Published online Feb 28, 2017. doi: 10.4254/wjh.v9.i6.310
Peer-review started: September 27, 2016
First decision: November 22, 2016
Revised: December 15, 2016
Accepted: February 8, 2017
Article in press: February 13, 2017
Published online: February 28, 2017
Processing time: 154 Days and 10 Hours
To evaluate the performance of FibroMeterVirus3G combined to the first generation tests aspartate aminotransferase-to-platelet ratio index (APRI) or Forns index to assess significant fibrosis in chronic hepatitis C (CHC).
First generation tests APRI or Forns were initially applied in a derivation population from Rio de Janeiro in Brazil considering cut-offs previously reported in the literature to evaluate significant fibrosis. FibroMeterVirus3G was sequentially applied to unclassified cases from APRI or Forns. Accuracy of non-invasive combination of tests, APRI plus FibroMeterVirus3G and Forns plus FibroMeterVirus3G was evaluated in the Brazilian derivation population. APRI plus FibroMeterVirus3G combination was validated in a population of CHC patients from Angers in France. All patients were submitted to liver biopsy staged according to METAVIR score by experienced hepatopathologists. Significant fibrosis was considered as METAVIR F ≥ 2. The fibrosis stage classification was used as the reference for accuracy evaluation of non-invasive combination of tests. Blood samples for the calculation of serum tests were collected on the same day of biopsy procedure or within a maximum 3 mo interval and stored at -70 °C.
Seven hundred and sixty CHC patients were included (222 in the derivation population and 538 in the validation group). In the derivation population, the FibroMeterVirus3G AUROC was similar to APRI AUROC (0.855 vs 0.815, P = 0.06) but higher than Forns AUROC (0.769, P < 0.001). The best FibroMeterVirus3G cut-off to discriminate significant fibrosis was 0.61 (80% diagnostic accuracy; 75% in the validation population, P = 0.134). The sequential combination of APRI or Forns with FibroMeterVirus3G in derivation population presented similar performance compared to FibroMeterVirus3G used alone (79% vs 78% vs 80%, respectively, P = 0.791). Unclassified cases of significant fibrosis after applying APRI and Forns corresponded to 49% and 54%, respectively, of the total sample. However, the combination of APRI or Forns with FibroMeterVirus3G allowed 73% and 77%, respectively, of these unclassified cases to be correctly evaluated. Moreover, this combination resulted in a reduction of FibroMeterVirus3G requirement in approximately 50% of the entire sample. The stepwise combination of APRI and FibroMeterVirus3G applied to the validation population correctly identified 74% of patients with severe fibrosis (F ≥ 3).
The stepwise combination of APRI or Forns with FibroMeterVirus3G may represent an accurate lower cost alternative when evaluating significant fibrosis, with no need for liver biopsy.
Core tip: Liver fibrosis assessment still poses a challenge when prioritizing hepatitis C treatment due to logistical and financial barriers in the use of direct acting antiviral drugs. We introduced a new stepwise combination of first generation fibrosis tests - aminotransferase-to-platelet ratio index (APRI) and Forns-followed by FibroMeterVirus3G whenever results remained unclassified after first generation tests to identify significant fibrosis. This combination presented similar accuracy to FibroMeterVirus3G used as the only test, reduced APRI and Forns grey zone, and spared FibroMeterVirus3G requirement in 50% of cases. This approach represents a lower-cost alternative to assess fibrosis, with no need for liver biopsy.
- Citation: Chindamo MC, Boursier J, Luiz RR, Fouchard-Hubert I, Pannain VLN, de Araújo Neto JM, Coelho HSM, de Mello Perez R, Calès P, Villela-Nogueira CA. Fibrosis assessment using FibroMeter combined to first generation tests in hepatitis C. World J Hepatol 2017; 9(6): 310-317
- URL: https://www.wjgnet.com/1948-5182/full/v9/i6/310.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i6.310
Fibrosis staging in chronic hepatitis C (CHC) has evolved in recent years with the introduction of blood tests for liver fibrosis as well as physical methods such as elastometry. Although liver biopsy has been classically considered the standard tool to evaluate fibrosis, it presents well-known inconveniences[1-3] and limitations[4-7] which make its use to assess fibrosis staging controversial amongst various authors[8-11]. However, even when considering the recent advances in CHC therapy, the diagnosis of significant fibrosis still represents a challenge to define which patients should have priority in treatment, mainly in resource limited countries. Thus, the development and improvement of alternative methods to identify candidates for an early treatment or intensive fibrosis monitoring is still recommended[11]. Most of the commonly used first generation non-invasive tests such as aspartate aminotransferase-to-platelet ratio index (APRI)[12], FIB-4[13] and Forns index[14] have been constructed and evaluated as binary diagnosis tools aiming to predict or exclude significant fibrosis, advanced fibrosis or cirrhosis at specific cut-offs. Although they are all free of charge, easily accessible and well validated for CHC, these non-invasive tests are limited to classify all patients[12-14].
The interest in detailed fibrosis class classification for non-invasive tests of fibrosis has recently grown[15-18], representing a more comprehensive and sophisticated approach to assess liver fibrosis. In this line, FibroMeters are a group of blood tests providing classifications intended to evaluate liver fibrosis in chronic viral hepatitis, alcoholic liver disease and non-alcoholic fatty liver disease[19-21]. FibroMeter dedicated for viral aetiology has recently evolved from FibroMeterVirus2G[20] to a less costly hyaluronic acid free test FibroMeterVirus3G[21], which discriminates seven different fibrosis classes. FibroMeters provide scores ranging from 0 to 1 which are correlated with METAVIR staging system[22]. Although this new non-invasive test represents a better strategy to evaluate fibrosis in CHC, it may signify an economic burden hindering easy access mainly in developing countries. Thus, in order to identify patients with significant fibrosis and optimize costs, we evaluated the performance of a stepwise combination using APRI and Forns followed by FibroMeterVirus3G in cases whose results remained unclassified after use of these first generation tests, always considering liver biopsy as reference.
A cross-sectional study with prospective inclusion of compensated CHC patients submitted to percutaneous liver biopsy was performed at the Federal University of Rio de Janeiro, Brazil, as part of a pre-treatment routine evaluation. This group represented the derivation population of the study. Patients with concomitant human immunodeficiency virus infection, hepatitis B virus, alcohol abuse, metabolic, autoimmune or biliary diseases, liver transplantation or those who had previously undergone antiviral treatment were excluded. The validation population was composed by an independent cohort of CHC patients from Angers in France, who fulfilled the same inclusion and exclusion criteria. All patients signed an informed consent form and the study was approved by the Ethics Committee of both Institutions.
In the derivation population, all consecutive biopsies were guided by ultrasonography using a 14 or 16 G disposable Tru Cut needle (Surecutw, TSK Laboratory, Akasaka, Japan) obtaining a maximum length of 20 mm for each pass. In validation population, Menghini needle was used. Samples were considered inappropriate when length presented < 10 mm or contained < 6 portal tracts. Serial sections 5 μm thick were cut from each paraffin block and routinely stained with hematoxylin and eosin, periodic acid-Schiff diastase, reticulin, Masson Trichrome and Picrosirius red. Liver fibrosis was staged from F0 to F4 according to METAVIR staging system[22] by an experienced hepatopathologist in each centre, blinded for biological and clinical results. METAVIR F ≥ 2 was considered significant fibrosis. This fibrosis stage classification was used as the reference for accuracy calculation of non-invasive tests.
Serum tests of fibrosis were performed with blood samples collected from fasting patients on the same day of biopsy procedure or within a maximum 3 mo interval and stored at -70 °C. APRI and Forns were selected due to their free accessibility and their good accuracy to discriminate significant fibrosis. The values of APRI[12], Forns[14] and FibroMeterVirus3G[21] tests were calculated according to the original studies: (1) APRI = AST level/ULN)/platelet counts (109/L) × 100; (2) Forns index = 7.811 - 3.131 × ln(platelet count) + 0.781 × ln(GGT) + 3.467 × ln(age) - 0.014 × cholesterol; and (3) FibroMeterVirus3G = patented formula including the biologic parameters prothrombin index, AST, ALT, Urea, GGT, alpha-2-macroglobulin and platelets. The calculations were performed by Echosens (Paris, France) laboratory.
Quantitative variables were expressed as mean ± SD values or proportions. Student’s t test or ANOVA were used to compare continuous variables, and McNemar χ2 test to compare paired proportions. The performance of APRI, Forns and FibroMeterVirus3G to predict significant fibrosis was expressed by the AUROC. In order to evaluate the applicability of FibroMeterVirus3G, considering an economic approach, we determined the best cut-off point of FibroMeterVirus3G to discriminate significant fibrosis using the Youden index that maximizes sensitivity and specificity. The performance of APRI and Forns were separately assessed to exclude or predict significant fibrosis respectively, at cut-offs already established in literature as follows: APRI: cut-off of 0.5 and 1.5[12]; Forns: cut-off of 4.2 and 6.9[14]. FibroMeterVirus3G was then sequentially tested in a stepwise use, considering the results allocated in unclassified APRI values (between 0.5 and 1.5) and Forns values (between 4.2 and 6.9), using histology as reference. The overall accuracy of the aforementioned approaches was calculated considering the sum of true positive and negative cases as a proportion of the total. Sensitivity, specificity, predictive positive (PPV) and negative values (NPV) of first generation tests and sequential use of APRI + FibroMeterVirus3G and Forns + FibroMeterVirus3G were evaluated.
Data were analyzed using the statistics software programs SPSS version 20 for Windows and MedCalc version 14.8.1. A P value < 0.05 was considered statistically significant.
The initial series of liver specimens in Rio population consisted of 231 biopsies, of which four (1.7%) were excluded, due to evidence of other hepatic diseases associated with hepatitis C, and five (2.0%) were considered inadequate for analysis. Thus, the final Rio population included 222 biopsies of CHC patients. The validation cohort was represented by 538 French CHC patients. Excepted for gender, demographic characteristics, laboratory data and histological features of derivation and validation cohort were similar and described in Table 1.
| Variables | All (n = 760) | Rio population (n = 222) | Angers population (n = 538) |
| Females, n (%) | 401 (54) | 134 (60) | 179 (35) |
| Age (yr, mean ± SD) | 46 ± 11 | 51 ± 11 | 46 ± 11 |
| AST, IU/L (mean ± SD) | 67 ± 58 | 68 ± 52 | 66 ± 60 |
| ALT, IU/L (mean ± SD) | 101 ± 84 | 100 ± 67 | 101 ± 90 |
| Platelet count, 106/mm3 (mean ± SD) | 208 ± 68 | 203 ± 63 | 210 ± 70 |
| GGT, IU/L (mean ± SD) | 144 ± 171 | 124 ± 135 | 110 ± 184 |
| APRI | 1.0 ± 1.2 | 0.9 ± 1.2 | 1.1 ± 1.3 |
| Forns | 6.0 ± 1.9 | ||
| FibroMeterVirus3G | 0.6 ± 0.3 | 0.6 ± 0.3 | 0.6 ± 0.3 |
| Biopsy length (mm, mean ± SD) | 22 ± 9 | 24 ± 5 | 22 ± 10 |
| METAVIR stage, n (%) | |||
| F0 | 22 (3) | 5 (2) | 17 (3) |
| F1 | 283 (37) | 91 (41) | 192 (36) |
| F2 | 215 (28) | 55 (25) | 160 (30) |
| F3 | 145 (19) | 54 (24) | 91 (17) |
| F4 | 95 (13) | 17 (8) | 78 (14) |
The mean length of liver fragments was 24 ± 5 mm in derivation population vs 22 ± 10 mm in validation cohort (P = 0.315). The prevalence of significant fibrosis was 57% vs 61% (P = 0.399) comparing derivation population to validation cohort, respectively, considering liver biopsy as reference.
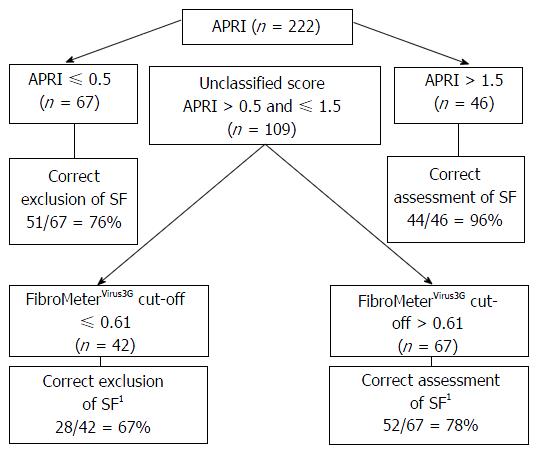
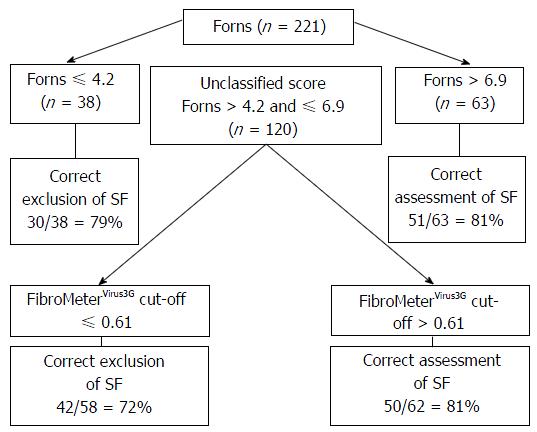
FibroMeterVirus3G applied as a class classification test presented an overall rate of correct diagnosis of 86% considering any of the results reported in FibroMeterVirus3G stage class in accordance with fibrosis scored by METAVIR (Table 2). The AUROCs of both tests comparing METAVIR F0-F1 vs F2-F4 were similar between FibroMeterVirus3G and APRI [0.855 (0.801-0.898) vs 0.815 (0.757-0.864)] but the difference was at the limit of significance (P = 0.06). The FibroMeterVirus3G AUROC was higher in comparison to Forns AUROC [0.855 (0.801-0.898) vs 0.769 (0.708-0.823); P < 0.001]. The best cut-off that predicted significant fibrosis was 0.61, demonstrating an accuracy of 80% compared to liver biopsy. The PPV, NPV and accuracy of all tests were shown in Table 3. The stepwise combination of APRI or Forns followed by FibroMeterVirus3G provided an overall accuracy of 79% (Figure 1) and 78% (Figure 2), respectively (P = 0.791), when identifying significant fibrosis. It also enabled 49% (n = 109) and 54% (n = 120) of the total sample, representing the grey zone of APRI and Forns for significant fibrosis, to be correctly classified in 73% and 77% of cases, respectively.
| FibroMeter stage | METAVIR fibrosis classification | Correct fibrosis class classification according to liver biopsy (%) | |||||
| 0 | 1 | 2 | 3 | 4 | n | ||
| F0/F1 | 0 | 10 | 0 | 0 | 0 | 10 | 10/10 = 100 |
| F1 | 0 | 6 | 1 | 0 | 0 | 7 | 6/7 = 86 |
| F1[F1-F2] | 3 | 21 | 4 | 2 | 0 | 30 | 25/30 = 83 |
| F2[F1-F2] | 1 | 26 | 10 | 4 | 1 | 42 | 36/42 = 86 |
| F2[F1-F3] | 1 | 15 | 12 | 10 | 0 | 38 | 37/38 = 97 |
| F2/F3 | 0 | 9 | 17 | 12 | 3 | 41 | 29/41 = 71 |
| F3[F2-F4] | 0 | 4 | 8 | 22 | 6 | 40 | 36/40 = 90 |
| F4[F3-F4] | 0 | 0 | 3 | 4 | 7 | 14 | 11/14 = 79 |
| Total | 5 | 91 | 55 | 54 | 17 | 222 | 190/222 = 86 |
| Serum fibrosis tests | AUROC (95%CI) | Cut-off value | Se (%) | Sp (%) | PPV (%) | NPV (%) | OA (%) |
| Derivation population (n = 222) | |||||||
| FM score | 0.855 (0.801-0.898) | 0.61 | 79 | 81 | 85 | 74 | 80 |
| APRI | 0.815 (0.757-0.864) | 0.51 | 87 | 53 | 71 | 76 | 72 |
| 1.52 | 35 | 98 | 96 | 53 | 62 | ||
| Forns | 0.769 (0.708-0.823) | 4.23 | 94 | 31 | 64 | 79 | 66 |
| 6.94 | 41 | 87 | 81 | 53 | 61 | ||
| Apri + FM | 76 | 82 | 85 | 72 | 79 | ||
| Forns + FM | 81 | 75 | 81 | 75 | 78 | ||
| Validation population (n = 538) | |||||||
| FM score | 0.854 (0.821-0.888) | 0.61 | 67 | 87 | 89 | 63 | 75 |
| Apri + FM | 57 | 88 | 87 | 57 | 69 |
Thus, diagnostic accuracy did not differ comparing the use of FibroMeterVirus3G test alone or combined with APRI or Forns (80% vs 79% vs 78%, respectively, P = 0.79), but represented a lower cost alternative since this procedure led to a 51% reduction of FibroMeterVirus3G test requirement using APRI + FibroMeterVirus3G and 46% reduction using Forns + FibroMeterVirus3G (P = 0.25). Rates of well classified patients applying the algorithm APRI + FibroMeterVirus3G, using METAVIR score as reference, were as follows: 100% for F0, 81% for F1, 67% for F2, 80% for F3 and 94% for F4.
The cut-off of 0.61 found in derivation population presented an overall accuracy of 75% when discriminating significant fibrosis in the validation cohort in comparison to 80% in derivation population (P = 0.13), considering histology as reference. The diagnostic accuracy of APRI + FibroMeterVirus3G combination in validation population to detect significant fibrosis and advanced fibrosis was respectively 69% and 74%. Rates of correct classification of this algorithm according to METAVIR score were as follows: 100% for F0, 88% for F1, 39% for F2, 64% for F3 and 85% for F4. When analysing the subgroup of false negative patients in this population we observed that 69% are represented by F2, 23% are F3, and only 8% are cirrhotic. The PPV, NPV and accuracy of APRI + FibroMeterVirus3G combination on validation population are shown in Table 3.
Although new treatment regimens with very high rates of sustained virologic response are now available to treat hepatitis C patients, the logistical and financial barriers to treat all infected patients represent an important limitation, even in resource-replete countries[23]. Thus, it is necessary to determine an optimal and practical approach to prioritize these highly efficacious, but extremely costly therapies, for a selected population at risk of disease progression or for those who require immediate therapy. There is a consensus that non-invasive evaluation of liver fibrosis may be useful to complement or even replace liver biopsy in CHC owing to its low risk of complications and good accuracy. However, non-invasive tests also present some limitations related to cost, fibrosis discrimination and accuracy.
The present study originally evaluated the combination of a more robust patented fibrosis test, FibroMeterVirus3G, with low cost first generation tests to enhance its applicability in the clinical practice. Although APRI and Forns are well established non-invasive tests to assess fibrosis in CHC, their main limitation is that when used alone, almost half of the patients could not be classified according to the possibility of presenting or not significant fibrosis. Thus, using a second test to improve discrimination would enhance the accuracy of these results in order to diagnose significant fibrosis. In the present study, the use of first generation tests combined with FibroMeterVirus3G demonstrated to be a lower cost strategy since it reduced FibroMeterVirus3G requirement, without loss of accuracy, eliminating the requirement for liver biopsy procedure.
When analyzed as a class classification test, FibroMeterVirus3G presented an overall accuracy of 86% similar to the rate of 87% described in FibroMeterVirus3G original report[21]. The AUROC for significant fibrosis was 0.85, comparable to previous reports ranging from 0.84 to 0.86[16,17,21]. Analysing under a practical point of view, and considering significant fibrosis as the criteria to treat or not the patient, we presented important results that may help hepatologists on clinical decision-making. We found a cut-off of 0.61 as the best numeric value to discriminate significant fibrosis for FibroMeterVirus3G, which is close to the value displayed in the manufacturer’s bar graph of FibroMeterVirus3G report of 0.63, representing the transition from F1 to F2 METAVIR stage[24]. The cut-off of 0.61 presented similar performance in comparison to manufacturer’s cut-off of 0.63 in the French validation cohort.
FibroMeterVirus3G applied as a numeric score also enables its association to other scores. Sebastiani et al[25] provided a sequential algorithm for fibrosis evaluation (SAFE) biopsy combining APRI and Fibrotest, another biomarker based on fibrosis class classification, resulting in a 46% reduction of liver biopsy requirement to identify significant fibrosis. In a more recent study performed with 1785 CHC patients, Boursier et al[26] reported that the diagnosis of significant fibrosis using SAFE still required liver biopsy in 64% of the cases. To our knowledge, to date the stepwise combination of APRI or Forns with FibroMeterVirus3G has never been evaluated. The sequential algorithm of either APRI or Forns combined with FibroMeterVirus3G represents a lower cost method with similar accuracy when compared to the isolated use of FibroMeterVirus3G test. This represents an advantage when reducing the number of unclassified APRI and Forns patients in the grey zone, without the need for liver biopsy. This is a useful and alternative approach in countries with less financial resources, considering the easy applicability and low cost of APRI and Forns for significant fibrosis. This procedure may represent a more comprehensive proposal to apply these non-invasive tests in the clinical setting.
Some limitations may be discussed in this study. The prevalence of significant fibrosis in our population was higher than the prevalence reported in a meta-analysis including more than 30000 CHC patients which showed a rate of 48% of significant fibrosis histologically assessed[27]. Our prevalence is greatly influenced by the fact that this study was carried out in a tertiary-care setting. Another limitation is that derivation and validation populations came from different racial ethnic backgrounds. Nevertheless, most patients included in the derivation population were Caucasians and both populations shared similar characteristics regarding laboratorial results and distribution of significant fibrosis.
The diagnostic accuracy of the APRI and FibroMeterVirus3G combination in validation population was 69%. A decrease in diagnostic accuracy is usually expected in the validation population when compared to the derivation population; however some points need to be emphasized. The PPV of the algorithm APRI and FibroMeterVirus3G in the validation population was high (87%). Consequently the algorithm enabled the selection of a subset of patients where very few false positive results were found. In other words, this algorithm allowed treatment to be given to those patients who really required antiviral drugs. The low sensitivity (57%) remains a limitation, since a considerable number of patients who need to be treated will not be correctly identified by the algorithm. Nevertheless, when analysing the subgroup of false negative patients in the validation population, we observed that the majority (69%) were represented by F2 and only 8% were cirrhotic. In the whole validation population, most of the F0 patients (100%), the F1 patients (88%) and the F4 (85%) were well classified by the algorithm, as well as two thirds of the F3. Therefore, the algorithm identifies the more severe patients (F3 and F4) while most of the misclassification concerns the F2 stage. In the derivation population, even though the accuracy of the algorithm was found to be better, the worst result was also observed in F2 stage. And lastly, even when considering a gold standard such as liver biopsy, there is a considerable misclassification of F2 patients[28]. In low income countries, where therapy is offered only to patients with advanced fibrosis, a close follow-up is required until these untreated patients fulfil the criteria for direct antiviral therapy. We may consider following the “missed” patients by reapplying the algorithm to better identify when patients change their fibrosis stage and require treatment[29]. Since most misclassified patients present F2 fibrosis stage, there is sufficient time before they become cirrhotic.
Our findings demonstrated that the association of a more robust non-invasive marker of fibrosis such as FibroMeterVirus3G and first generation tests may represent a useful alternative for fibrosis staging in CHC without loss of accuracy and without the need for liver biopsy. This might be an attractive approach mainly in limited resource countries.
Fibrometer tests results were granted by Echosens, Paris, Fr.
Despite recent advances in chronic hepatitis C therapy, diagnosis of significant fibrosis still represents a challenge when defining which patients should have priority in treatment, mainly in resource limited countries. Although liver biopsy has been classically considered the standard tool to evaluate fibrosis, it presents well-known inconveniences and limitations which make its use controversial amongst various authors. Thus, the development and improvement of alternative methods to identify candidates for an early treatment or intensive fibrosis monitoring is still recommended. First generation non-invasive tests such as aminotransferase-to-platelet ratio index (APRI), FIB4 and Forns are free of charge, easily accessible and well validated for chronic hepatitis C, however are limited when classifying all patients. Therefore, in order to increase assessment availability for patients with significant fibrosis, the authors evaluated the performance of a stepwise combination of first generation tests of fibrosis - APRI and Forns - followed by FibroMeterVirus3G, a more robust test, whenever results remained unclassified after first generation tests, always using liver biopsy as reference. This proposed combination allows costs to be optimized with no loss of accuracy and no need of liver biopsy, thus representing a favorable economic approach in resource limited areas.
This study introduces alternative approaches to evaluate significant fibrosis in chronic hepatitis C using a stepwise algorithm with first generation tests and FibroMeterVirus3G both to improve clinical decision-making and reduce costs. The authors considered this topic of great interest for clinicians and hepatologists in the daily practice management of chronic hepatitis C.
The present study demonstrated that the association of a more robust non-invasive marker of fibrosis such as FibroMeterVirus3G and first generation tests such as APRI and Forns may represent a useful alternative for fibrosis staging in chronic hepatitis C. This might be an attractive non-invasive approach to evaluate liver fibrosis and to optimize the access to potent but expensive direct-acting antiviral agents. To our knowledge, to date the stepwise combination of APRI or Forns with FibroMeterVirus3G has never been evaluated.
The sequential algorithm of either APRI or Forns combined with FibroMeterVirus3G represents an alternative approach to recognize and prioritize patients with chronic hepatitis C to antiviral therapy. It reduces the number of unclassified APRI and Forns patients allocated in the grey zone and reduces the total FibroMeterVirus3G requirement in 50%, representing an alternative approach in countries with less financial resources, without loss of accuracy, eliminating the requirement for liver biopsy procedure. This procedure may represent a more comprehensive proposal to apply these non-invasive tests in the clinical setting.
FibroMeterVirus3G is a non-invasive test to evaluate liver fibrosis in chronic hepatitis C represented by a patented formula including the biologic parameters prothrombin index, AST, ALT, Urea, GGT, alpha-2-macroglobulin and platelets.
It is a carefully planed study, it takes in to consideration the Liver biopsy and the Fibrometer Virus2 and Virus3 and makes a head to head comparison of the three, as to discover the safety profile and the accuracy when it comes to clinical use especially for the group patients with cirhossis and to evaluate the change in fibrosis stage in pts with cirrhosis that are unable to undergo liver biopsy with a non-invasive procedure. The Fibre meter requires more wide use in the clinical setting as to prove its self as a reliable and non-invasive method of estimating the fibrosis stage.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Chiang TA, Samy Kohla MA, Savopoulos CG S- Editor: Qi Y L- Editor: A E- Editor: Li D
| 1. | Piccinino F, Sagnelli E, Pasquale G, Giusti G. Complications following percutaneous liver biopsy. A multicentre retrospective study on 68,276 biopsies. J Hepatol. 1986;2:165-173. [PubMed] |
| 2. | Huang JF, Hsieh MY, Dai CY, Hou NJ, Lee LP, Lin ZY, Chen SC, Wang LY, Hsieh MY, Chang WY. The incidence and risks of liver biopsy in non-cirrhotic patients: An evaluation of 3806 biopsies. Gut. 2007;56:736-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Myers RP, Fong A, Shaheen AA. Utilization rates, complications and costs of percutaneous liver biopsy: a population-based study including 4275 biopsies. Liver Int. 2008;28:705-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 203] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 4. | Maharaj B, Maharaj RJ, Leary WP, Cooppan RM, Naran AD, Pirie D, Pudifin DJ. Sampling variability and its influence on the diagnostic yield of percutaneous needle biopsy of the liver. Lancet. 1986;1:523-525. [PubMed] |
| 5. | Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, Feng ZZ, Reddy KR, Schiff ER. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614-2618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1504] [Cited by in RCA: 1569] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 6. | Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology. 1994;20:15-20. [PubMed] |
| 7. | Goldin RD, Goldin JG, Burt AD, Dhillon PA, Hubscher S, Wyatt J, Patel N. Intra-observer and inter-observer variation in the histopathological assessment of chronic viral hepatitis. J Hepatol. 1996;25:649-654. [PubMed] |
| 8. | Afdhal NH. Diagnosing fibrosis in hepatitis C: is the pendulum swinging from biopsy to blood tests? Hepatology. 2003;37:972-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 93] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 9. | Dienstag JL. The role of liver biopsy in chronic hepatitis C. Hepatology. 2002;36:S152-S160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 72] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Carey E, Carey WD. Noninvasive tests for liver disease, fibrosis, and cirrhosis: Is liver biopsy obsolete? Cleve Clin J Med. 2010;77:519-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 11. | Trifan A, Stanciu C. Checkmate to liver biopsy in chronic hepatitis C? World J Gastroenterol. 2012;18:5514-5520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2762] [Cited by in RCA: 3245] [Article Influence: 147.5] [Reference Citation Analysis (0)] |
| 13. | Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, Fontaine H, Pol S. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1288] [Cited by in RCA: 1609] [Article Influence: 89.4] [Reference Citation Analysis (0)] |
| 14. | Forns X, Ampurdanès S, Llovet JM, Aponte J, Quintó L, Martínez-Bauer E, Bruguera M, Sánchez-Tapias JM, Rodés J. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology. 2002;36:986-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 721] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 15. | Poynard T, Imbert-Bismut F, Munteanu M, Messous D, Myers RP, Thabut D, Ratziu V, Mercadier A, Benhamou Y, Hainque B. Overview of the diagnostic value of biochemical markers of liver fibrosis (FibroTest, HCV FibroSure) and necrosis (ActiTest) in patients with chronic hepatitis C. Comp Hepatol. 2004;3:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 280] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 16. | Leroy V, Halfon P, Bacq Y, Boursier J, Rousselet MC, Bourlière M, de Muret A, Sturm N, Hunault G, Penaranda G. Diagnostic accuracy, reproducibility and robustness of fibrosis blood tests in chronic hepatitis C: a meta-analysis with individual data. Clin Biochem. 2008;41:1368-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Zarski JP, Sturm N, Guechot J, Paris A, Zafrani ES, Asselah T, Boisson RC, Bosson JL, Guyader D, Renversez JC. Comparison of nine blood tests and transient elastography for liver fibrosis in chronic hepatitis C: the ANRS HCEP-23 study. J Hepatol. 2012;56:55-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 158] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 18. | Boursier J, Bertrais S, Oberti F, Gallois Y, Fouchard-Hubert I, Rousselet MC, Zarski JP, Calès P. Comparison of accuracy of fibrosis degree classifications by liver biopsy and non-invasive tests in chronic hepatitis C. BMC Gastroenterol. 2011;11:132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Calès P, Boursier J, Oberti F, Hubert I, Gallois Y, Rousselet MC, Dib N, Moal V, Macchi L, Chevailler A. FibroMeters: a family of blood tests for liver fibrosis. Gastroenterol Clin Biol. 2008;32:40-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Calès P, de Ledinghen V, Halfon P, Bacq Y, Leroy V, Boursier J, Foucher J, Bourlière M, de Muret A, Sturm N. Evaluating the accuracy and increasing the reliable diagnosis rate of blood tests for liver fibrosis in chronic hepatitis C. Liver Int. 2008;28:1352-1362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 21. | Calès P, Boursier J, Bertrais S, Oberti F, Gallois Y, Fouchard-Hubert I, Dib N, Zarski JP, Rousselet MC. Optimization and robustness of blood tests for liver fibrosis and cirrhosis. Clin Biochem. 2010;43:1315-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2860] [Cited by in RCA: 3082] [Article Influence: 106.3] [Reference Citation Analysis (0)] |
| 23. | Konerman MA, Yapali S, Lok AS. Systematic review: identifying patients with chronic hepatitis C in need of early treatment and intensive monitoring--predictors and predictive models of disease progression. Aliment Pharmacol Ther. 2014;40:863-879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | FibroMeter est une famille de tests sanguins dédiés à l’évaluation de la fibrose hépatique et de la cirrhose. Available from: http://www.FibroMeter.com. |
| 25. | Sebastiani G, Halfon P, Castera L, Pol S, Thomas DL, Mangia A, Di Marco V, Pirisi M, Voiculescu M, Guido M. SAFE biopsy: a validated method for large-scale staging of liver fibrosis in chronic hepatitis C. Hepatology. 2009;49:1821-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 126] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 26. | Boursier J, de Ledinghen V, Zarski JP, Fouchard-Hubert I, Gallois Y, Oberti F, Calès P. Comparison of eight diagnostic algorithms for liver fibrosis in hepatitis C: new algorithms are more precise and entirely noninvasive. Hepatology. 2012;55:58-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 27. | Thein HH, Yi Q, Dore GJ, Krahn MD. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology. 2008;48:418-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 623] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 28. | Chindamo MC, Nunes-Pannain VL, Araújo-Neto JM, Moraes-Coelho HS, Luiz RR, Villela-Nogueira CA, Perez RM. Intermediate fibrosis staging in hepatitis C: a problem not overcome by optimal samples or pathologists’ expertise. Ann Hepatol. 2015;14:652-657. [PubMed] |
| 29. | Vergniol J, Boursier J, Coutzac C, Bertrais S, Foucher J, Angel C, Chermak F, Hubert IF, Merrouche W, Oberti F. Evolution of noninvasive tests of liver fibrosis is associated with prognosis in patients with chronic hepatitis C. Hepatology. 2014;60:65-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |