Published online Oct 28, 2017. doi: 10.4254/wjh.v9.i30.1190
Peer-review started: April 10, 2017
First decision: May 19, 2017
Revised: July 14, 2017
Accepted: September 3, 2017
Article in press: September 4, 2017
Published online: October 28, 2017
Processing time: 203 Days and 12.4 Hours
To evaluate the safety and efficacy of ledipasvir/sofosbuvir on hepatitis C eradication in patients with hepatitis C virus (HCV)/human immunodeficiency virus (HIV) co-infection in an urban HIV clinic.
A retrospective cohort study of 40 subjects co-infected with HIV-1 and HCV treated with the fixed-dose combination of ledipasvir and sofosbuvir for 12 wk from 2014 to 2016. All patients included were receiving antiretroviral therapy (ART) with HIV RNA values of 100 copies/mL or fewer regardless of baseline HCV RNA level. The primary end point was a sustained virologic response of HCV at 12 wk (SVR12) after the end of therapy.
Of the 40 patients enrolled, 55% were black, 22.5% had been previously treated for HCV, and 25% had cirrhosis. The patients were on a wide range of ART. Overall, 39 patients (97.5%) had a SVR 12 after the end of therapy, including rates of 97.1% in patients with HCV genotype 1a and 100% in those with HCV genotype 1b. One patient with HCV genotype 3a was included and achieved SVR12. Rates of SVR12 were similar regardless of previous treatment or the presence of compensated cirrhosis. Only 1 patient experienced relapse at week 12 following treatment and deep sequencing didn’t reveal any resistance associated mutation in the NS5A or NS5B region. Interestingly, 7 (17.5%) patients who were adherent to ART experienced HIV viral breakthrough which resolved after continuing the same ART regimen. Two (5%) patients experienced HIV-1 virologic rebound due to noncompliance with HIV therapy, which resolved after resuming the same ART regimen. No severe adverse events were observed and no patient discontinued treatment because of adverse events. The most common adverse events included headache (12.5%), fatigue (10%), and diarrhea (2.5%).
This retrospective study demonstrated the high rates of SVR12 of ledipasvir/sofosbuvir on HCV eradication in patients co-infected with HCV and HIV, regardless of HCV baseline levels, HCV treatment history or cirrhosis condition. The oral combination of ledipasvir/sofosbuvir represents a safe and well tolerated HCV treatment option that does not require modification for many of the common HIV ART. Occasional HIV virologic rebound occurred but later resolved without the need to change ART.
Core tip: This is a retrospective study to evaluate the safety and efficacy of ledipasvir/sofosbuvir on hepatitis C eradication in patients with hepatitis C virus (HCV) and human immunodeficiency virus (HIV) co-infection in an urban HIV clinic. It demonstrated the high rates of SVR12 of ledipasvir/sofosbuvir on HCV eradication in patients co-infected with HCV and HIV, regardless of HCV baseline levels, HCV treatment history or cirrhosis condition. The oral combination of ledipasvir/sofosbuvir represents a safe and well tolerated HCV treatment option that does not require modification for many of the common HIV antiretroviral therapy (ART). Occasional HIV virologic rebound occurred but later resolved without the need to change ART.
- Citation: He X, Hopkins L, Everett G, Carter WM, SchroppDyce C, Abusaada K, Hsu V. Safety and efficacy of ledipasvir/sofosbuvir on hepatitis C eradication in hepatitis C virus/human immunodeficiency virus co-infected patients. World J Hepatol 2017; 9(30): 1190-1196
- URL: https://www.wjgnet.com/1948-5182/full/v9/i30/1190.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i30.1190
More than 185 million people around the world are infected with the hepatitis C virus (HCV), 350000 of whom die each year[1,2]. Human immunodeficiency virus (HIV) and HCV have common routes of transmission, and it is estimated that 4-5 million persons out of the 185 million infected by HCV are also co-infected by HIV. On the other hand, up to 30% of HIV positive patients are infected with HCV[2,3]. There is increasing evidence that HCV coinfection has a harmful effect on the progression of HIV infection with increased risk of mortality[4]. Liver disease has become a major cause of morbidity and mortality in HIV-infected persons.
Sustained viral response (SVR) (equivalent to eradication of HCV) after administering anti-HCV therapy is associated with improved survival and reduced liver decompensation in patients with chronic hepatitis C with HIV infection[5,6]. It may also decrease the progression of HIV infection and mortality not related to liver disease[7]. The mainstay of therapy over the last two decades involved a combination of interferon α and ribavirin (RBV). SVR rates with pegylated interferon and RBV were very low, averaging between 40% and 50% and the treatment duration required is long, ranging from 24 to 48 wk[1]. In addition, peginterferon has many side effects and contraindications. Many patients with HIV infection are unwilling to take interferon. The availability of an effective HCV interferon free regimen is highly needed for the management of hepatitis C in HIV infected patients.
In recent years, the management of chronic hepatitis C has been revolutionized by the development of direct-acting antiviral agents (DAAs) which significantly improved rates of cure in chronic HCV infection. Ledipasvir is an inhibitor of nonstructural protein 5A (NS5A), which has an important role in HCV RNA replication[8]. Sofosbuvir (SOF), a uridine nucleotide analog prodrug, was approved by the US FDA in December 2013. The active metabolite of SOF, is incorporated by the NS5B polymerase into HCV RNA, resulting in chain termination[3]. The fixed-dose combination of ledipasvir and sofosbuvir has demonstrated minimal toxicities and high efficacy, with an overall SVR of over 91%, in patients infected with HCV genotype 1, without the need for either interferon or RBV[8-10]. Osinusi et al[11] for the first time, reported that the combination of ledipasvir and sofosbuvir was associated with SVR rate of 98% in patients co-infected with HCV genotype 1 and HIV in a phase 2 study. Later, a larger phase 3 trial (ION-4 study) demonstrated that 12 wk of treatment with ledipasvir/sofosbuvir resulted in a SVR rate of 96% in patients who were co-infected with HIV and HCV genotype 1 or 4[12]. Harvoni, the fixed dose combination of ledipasvir and sofosbuvir, became the first approved once daily Single-tablet-regimen (STR) for treatment of chronic HCV in HIV positive patients in Nov 2015. This combination may have additional mental health benefits in HIV/HCV co-infected patients[13].
Currently, there are few published data on the experience with this newly approved combination of ledipasvir/sofosbuvir in HCV/HIV co-infected patients. Here, we reported a single-center, retrospective study evaluating the safety and efficacy of this combination on HCV eradication in the patients co-infected with HCV and HIV with or without previous treatment for HCV.
This was a retrospective cohort study. All of the research reviews were conducted under protocols approved by the institutional independent ethics committee and all data were collected and analyzed in a Health Insurance Portability and Accountability Act-compliant manner to ensure patient privacy and data integrity. The study was conducted in Sunshine Care Center (Florida Department of Health in Orange County, Orlando, FL), an urban HIV clinic in Orlando.
Patients older than 18 years diagnosed as HIV/HCV co-infection at Sunshine Care Center between 2014 and 2016 and treated with the fixed dose combination of ledipasvir/sofosbuvir for 12 wk were included. Charts were reviewed and data were collected by a trained internal medicine resident. The presence of cirrhosis was determined by liver biopsy with a Metavir score of F4; or a score of more than 12.5 kPa on transient elastography testing (Fibroscan); or Radiological imaging consistent with cirrhosis.
For each patient included in the study, the demographic data were collected through manual chart review, including age, race, sex, body-mass index (BMI), smoking history, HCV genotype, and medical history (Table 1).
| Characteristic | Ledipasvir/sofosbuvir for 12 wk (n = 40) |
| Median age (IQR) - yr | 53 (51-57) |
| Male sex | 25 (62.5) |
| Race1 | |
| White | 13 (32.5) |
| Black | 22 (55.0) |
| Asian | 1 (2.5) |
| Other or unknown | 4 (10.0) |
| Mean body-mass index (IQR)2 | 26.2 (22.7-28.7) |
| Smoking | 13 (32.5) |
| HCV genotype | |
| 1a | 34 (85.0) |
| 1b | 5 (12.5) |
| 3a | 1 (2.5) |
| Baseline HCV RNA (IQR), log10 IU/mL | 6.3 (6.0-6.6) |
| HCV RNA > 6 million IU/mL | 5 (12.5) |
| Cirrhosis | 10 (25.0) |
| Baseline creatinine, mean (range), mg/dL | 0.95 (0.56-1.48) |
| Baseline eGFR, mean (range), mL/min | 90.0 (52-134) |
| CD4, cells/mm3 | |
| < 200 | 1 (2.5) |
| 200-350 | 7 (17.5) |
| > 350 | 32 (80) |
| Mean CD4+ cell count (IQR), cells/μL | 638 (366-857) |
| Antiviral regimen | 40 (100) |
| HCV treatment history | |
| No previous treatment | 31 (77.5) |
| Previous treatment | 9 (22.5) |
The primary efficacy end point was sustained virologic response (HCV RNA level < 15 IU/mL by real-time HCV assay) at 12 wk after treatment completion (SVR12) among all patients enrolled in the study.
Pre-treatment, during treatment, and post-treatment data of the standard laboratory testing (complete blood count (CBC), levels of albumin, bilirubin, alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen, creatinine) and measurements of plasma HCV RNA and HIV-1 RNA levels, along with evaluations of adherence were collected. Plasma HCV RNA levels were measured using the real-time HCV assay (Abbott), with the Lower Limit of Quantitation (LLOQ) of 15 IU/mL. Plasma HIV RNA levels were measured at all points using reverse transcription polymerase chain reaction (real-time HIV assay), with an LLOQ of 40 copies/mL. Adherence to ledipasvir and sofosbuvir was measured by pill counts and patient self-report. All adverse events were recorded and graded according to the NIAID Division of AIDS toxicity table (version 1.0 2009 clarification).
Hepatitis C viral relapse was defined as an HCV RNA level higher than the LLOQ at any posttreatment point after having an HCV RNA level lower than the LLOQ at the end of treatment. Hepatitis C viral breakthrough was defined as an HCV RNA level at the LLOQ or higher during treatment after having previously had an HCV RNA level lower than the LLOQ while taking study drugs, confirmed with 2 consecutive values. HIV viral breakthrough was defined as an HIV RNA level at the LLOQ or higher during treatment after having previously had an HIV RNA level lower than the LLOQ while taking ART, confirmed with 2 consecutive values. Patients with plasma HIV-1 RNA levels of 400 copies per milliliter or higher at two or more consecutive post-baseline visits at least 2 wk apart were considered to have HIV-1 virologic rebound.
Deep sequencing of the HCV NS5A and NS5B regions was performed only for the patient with virologic failure, from samples collected at the time of virologic failure, using DDL (DDL Diagnostics Laboratory). Variants that were present in at least 1% of the viral population were reported.
We calculated the proportion of patients who had a sustained virologic response along with exact two-sided 95%CI using the Clopper-Pearson method. Statistical differences were analyzed by χ2 tests for categorical variables and t-test for continuous variables with significance defined as a P value less than 0.05.
A total of 40 patients were enrolled. Eighty-five percent of patients were infected with HCV genotype 1a, 12.5% with HCV genotype 1b, and 2.5% with HCV genotype 3a (Table 1). Overall, 55% of patients were black, 62.5% were male, 25% had compensated cirrhosis, and 22.5% had received previous unsuccessful treatment for HCV. Among the 10 patients with cirrhosis, the mean baseline albumin level was 4.3 g per deciliter, the mean platelet count was 110940 per microliter, the mean Bilirubin level was 1.0 milligrams per deciliter (mg/dL), the mean ALP level was 101 units per liter (U/L), the mean ALT level was 89 U/L, and the mean AST level was 67 U/L. Nine patients received previous treatments for HCV with pegylated interferon (peginterferon) plus ribavirin. All patients were receiving ART with a wide range of regimen (Table 2).
| Antiviral regimen | n (%) |
| Efavirenz-emtricitabine-tenofovir DF | 1 (2.5) |
| Tivicay-emtricitabine-tenofovir DF | 1 (2.5) |
| Rilpivirine-emtricitabine-tenofovir DF | 5 (12.5) |
| Raltegravir- Rilpivirine-emtricitabine-tenofovir DF | 2 (5) |
| Raltegravir-emtricitabine-tenofovir DF | 6 (15) |
| Ritonavir- Raltegravir-emtricitabine-tenofovir DF | 1 (2.5) |
| Dolutegravir-emtricitabine-tenofovir DF | 1 (2.5) |
| Raltegravir-telaprevir | 4 (10) |
| Abacavir-dolutegravir-lamivudine | 8 (20) |
| Abacavir-etravirine-lamivudine | 1 (2.5) |
| Darunavir-ritonavir-etravirine-raltegravir | 3 (7.5) |
| Abacavir-lamivudine-darunavir-ritonavir | 3 (7.5) |
| Abacavir-lamivudine-darunavir-ritonavir-etravirine-raltegravir | 1 (2.5) |
| Elvitegravir, cobicistat, emtricitabine, tenofovir alafenamide | 3 (7.5) |
Among the 40 patients who were enrolled and treated, 39 [97.5%; 95% confidence interval (CI), 90 to 100] had a sustained virologic response 12 wk after the end of therapy (Table 3). The rates of response at 12 wk were similar in patients with genotype 1a (97%) and those with 1b (100%), in men (96%) and women (100%), in black patients (100%) and other races (94.4%), in patients who had undergone previous treatment (100%) and those who had not (96.8%), in patients with cirrhosis (100%) and those without cirrhosis (96.7%).
Only 1 patient did not achieve SVR12 and experienced relapse by week 12 after treatment completion. This was a 53-year-old white male, with HCV genotype 1a infection and stage 1 liver disease. The baseline HCV viral load was 11370594 IU/mL as determined by real-time PCR assay. The medications that he received against HIV infection included raltegravir, etravirine, ritonavir and darunavir. HCV viral suppression was achieved by week 8 with viral load lower than the LLOQ, which was maintained through 12 wk. However, HCV viral load increased to 7043 IU/mL at week 12 after treatment completion and was 7165187 at week 16 after treatment completion. Deep sequencing failed to reveal any mutation was seen in the NS5A or NS5B region.
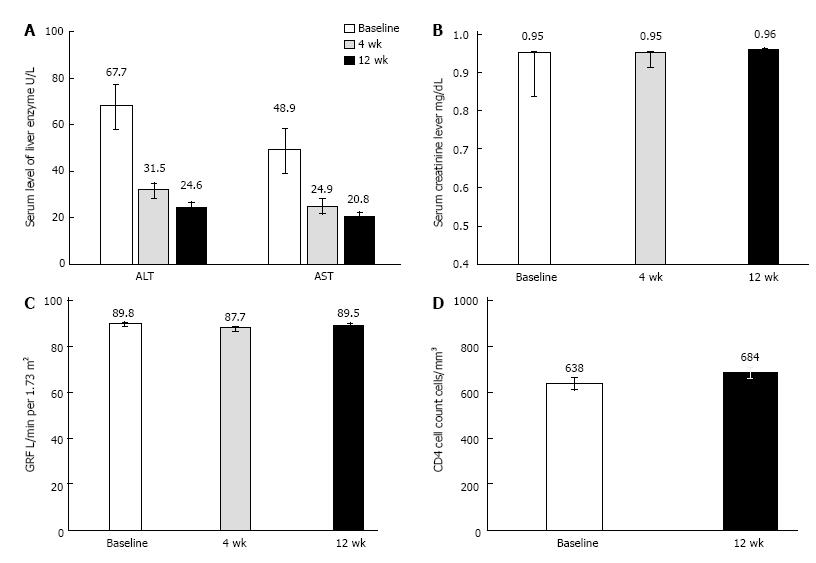
Levels of ALT and AST became normal rapidly with treatment (Figure 1A). There were no significant changes in estimated GFR or serum creatinine levels over time (Figure 1B and C. No participants were identified as having a treatment-emergent eGFR less than 50 mL/min or a decrease in eGFR (mL/min) greater than 25%.
The mean CD4+ cell count at baseline was 638 cells per microliter; the CD4+ count was under 200 cells per microliter in 1 patient and under 350 cells per microliter in 7 patients. There were no significant changes in CD4 cell counts with treatment (Figure 1D).
Two patients experienced HIV-1 virologic rebound. One had missed 2 wk ART (emtricitabine/rilpivirine/tenofovir DF) and the other had missed 5 d of ART (emtricitabine/tenofovir DF/raltegravir). They continued the same regimen and the HIV viral load was less than 20 copies/mL by the next visit (4 wk later). Moreover, 7 patients experienced HIV breakthrough, a transient increase in HIV viral load (HIV-1 RNA ≥ 40 copies/mL) while in the study. All of them denied non-compliance with ART. They continued the same regimen and the HIV viral load was less than 40 copies/mL 4 to 8 wk later. All of these 9 patients achieved SVR12 for HCV treatment.
There were no deaths or serious adverse events observed in this study. The most common adverse events were mild to moderate headache (12.5%), fatigue (10%), and diarrhea (2.5%). Symptoms resolved while the patient was receiving study drug.
Adherence to ledipasvir and sofosbuvir, as measured by pill counts, was high over the course of treatment. Ninety-five percent of all participants had no missed doses. Five percent of patients missed 1 to 4 doses of study drug, for an adherence rate greater than 95%. As determined by pill count at the end of study, the participant who experienced HCV viral relapse by week 12 after treatment completion reported no missed doses.
In this retrospective study, the combination of ledipasvir and sofosbuvir was associated with a high rate of SVR (97.5%) in HIV and HCV co-infected patients, comparable with SVR rates observed in the previous clinical trials[11-13]. Our study included HCV treatment-naïve (77.5%) and treatment-experienced (22.5%) patients, including patients with compensated cirrhosis (25%). Consistent with the previous reports[11,12], HCV treatment history, baseline HCV RNA levels, and cirrhosis didn’t appear to have any effect on SVR12 rates. All 9 treatment experienced patients and 30 of 31 treatment naive patients achieved SVR, regardless of HCV baseline levels. One patient with HCV 3a genotype was also allowed to enroll in the study and successfully achieved SVR12. In the recent ION 4 study, black patients with HCV and HIV co-infection were reported to have lower rates of SVR compared to non-black patients who received 12 wk treatment of ledipasvir/sofosbuvir[12]. However, no differences in efficacy were observed in patients when stratified by race in our study. Using data from the three open-label ION clinical trials, Wilder et al[14] evaluated the efficacy of ledipasvir/sofosbuvir in 308 black patients. Consistent with our result, they found that an once daily dosage of ledipasvir/sofosbuvir was similarly effective in black and non-black patients with genotype 1 HCV infection. In the 1 participant who experienced relapse, HCV sequencing data didn’t detect any mutation in the NS5A or NS5B region at the time of relapse and the patient was adherent to his medications, suggesting that the underlying mechanism contributing to the resistance to this regimen is unknown and needs future investigation.
All patients enrolled in this study were receiving an antiretroviral regimen for HIV-1 with evidence of HIV-1 viral suppression to a level less than 100 copies per milliliter. The main strength of this study was that the patients were on a wide range of antiretrovirals, including complex antiretroviral regimens that contained drugs from 3 or more antiretroviral classes. Drug-drug interactions between certain directly acting antiviral agents such as boceprevir, telaprevir, and antiretrovirals could result in adverse events or antiretroviral failures, restricting the wider use of these medications in patients with HIV[15,16]. Although Ledipasvir-sofosbuvir has limited potential for clinically significant drug interactions with most antiretroviral agents[17], the results from the phase 1[17] and 3[12] evaluations suggested potential drug interaction between ledipasvir/sofosbuvir and tenofovir resulting in increased exposure of tenofovir. Four patients developed treatment-emergent worsening of renal function which might be related to increased exposure of tenofovir[12]. In our study, evaluation of renal function didn’t reveal significant changes in GFRs and serum creatinine levels throughout this study and no patients taking tenofovir were required to modify HIV treatment due to tenofovir-induced complications. In the previous study, patients taking ritonavir-boosted HIV-1 protease inhibitors or cobicistat-boosted elvitegravir with tenofovir disoproxil fumarate were excluded, so the safety of this HCV combination in patients with HIV-1 infection who are receiving these antiretroviral regimens is unknown[12]. Interestingly, 11 patients enrolled in this study were on ritonavir-boosted or cobicistat-boosted ART and no severe adverse effects were noticed with SVR12 rates of 91.0% (the patient who experienced relapse was receiving the combination of darunavir-ritonavir-etravirine-raltegravir for ART). Thus, ledipasvir/sofosbuvir treatment represents a safe HCV treatment option that does not require modification for many of the common antiretroviral regimens.
In our study, ledipasvir/sofosbuvir treatment in HIV-HCV-coinfected patients did not compromise HIV control. CD4 cell counts remained stable and HIV RNA remained suppressed for the majority of participants throughout the study. Seven patients who were adherent to the medications experienced transient mild HIV viral breakthrough with maximum HIV RNA less than 250 copies. The viral breakthrough resolved spontaneously 4 to 8 wk after the patients were continuing the same ART regimen. HIV viral rebound documented in 2 participants was associated with nonadherence to antiretroviral treatment, which also resolved after resuming the same ART treatment.
In this study, there were no deaths, medication discontinuations, or severe adverse events attributable to ledipasvir/sofosbuvir treatment. Most adverse events associated with combined ledipasvir and sofosbuvir in participants co-infected with HCV and HIV were mild.
In conclusion, excellent treatment outcomes among our cohort of HIV/HCV co-infected patients were achieved with the FDA approved combination of ledipasvir/sofosbuvir for HCV. The main strength of this study was that a broad range of antiretrovirals were included in this study which demonstrated that ledipasvir/sofosbuvir was generally well tolerated when coadministered with a broad range of ART. Larger studies are required to further understand the efficacy and safety of the combination of ledipasvir/sofosbuvir in HIV/HCV co-infected patients.
Hepatitis C virus (HCV) coinfection has a harmful effect on the progression of human immunodeficiency (HIV) infection with increased risk of mortality. In recent years, the management of chronic hepatitis C has been revolutionized by the development of direct-acting antiviral agents (DAAs) which significantly improved rates of cure in chronic HCV infection.
The fixed dose combination of ledipasvir and sofosbuvir demonstrated high SVR rate in patients infected with HCV and recently became the first approved once daily Single-tablet-regimen for treatment of chronic HCV in HIV positive patients. Currently, there are few published data on the experience with this newly approved combination of ledipasvir/sofosbuvir in HCV/HIV co-infected patients. The drug interaction is always a safety concern while treating HCV/HIV co-infected patients.
The authors conducted a single-center, retrospective study evaluating the safety and efficacy of the combination of ledipasvir/sofosbuvir on HCV eradication in the patients co-infected with HCV and HIV with or without previous treatment for HCV. Overall, the rate of SVR12 was 97.5% and only 1 (2.5%) patient experienced relapse at week 12 following treatment. No severe adverse events were observed and no patient discontinued treatment because of adverse events.
The results demonstrated that this combination represents a safe and well tolerated HCV treatment option that does not require modification for many of the common HIV ART.
SVR: Sustained viral response. SVR is specific to hepatitis C and is the absence of HCV RNA for 12 wk after the cessation of treatment.
Clearly written and stylish manuscript. The approach is not very original (in the last years several papers regarding the efficacy of DDA in real life settings have been published) but since in this subgroup of patients there are few publications, it is still interesting.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Dirchwolf MM, Zheng SJ S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
| 1. | Lam BP, Jeffers T, Younoszai Z, Fazel Y, Younossi ZM. The changing landscape of hepatitis C virus therapy: focus on interferon-free treatment. Therap Adv Gastroenterol. 2015;8:298-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 2. | Puoti M, Panzeri C, Rossotti R, Baiguera C. Efficacy of sofosbuvir-based therapies in HIV/HCV infected patients and persons who inject drugs. Dig Liver Dis. 2014;46 Suppl 5:S206-S211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | McQuaid T, Savini C, Seyedkazemi S. Sofosbuvir, a Significant Paradigm Change in HCV Treatment. J Clin Transl Hepatol. 2015;3:27-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 4. | Puoti M, Rossotti R, Travi G, Panzeri C, Morreale M, Chiari E, Cocca G, Orso M, Moioli MC. Optimizing treatment in HIV/HCV coinfection. Dig Liver Dis. 2013;45 Suppl 5:S355-S362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Limketkai BN, Mehta SH, Sutcliffe CG, Higgins YM, Torbenson MS, Brinkley SC, Moore RD, Thomas DL, Sulkowski MS. Relationship of liver disease stage and antiviral therapy with liver-related events and death in adults coinfected with HIV/HCV. JAMA. 2012;308:370-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 159] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 6. | Berenguer J, Alvarez-Pellicer J, Martín PM, López-Aldeguer J, Von-Wichmann MA, Quereda C, Mallolas J, Sanz J, Tural C, Bellón JM. Sustained virological response to interferon plus ribavirin reduces liver-related complications and mortality in patients coinfected with human immunodeficiency virus and hepatitis C virus. Hepatology. 2009;50:407-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 238] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 7. | Berenguer J, Rodríguez E, Miralles P, Von Wichmann MA, López-Aldeguer J, Mallolas J, Galindo MJ, Van Den Eynde E, Téllez MJ, Quereda C. Sustained virological response to interferon plus ribavirin reduces non-liver-related mortality in patients coinfected with HIV and Hepatitis C virus. Clin Infect Dis. 2012;55:728-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 129] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 8. | Afdhal N, Reddy KR, Nelson DR, Lawitz E, Gordon SC, Schiff E, Nahass R, Ghalib R, Gitlin N, Herring R. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370:1483-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1065] [Cited by in RCA: 1064] [Article Influence: 96.7] [Reference Citation Analysis (0)] |
| 9. | Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, Romero-Gomez M, Zarski JP, Agarwal K, Buggisch P. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889-1898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1065] [Cited by in RCA: 1064] [Article Influence: 96.7] [Reference Citation Analysis (0)] |
| 10. | Kowdley KV, Gordon SC, Reddy KR, Rossaro L, Bernstein DE, Lawitz E, Shiffman ML, Schiff E, Ghalib R, Ryan M. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370:1879-1888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 911] [Cited by in RCA: 928] [Article Influence: 84.4] [Reference Citation Analysis (0)] |
| 11. | Osinusi A, Townsend K, Kohli A, Nelson A, Seamon C, Meissner EG, Bon D, Silk R, Gross C, Price A. Virologic response following combined ledipasvir and sofosbuvir administration in patients with HCV genotype 1 and HIV co-infection. JAMA. 2015;313:1232-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 172] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 12. | Naggie S, Cooper C, Saag M, Workowski K, Ruane P, Towner WJ, Marks K, Luetkemeyer A, Baden RP, Sax PE. Ledipasvir and Sofosbuvir for HCV in Patients Coinfected with HIV-1. N Engl J Med. 2015;373:705-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 379] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 13. | Tang LS, Masur J, Sims Z, Nelson A, Osinusi A, Kohli A, Kattakuzhy S, Polis M, Kottilil S. Safe and effective sofosbuvir-based therapy in patients with mental health disease on hepatitis C virus treatment. World J Hepatol. 2016;8:1318-1326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Wilder JM, Jeffers LJ, Ravendhran N, Shiffman ML, Poulos J, Sulkowski MS, Gitlin N, Workowski K, Zhu Y, Yang JC. Safety and efficacy of ledipasvir-sofosbuvir in black patients with hepatitis C virus infection: A retrospective analysis of phase 3 data. Hepatology. 2016;63:437-444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 15. | Sulkowski MS, Sherman KE, Dieterich DT, Bsharat M, Mahnke L, Rockstroh JK, Gharakhanian S, McCallister S, Henshaw J, Girard PM. Combination therapy with telaprevir for chronic hepatitis C virus genotype 1 infection in patients with HIV: a randomized trial. Ann Intern Med. 2013;159:86-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 16. | Sulkowski M, Pol S, Mallolas J, Fainboim H, Cooper C, Slim J, Rivero A, Mak C, Thompson S, Howe AY. Boceprevir versus placebo with pegylated interferon alfa-2b and ribavirin for treatment of hepatitis C virus genotype 1 in patients with HIV: a randomised, double-blind, controlled phase 2 trial. Lancet Infect Dis. 2013;13:597-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 154] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 17. | Harvoni (ledipasvir-sofosbuvir) tablets: U. S. prescribing information. Foster City, CA: Gilead Sciences; 2015; Available from: http://www.gilead.com/~/media/Files/pdfs/medicines/liver-disease/harvoni/harvoni_pi.pdf. |