Published online Feb 28, 2017. doi: 10.4254/wjh.v9.i6.326
Peer-review started: September 9, 2016
First decision: October 20, 2016
Revised: January 3, 2017
Accepted: February 8, 2017
Article in press: February 13, 2017
Published online: February 28, 2017
Processing time: 172 Days and 9.6 Hours
To perform a systematic review to evaluate the incidence and prevalence of non-alcoholic fatty liver disease (NAFLD) in adult patients with sarcopenia.
Randomized clinical trials, cross-sectional or cohort studies including adult patients (over 18 years) with sarcopenia were selected. The primary outcomes of interest were the prevalence or incidence of NAFLD in sarcopenic patients. In the screening process, 44 full-text articles were included in the review and 41 studies were excluded.
Three cross-sectional studies were included. The authors attempted to perform a systematic review, but due to the differences between the studies, a qualitative synthesis was provided. The diagnosis of NAFLD was made by non-invasive methods (image methods or any surrogate markers) in all three evaluated studies. All the studies suggested that there was an independent association between sarcopenia and NAFLD.
Sarcopenia is independently associated with NAFLD and possibly to an advanced fibrosis.
Core tip: The aim of the present study was to perform a systematic review evaluating the incidence and prevalence of non-alcoholic fatty liver disease (NAFLD) in adult patients with sarcopenia. Randomized clinical trials, cross-sectional or cohort studies including adult patients (over 18 years) with sarcopenia were selected. The primary outcomes of interest were the prevalence or incidence of NAFLD in sarcopenic patients, and three cross-sectional studies were finally included. There was an independent association between sarcopenia and NAFLD in all the studies. In conclusion, sarcopenia is independently associated with NAFLD and possibly to an advanced fibrosis.
- Citation: Tovo CV, Fernandes SA, Buss C, de Mattos AA. Sarcopenia and non-alcoholic fatty liver disease: Is there a relationship? A systematic review. World J Hepatol 2017; 9(6): 326-332
- URL: https://www.wjgnet.com/1948-5182/full/v9/i6/326.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i6.326
Non-alcoholic fatty liver disease (NAFLD) is defined as a set of liver diseases that can range from simple steatosis to steatohepatitis (NASH), which can progress to fibrosis or even cirrhosis[1] and complications such as hepatocellular carcinoma[2,3]. It will soon become the most common liver disease worldwide[4], with an estimated prevalence in the general population of Western countries about 20% to 30%[5]. In specific populations, its prevalence can be much higher and may reach 90% in morbidly obese patients eligible for bariatric surgery, 69% in type 2 diabetes mellitus patients and 50% in dislipidemic ones[4]. In our experience, the prevalence of NASH when obese individuals without diabetes mellitus with high aminotransferases levels were evaluated in a nutrition outpatient clinic was 88%[6]. On the other hand, when we evaluated morbidly obese patients submitted to bariatric surgery, the prevalence of steatosis was 90.4% and NASH 70.4%[7]. NAFLD patients present higher mortality than the general population, being the cardiovascular disease the most common cause of death. In patients presenting NASH, however, the mortality is associated more often to hepatic causes[4].
Sarcopenia is well characterized by the progressive loss of strength and skeletal muscle mass, generally associated with functional limitations, morbidity, and mortality[3,8,9]. The European consensus on definition and diagnosis of sarcopenia recommends using the low muscle mass and muscle function (strength or performance) for its diagnosis. Assessment of different stages of sarcopenia may help to establish the best treatment to be administered in different contexts and set appropriate recovery targets[9].
There is some concern about whether NAFLD results in sarcopenia through the activation of myostatin in the skeletal muscle, or if is sarcopenia the initial abnormality resulting in the activation of the stellate cells with fibrogenic properties in the liver. Considering the hypothesis that myostatin increases adipose tissue mass that will result in the decrease of adiponectin secretion, the original defect may actually begin in the skeletal muscle[10].
Sarcopenia may occur simultaneously with obesity, particularly the accumulation of visceral fat, which can be related to inflammation, insulin resistance (IR), and further reduction in the skeletal muscle mass, consequently causing muscle catabolism[11]. In some conditions, lean body mass is lost while fat mass may be preserved or even increased[12]; this state is called sarcopenic obesity[9]. The prevalence of sarcopenic obesity increases with age, depending on definitions and reference populations[13-15].
Although sarcopenia has been independently related to an increased risk of NAFLD and advanced fibrosis, and that sarcopenia may be associated with worse liver related clinical outcomes, this is an understudied issue, and its role on NAFLD or NASH has not been fully established[16]. The aim of this study was to perform a systematic review identifying original studies that evaluated the association between sarcopenia and NAFLD in adults.
This systematic review was registered at the international prospective register of systematic reviews platform (PROSPERO) (https://www.crd.york.ac.uk/PROSPERO/), number CRD42015027083. This study followed the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses[17].
Randomized clinical trials (RCTs), cross-sectional or cohort studies including adult patients (over 18 years) with sarcopenia were selected.
The primary outcomes of interest were the prevalence or incidence of NAFLD in sarcopenic patients, liver fibrosis and NASH activity index assessed by biopsy or non-invasive methods. Studies in which one or more of these outcomes were assessed were included in the present systematic review.
The search for eligible studies was performed in PubMed, Lilacs, EMBASE and Cochrane in October, 2016, without a limiting period. The search strategy included the following set of keywords: “Sarcopenia”(Mesh) OR “Sarcopenia” OR “Loss of skeletal muscle” OR “Loss of muscle mass and strength” OR “Reduced muscle mass and strength” OR “Intra-abdominal fat” OR “Muscle wasting” OR “Sarcopenic obesity” and (additional keyword). The last gap was changed at each search using the keywords “Non-alcoholic fatty liver disease”(Mesh) OR “Non-alcoholic fatty liver disease” OR “NAFLD” OR “NASH” OR “Non-alcoholic fatty liver disease” OR “Nonalcoholic fatty liver disease” OR “Nonalcoholic fatty liver” OR “Nonalcoholic fatty livers” OR “Nonalcoholic steatohepatitis” OR “Nonalcoholic steatohepatitides” OR “Fatty liver index”. The searches were performed without limiting the types of articles (RCTs, clinical trial, comparative study). The selection of eligible studies was performed by title and abstract reading. When abstracts regarding subjects or outcomes of interest were not clear, the full text of the article was read.
Data was collected by two independent investigators for the following variables: Design of the study, age and sex of participants, and the presence of NAFLD. The methodological quality assessment criteria followed the guidelines according to the study design - CONSORT[18] or STROBE[19].
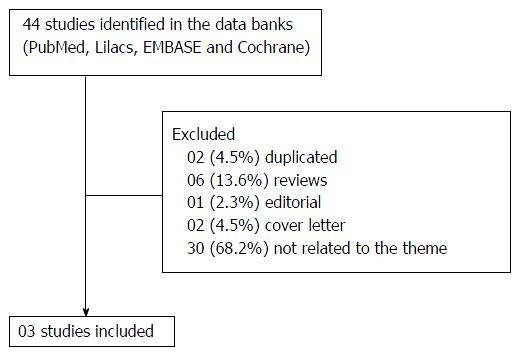
In the initial screening process (Figure 1), 44 full-text articles were included in the present review, of which 41 studies were finally excluded, remaining three cross-sectional studies for analysis. The authors attempted a systematic review with meta-analysis, but due to the variance amongst the three studies, a qualitative synthesis is provided. The main results of the studies with the respective comparisons within and between groups (when available) are shown in Table 1.
| Ref. | Year | Design of the study | Sample size | Mean age (± SD) | Gender | Method of diagnosis of sarcopenia | Independent variable | Method of diagnosis of NAFLD | Frequency of NAFLD | Results of the studies |
| Hong et al[15] | 2014 | Cross-sectional | 452 | 49.5 ± 10.3 | 285 women (63.1%) | DXA | SMI/weight (quartiles) | CT (LAI) | Prevalence | OR of having NAFLD by quartiles of SMI after adjusting for potential confounding factors: OR = 5.16 (95%CI: 1.63-16.33) |
| P = 0.041 after adjustment for age, sex, smoking status, phisical activity, HOMA-IR, hsCRP and 25[OH]D levels | ||||||||||
| Lee et al[16] | 2015 | Cross-sectional | 15132 | 49.7 ± 16.5 | 9515 women (62.9%) | DXA | SMI: < 32.2% for men and < 25.5% for women | HSI, CNS and LFS BARD and FIB-4 for advanced fibrosis | Prevalence: 22%-29% | Sarcopenic vs non-sarcopenic patients according to the NAFLD assessment method: OR = 1.18-1.22 (95%CI: 1.02-1.39) |
| P < 0.001 when adjusted for age, sex, regular exercise, HOMA-IR, smoking and HT | ||||||||||
| Moon et al[20] | 2013 | Cross-sectional | 9565 | 47 ± 10.3 | 5293 men (55.3%) | BIA multi frequencies | SVR (quartiles) | Surrogate marker: FLI ≥ 60 | Prevalence: 19.32% | OR for NAFLD among the quartiles of SVR using multiple logistic regression analysis: OR = 0.037 (95%CI: 0.029-0.049) |
| P < 0.001 when adjusted for age, sex, total cholesterol, low-density lipoprotein cholesterol, DM, HTN, hsCRP |
The diagnosis of NAFLD was made by non-invasive methods (image methods or any surrogate markers) in all three evaluated studies. The liver attenuation index (LAI) was evaluated by computed tomography in the study of Hong et al[15]. The fatty liver index (FLI) was calculated from waist circumference, body mass index, gama-glutamyl transpeptidadase and triglyceride levels in the study of Moon et al[20]. The NAFLD fibrosis score (NFS), hepatic steatosis index and the liver fat score were non-invasive scores used in the studies of Lee et al[16].
The diagnosis of sarcopenia was defined by the skeletal muscle mass index (SMI) as follow: Total skeletal muscle mass (kg)/weight (kg) × 100, and was evaluated by dual energy X-ray absorptiometry (DXA) in three of the studies[15,16] or by bioelectric impedance analysis (BIA) in one[20].
Moon et al[20] evaluated the effects of skeletal muscle mass to visceral fat area ratio by BIA on NAFLD (diagnosed using FLI). Of all the 9565 individuals who underwent a routine health examination, 1848 (19.3%) presented NALFD (FLI ≥ 60). The group with low FLI showed the lowest visceral fat area and highest skeletal muscle mass, and the SMI presented inverse correlations with FLI. In the multivariate analysis, skeletal muscle mass to visceral fat ratio was negatively associated with FLI. Considering the quartiles of the skeletal muscle mass to visceral fat ratio, the highest one showed the lowest risk of NAFLD, adjusted for age, gender, diabetes mellitus, hypertension, C-reactive protein and lipid profile (odds ratio, 0.037).
The study of Hong et al[15] performed a cross-sectional analysis between sarcopenia and NAFLD in the Korean Sarcopenic Obesity Study, a prospective observational cohort study. The authors included 452 healthy adults by LAI (evaluated by computed tomography), used as a parameter for the diagnosis of NAFLD. Both SMI and LAI were negatively correlated with the homeostasis model assessment of insulin resistance (P < 0.001). After using the multiple logistic regression analysis, the odds ratio for NAFLD was 5.16 in the lowest quartile of SMI (adjusting for potential confounding factors).
Lee et al[16] used a representative sample of 15132 subjects from the Korea National Health and Nutrition Examination Surveys (2008-2011), a population-based study. Non-invasive scores as the body mass index, aspartate aminotransferase/alanine aminotransferase ratio and diabetes mellitus (BARD) and fibrosis-4 (FIB-4) were used to define advanced fibrosis in subjects with NAFLD. The prevalence of NAFLD in non-sarcopenic patients ranged from 4% to 14% (non-obese) and from 50% to 72% (obese), depending on the hepatic steatosis score employed. The prevalence of NAFLD in sarcopenic patients ranged from 9% to 30% (non-obese) and from 61% to 83% (obese). The SMI was inversely correlated with the NAFLD predicting scores (P < 0.001). Sarcopenic subjects had an increased risk of NAFLD regardless of obesity (odds ratio 1.55-3.02; P < 0.001) or metabolic syndrome (odds ratio 1.63-4.00; P < 0.001) than those non-sarcopenic. Furthermore, it was demonstrated an independent association between sarcopenia and NAFLD when analysed by multiple logistic regression analysis. Among the individuals with NAFLD, the lower the SMI, the more chance of advanced fibrosis when compared with the non-sarcopenic (P < 0.001).
In the present review, all the studies[15,16,20] concluded that that there was an independent association between sarcopenia and NAFLD. The association of sarcopenia with NAFLD seems to be independent of IR[15,16] or obesity[16]. However, it is not possible to establish whether the association between sarcopenia and NAFLD is a cause or an effect. The skeletal muscle is now recognized as an endocrine organ secreting myokines, and this fact may help to understand its role in the pathogenesis of NAFLD[21] as well as contribute to the development of effective therapeutic options[10].
The association between fat accumulation in the liver and in the muscle has recently been established. The fat content in the paravertebral muscles analyzed by computed tomography may be correlated with aging and steatosis, and a reduction in muscle fat may be associated with an decrease of the liver fat content[22].
Insulin resistance and metabolic syndrome has been consistently associated with sarcopenia and NAFLD, as both conditions may share pathophysiological mechanisms[23-26]. However, the association between sarcopenia and NAFLD seems to be independent of IR, raising the possibility that the loss of muscle mass may contribute to the development of NAFLD[27].
The study of Moon et al[20] showed that the FLI was lower in the group with higher skeletal muscle mass, and the group with NAFLD (high FLI) presented lower SMI and higher visceral fat area when compared with the lower FLI group, suggesting that the incidence of NAFLD increases as the muscle mass relative to visceral fat decreases. Therefore, this fact could support a favorable role for skeletal muscle in IR and in the development of NAFLD.
Hong et al[15] evaluated the relationship between sarcopenia and NAFLD, demonstrating a higher risk of NAFLD in those with lower muscle mass after adjusting for confounding factors as IR and inflammation. The individuals with sarcopenia presented more metabolic syndrome, higher C-reactive protein levels and higher body fat mass when compared to those without sarcopenia.
The study of Lee et al[16] compared sarcopenic and non-sarcopenic patients within obese and non-obese groups of patients. The analysis made it possible to control the effect of obesity on NAFLD and it was the only study that clearly presented an association of sarcopenia and hepatic steatosis. The prevalence of NAFLD in non-obese sarcopenic patients was more than twice as high as in non-obese non-sarcopenic patients. The proportion of increase in the prevalence of NAFLD comparing obese sarcopenic patients and obese non-sarcopenic patients was remarkably lower. This demonstrates the strong association of sarcopenia and NAFLD in non-obese patients, as well as with fibrosis.
It is worth noting that all three studies included representative samples and performed differing methods of analysis of the outcome, i.e., the relationship between sarcopenia and NAFLD. Even though all three presented multivariable logistic regression analysis, the predictive models were different in all of them, illustrating the complexity and lack of consensus on the factors affecting NAFLD risk. Regardless the model, all of them showed increased risk of NAFLD in the presence of sarcopenia.
More recently, Lee et al[28] investigated whether sarcopenia was associated with significant liver fibrosis in the same population. Liver fibrosis was assessed by non-invasive scores as Forns, FIB-4 and NFS. It was observed that sarcopenia was significantly associated with significant liver fibrosis (odds ratio 0.52-0.67; P < 0.01) in subjects with NAFLD, independently of obesity and IR.
As possible limitations of the studies, the use of a cross-sectional design limits the possibility to infer causality between skeletal muscle mass loss and NAFLD or NASH[15,16,20]; and there was no information regarding the use of smoking status or alcohol consumption[15], which may allow for a bias. Also, no study performed liver biopsy to establish the diagnosis of NAFLD, considered the gold standard in the respective diagnosis[4,9,15,20]. Furthermore, the BMI of the patients included in the studies was not so high, varying from 21.4[20] to 27.9[16], characterizing overweight and not obesity, and being lower than the BMI of the occidental population[29]. This point may be explained by the local ethnic characteristics (all three studies reviewed are Korean studies), limiting the external validity of such studies.
The European consensus[9] defined that the CT scan and the magnetic resonance imaging are considered the gold standard to estimate muscle mass. DXA is considered the preferred alternative method, and BIA is a portable alternative to DXA. All the three studies included in the present analysis used the gold standard methods for the diagnosis of sarcopenia, being BIA[20] or DXA[15,16].
Of the three articles included in the present systematic review, only the one of Lee et al[16] reported the exclusion of approximately 25% of the patients because of missing information about the main variables evaluated (skeletal muscle mass and NAFLD).
Two additional studies were published in 2016, however they were excluded of the present systematic review because of the different primary outcomes of interest. The first was the cross-sectional study of Kim et al[30], evaluating 3739 Korean people, showing that the risk of NAFLD is associated with a low SMI independent of metabolic risk factors, and may differ according to the age or menopausal status. The other study, of Koo et al[31], evaluated 309 Korean subjects, where the prevalence of sarcopenia was 8.7%, 17.9% and 35.0% in subjects without NAFLD, with NAFLD and with NASH respectively (P < 0.001).
There is an independent association between sarcopenia and NAFLD and possibly to an advanced fibrosis. A higher skeletal muscle mass may have a beneficial effect in the prevention of NAFLD, which might be explored by future standardized experimental studies.
Non-alcoholic fatty liver disease (NAFLD) is becoming the most common liver disease worldwide, presenting a higher mortality than the general population. Sarcopenia has been related to an increased risk of NAFLD and advanced fibrosis, and may be associated with worse liver related clinical outcomes. However, this is an understudied issue, and its role on NAFLD has not been fully established. The aim of this study was to perform a systematic review identifying original studies that evaluated the association between sarcopenia and NAFLD in adults.
Sarcopenia may occur simultaneously with obesity, particularly the accumulation of visceral fat, which can be related to inflammation, insulin resistance and further reduction in the skeletal muscle mass, consequently causing muscle catabolism. In some conditions, lean body mass is lost while fat mass may be preserved or even increased; this state is called sarcopenic obesity. The skeletal muscle is now recognized as an endocrine organ secreting myokines, and this fact may help to understand its role in the pathogenesis of NAFLD as well as contribute to the development of effective therapeutic options.
In the present review, all the studies concluded that there was an independent association between sarcopenia and NAFLD. The association of sarcopenia with NAFLD seems to be independent of insulin resistance or obesity. However, it is not possible to establish whether the association between sarcopenia and NAFLD is a cause or an effect.
The association between fat accumulation in the liver and in the muscle has just recently been established. The fat content in the paravertebral muscles analyzed by computed tomography may be correlated with aging and steatosis, and a reduction in muscle fat may be associated with an decrease of the liver fat content.
Dual energy X-fay absorptimetry and bioelectric impedance analysis are methods of diagnosis of sarcopenia. Computed tomography using liver attenuation index, as well as the comprehensive NAFLD score, the hepatic steatosis index, the liver fat score and the fatty liver index are non-invasive methods of diagnosis of NAFLD.
This review is timely as there is emerging evidence and understanding of the association between NAFLD and sarcopenia.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Hamaguchi M, Qu BG, Tan CK S- Editor: Song XX L- Editor: A E- Editor: Li D
| 1. | Vajro P, Lenta S, Pignata C, Salerno M, D’Aniello R, De Micco I, Paolella G, Parenti G. Therapeutic options in pediatric non alcoholic fatty liver disease: current status and future directions. Ital J Pediatr. 2012;38:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413-1419. [PubMed] |
| 3. | Kim JH, Lim S, Choi SH, Kim KM, Yoon JW, Kim KW, Lim JY, Park KS, Jang HC. Sarcopenia: an independent predictor of mortality in community-dwelling older Korean men. J Gerontol A Biol Sci Med Sci. 2014;69:1244-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 4. | Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2413] [Cited by in RCA: 2613] [Article Influence: 201.0] [Reference Citation Analysis (1)] |
| 5. | Bellentani S, Scaglioni F, Marino M, Bedogni G. Epidemiology of non-alcoholic fatty liver disease. Dig Dis. 2010;28:155-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 653] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 6. | Zamin I, de Mattos AA, Zettler CG. Nonalcoholic steatohepatitis in nondiabetic obese patients. Can J Gastroenterol. 2002;16:303-307. [PubMed] |
| 7. | Losekann A, Weston AC, de Mattos AA, Tovo CV, de Carli LA, Espindola MB, Pioner SR, Coral GP. Non-Alcoholic Steatohepatitis (NASH): Risk Factors in Morbidly Obese Patients. Int J Mol Sci. 2015;16:25552-25559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Batsis JA, Mackenzie TA, Barre LK, Lopez-Jimenez F, Bartels SJ. Sarcopenia, sarcopenic obesity and mortality in older adults: results from the National Health and Nutrition Examination Survey III. Eur J Clin Nutr. 2014;68:1001-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 343] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 9. | Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412-423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6987] [Cited by in RCA: 8472] [Article Influence: 564.8] [Reference Citation Analysis (0)] |
| 10. | Merli M, Dasarathy S. Sarcopenia in non-alcoholic fatty liver disease: Targeting the real culprit? J Hepatol. 2015;63:309-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Lim S, Kim JH, Yoon JW, Kang SM, Choi SH, Park YJ, Kim KW, Lim JY, Park KS, Jang HC. Sarcopenic obesity: prevalence and association with metabolic syndrome in the Korean Longitudinal Study on Health and Aging (KLoSHA). Diabetes Care. 2010;33:1652-1654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 410] [Cited by in RCA: 435] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 12. | Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, Baracos VE. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9:629-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1822] [Cited by in RCA: 2380] [Article Influence: 140.0] [Reference Citation Analysis (0)] |
| 13. | Batsis JA, Barre LK, Mackenzie TA, Pratt SI, Lopez-Jimenez F, Bartels SJ. Variation in the prevalence of sarcopenia and sarcopenic obesity in older adults associated with different research definitions: dual-energy X-ray absorptiometry data from the National Health and Nutrition Examination Survey 1999-2004. J Am Geriatr Soc. 2013;61:974-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 239] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 14. | Kim YS, Lee Y, Chung YS, Lee DJ, Joo NS, Hong D, Song Ge, Kim HJ, Choi YJ, Kim KM. Prevalence of sarcopenia and sarcopenic obesity in the Korean population based on the Fourth Korean National Health and Nutritional Examination Surveys. J Gerontol A Biol Sci Med Sci. 2012;67:1107-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 258] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 15. | Hong HC, Hwang SY, Choi HY, Yoo HJ, Seo JA, Kim SG, Kim NH, Baik SH, Choi DS, Choi KM. Relationship between sarcopenia and nonalcoholic fatty liver disease: the Korean Sarcopenic Obesity Study. Hepatology. 2014;59:1772-1778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 314] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 16. | Lee YH, Jung KS, Kim SU, Yoon HJ, Yun YJ, Lee BW, Kang ES, Han KH, Lee HC, Cha BS. Sarcopaenia is associated with NAFLD independently of obesity and insulin resistance: Nationwide surveys (KNHANES 2008-2011). J Hepatol. 2015;63:486-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 272] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 17. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [PubMed] |
| 18. | Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, Pitkin R, Rennie D, Schulz KF, Simel D. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA. 1996;276:637-639. [PubMed] |
| 19. | von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335:806-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3438] [Cited by in RCA: 6246] [Article Influence: 347.0] [Reference Citation Analysis (0)] |
| 20. | Moon JS, Yoon JS, Won KC, Lee HW. The role of skeletal muscle in development of nonalcoholic Fatty liver disease. Diabetes Metab J. 2013;37:278-285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 21. | Henningsen J, Rigbolt KT, Blagoev B, Pedersen BK, Kratchmarova I. Dynamics of the skeletal muscle secretome during myoblast differentiation. Mol Cell Proteomics. 2010;9:2482-2496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 230] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 22. | Kitajima Y, Eguchi Y, Ishibashi E, Nakashita S, Aoki S, Toda S, Mizuta T, Ozaki I, Ono N, Eguchi T. Age-related fat deposition in multifidus muscle could be a marker for nonalcoholic fatty liver disease. J Gastroenterol. 2010;45:218-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 23. | Lonardo A, Caldwell SH, Loria P. Clinical physiology of NAFLD: a critical overview of pathogenesis and treatment. Expert Rev Endocrinol Metab. 2010;5:403-423. |
| 24. | Abbatecola AM, Paolisso G, Fattoretti P, Evans WJ, Fiore V, Dicioccio L, Lattanzio F. Discovering pathways of sarcopenia in older adults: a role for insulin resistance on mitochondria dysfunction. J Nutr Health Aging. 2011;15:890-895. [PubMed] |
| 25. | Bertolotti M, Lonardo A, Mussi C, Baldelli E, Pellegrini E, Ballestri S, Romagnoli D, Loria P. Nonalcoholic fatty liver disease and aging: epidemiology to management. World J Gastroenterol. 2014;20:14185-14204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 232] [Cited by in RCA: 226] [Article Influence: 20.5] [Reference Citation Analysis (1)] |
| 26. | Sanada K, Iemitsu M, Murakami H, Gando Y, Kawano H, Kawakami R, Tabata I, Miyachi M. Adverse effects of coexistence of sarcopenia and metabolic syndrome in Japanese women. Eur J Clin Nutr. 2012;66:1093-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 27. | Guichelaar MM, Charlton MR. Decreased muscle mass in nonalcoholic fatty liver disease: new evidence of a link between growth hormone and fatty liver disease? Hepatology. 2014;59:1668-1670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Lee YH, Kim SU, Song K, Park JY, Kim do Y, Ahn SH, Lee BW, Kang ES, Cha BS, Han KH. Sarcopenia is associated with significant liver fibrosis independently of obesity and insulin resistance in nonalcoholic fatty liver disease: Nationwide surveys (KNHANES 2008-2011). Hepatology. 2016;63:776-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 274] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 29. | Vigitel Brasil 2014 Supplementary Health: surveillance of risk factors and protection for chronic diseases by telephone survey. Ministry of Health, National Supplementary Health Agency. Brasília, Brazil: Ministry of Health, 2015. ISBN 978-85-334-2322-0. Available from: http://www.ans.gov.br/images/stories/Materiais_ para_pesquisa/Materiais_por_assunto/2015_vigitel.pdf. |
| 30. | Kim HY, Kim CW, Park CH, Choi JY, Han K, Merchant AT, Park YM. Low skeletal muscle mass is associated with non-alcoholic fatty liver disease in Korean adults: the Fifth Korea National Health and Nutrition Examination Survey. Hepatobiliary Pancreat Dis Int. 2016;15:39-47. [PubMed] |
| 31. | Koo BK, Kim D, Joo SK, Kim JH, Chang MS, Kim BG, Lee KL, Kim W. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J Hepatol. 2017;66:123-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 341] [Article Influence: 42.6] [Reference Citation Analysis (0)] |