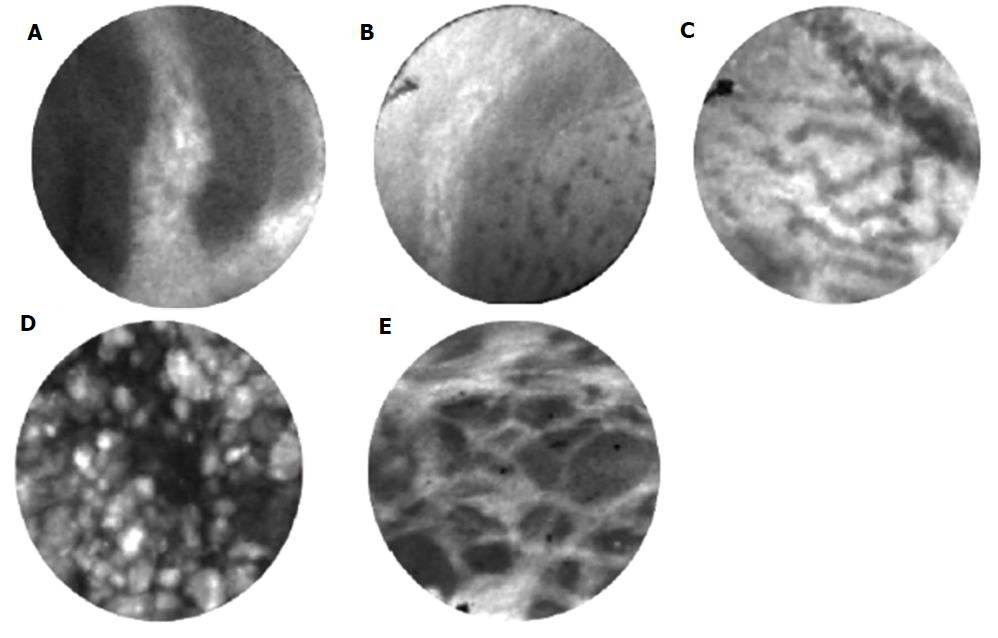
Multiple clinical trials have identified characteristic nCLE findings of various pancreatic cystic lesions (Table 1). For IPMN and MCN, characteristic findings include finger-like papillae and a single or layers of band-like epithelium, respectively[15-17]. In vivo and ex vivo nCLE findings for IPMN have been validated compared to surgical pathology as gold standard[18]. The finding of a “superficial vascular network” or “fern pattern” is highly specific for SCA[19,20]. Pseudocysts contain bright particles, corresponding to inflammatory cells, against a dark background due to the lack of a true cyst wall[17]. Cystic neuroendocrine tumors demonstrate high cellularity demonstrating trabeculae or cords of cells separated by fibrous bands[18]. More rare cystic lesions, such as those lined by squamous epithelium (lymphoepithelial cysts) have been characterized in case reports[21,22].
The INSPECT study was a pilot to assess the feasibility of nCLE in differentiating mucinous PCLs and establish safety[15]. The DETECT study’s aim was to identify the feasibility, safety, diagnostic yield of cystoscopy and nCLE to diagnose PCLs using the consensus criteria developed for the INSPECT trial. The patients included in the study had clinical diagnoses of IPMN, MCN, pseudocyst, lymphoepithelial cyst, and retention cyst. The diagnosis of IPMN was supported by the identification of finger-like papillae[23]. The CONTACT-1 trial enrolled 31 patients with solitary pancreatic cystic lesions who underwent EUS-nCLE. The nCLE finding of a superficial vascular network, which correlated microscopically to a dense and subepithelial capillary vascularization, was only seen in SCA[19]. The CONTACT-2 study identified new nCLE criteria for MCN (epithelial bands), pancreatic pseudocysts (field of bright particles), and cystic neuroendocrine neoplasm (black cell clusters with white fibrous areas), which correlated with histologic features[17]. The INDEX trial validated the previously described nCLE findings in ex vivo CLE of resected PCLs; demonstrated substantial interobserver agreement for mucinous PCLs among nCLE-naïve observers; and established an ”almost perfect“ interobserver agreement and intraobserver reliability among external blinded observers for the detection of mucinous PCLs[24]. Based on the above studies and our experience, we have suggested an algorithm for evaluation of a PCL utilizing EUS-nCLE (Figure 1).
Over the last decade, DNA-based molecular testing has emerged as a potent diagnostic modality for the assessment of PCLs. Analyzing the DNA present in the cyst fluid for the pattern of genetic alterations can provide both diagnostic and prognostic data regarding likelihood of progression to pancreatic adenocarcinoma[25,26].
There are three main components of molecular analysis: DNA quantity and quality, oncogenic mutations, loss of heterozygosity (LOH) of tumor suppressor genes. DNA quantity is determined by spectrophotometric analysis. By exposing the DNA sample to ultraviolet light, a photo-detector can be used to determine the quantity of nucleic acid in the sample. The concentration of DNA can be determined using the optical density ratio at a certain wavelength (260 of 280) light after extracting DNA from fluid. In a study of 113 patients with pancreatic cysts, elevated amounts of cyst fluid DNA were associated with malignancy[27]. Loss of heterozygosity results in loss of the entire gene and the surrounding chromosomal region. The detection of LOH by using microsatellite markers closely linked to key tumor suppressor genes correlates with gene inactivation and mutation, resulting in loss of tumor suppressor activity and development of malignancy[28].
Prior studies evaluating DNA testing of PCL fluid were limited by insensitive detection strategies (conventional Sanger sequencing). The use of next-generation sequencing (NGS) has revealed specific molecular markers that aid in the diagnosis of mucinous cysts as well as detection of advanced neoplasia. NGS refers to DNA sequencing technologies that allow sequencing of numerous small fragments of DNA in parallel, which are then pieced together by mapping individual reads to the reference genome. This allows rapid sequencing of entire genomes compared to conventional Sanger sequencing. Whole exome and targeted sequencing studies of PCL fluid have revealed certain mutational profiles of major cyst subtypes as well as markers of advanced neoplasia (high-grade dysplasia/pancreatic adenocarcinoma).
More widespread utilization of NGS is limited by suboptimal identification of specific PCL types, including MCN (low sensitivity) and cystic neuroendocrine tumor (lack of DNA) as well as poor sensitivity for detection of the VHL gene (as seen in SCAs) requiring Sanger sequencing[8,29]. A proposed algorithm for evaluation of PCLs based on cyst fluid molecular markers is shown in Figure 2.
KRAS mutations are seen in both IPMN and MCN, although less sensitive for detection of MCN[30]. GNAS mutations are found in IPMN but not MCN[25,31]. RNF43 mutations occur in 14%-38% of IPMNs[25,31]. VHL gene mutations have been identified in SCA but not in other pancreatic cystic lesions[25,29]. CTNNB1 gene mutations are the most commonly seen alteration in SPN[25].
EUS guided evaluation of PCLs permits integrated evaluation with imaging (nCLE) and molecular (cyst fluid) biomarkers. Table 2 and Figure 3 summarize the key imaging and molecular biomarkers for different types of PCLs.
| IPMN | MCN | SCA | SPN | PC | NEN | |
| Imaging biomarker | ||||||
| nCLE patterns | Finger-like Papillae[17,24] | Epithelial bands (single or multiple)[17] | Fern pattern or superficial vascular network[17,19] | Not well defined | Bright particles against dark background[17] | Trabecular pattern[17] |
| Rope ladder or branched type vascularity[49] | Rope ladder or branched type vascularity[49] | |||||
| Molecular biomarker | ||||||
| Cyst fluid molecular analysis | KRAS, GNAS, RNF43 positive[25,31,34] | KRAS, RNF43 positive, GNAS negative[25,31] | VHL positive[29] | CTNNB1 positive[25] | Negative | Not well characterized |
| Cysts with advanced neoplasia | TP53, SMAD4, PIK3CA, PTEN, CKDN2A positive[35,38,37] | TP53, SMAD4, PIK3CA, PTEN, CKDN2A positive[31] | ||||
| p16, p53 positive[37] |
Intra-ductal papillary mucinous neoplasm: IPMNs are epithelial neoplasms that produce mucin. They are classified based on involvement of the main pancreatic duct: main duct IPMN (MD-IPMN), branch duct IPMN (BD-IPMN), mixed (both main and branch duct) IPMN. MD-IPMN is characterized by either segmental or diffuse dilation of the main pancreatic duct greater than 5 mm without other causes of obstruction. BD-IPMN is characterized by cyst diameter greater than 5 mm that communicates with the main pancreatic duct. Mixed-IPMN meets criteria for both MD-IPMN and BD-IPMN. MD-IPMN and mixed IPMN are associated with significantly higher incidence of malignancy compared to BD-IPMN (60% vs 25%)[9,32]. They are also classified into gastric, intestinal, pancreaticobiliary, oncocytic subtypes[33].
Patterns of papillae or epithelial bands on nCLE have high correlation with mucinous cysts[15,17]. The epithelial bands typically seen in MCNs do not have papillary morphology. On the other hand, IPMNs have complete papillae[24]. Analysis of performance of nCLE criteria for IPMN showed an accuracy 90%, sensitivity 80%, specificity 92%, positive predictive value 67%, and 96% negative predictive value[17].
The oncogenic KRAS and GNAS mutations have been extensively studied in IPMNs. The KRAS mutation is seen in 80% of IPMNs while 65% of IPMNs have mutations in the GNAS oncogene[34]. KRAS mutations are associated with branch duct location[30], while GNAS mutations are associated with main duct location[29]. KRAS and GNAS are considered early events in the progression to PDAC and mutations in either KRAS or GNAS are seen in over 96% of IPMNs[29].
In addition, inactivating mutations of the tumor suppressor gene RNF43 occur in 14%-38% of IPMNs[25,31]. Additional molecular markers present in IPMNs include p16 (lost earlier compared to p53), SMAD4, p53, and TP53[35-38].
IPMNs with advanced neoplasia may have TP53, PIK3CA, PTEN, and/or AKT1 mutations[36,39-43]. A prospective single center study showed that a combination of KRAS/GNAS mutations and changes in TP53/PIK3CA/PTEN had 78% sensitivity and 97% specificity for advanced neoplasia[44]. Studies combining DNA quantity, KRAS mutations, and LOH mutations have shown variable sensitivities: 50%[45] vs 83%[46]. An additional study found that both KRAS and LOH was present in 50% of carcinoma or high grade dysplasia compared to 8% of premalignant IPMNs, indicating the progression of neoplasia may correlate with accumulation of genetic disturbances[38].
Mucinous cystic neoplasm: Like IPMNs, MCNs are also mucin-producing epithelial neoplasms. Typically they are located in the body or tail of the pancreas and are not associated with the main pancreatic duct[47]. They are more commonly seen in women and typically occur between the ages of 30 to 50 years of age[34]. Microscopically, MCNs are composed of columnar mucinous epithelium and characteristic dense ovarian-type stroma, which express hormone receptors.
During EUS-nCLE, MCNs typically demonstrate single or layers of epithelial bands rather than papillae[17]. In a minority of patients, some MCN show evidence of chronic inflammation with bright fluorescent inflammatory cells[24].
Similar to IPMNs, the most common mutation in MCNs is the KRAS gene. The prevalence of KRAS mutations increases with the degree of dysplasia: 26% in low-grade MCNs but 89% in advanced neoplasia[25]. Mutations or deletions in TP53, PIK3CA, PTE, CDKN2A, SMAD4 are associated with advanced neoplasia in MCN[31]. Unlike IPMNs, the GNAS mutation is not seen in MCNs[25,31].
Although the KRAS mutation is seen in both IPMN and MCN, it is much less sensitive for detection of MCN (sensitivity of 14%) than IPMN[30]. Other genetic alterations in MCNs include KRAS, TP53, and SMAD4. Additional associations with PIK3CA, PTEN, and CKDN2A have also been published[25,31,40].
Serous cystadenomas are benign cystic neoplasms that are more common in women[48]. A large retrospective, multinational study of over 2600 patients diagnosed with serous cystic neoplasms showed minimal risk of clinically relevant symptoms over a three-year follow up period. Given their lack of malignant potential, surgical management is only needed if they are symptomatic (causing pancreatitis or jaundice)[48].
A report from the CONTACT study identified a superficial vascular network (subepithelial vessels uniformly distributed in the cyst wall) or fern pattern as a characteristic of SCA[19,49]. The presence of this pattern is highly specific for SCA. On the other hand, sensitivity for diagnosis of SCA is low in the absence of this pattern (69% to 75%)[17,19].
VHL gene mutations have been identified in SCA cyst fluid[29] but not in IPMN, MCN, or SPN[25]. However, VHL mutations are also seen in pancreatic neuroendocrine tumors and are not specific to SCAs. TP53 and PIK3CA have been rarely described. KRAS, GNAS, and RNF43 mutations, which can be seen in mucinous cysts, have not been identified[25,29].
Solid pseudopapillary neoplasms are typically well-defined solitary lesions often found in younger women[50]. Microscopically, they are composed of poorly cohesive cells forming a mixed pattern of solid, pseudopapillary, and hemorrhagic cystic structures[34]. They do not communicate with the main pancreatic duct and contain myxoid stroma on cytology[47].
The nCLE findings of solid pseudopapillary neoplasms are not well defined due to their rarity.
Mutations of the B-catenin gene (CTNNB1) are the most commonly seen alteration in SPN[25]. This results in cytoplasmic and nuclear accumulation of B-catenin. VHL, GNAS, RNF43 mutations have not been identified in these cysts[25,29]. Therefore, the presence of CTNNB1 in the absence of KRAS, GNAS, and RNF43 mutations is confirmatory for diagnosing SPNs[25].
Pancreatic pseudocysts are an encapsulated collections of fluid with a well-defined inflammatory wall with minimal or no necrosis[51]. They are histologically composed of fibro-inflammatory tissue surrounding necrotic adipocytes without epithelial lining. No vasculature is seen because pseudocysts do not have an epithelium. On nCLE, this is characterized by bright inflammatory cells against a dark background[17]. As pseudocysts are not neoplastic, molecular markers related to malignancy are not found.
Microscopically, cystic neuroendocrine neoplasms are characterized by a neoplastic monomorphic cell proliferation with variations in cellular architecture. Characteristic nCLE appearance of pancreatic neuroendocrine tumors have been described[21]. Endomicroscopy demonstrates dark, irregular clusters or trabeculae of compact cells (neoplastic cells) surrounded by gray tissue (fibrovascular stroma)[17]. Neuroendocrine neoplasms have not been well characterized on molecular studies and further research is needed.
This review summarizes the current status of new technologies for the evaluation of PCLs including confocal endomicroscopy and molecular markers. Both EUS-nCLE and cyst fluid molecular analysis of PCLs represent promising new modalities to improve the diagnostic evaluation of PCLs by supplementing the standard evaluation of pancreatic cysts which includes imaging (MRI, CT) and endoscopy (EUS). Given the limitations of current diagnostic algorithms, these imaging and molecular biomarkers can increase diagnostic accuracy and improve management of PCLs. Prospective multicenter studies are needed to determine how to integrate nCLE and molecular analysis into existing management protocols and clinical practice. In clinical practice, these technologies may especially be applied in the setting of cases with diagnostic uncertainty in order to improve accuracy and allow for appropriate risk stratification. Expertise in these technologies may not be widespread and referral to centers with experience may be necessary.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and Hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Agrawal S, Amornyotin S, Lee CL, Toshniwal JJ S- Editor: Cui LJ L- Editor: A E- Editor: Song XX
| 1. | de Jong K, Nio CY, Hermans JJ, Dijkgraaf MG, Gouma DJ, van Eijck CH, van Heel E, Klass G, Fockens P, Bruno MJ. High prevalence of pancreatic cysts detected by screening magnetic resonance imaging examinations. Clin Gastroenterol Hepatol. 2010;8:806-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 376] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 2. | Laffan TA, Horton KM, Klein AP, Berlanstein B, Siegelman SS, Kawamoto S, Johnson PT, Fishman EK, Hruban RH. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol. 2008;191:802-807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 724] [Cited by in RCA: 658] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 3. | Zhang XM, Mitchell DG, Dohke M, Holland GA, Parker L. Pancreatic cysts: depiction on single-shot fast spin-echo MR images. Radiology. 2002;223:547-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 277] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 4. | Matthaei H, Schulick RD, Hruban RH, Maitra A. Cystic precursors to invasive pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2011;8:141-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 144] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 5. | Zamboni G, Klöppel G, Hruban R, Longnecker D, Adler G. Mucinous cystic neoplasms of the pancreas: IARC Press, 2000. |
| 6. | Brugge WR, Lewandrowski K, Lee-Lewandrowski E, Centeno BA, Szydlo T, Regan S, del Castillo CF, Warshaw AL. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1016] [Cited by in RCA: 901] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 7. | Gaddam S, Ge PS, Keach JW, Mullady D, Fukami N, Edmundowicz SA, Azar RR, Shah RJ, Murad FM, Kushnir VM. Suboptimal accuracy of carcinoembryonic antigen in differentiation of mucinous and nonmucinous pancreatic cysts: results of a large multicenter study. Gastrointest Endosc. 2015;82:1060-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 8. | Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, Yamaguchi K, Yamao K, Matsuno S; International Association of Pancreatology. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1539] [Cited by in RCA: 1441] [Article Influence: 75.8] [Reference Citation Analysis (0)] |
| 9. | Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, Kimura W, Levy P, Pitman MB, Schmidt CM. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1714] [Cited by in RCA: 1614] [Article Influence: 124.2] [Reference Citation Analysis (0)] |
| 10. | Vege SS, Ziring B, Jain R, Moayyedi P; Clinical Guidelines Committee; American Gastroenterology Association. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148:819-822; quize12-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 629] [Cited by in RCA: 759] [Article Influence: 75.9] [Reference Citation Analysis (1)] |
| 11. | Pelaez-Luna M, Chari ST, Smyrk TC, Takahashi N, Clain JE, Levy MJ, Pearson RK, Petersen BT, Topazian MD, Vege SS. Do consensus indications for resection in branch duct intraductal papillary mucinous neoplasm predict malignancy? A study of 147 patients. Am J Gastroenterol. 2007;102:1759-1764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 205] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 12. | Tang RS, Weinberg B, Dawson DW, Reber H, Hines OJ, Tomlinson JS, Chaudhari V, Raman S, Farrell JJ. Evaluation of the guidelines for management of pancreatic branch-duct intraductal papillary mucinous neoplasm. Clin Gastroenterol Hepatol. 2008;6:815-819; quiz 719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 132] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 13. | Kaimakliotis P, Riff B, Pourmand K, Chandrasekhara V, Furth EE, Siegelman ES, Drebin J, Vollmer CM, Kochman ML, Ginsberg GG. Sendai and Fukuoka Consensus Guidelines Identify Advanced Neoplasia in Patients With Suspected Mucinous Cystic Neoplasms of the Pancreas. Clin Gastroenterol Hepatol. 2015;13:1808-1815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 14. | Singhi AD, Zeh HJ, Brand RE, Nikiforova MN, Chennat JS, Fasanella KE, Khalid A, Papachristou GI, Slivka A, Hogg M. American Gastroenterological Association guidelines are inaccurate in detecting pancreatic cysts with advanced neoplasia: a clinicopathologic study of 225 patients with supporting molecular data. Gastrointest Endosc. 2016;83:1107-1117.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 118] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 15. | Konda VJ, Meining A, Jamil LH, Giovannini M, Hwang JH, Wallace MB, Chang KJ, Siddiqui UD, Hart J, Lo SK. A pilot study of in vivo identification of pancreatic cystic neoplasms with needle-based confocal laser endomicroscopy under endosonographic guidance. Endoscopy. 2013;45:1006-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 143] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 16. | Modi RM, Kamboj AK, Swanson B, Conwell DL, Krishna SG. Novel technique for diagnosis of mucinous cystic neoplasms: in vivo and ex vivo confocal laser endomicroscopy. VideoGIE. 2017;2:55-56. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Napoleon B, Lemaistre AI, Pujol B, Caillol F, Lucidarme D, Bourdariat R, Morellon-Mialhe B, Fumex F, Lefort C, Lepilliez V. In vivo characterization of pancreatic cystic lesions by needle-based confocal laser endomicroscopy (nCLE): proposition of a comprehensive nCLE classification confirmed by an external retrospective evaluation. Surg Endosc. 2016;30:2603-2612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 18. | Krishna SG, Swanson B, Conwell DL, Muscarella P 2nd. In vivo and ex vivo needle-based confocal endomicroscopy of intraductal papillary mucinous neoplasm of the pancreas. Gastrointest Endosc. 2015;82:571-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Napoléon B, Lemaistre AI, Pujol B, Caillol F, Lucidarme D, Bourdariat R, Morellon-Mialhe B, Fumex F, Lefort C, Lepilliez V. A novel approach to the diagnosis of pancreatic serous cystadenoma: needle-based confocal laser endomicroscopy. Endoscopy. 2015;47:26-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 20. | Modi RM, Swanson B, Muscarella P 2nd, Conwell DL, Krishna SG. Novel techniques for diagnosis of serous cystadenoma: fern pattern of vascularity confirmed by in vivo and ex vivo confocal laser endomicroscopy. Gastrointest Endosc. 2017;85:258-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Kamboj AK, Swanson B, Dillhoff ME, Conwell DL, Krishna SG. Cystic pancreatic neuroendocrine tumors: correlation of in vivo needle-based confocal endomicroscopic findings by ex vivo analysis. Gastrointest Endosc. 2017;85:259-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Modi RM, Kamboj AK, Swanson B, Conwell DL, Krishna SG. Epidermoid cyst within an intrapancreatic accessory spleen: endosonography and confocal endomicroscopy of an unusual pancreatic cystic lesion. Endoscopy. 2016;48:E332-E333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Nakai Y, Iwashita T, Park DH, Samarasena JB, Lee JG, Chang KJ. Diagnosis of pancreatic cysts: EUS-guided, through-the-needle confocal laser-induced endomicroscopy and cystoscopy trial: DETECT study. Gastrointest Endosc. 2015;81:1204-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 134] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 24. | Krishna SG, Swanson B, Hart PA, El-Dika S, Walker JP, McCarthy ST, Malli A, Shah ZK, Conwell DL. Validation of diagnostic characteristics of needle based confocal laser endomicroscopy in differentiation of pancreatic cystic lesions. Endosc Int Open. 2016;4:E1124-E1135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 25. | Springer S, Wang Y, Dal Molin M, Masica DL, Jiao Y, Kinde I, Blackford A, Raman SP, Wolfgang CL, Tomita T. A combination of molecular markers and clinical features improve the classification of pancreatic cysts. Gastroenterology. 2015;149:1501-1510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 327] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 26. | Khalid A, Brugge W. ACG practice guidelines for the diagnosis and management of neoplastic pancreatic cysts. Am J Gastroenterol. 2007;102:2339-2349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 209] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 27. | Khalid A, Zahid M, Finkelstein SD, LeBlanc JK, Kaushik N, Ahmad N, Brugge WR, Edmundowicz SA, Hawes RH, McGrath KM. Pancreatic cyst fluid DNA analysis in evaluating pancreatic cysts: a report of the PANDA study. Gastrointest Endosc. 2009;69:1095-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 314] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 28. | Khalid A, Pal R, Sasatomi E, Swalsky P, Slivka A, Whitcomb D, Finkelstein S. Use of microsatellite marker loss of heterozygosity in accurate diagnosis of pancreaticobiliary malignancy from brush cytology samples. Gut. 2004;53:1860-1865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Wu J, Matthaei H, Maitra A, Dal Molin M, Wood LD, Eshleman JR, Goggins M, Canto MI, Schulick RD, Edil BH. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med. 2011;3:92ra66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 596] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 30. | Nikiforova MN, Khalid A, Fasanella KE, McGrath KM, Brand RE, Chennat JS, Slivka A, Zeh HJ, Zureikat AH, Krasinskas AM. Integration of KRAS testing in the diagnosis of pancreatic cystic lesions: a clinical experience of 618 pancreatic cysts. Mod Pathol. 2013;26:1478-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 31. | Wu J, Jiao Y, Dal Molin M, Maitra A, de Wilde RF, Wood LD, Eshleman JR, Goggins MG, Wolfgang CL, Canto MI. Whole-exome sequencing of neoplastic cysts of the pancreas reveals recurrent mutations in components of ubiquitin-dependent pathways. Proc Natl Acad Sci USA. 2011;108:21188-21193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 482] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 32. | Crippa S, Fernández-Del Castillo C, Salvia R, Finkelstein D, Bassi C, Domínguez I, Muzikansky A, Thayer SP, Falconi M, Mino-Kenudson M. Mucin-producing neoplasms of the pancreas: an analysis of distinguishing clinical and epidemiologic characteristics. Clin Gastroenterol Hepatol. 2010;8:213-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 233] [Article Influence: 15.5] [Reference Citation Analysis (2)] |
| 33. | Machado NO, Al Qadhi H, Al Wahibi K. Intraductal Papillary Mucinous Neoplasm of Pancreas. N Am J Med Sci. 2015;7:160-175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 34. | Singhi AD, Nikiforova MN, McGrath K. DNA testing of pancreatic cyst fluid: is it ready for prime time? Lancet Gastroenterol Hepatol. 2017;2:63-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 35. | Biankin AV, Biankin SA, Kench JG, Morey AL, Lee CS, Head DR, Eckstein RP, Hugh TB, Henshall SM, Sutherland RL. Aberrant p16(INK4A) and DPC4/Smad4 expression in intraductal papillary mucinous tumours of the pancreas is associated with invasive ductal adenocarcinoma. Gut. 2002;50:861-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 142] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 36. | Kanda M, Sadakari Y, Borges M, Topazian M, Farrell J, Syngal S, Lee J, Kamel I, Lennon AM, Knight S. Mutant TP53 in duodenal samples of pancreatic juice from patients with pancreatic cancer or high-grade dysplasia. Clin Gastroenterol Hepatol. 2013;11:719-730.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 133] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 37. | Sasaki S, Yamamoto H, Kaneto H, Ozeki I, Adachi Y, Takagi H, Matsumoto T, Itoh H, Nagakawa T, Miyakawa H. Differential roles of alterations of p53, p16, and SMAD4 expression in the progression of intraductal papillary-mucinous tumors of the pancreas. Oncol Rep. 2003;10:21-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 38. | Schoedel KE, Finkelstein SD, Ohori NP. K-Ras and microsatellite marker analysis of fine-needle aspirates from intraductal papillary mucinous neoplasms of the pancreas. Diagn Cytopathol. 2006;34:605-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 77] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 39. | Pea A, Yu J, Rezaee N, Luchini C, He J, Dal Molin M, Griffin JF, Fedor H, Fesharakizadeh S, Salvia R. Targeted DNA Sequencing Reveals Patterns of Local Progression in the Pancreatic Remnant Following Resection of Intraductal Papillary Mucinous Neoplasm (IPMN) of the Pancreas. Ann Surg. 2017;266:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 103] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 40. | Garcia-Carracedo D, Chen ZM, Qiu W, Huang AS, Tang SM, Hruban RH, Su GH. PIK3CA mutations in mucinous cystic neoplasms of the pancreas. Pancreas. 2014;43:245-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 41. | Yu J, Sadakari Y, Shindo K, Suenaga M, Brant A, Almario JAN, Borges M, Barkley T, Fesharakizadeh S, Ford M. Digital next-generation sequencing identifies low-abundance mutations in pancreatic juice samples collected from the duodenum of patients with pancreatic cancer and intraductal papillary mucinous neoplasms. Gut. 2017;66:1677-1687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 119] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 42. | Garcia-Carracedo D, Turk AT, Fine SA, Akhavan N, Tweel BC, Parsons R, Chabot JA, Allendorf JD, Genkinger JM, Remotti HE. Loss of PTEN expression is associated with poor prognosis in patients with intraductal papillary mucinous neoplasms of the pancreas. Clin Cancer Res. 2013;19:6830-6841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 43. | Schönleben F, Qiu W, Ciau NT, Ho DJ, Li X, Allendorf JD, Remotti HE, Su GH. PIK3CA mutations in intraductal papillary mucinous neoplasm/carcinoma of the pancreas. Clin Cancer Res. 2006;12:3851-3855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 134] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 44. | Singhi AD, McGrath K, Brand RE, Khalid A, Zeh HJ, Chennat JS, Fasanella KE, Papachristou GI, Slivka A, Bartlett DL. Preoperative next-generation sequencing of pancreatic cyst fluid is highly accurate in cyst classification and detection of advanced neoplasia. Gut. 2017; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 266] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 45. | Al-Haddad M, DeWitt J, Sherman S, Schmidt CM, LeBlanc JK, McHenry L, Coté G, El Chafic AH, Luz L, Stuart JS. Performance characteristics of molecular (DNA) analysis for the diagnosis of mucinous pancreatic cysts. Gastrointest Endosc. 2014;79:79-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 46. | Shen J, Brugge WR, Dimaio CJ, Pitman MB. Molecular analysis of pancreatic cyst fluid: a comparative analysis with current practice of diagnosis. Cancer. 2009;117:217-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 47. | 47 Lennon AM, Wolfgang C. Cystic neoplasms of the pancreas. J Gastrointest Surg. 2013;17:645-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 48. | Jais B, Rebours V, Malleo G, Salvia R, Fontana M, Maggino L, Bassi C, Manfredi R, Moran R, Lennon AM. Serous cystic neoplasm of the pancreas: a multinational study of 2622 patients under the auspices of the International Association of Pancreatology and European Pancreatic Club (European Study Group on Cystic Tumors of the Pancreas). Gut. 2016;65:305-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 213] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 49. | Krishna SG, Brugge WR, Dewitt JM, Kongkam P, Napoleon B, Robles-Medranda C, Tan D, El-Dika S, McCarthy S, Walker J. Needle-based confocal laser endomicroscopy for the diagnosis of pancreatic cystic lesions: an international external interobserver and intraobserver study (with videos). Gastrointest Endosc. 2017;86:644-654.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 50. | Law JK, Ahmed A, Singh VK, Akshintala VS, Olson MT, Raman SP, Ali SZ, Fishman EK, Kamel I, Canto MI. A systematic review of solid-pseudopapillary neoplasms: are these rare lesions? Pancreas. 2014;43:331-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 295] [Cited by in RCA: 241] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 51. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4330] [Article Influence: 360.8] [Reference Citation Analysis (45)] |