Published online Dec 8, 2017. doi: 10.4254/wjh.v9.i34.1270
Peer-review started: June 1, 2017
First decision: July 4, 2017
Revised: August 2, 2017
Accepted: October 15, 2017
Article in press: October 16, 2017
Published online: December 8, 2017
Processing time: 189 Days and 17.4 Hours
To determine whether ribavirin (RBV) concentrations differ according to cirrhosis stage among cirrhotic patients treated with interferon-free regimens.
We included patients with hepatitis C virus and cirrhosis [Child-Pugh (CP) A or B], Glomerular Filtration Rate ≥ 60 mL/min, who started therapy with DAAs and weight-based RBV between October 2014 and February 2016. RBV plasma levels were assessed during the treatment. We focused our analysis on the first 8 wk of therapy.
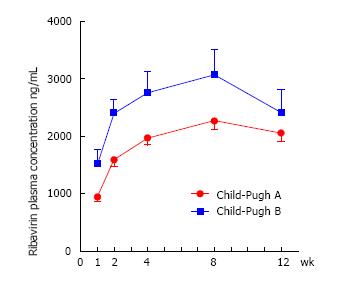
We studied 68 patients: 54 with compensated (CP-B) and 14 with decompensated (CP-A) cirrhosis. Patients with decompensated cirrhosis displayed significantly higher RBV concentrations than those with compensated cirrhosis at week 1, 2, 4 and 8 (P < 0.035). RBV levels were positively correlated with Hb loss over the treatment (P < 0.04). Majority (71%) of CP-B patients required a RBV dosage reduction during the treatment. After adjustment for confounders, Child-Pugh class remained significantly associated (95%CI: 35, 348, P = 0.017) to RBV levels, independently from baseline per-Kg RBV dosage.
Liver decompensation might affect RBV clearance leading to an overexposure and increased related toxicities in decompensated cirrhosis. Our findings underscore the importance of an early ribavirin therapeutic drug monitoring and suggest that an initial lower RBV dose, rather than weight-based, might be considered in those with advanced liver disease (CP-B) treated with direct-acting antivirals.
Core tip: In this study, patients with decompensated cirrhosis displayed higher plasma ribavirin concentrations in comparison to compensated patients, when treated with Interferon-free regimens for hepatitis C. Higher ribavirin levels were found to lead to greater rates of related toxicities and Child-Pugh class resulted independently associated with ribavirin plasma levels, in our population. Our findings suggest that ribavirin concentrations should be strictly monitored in subjects with advanced liver disease, during direct-acting antivirals-treatment. An early dosage adjustment of ribavirin should be performed when high levels of this antiviral are detected in patients’ plasma, in order to avoid toxicities among these frail individuals.
- Citation: Guardigni V, Badia L, Conti M, Rinaldi M, Mancini R, Viale P, Verucchi G. Liver decompensation predicts ribavirin overexposure in hepatitis C virus patients treated with direct-acting antivirals. World J Hepatol 2017; 9(34): 1270-1277
- URL: https://www.wjgnet.com/1948-5182/full/v9/i34/1270.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i34.1270
Before the advent of direct-acting antivirals (DAAs), the association of Peg-interferon (Peg-IFN) and ribavirin (RBV) represented the standard of care (SOC) for the treatment of chronic C hepatitis, regardless of hepatitis C virus (HCV) genotype and stage of liver disease[1]. Although HCV-induced cirrhosis represents a major cause of liver-related morbidity and mortality and successful therapy of individuals with advanced fibrosis and liver cirrhosis is associated with better outcomes [e.g., decreased incidence of hepatocellular carcinoma (HCC), decompensation][2], patients with cirrhosis were rarely treated because of the high risk of decompensation due to Peg-IFN administration.
New DAAs-regimens have dramatically changed this scenario, leading to the achievement of high rate of HCV eradication, even in individuals with compensated and decompensated cirrhosis. Although the role of RBV in the era of DAAs will probably decrease, it is currently still recommended for difficult-to-treat patients (i.e., experienced and cirrhotic), to optimize sustained virological response (SVR) in many treatment regimens[3].
Monitoring of ribavirin plasma concentration, given its interindividual variability, was used during combination therapy with Peg-IFN, since RBV exposure had been shown to be associated with treatment efficacy (SVR) and side effects (i.e., anaemia)[4,5]. In particular, trough RBV concentration (C trough) at week 4 and 8 of treatment was commonly used[5-7]. Indeed, steady-state concentrations are reached after the first 4 wk of therapy according to scientific literature[8].
Although higher RBV concentration levels have been associated to higher rates of SVR and therapeutic ranges at week 8 have been established also for first-generation DAAs-based HCV therapies (with telaprevir or boceprevir)[9], role of RBV therapeutic drug monitoring (TDM) in the era of second-generation DAAs regimens has not been investigated and elucidated yet.
Thus far, data on ribavirin TDM among cirrhotic patients with advanced liver disease, treated by the combination of RBV and last generation DAAs are lacking.
Multiple factors are involved in RBV pharmacokinetics consequently affecting RBV plasma concentrations, such as creatinine clearance, gender, age, diet, HIV infection, weight[3,10]. Child-Pugh score, a marker of cirrhosis severity, used to estimate the prognosis in these patients, has never been investigated as a determinant of RBV plasma concentrations during HCV treatment.
The aim of our retrospective study was to determine whether ribavirin plasma levels differ according to cirrhosis stage (defined by Child-Pugh class) in a cohort of subjects with liver cirrhosis, treated for HCV with IFN-free DAA-regimens containing RBV and to define effects of plasma RBV levels.
We retrospectively included in this study all the patients with chronic C hepatitis and cirrhosis (Child-Pugh A or B), without significant renal impairment [Estimated Glomerular Filtration Rate (eGFR) ≥ 60 mL/min], who started HCV treatment with DAAs in association with ribavirin (weight-based dose ≥ 11 mg/kg per die) between October 2014 and February 2016 at Infectious Diseases Department of University Hospital of Bologna (Italy). We excluded from the analysis two subjects who had less than two available plasma ribavirin level measurements throughout the antiviral therapy. Patients who received Peg-IFN in combination with DAAs and RBV were excluded from the study. All the patients were treated for 12 or 24 wk according to the national and international guidelines and were afterwards monitored for at least 12 wk after completing the therapy to assess SVR (SVR12). Patients were categorized into two groups (compensated or decompensated cirrhosis) according to their Child-Pugh score (< 7 or ≥ 7, respectively) to perform the analysis of interest.
For each subject, we collected the following data at baseline (corresponding with day of treatment start): Demographics (sex, age, race), medical history on previous HCV treatment (naïve or experienced), on chronic C hepatitis (HCV genotype, HCV RNA quantification) and on liver diseases (fibrosis stage, history of previous HCC, Child-Pugh class), HIV and HBV coinfections, creatinine level, eGFR, haemoglobin (Hb), bilirubin, current anti-HCV regimen (administered DAAs and Ribavirin initial dosage).
Data on RBV dose reduction over the course of treatment, therapy duration and response to the HCV treatment (as SVR12) were also recorded. Liver fibrosis was assessed according to the Metavir score either from elastographic measurements or from liver biopsy prior to HCV treatment. HCV RNA levels were detected by using Roche COBAS AmpliPrep/COBAS TaqMan HCV Test v 2.0 (Roche Molecular Systems, Inc., Pleasanton, CA). eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration formula.
Reasons for RBV dosage adjustment were the following: Occurrence of anaemia (Hb decline of more than 20 g/L or Hb less than 100 g/L), high RBV trough concentration (> 2500 ng/mL), that was considered at high risk for significant Hb decline[11,12], and other adverse effects (e.g., cutaneous rash, itch).
All the subjects’ visits and blood samples were standardized according to treatment schedule and performed at baseline and at week 1, 2, 4, 8, 12, 16, 20, 24 over treatment period. For each patient RBV concentrations were quantified using plasma samples that were collected multiple times during the treatment (average of 6.5 samples per subject), although we mainly focused our analysis on the first 8 wk of treatment because steady concentrations are usually reached between week 4 and 8[8] and because changes in RBV concentrations after week 8 (due to dosage adjustment) were expected to occur in our population.
RBV concentrations were measured in plasma samples, collected before taking the next dose of the drug (C trough), using a novel analytical method, validated according to the ISO 15089 predicaments. In brief, Ribavirin is extracted from plasma by means of a methanol-water (20%-80% mL/L) solution containing ZnSO4 (0.1 mol/L in water). The drug is then resolved chromatographically from endogenous and exogenous isobaric interferences by means of a binary chromatographic gradient. Specific signals for the drug are obtained in multiple reaction monitoring mode, recording the 245.1 > 113.2 and 245.1 > 96.2 m/z mass transitions. Ion extraction chromatograms enable drug determination with great specificity and sensitivity (Limit of Quantification = 1 μg/L).
Variables were expressed as mean ± SD or median and range interquartile. Continuous variables were compared by t-test or by Mann-Whitney-U test, when distribution was normal or not normal, respectively. Categorical variables were compared by chi-square test or Fisher’s exact as appropriate. Bivariate association between RBV plasma levels and continuous variables of interest were tested by Spearman rank correlations. Linear regression was first performed to evaluate factors associated with RBV plasma levels in the univariate analysis and afterward to determine independent predictors of RBV plasma levels among variables with significant result at the univariate. All statistical analysis were performed using SPSS software version 21. Tests were considered significant for a P value < 0.05.
We totally evaluated 68 cirrhotic patients who underwent anti-HCV treatment with DAAs in association with RBV in the study period: 54 had a compensated liver cirrhosis and 14 a decompensated cirrhosis (Child-Pugh A, scored < 7 and B, scored ≥ 7, respectively). Baseline characteristics of our population are shown in Table 1. No differences in demographics were found between the two groups. Fifty-seven point four percent of the population was composed by HIV co-infected patients who showed a greater, but not significantly, prevalence in decompensated subjects (53.7% vs 71.4%). Most of the subjects (63.2%) had been previously treated with Peg-IFN-based regimen, unsuccessfully. Genotype 1a was the most common HCV genotype (33.8%), as well as the association of Sofosbuvir (SOF, 400 mg/die) and Daclatasvir (DCV, 60 mg/die, or 30 mg/die in 13 HIV patients taking atazanavir/rtv-containing antiretroviral regimens) was the most frequently used (42.6%) in the overall population, without any difference according to Child-Pugh class. As expected, significant differences in haemoglobin and total bilirubin levels were detected, before starting antiviral therapy between the two groups: Indeed compensated cirrhotic patients showed higher mean Hb (146 ± 16 g/L vs 129 ± 17 g/L, P = 0.002) and lower median bilirubin levels [0.47, interquartile range (IQR) 2.8-8.2 mg/L vs 7.7 mg/L, IQR 5.3-16, P < 0.001] compared to decompensated patients. Creatinine values resulted higher in compensated patients (P = 0.011), although eGFR was similar in the two groups. 86.8% of subjects was treated for 24 wk, 13.2% for 12 wk.
| Characteristics | Overall (n = 68) | Child pugh A (n = 54) | Child pugh B (n = 14) | P value |
| Age (yr), mean ± SD | 55 ± 8.6 | 55.2 ± 9.5 | 54.6 ± 5 | 0.8 |
| Male, n (%) | 52 (76.5) | 43 (79.6) | 9 (64.3) | 0.2 |
| White race, n (%) | 66 (97.1) | 53 (98.1) | 13 (92.9) | 0.3 |
| HIV infection, n (%) | 39 (57.4) | 29 (53.7) | 10 (71.4) | 0.2 |
| HBsAg positive, n (%) | 1 (1.5) | 1 (1.9) | 0 | - |
| History of HCC, n (%) | 6 (8.8) | 4 (7.4) | 2 (14.3) | 0.6 |
| HCV treatment experienced, n (%) | 43 (63.2) | 33 (61.1) | 10 (71.4) | 0.5 |
| Liver stiffness (kPa), median (IQR) | 21.4 (15.9-34.4) | 21.2 (15.6-33.1) | 31.1 (17.8-54.3) | 0.1 |
| HCV genotype, n (%) | 0.7 | |||
| 1a | 23 (33.8) | 18 (33.3) | 5 (35.7) | |
| 1b | 11 (16.2) | 9 (16.7) | 2 (14.3) | |
| 2 | 6 (8.8) | 6 (11.1) | - | |
| 3 | 22 (32.4) | 17 (31.5) | 5 (35.7) | |
| 4 | 6 (8.8) | 4 (7.4) | 2 (14.3) | |
| Weight (kg), mean ± SD | 73 ± 14.1 | 73.2 ± 15.3 | 72.1 ± 8.5 | 0.7 |
| Hb baseline (g/L), mean ± SD | 143 ± 18 | 146 ± 16 | 129 ± 17 | 0.002 |
| Bilirubin (mg/L), median (IQR) | 9.5 (5.6-19) | 4.7 (2.8-8.2) | 7.7 (5.3-16) | < 0.001 |
| Creatinine (mg/L), mean ± SD | 8 ± 1.3 | 8.2 ± 1.5 | 7.5 ± 0.8 | 0.011 |
| eGFR (mL/min per 1.73 m2), median (IQR) | 101 (90-109) | 99 (88-105) | 102 (98-107) | 0.3 |
| HCV-RNA baseline (IU/mL) | 933559 (25667-2128936) | 1228876 (410822-2940050) | 246455 (126308-712307) | 0.007 |
| 1DAAs regimen, n (%) | 0.2 | |||
| SOF, n (%) | 6 (8.8) | 6 (11.1) | - | |
| SOF + DCV, n (%) | 29 (42.6) | 21 (38.9) | 8 (57.1) | |
| SOF + SMP, n (%) | 1 (1.5) | - | 1 (7.1) | |
| DCV + SMP, n (%) | 4 (5.9) | 3 (5.6) | 1 (7.1) | |
| SOF + LDV, n (%) | 12 (17.6) | 11 (20.4) | 1 (7.1) | |
| OBV/PTV/r + DSV, n (%) | 12 (17.6) | 9 (16.7) | 3 (21.4) | |
| OBV/PTV/r, n (%) | 4 (5.9) | 4 (7.4) | - |
There was no difference in initial mean dose of RBV (mg/kg) between the two groups (P = 0.3). Significant differences in RBV plasma concentrations between the two groups were found at each considered time-point (week 1, 2, 4 and 8) with much higher Ctrough levels among decompensated (Child-Pugh B) than among compensated (Child-Pugh A) patients. Otherwise, no remarkable difference in overall RBV mean values (measured during all the course of treatment) was revealed (Table 2).
| Parameters | Overall (n = 68) | Child pugh A (n = 54) | Child pugh B (n = 14) | P value |
| RBV (mg/kg pre die), mean ± SD | 14.4 ± 1.7 | 14.4 ± 1.36 | 14.9 ± 1.6 | 0.3 |
| 1RBV week 1 (ng/L) median (IQR) | 9105 (6130-1310) | 8750 (5700-11680) | 14050 (9010-22280) | 0.007 |
| 1RBV week 2 (ng/L) median (IQR) | 15000 (11600-27680) | 14150 (11150-20000) | 23550 (17200-28930) | 0.001 |
| 2RBV week 4 (ng/L) median (IQR) | 18300 (12900-27680 ) | 17850 (12200-26500) | 26250 (17700-35130) | 0.024 |
| 3RBV week 8 (ng/L) median (IQR) | 23100 (17050-31000) | 21500 (16100-27950) | 33000 (19800-41380) | 0.0034 |
| Mean RBV 1, 2, 4 wk, (ng/L) median (IQR) | 14240 (10770-19450) | 13280 (10480-18200) | 19600 (14670-26870) | 0.005 |
| Mean RBV 1, 2, 4, 8 wk, (ng/L) median (IQR) | 16490 (12220-21750) | 15135 (11850-19830) | 21700 (15090-29280) | 0.006 |
| Mean RBV, full treatment duration (ng/L), mean ± SD | 18079 ± 6609 | 17528 ± 6020 | 20327 ± 7700 | 0.2 |
| RBV dosage reduction, n (%) | 37 (54.4) | 27 (50) | 10 (71.4) | 0.2 |
| Weeks before reduction, median (IQR) | 6.1 (4-9.8) | 5 (4-9.7) | 7 (4-10) | 0.6 |
| Hb loss week 4 (g/L), mean ± SD | 19.6 ± 1.8 | 19 ± 13 | 21 ± 32 | 0.54 |
| Hb loss week 8 (g/L), mean ± SD | 21 ± 19 | 20 ± 13 | 25 ± 35 | 0.34 |
Figure 1 shows the significant different trends of RBV plasma concentration from baseline until week 12 in the two cirrhotic groups.
In almost three-quarters (71.4%) of Child-Pugh B patients a reduction of RBV dosage was required during the treatment period, instead only 50% of Child-Pugh A subjects experienced a dosage adjustment (P = 0.15), after an average of 6.8 and 7.1 wk from treatment start among Child-Pugh A and B, respectively. Most common reasons for RBV dosage reduction were onset of anaemia (36.1%) and high RBV concentration revealed by TDM (36.1%), data not shown. Although decompensated patients experienced greater Hb loss (at both week 4 and week 8) than those with compensated cirrhosis, the differences were not significant (Table 2).
Multiple factors potentially associated with RBV concentration were investigated at univariate analysis. The significant predictors of RBV concentrations (as a treatment average between week 1 and 8) were baseline RBV dose per kilogram (β value 205, 95%CI: 77; 332, P = 0.002), male gender (β value -539, 95%CI: -975; -103, P = 0.016) and Child-Pugh class (β value 232, 95%CI: 66; 399, P value= 0.007). After adjusting for all these significant variables, only baseline RBV dosage (β value 161.1, 95%CI: 35.6; 286, P = 0.013) and Child-Pugh class (β value 191.7, 95%CI: 35; 348, P = 0.017) remained significantly associated to RBV levels (Table 3). To investigate whether the association with Child-Pugh class was driven by single items of this composite index, we analysed each of them (i.e., Encephalopathy, Ascites, Bilirubin, INR, Albumin at baseline) in relationship to RBV concentrations, but no significant differences in RBV overexposure were observed. Moreover, clearance of RBV (indirectly assessed by its plasma concentrations) did not seem to be related to portal hypertension signs (e.g., platelet count, esophageal varices), data not shown.
| Univariate analysis | Multivariate analysis | |||||
| β value | 95%CI | P value | β value | 95%CI | P value | |
| Baseline ribavirin dosage (mg/kg) | 205 | 77; 332 | 0.002 | 161 | 36; 286 | 0.013 |
| IFN-experienced | 97 | -303; 497 | 0.6 | |||
| Male gender | -539 | -975; -103 | 0.016 | -349 | -765; 68 | 0.099 |
| Age (yr) | 13 | -9; 34 | 0.3 | |||
| White race | 424 | -714; 1563 | 0.5 | |||
| Bilirubin (mg/L) | 870 | -920; 2670 | 3 | |||
| Creatinine (mg/L) | -2890 | -16830; 11040 | 7 | |||
| Child-Pugh class | 232 | 66; 399 | 0.007 | 192 | 35; 348 | 0.017 |
| Liver stiffness (kPa) | -1.6 | -14; 11 | 0.8 | |||
| HIV infection | 76 | -314; 466 | 0.7 | |||
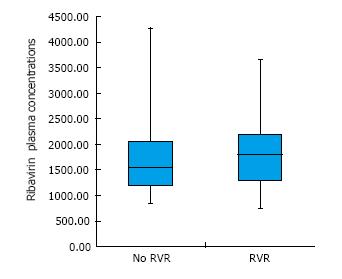
Remarkably, there were no significant differences in RBV concentration (as an average value obtained from the first 8 wk measurements) between subjects who achieved a rapid virological response (RVR, namely HCV undetectability within week 4 of treatment) and those who did not (Figure 2). Furthermore, no correlation between RBV concentrations (at any time-point) and time to reach HCV undetectability was observed in our population (data not shown). The majority of patients included in this study (92.6%) achieved SVR12, without any difference in SVR rate based on Child-Pugh class and RBV levels at week 4 and 8 (data not shown).
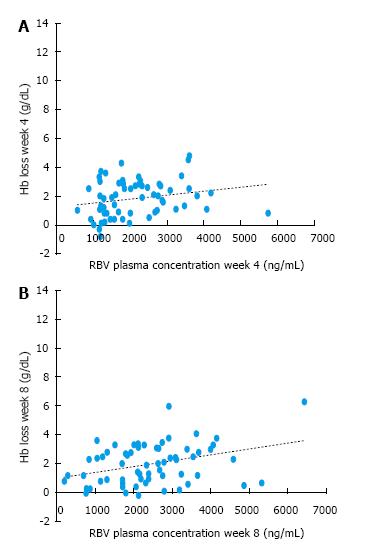
As depicted in Figure 3, Hb loss (g/L) in the first two months of treatment (at the two therapy time-points: Week 4 and 8) was correlated to RBV concentrations measured at the corresponding week (R = + 0.272, P value= 0.033 at week 4, R = + 0.279, P = 0.025 at week 8), with higher RBV levels associated with greater Hb decline. The relationship between Hb loss at week 8 and RBV levels at that specific week was independent from Child-Pugh class (in a multivariate linear regression, P = 0.002).
The advent of new IFN-free DAAs regimens has allowed to achieve very high rates of HCV eradication, even in patients with advanced liver disease. Although the role of RBV is now discussed and its relevance will probably decrease over time, this antiviral drug is still recommended in combination with DAAs in several challenging situations: For treatment-experienced but DAAs-naïve patients without cirrhosis or with compensated cirrhosis (Child-Pugh A) to reduce weeks of therapy, for subjects who failed to achieve SVR on prior antiviral therapy containing DAAs and for those with decompensated cirrhosis (Child-Pugh B or C) to increase SVR rates[13].
To date, specific data regarding ribavirin TDM in cirrhotic patients treated with IFN-free regimens are lacking and daily weight-based ribavirin remains the SOC. In particular, a daily dose of ribavirin higher than 10 mg/kg of body weight had been previously associated to higher rate of SVR among subjects treated with Peg-IFN and RBV[14] and it is still used with DAAs, although the use of RBV according body weight has been occasionally disapproved due to the variability in this antiviral elimination phase[4].
In our study, patients with decompensated cirrhosis (Child-Pugh B) remarkably displayed higher plasma levels of RBV during the first 8 wk of therapy with DAAs compared to those with compensated cirrhosis (Child-Pugh A), although initial weight-dosages were similar. This raises the hypothesis that liver failure might play a meaningful role in RBV clearance, not observed before.
The fact that overall RBV mean plasma concentrations did not differ based on Child-Pugh class in our cohort might be explained by the greater rate of RBV dose reduction (but no discontinuation) and the following RBV exposure decline in those with decompensated cirrhosis, over the course of treatment.
Reduced dosages of ribavirin are currently recommended in patients with moderate or severe renal impairment (Creatinine Clearance ≤ 60 mL/min), since RBV exposure is predicted to increase up to 17% in this subsets of patients, due to its renal excretion[15]. Also weight, gender, age, fat meals have been shown to clearly affect RBV metabolism[3]. Gastrointestinal tract is another potential site of RBV clearance[16].
To date, no adjustment in ribavirin dosage is required in liver dysfunction, which instead affects metabolism of many drugs[17], since so far liver failure did not seem to impact on ribavirin clearance.
Only Glue[18] assessed the single dose pharmacokinetics of this antiviral in subjects with various degrees of chronic liver disease: In agreement to our results, they observed that Cmax significantly increased with the severity of liver dysfunction, although there was a remarkable overlap in individual Cmax values among the groups and they eventually did not propose any dosage reduction in these patients.
Contrarily, we first evaluated RBV pharmacokinetics in patients with liver disease over the course of the treatment in a real-world setting, obtaining then different findings. One could argue that a slower clearance of the drug in our population was due to a cirrhosis-related renal dysfunction (i.e., hepatorenal syndrome), but we included only subjects with eGFR ≥ 60 mL/min and unexpectedly creatinine was not found to be associated to RBV concentrations over the first 8 wk at univariate analysis in our population.
Conversely, a higher Child-Pugh score determined a RBV overexposure, leading to high rate of dose adjustment in those with Child-Pugh ≥ 7. To our knowledge, this is the first study exploring ribavirin TDM in decompensated cirrhotic individuals treated with new DAAs regimens and showing an overexposure in this group of patients. Indeed, while few data report RBV plasma levels in therapeutic range in patients with Child-Pugh A treated with a fixed dose (800 mg daily) of RBV associated with SOF and DCV[19], no information about Child-Pugh B or C patients are currently available. These findings might be useful to clinicians, since an overexposure can be modulated by lowering the initial RBV dosage from the weight-based dosage in certain patients with advanced liver disease, avoiding secondary and detrimental effects.
Furthermore, our data show a positive correlation between RBV concentrations and Hb loss, as already widely reported in literature, even in the context of IFN-free regimens[20]. This finding strengthens the importance of considering a lower RBV dosage in subjects with decompensated cirrhosis, also considering their higher rate of baseline anaemia characterizing this group of patients (confirmed also in our population). Indeed, anaemia occur in about 75% of patients with chronic liver disease and can be linked to different aetiologies (e.g., haemorrhage, splenomegaly)[21], regardless of the use of anti-HCV drugs, such as ribavirin, and represents a condition to be taken into account in the management of cirrhotic patients undergoing anti-HCV treatment.
According to our results, higher RBV concentrations in the first weeks of therapy did not seem to enhance RVR rate in cirrhotic patients treated with IFN-free regimens and no relationship between RBV plasma concentration at week 4 or 8 and rate of SVR was found. This might appear in contrast with prior data regarding Peg-IFN/RBV[22] or, more recently, SOF/RBV treatment (e.g., FISSION, NEUTRINO and FUSION trials)[23,24], in which RBV exposure was one of the factor associated with SVR, with suggested RBV plasma concentration threshold between 2000 and 3000 ng/mL[25,26]. In our cohort, the low rate of patients failing to achieve SVR (7.4%) could explain the lack of relationship between RBV levels and SVR rate: It is in general difficult to identify statistically significant predictive markers with such a high response rate.
This study has some limitation to be considered. The main limitation is the absence of Cmax and AUC data, due to the observational nature of the study; however Ctrough can be considered in clinical practice as a surrogate of plasma exposure and its values correlate with drug efficacy and toxicity in other studies[27]. Moreover, this is a retrospective study with a limited population size and this suggests caution prior to extend our results to the all the population with liver cirrhosis treated with RBV and DAAs. The inclusion of patients with mild renal impairment (eGFR 60-89 mL/min) could represent a confounding factor, although baseline creatinine was not found to affect RBV concentrations in our population.
In conclusion, liver failure might affect RBV clearance leading to an overexposure and increased related toxicities (e.g., anaemia) in subjects with decompensated cirrhosis who are now being treated with last-generation DAAs. Our findings underscore the importance of RBV plasma monitoring and early dose adjustments in these patients and suggest that an initial lower dose, rather than weight-based, of RBV might be considered in individuals with advanced liver disease (CP B class) treated with new DAAs in order to reduce toxicities without increasing virological failure rates.
This might improve the knowledge on the tailored use of ribavirin within IFN-free regimens for HCV treatment, in subjects with advanced liver disease. Further studies will be needed to confirm our results in order to determine the optimal dosage of ribavirin in patients with advanced cirrhosis in the era of DAAs-based regimens, since difficult-to-treat patients remain a challenge in HCV treatment and maintaining the benefit of this effective antiviral is still a relevant issue.
In the era of new direct-acting antivirals (DAAs), ribavirin (RBV) is still recommended for difficult-to-treat hepatitis C virus (HCV) positive patients, but data on RBV therapeutic drug monitoring (TDM) among patients with advanced liver disease treated with RBV and DAAs are lacking.
TDM represent an innovative technique and is currently widely used approach to personalize and ameliorate anti-infective treatments.
This is the first study showing that patients with decompensated cirrhosis displayed significantly higher RBV concentrations than those with compensated cirrhosis, when treated with new Interferon-free regimens.
These findings might improve the knowledge on the tailored use of ribavirin within IFN-free regimens for HCV treatment in subjects with advanced liver disease, for whom a lower initial dosage of RBV might be considered, in order to reduce risks related to RBV overexposure.
DAAs: Direct-acting antivirals represent the current standard of care for Chronic C Hepatitis; RBV: Ribavirin is an old-fashioned antiviral used in combination with last generation DAAs for difficult-to-treat HCV patients; TDM: Therapeutic drug monitoring by means of blood measurement of a drug concentration; C trough: Plasma concentration of a drug measured before taking the next dose of the drug.
This paper offers important approach to the topic and treatment algorithm.
The authors thank Dr. Mario Luca Morieri for his relevant contribution to this manuscript.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Han ZG, Ozenirler S, Solinas A S- Editor: Cui LJ L- Editor: A E- Editor: Lu YJ
| 1. | Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL Jr, Häussinger D, Diago M, Carosi G, Dhumeaux D, Craxi A, Lin A, Hoffman J, Yu J. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4847] [Cited by in RCA: 4747] [Article Influence: 206.4] [Reference Citation Analysis (0)] |
| 2. | Van der Meer AJ, Veldt BJ, Feld JJ, Wedemeyer H, Dufour JF, Lammert F, Duarte-Rojo A, Heathcote EJ, Manns MP, Kuske L. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308:2584-2593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1165] [Cited by in RCA: 1167] [Article Influence: 89.8] [Reference Citation Analysis (0)] |
| 3. | Pradat P, Virlogeux V, Gagnieu M-C, Zoulim F, Bailly F. Ribavirin at the Era of Novel Direct Antiviral Agents for the Treatment of Hepatitis C Virus Infection: Relevance of Pharmacological Monitoring. Adv Hepatol. 2014;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Brochot E, Castelain S, Duverlie G, Capron D, Nguyen-Khac E, François C. Ribavirin monitoring in chronic hepatitis C therapy: Anaemia versus efficacy. Antivir Ther. 2010;15:687-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Kuntzen T, Kuhn S, Kuntzen D, Seifert B, Mullhaupt B, Geier A. Influence of Ribavirin Serum Levels on Outcome of Antiviral Treatment and Anemia in Hepatitis C Virus Infection. PLoS One. 2016;11:e0158512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Loustaud-Ratti V, Debette-Gratien M, Jacques J, Alain S, Marquet P, Sautereau D, Rousseau A, Carrier P. Ribavirin: Past, present and future. World J Hepatol. 2016;8:123-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (1)] |
| 7. | Marucco DA, de Requena DG, Bonora S, Tettoni C, Bonasso M, De Blasi T, D’Avolio A, Sciandra M, Siccardi M, Baietto L. The use of trough ribavirin concentration to predict sustained virological response and haematological toxicity in HIV/HCV-co-infected patients treated with ribavirin and pegylated interferon. J Antimicrob Chemother. 2008;61:919-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Glue P. The clinical pharmacology of ribavirin. Semin Liver Dis 19 Suppl. 1999;1:17-24. [PubMed] |
| 9. | De Kanter CTMM, Buti M, DeMasi R, Ouwerkerk-Mahadevan S, Dofferhoff ASM, Witek J, Drenth JPH, Zeuzem S, Burger DM. Ribavirin concentration determines treatment success of first-generation DAA-based chronic HCV therapy. Antivir Ther. 2016;21:153-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Hatu G, Bailly F, Pourcelot E, Pradat P1, Miailhes P, Maynard M, Parant F, Chiarello P, Livrozet JM, Zoulim F. Lower ribavirin biodisponibility in patients with HIV-HCV coinfection in comparison with HCV monoinfected patients. BMC Infect Dis. 2014;14:150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Bonora S, Calcagno A, Cometto C, Fontana S, Aguilar D, D’Avolio A, Gonzalez de Requena D, Maiello A, Dal Conte I, Lucchini A. Short-term additional enfuvirtide therapy is associated with a greater immunological recovery in HIV very late presenters: A controlled pilot study. Infection. 2012;40:69-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Rendon AL, Nunez M, Romero M, Barreiro P, Martin-Carbonero L, Garcia-Samaniego J, Jimenez-Nacher I, Gonzalez-Lahoz J, Soriano V. Early monitoring of ribavirin plasma concentrations may predict anemia and early virologic response in HIV/hepatitis C virus-coinfected patients. J Acquir Immune Defic Syndr. 2005;39:401-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C. J Hepatol. 2017;66:153-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 821] [Cited by in RCA: 804] [Article Influence: 100.5] [Reference Citation Analysis (0)] |
| 14. | Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4736] [Cited by in RCA: 4558] [Article Influence: 189.9] [Reference Citation Analysis (0)] |
| 15. | Polepally AR, Badri PS, Eckert D, Mensing S, Menon RM. Effects of Mild and Moderate Renal Impairment on Ombitasvir, Paritaprevir, Ritonavir, Dasabuvir, and Ribavirin Pharmacokinetics in Patients with Chronic HCV Infection. Eur J Drug Metab Pharmacokinetics. 2017;42:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Dixit NM, Perelson AS. The metabolism, pharmacokinetics and mechanisms of antiviral activity of ribavirin against hepatitis C virus. Cell Mol Life Sci. 2006;63:832-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Tolman KG. Drugs and the liver. Med J Aust. 1977;2:655-656. [PubMed] |
| 18. | Glue P, Schenker S, Gupta S, Clement RP, Zambas D, Salfi M. The single dose pharmacokinetics of ribavirin in subjects with chronic liver disease. Br J Clin Pharmacol. 2000;49:417-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Parisi SG, Loregian A, Andreis S, Nannetti G, Cavinato S, Basso M, Scaggiante R, Dal Bello F, Messa L, Cattelan AM. Daclatasvir plasma level and resistance selection in HIV patients with hepatitis C virus cirrhosis treated with daclatasvir, sofosbuvir, and ribavirin. Int J Infect Dis. 2016;49:151-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Rower JE, Meissner EG, Jimmerson LC, Osinusi A, Sims Z, Petersen T, Bushman LR, Wolfe P, McHutchison JG, Kottilil S. Serum and cellular ribavirin pharmacokinetic and concentration-effect analysis in HCV patients receiving sofosbuvir plus ribavirin. J Antimicrob Chemother. 2015;70:2322-2329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Gonzalez-Casas R, Jones EA, Moreno-Otero R. Spectrum of anemia associated with chronic liver disease. World J Gastroenterol. 2009;15:4653-4658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 113] [Cited by in RCA: 135] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 22. | Larrat S, Stanke-Labesque F, Plages A, Zarski JP, Bessard G, Souvignet C. Ribavirin quantification in combination treatment of chronic hepatitis C. Antimicrob Agents Chemother. 2003;47:124-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 83] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, Schultz M, Davis MN, Kayali Z, Reddy KR. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J of Med. 2013;368:1878-1887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1322] [Cited by in RCA: 1325] [Article Influence: 110.4] [Reference Citation Analysis (0)] |
| 24. | Jacobson IM, Gordon SC, Kowdley K V, Yoshida EM, Rodriguez-Torres M, Sulkowski MS, Shiffman ML, Lawitz E, Everson G, Bennett M. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J of Med. 2013;368:1867-1877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 846] [Cited by in RCA: 838] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 25. | Maynard M, Pradat P, Gagnieu MC, Souvignet C, Trepo C. Prediction of sustained virological response by ribavirin plasma concentration at week 4 of therapy in hepatitis C virus genotype 1 patients. Antivir Ther. 2008;13:607-611. [PubMed] |
| 26. | Maeda Y, Kiribayashi Y, Moriya T, Maruhashi A, Omoda K, Funakoshi S, Murakami T, Takano M. Dosage adjustment of ribavirin based on renal function in Japanese patients with chronic hepatitis C. Ther Drug Monit. 2004;26:9-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Arase Y, Ikeda K, Tsubota A, Suzuki F, Suzuki Y, Saitoh S, Kobayashi M, Akuta N, Someya T, Hosaka T. Significance of serum ribavirin concentration in combination therapy of interferon and ribavirin for chronic hepatitis C. Intervirology. 2005;48:138-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |