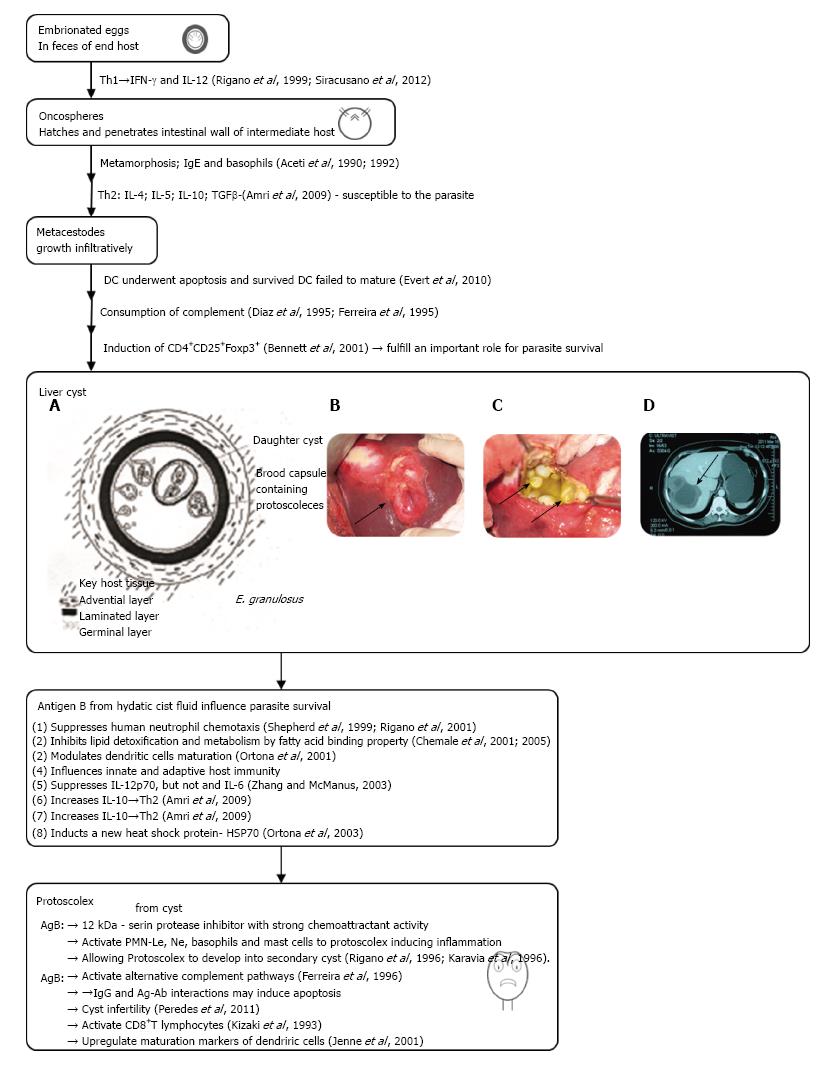
INTRODUCTION
The dog tapeworm Echinococcus granulosus (E. granulosus) is the main challenger of a global parasitic zoonosis cystic echinococcosis (CE) in humans caused by the larvae from the infected dog[1]. After incidental ingestion the larvae of parasite, the oncosphere/exacanth larvae is releasing from the keratinized embryophore in the stomach and intestine of the intermediate host (herbivores or humans). Embriophores in the intestine of man via hook movements penetrate into the small intestine of host. Its life cycle develops in dogs and other canids (wolves, foxes, coyotes, jakals) that harbor the adult tapeworm. The larval metacestode form develops in different organs of the intermediate host[2]. Hydatid cyst is mainly located in the liver (70%) or lungs (20%), but occasionally they may find their way to other organs (kidney 2%, spleen 2% and brain less than 2%)[3]. In the intermediate host the eggs cross intestinal wall and develop into larvae. The oncosphere is then carried out via portal vein flow into the liver and other organs. There oncospheres undergoes a metamorphosis towards the metacestodes. The metacestodes implant into the organ and grow into cysts, with all characteristic layers: Germinal, outer and laminated (Figure 1)[4,5]. Organs may also be reached through the lymphatic system[6]. Echinococcal cysts is surrounded by pericyst (adventia) from the periparasitic host tissue, which surround the larval endocyst (Figure 1A and B). The endocyst is also composed of a cuticular or laminal acellular outer layer and an inner germinal proligerous, which gives rise in a fertile cyst to root capsules and protoscoleces (PSCs)[7]. Some cysts may also harbor daughter cysts of variable sizes (arrows in Figure 1C). Cysts also contain developing PSCs, which constitute an infectious agent. PSC is developing into the adult tapeworm if will be ingested by a suitable definitive host (sheep, cattle, goat). Some vesicles adhere to the walls by means of a peduncle or remain free within the hydatid fluid. A large number of these vesicles (endogenous daughter vesicles) and free protoscoleces float in the hydatid fluid, together forming the co-called “hydatid sand”. New offspring vesicles in the hydatid fluid play the same role and have the same constitution of the vesicle mother. The hydatic liquid is clean and clear. It contain all secreted molecules from parasite and host, which is very similar even identical to that of the host’s serum containing Na, K, Cl, and CO2), a density between 1.008 and 1.015, and alkaline pH[8]. Thus, in this way, protoscoleces may develop into either a new cyst or in adult parasite. As the cyst later becomes a successful xenograft in the host, it progressively enlarges until symptoms or complications appear[9,10]. Therefore, the clinical manifestation of infected humans with E. granulosus, could be expressed from asymptomatic infection to severe, potentially fatal disease. The parasite die due to dysfunction of germinal membrane (detached, aging or micrortaumatisms) but the scolex may transform into vesicle trying to preserve the species[11].
Figure 1 Diagrammatic representation of the development of the Echinococcus granulosus liver cyst.
A, B: Liver cystic echinococcosis, our surgical material; C: Computed tomography imaging of hepatic cystic echinococcosis; D: Abdominal scan of a female patient with a CE3b cyst in the VI liver segment (adapted from Ref. [120]). IL: Interleukin; IFN: Interferon; TGF: Transforming growth factor; PSC: Protoscolece; PMN: Polymorphonuclear.
The survival of Echinococcus within the host tissues, despite the development of specific antibodies (Abs), is possibly the result of specific immunomodulation induced by the parasite[12]. This phenomenon has been the subject of study by many researchers during the last two decades. They aimed to investigate the host responses to the parasite. The final goal of the present study was to review these modifications of the immune and autoimmune responses induced by E. granulosus. For better understanding of host-parasite interactions in this review human clinical study used complementary to animal studies. However, although some of the mutual interactions between parasite and human host in infection have been resolved, essence of protective mechanisms of human host are still unclear.
IMMUNE RESPONSE TO E. GRANULOSUS INFECTION OF THE HOST
Effects on innate immunity
Almost exclusively within the intermediate host’s liver, parasitic metacestode vesicles grow infiltratively, very similar to that of malignant tumor. The host immune system reacts to these formations. But there is no data of granulosis-induced immune suppression in echinococcosis on the molecular and cellular level, particularly in the early stages of the infection. On the other hand, there is no doubt that the defensive immune reaction is missing and parasite survive. Future survival of parasites indicates that they have developed some mechanisms of evasion from host protective immune mechanisms to preserve their expansion[13]. Many studies in humans and mice showed that after parasite infections at the beginning dominates T helper 1 (Th1) immune responses (Figure 2). Th1 immune responses is characterized by the release of interferon-γ (IFN-γ) and after priming by dendritic cells (DCs) with IL-12[11,14]. Both are effective in the elimination of the parasite at an early stage. However, it has become clear that the parasite, probably by its excretory/secretory products, actively influences the host immune response, leading it to the Th2 response and parasite survival. Namely, the Th2 cytokine profile of IL-4, IL-5, immunosuppressive IL-10 and transforming growth factor beta (TGF-β) are generally associated with receptive capability of the parasite that leading to progressive disease (https://http://www.ncbi.nlm.nih.gov/pmc/articles/pmc3283565/ - pntd.0001516-Vuitton1)[15]. On the other hand, the polymorphonuclear (PMN) leukocyte, basophil-mast cell and monocyte participation showed intense local inflammatory reaction to protoscoleces (PSCs)[16]. Significant increases in the chemiluminescence response, superoxide (O2) production and phagocyte index have been detected in patients with dead cysts compared with healthy subjects, whereas a marked reduction in all the above markers was observed in patients with liver cysts[17]. Functionally and metabolically the PMN leukocytes of infected patients are in an activated state[18]. Regarding basophils, the human basophil degranulation test was found to be positive in 33% of patients with hydatid disease (HD)[19]. Furthermore, evidence of increased histamine release from hydatid patient basophils following a challenge with anti-human IgE has also been obtained[20]. It can be concluded that both the generation of histamine releasing factor (HiRF) and production of IgE, which can bind cytokines, may be involved in this stage of infection[21]. This histamine releasing factor was found to activate basophils through surface-bound IgE, cytokine production and Th2 cell activation[16,21-23]. Method of antibody-dependent cell-mediated cytotoxicity is well established as an important mechanism by which the host can damage a multicellular parasite, but why it is not happening?
Figure 2 Subversive strategies of Echinococcus granulosus.
DC: Dendritic cell; IL: Interleukin; IFN: Interferon; TGF: Transforming growth factor.
Additionally, it is well known that the surface of many parasitic helminths, including E. granulosus, is able to activate the alternative pathway of the complement system[24,25]. Although the complement can lyse protoscoleces of E. granulosus, parasite with some secretion products could be able to consume the complement, which is an ability that has been proposed as the basis of an invasion mechanism by the parasite[25]. However, the levels of component 3 of complement and chemolitic complement in mice showed no evidence of complement consumption[25]. Moreover, C3 levels were significantly increased in patients with hydatid disease compared to controls[16,26]. Thus, it is possible that local consumption at the site of infection may exist, leading to systemic consumption in the more active cysts. Finally, the existence of several mechanisms of complement modulation was found when comparing complement activation in vitro by different E. granulosus extracts[27]. These findings further enhanced the possibility of their significant role in the susceptibility of infection and/or maintenance of the disease.
The parasite must be able to adapt metabolically to the host microenvironment, and antigen B (AgB) could be involved in this process. The termostabile AgB (166 kDa) resists boiling for 15 min without losing antigenicity. Thus, AgB proteins as highly immunogenic acts directly to innate and adaptive immunity. Additionally, many antigen B (AgB) molecules in the hydatid cyst fluid possibly guarantee parasite survival. The gene family of AgB comprising at least 10 unique genes in five subclasses differentialy expressed in its life cycle, except EgAgB3 which is expressing predominantly in all cell stages[28,29]. Because of their fatty acid binding property, some of them are involved in lipid detoxification, transport and metabolism[30,31]. Also it is well known that its 12 kDa unit is able to inhibit human neutrophil chemotaxis[32,33]. This unit is a serine protease inhibitor with strong chemoattractant activity. This is a reason for released protoscoleces to develop into secondary cysts[10]. Co-incubated with Echinococcus primary cells, AgB functioning similar to invading oncosphere or metacestode vesicles. In these conditions, some dendritic cells (DCs) dead, and the survived fail to mature[34]. But, DCs exposed to protoscoleces up-regulated maturation markers and stay functional. Pre-incubation with primary cells and metacestode vesicles, impaired ability of DCs to be activated by the Tall-like receptor ligand LPS. This was not observed in those pre-treated with protoscolex excretory/secretory products[35]. The induction of CD4+CD25+Foxp3+ T cells to metacestode E/S-products suggests that these cells play important role for parasite survival. The immunomodulatory products from parasites are therefore of high interest for understanding by infections induced immune pathology and treatment of allergy.
E. GRANULOSUS EVASION MECHANISMS IN THE HOST
Characterization of molecules involved in evasion
In intermediate hosts, protoscoleces develop exclusively in fertile cysts. This formation also consists all of three membranes (inner cellular, other glicin rich and laminated acellular)[36]. Nevertheles, E. granulosus cystic form can induce IgG that is able to cross the tegument and plasma membranes between laminar and germinal layers of the cyst. On the other side, method of antibody-dependent cell-mediated cytotoxicity is well established as an important mechanism by which the host can damage a multicellular parasite. There IgG recognize specific cystic antigens, and antigen-antibody complex may inhibit proliferative process of protoscoleces, but why it is not happening? Due to germinal layer of the cyst is a barrier for immune competent cells of the host[36]. Except this the parasite evolving other immune evasion strategies[37]. E. granulosus using two main mechanisms to undermine the host immune response: First is passive escape, in which during the development of hydatid cyst, parasites avoids the damaging effects of host immune reaction; and second is immunomodulation, in which the parasite is actively included in the immune reaction to trigger the host’s profit[38,39].
Circulating antibodies as immunological markers in CE
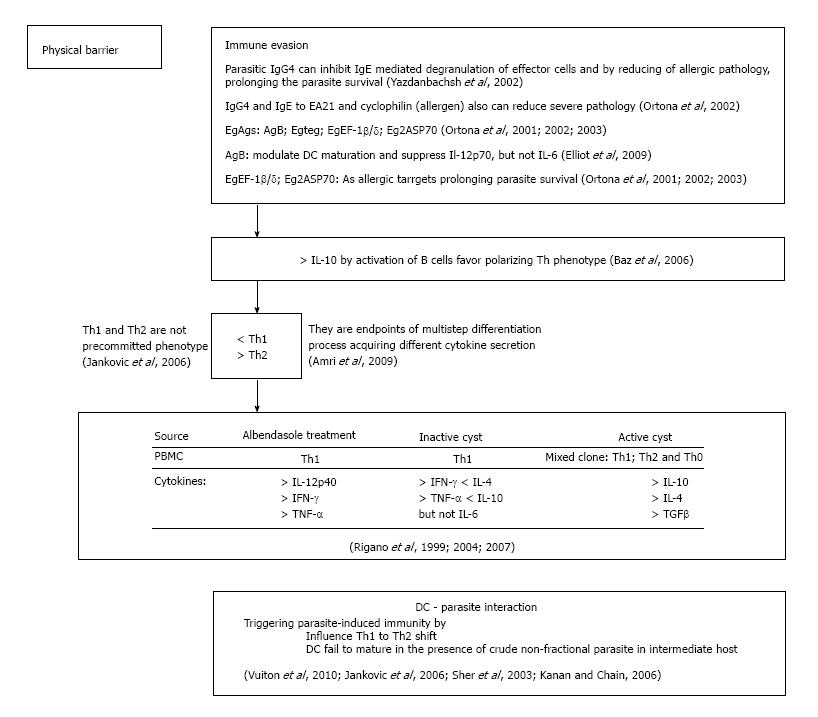
Although patients with hydatidosis releasing amount of circulating IgG, IgM, IgA and IgE antibodies (Abs) to E. granulosus, no one is associated with the host protection[40]. IgG4 in echinococcosis is not able to complement fixation neither is cytophilic, also weekly binding to Fc fragment of immunoglobulins, then is not functional. All of these will support the parasite evasion of host immune response[41]. Even, parasite-specific IgG4 antibodies by inhibiting IgE mediated degranulation of effector cells, reducing allergic pathology in the host to prolong parasite survival[42]. In agreement with this study are findings that significantly lower levels of serum IgG4 antibodies is detected in albendazole-treated patients who exhibited a good therapeutic and clinical response compared to that in poor responders or non-responders. Additionally, data obtained in various countries showed reverse trend of IgG1 levels[40,42]. Beside that patients showing differences in IgG1 expression. Those without allergic manifestations releasing IgG4 antibodies specific to EA21, whereas patients with allergic manifestations showed IgE specific to the same antigen. Authors have suggested that CE IgG4 obviously work to block pathogenic processes and to, minimize severe pathology in the host providing parasite survival[42] (Figure 2).
Antigen B and other new antigens in immunomodulation
In CE characterized with Th2 polarized microenvironment, besides AgB, EgTeg and EgEF-1 β/δ, several other parasite molecules can elicit this phenotype. More sophisticated approach such as proteomic pronounce the presence of a large number of antigenic proteins associated with parasites. Antigen B (AgB) modulates DC maturation and suppresses IL-12p70, but not IL-6 release[43]. As allergic targets in CE in acute cutaneous allergic manifestation, three conserved constitutive proteins at a molecular level have been identified: EgEF-1 β/δ, EA21 and Eg2ASP70[44-47]. At least two of three appear to have immune modulatory properties. EgEF-1 β/δ influences immune modulation and is released after the death or degeneration of protoscoleces[45]. Furthermore, appearance of CD4+CD25+Foxp3+ T cells in excretory/secretory products of metacestode suggests that these cells play important role in survival of parasite in chronic echinococcosis[35,48]. The secretory and excretory products of parasite helminthes are therefore extremely important for better understanding of immunopathology of parasite infection and for allergy treatment as well. Future immunological studies will give us opportunity to investigate their role and immunomodulatory effects on parasite infections in humans. However, the long- time (years) development of CE highlights the difficulty in understanding the host-parasite relationship[49,50]. More investigations are necessary for integration of these studies with previous obtained results to recognize and understand well the extreme complexity of the host-parasite interactions. These findings are extremely important for the development and improvement of CE diagnosis and treatment. Finally, it is indispensable for control strategies and vaccine development.
Immunomodulation by cytokine production
Plasticity of both the nature and magnitude of immune host responses depend on infective agents that permit the immune system to tailor its defense strategy. Th1 and Th2 cells are not pre-committed cells with defined phenotype, their phenotype is result of a multistep differentiation process, thus a precursor population acquires secretion of different cytokines profiles[47]. How E. granulosus antigens (Ags) encountering the human immune system can influence the differentiation decision in human echinococcosis? First of all it is well known that the immune response established in E. granulosus infection is mainly of Th2-phenotype. Also E. granulosus antigens modulate polarized T-cells (Figure 2). Furthermore, increased level of IgG4 and IgE antibodies and induced eosinophilia supporting assertion that the immune response established in E. granulosus infection is Th2-dominated. Findings from experimental echinococcosis confirm the hypothesis that early IL-10, secreted by B cells in response to mitogens, may favor parasitic survival by established type-2 cytokine response[50]. There are many evidence of molecular studies where reciprocity of IL-4/IL-10 is that impairs the Th1 protective response allowing the parasite survival in human host[51]. In addition, patients responsive to albendazole in peripheral blood monocyte cells (PBMCs) showed high amounts of IFN-γ (Th1-derived), whereas PBMCs from patients who did not respond to albendazol therapy produced a higher level of IL-4 and IL-10 (Th2 derived)[14]. These findings are in coordination with a molecular studies that detected IL-12p40 mRNA in 86% of successfully albendazole-treated patients at the end of chemotherapy who expressed a high level of IFN-γ and TNF-α DNA[15,52]. Finally, patient with an inactive cyst expressing Th1 phenotype, while patients with active and transitional cysts showed mixed Th1/Th2 and Th0 phenotype[53]. No IL-5 and scarce IL-4 and IL-10 is detected in seronegative patients[54]. Seronegativity occurs due to the host or parasite factors or both preclude the possibility of Th2 cell activation that limiting the production of IL-5, crucial for immunoglobulin expression.
It has been shown recently that bone marrow-derived dendritic cells (DCs) from non-lymphoid tissues show capability for antigen presentation and antigen processing[55]. Also there are findings that inflammatory mediators or microbial agents promote the migration of DCs into the lymph nodes and other secondary lymphoid organs. By maturation, DCs lose their ability for antigen presentation and gain an increased capability to prime T-cells[56]. Thus, it is no doubt that DC-parasite interactions are very important for triggering and regulation of parasite-induced immunity. On the other side, E. granulosus cystic fluid modulates differentiation and cytokine secretion of dendritic cells[57]. Finally, these cellular findings established that E. granulosus except for modulation of DC maturation is included in the polarization of T lymphocytes toward Th2 phenotype[58,59].
ADAPTIVE IMMUNITY
Role of dendritic cells in parasite evasion
Dendritic cells (DCs) as an antigen presenting cells, no doubt represent a link between innate and adaptive immune systems. They are inducing immune responses with Th1, Th2 or Th17-dominated phenotype (https://http://www.ncbi.nlm.nih.gov/pmc/articles/pmc3283565/ - pntd. 0001516-Everts1)[60]. After contact with parasite, DCs take up the recognized antigens and undergo maturation in the presence of up regulated MHC/HLA mice/man) complex and co-stimulatory molecules CD86 and CD80[34,60]. Nevertheless, after migration in the lymph nodes, DCs interact with naïve T cells to promote adaptive immune responses with Th1, Th2, and Th17 cell phenotype (https://http://www.ncbi.nlm.nih.gov/pmc/articles/pmc3283565/ - pntd.0001516-Banchereau1)[61,62]. There by activation of T regulatory cells, DCs becoming targets for parasite evasion. Mejri et al[63] showed that peritoneal DCs from chronically infected mice, representing the late stage of alveolar echinococcosis. The same authors have reported that DCs in infected mice specifically modulates CD4+ and CD8+ T cell responses, suggesting their immunosuppressive T regulatory function in echinococcosis[63]. In intermediate host parasitic larvae migrate from the intestinal entry site to the liver and late metastasis to other organs (lung, kidney, spleen or brain)[64]. This finding suggest that parasitic larvae encounter DCs in vivo[64]. Despite the general importance of DCs in cellular host-parasite interaction, immunomodulatory molecules that are released by Echinococcus larvae and have an influence on DC function, are still not characterized. Compared to other helminthic infections, immunomodulatory functions of DCs in E. granulosus infection in human host provided less attention, although this is an emerging and important field[65]. In two reports, Reyes et al[66] and Terrazas et al[67] investigated the effects of excretory/secretory products of Taenia crassiceps cysticerci on the activation of murine DCs, representing the metacestode larval stage of by Taenia infection. DC of susceptible mouse strain when preincubated with parasite excretory/secretory product, authors showed decreased DC maturation because of impaired susceptibility to TLR-dependent stimulus[66,67]. Whether these interactions are relevant in vivo is not clear. First of all due to the spectrum of protein products from metacestode excretion and secretion does not necessary overlap the spectrum of proteins in the hydatid cyst fluid[68]. For example, AgB is not detected in excreted or secreted products in vitro cultivated metacestode vesicles, but this component is well expressed in hydatic cystic fluid[69]. Second, the intact parasite tissue is showed usually prevents direct contact between hydatid cystic fluid and host immune effector cells. Dendritic cells react with unfractionated helminthic proteins generating anti-parasitic cytotoxic T lymphocyte[70]. Thus, crude methods of in vitro preparation of metacestode antigen, insufficient purified which contain vesicle fluid somatic parasite proteins and contaminating host components, tested concerning their effects on DCs, failed to induce maturation as did a purified mucin-type glycoprotein (Em2) that is usually expressing at the surface of LL-containing metacestode vesicles (https://http://www.ncbi.nlm.nih.gov/pmc/articles/pmc3283565/ - pntd.0001516-Hlsmeier1)[71,72]. However, it is well known that extrinsically triggered infectious with viruses, bacteria and parasites, usually results in a bystander effect of induced immunosuppression[73].
Bystander effects of parasite-induced immunosuppression
Dendritic cell apoptosis induction has been reported in nematodes in which their capacity for releasing of pro-inflammatory IL-12 is limited and that prevents activation and proliferation of T cells[74]. Different from others investigator accept the possibility that the diminished function of DCs in metacestode infection is by induction of apoptosis of immature cells rather than due to inhibition of its maturation. It could be a reason for establishing of an immunosuppressive environment around the parasite lesions. Transforming growth factor beta (TBF-β) signaling is involved very early in this process because in animal evolution they are expressed very early in all invertebrate. Therefore, it is understandable that diminished ability of DCs pre-incubated with excretory/secretory-products of primary cells in metacestode is indirectly mediated by the induction of apoptosis of immature DCs, rather than by direct inhibition of DC maturation. Imature DCs secrete TGF-β, which induces differentiation of naïve T cells into FOXP3+ T-regulatory cells, and subsequently immunosuppression around the parasite lesion[73]. TGF-β signaling is involved very early in animal evolution, thus TGF like cytokines are expressed very early in many invertebrate, and in parasites as well[75,76].
Different from DCs incubated with primary cells of metacestode vesicles, those exposed to E/S products of protoscoleces showed up-regulation of surface markers MHC-II and CD86, increased secretion of IL-6, but not IL-10 and impaired ability of DCs to produce IL-12 by tall-like receptor lipopolisacharides (LPS) stimulated[74].
Presented phenotypes are similar to that obtained when DCs are incubated with E. granulosus hydatid cyst fluid (HCF), and with isolated AgB[55,59].
In contrast to these investigations, DCs incubated with protoscolex compounds as presented in their study Rigano et al[55], 2007 and Kanan and Chain[59], 2006, dendritic cells did not release elevated levels of IL-10 or IL-12 (https://http://www.ncbi.nlm.nih.gov/pmc/articles/pmc3283565/ - pntd. 0001516-Kanan1). Presented phenotypes are similar to the obtained when DCs are incubated with E. granulossus hydatid cyst fluid (HCF) and with its isolated AgB. It seems that only protoscoleces weakly expressing AgB[30,77]. Finally, DCs phenotype upon co-incubation with E/S-products of protoscoleces is largely comparable with those incubated with certain Trypanosoma antigens, closely associated with the induction of Th2 immune response[78]. The differences obtained between the responses of DCs to E/S-products of early versus late developmental stages of E. multilocularis clearly demonstrates that an induction of tolerance of DCs is not a general characteristic of Echinococcus material. It is the results of excretory and secretory repertoire of primary cells. Metacestodes are specifically evolved to carry out these purposes[79]. The interpretation of obtained results concerning the immune response in echinococcosis by using of in vitro co-incubation-systems of Echinococcus protoscoleces with host cells[80-86] or in the mouse model of peritoneal, protoscoleces, should be presented very careful, because of not to provoke suspicious of their verification[87]. The oncosphere that undergo as metamorphosis toward metacestode could induce impaired response of IL-10 secreting DCs in vitro[87]. These findings suggests that similar mechanisms might also further investigation by methods of primary cells could resolve the nature of echinococcal products responsible for these effects. But the effects at the begining of infection with E. granulosus and that in chronic phases have not the same nature. They are late reduced, and leading to disappearence of FOXP3+-T-reg from microenvironment and decreased number of immature DCs in protoscolex stage. Beside that the results are concentrated about in vitro interactions between parasite larvaes and DCs in response to primary cells. No doubt that similar mechanisms operate in surrounding tissue in response to primary cells. This process might be important for early establishment of the parasite due to higher vulnerability of the host immune system. Later, after production of the laminar layer (LL) and activation of Treg cells, a slightly altered profile of excreted/secreted products could support long-term persistence and growth of the metacestode (https://http://www.ncbi.nlm.nih.gov/pmc/articles/pmc3283565/ - pntd.0001516-Mejri1)[88]. Future investigations by using of genome sequence information[5,89], and other genetic manipulation of primary cells[90], will give us opportunity for better understanding of the molecular mechanisms of parasite-host interactions.
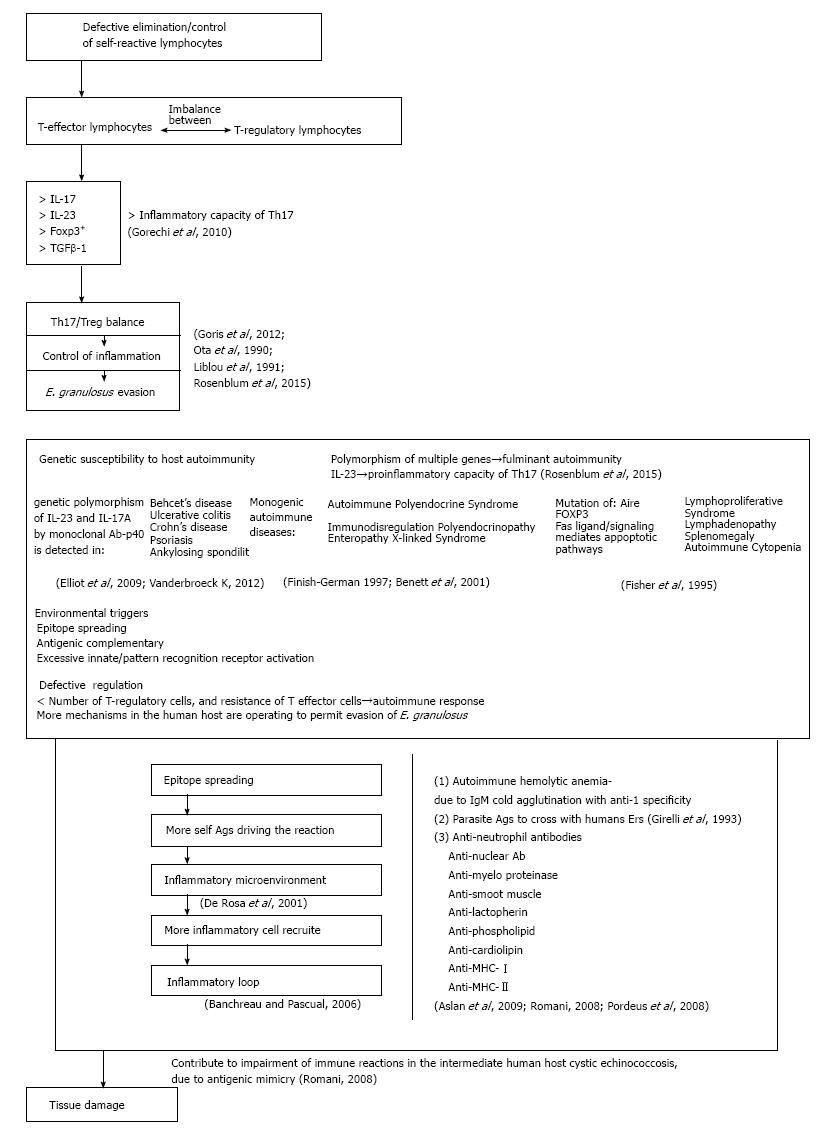
CE AND AUTOIMMUNITY
Genetic predisposition in combination to others environmental factors have a decisive role in the induction of autoimmune reactions to E. granulosus and others parasitic infections. Genetically predisposed person with defective regulation of immune functions could be more receptive for autoimmune follow-up after parasite infections. A combination of autoimmunity environmental triggers and genetic factors can lead to immune imbalance and could influence the appearance of autoimmune diseases (Figure 3). CE and others parasitic disorders at their source will request the answer on question, why in some persons abnormal immune reactions arise more extensively than in others infected with the same parasite? To answer this question understanding of intrinsic mechanisms responsible for immune suppression need to be resolved. The well-known part of this mechanism is uncontrolled synthesis and elimination of self-reactive lymphocytes. Patients with CE showed increased number of T regulatory (Treg) cells, related cytokines such as IL-17 and IL-23, transcription factor FOXP3 and TGFβ-1 level compared to control healthy subjects[91]. The Th17/Treg balance controls inflammation and thus may play an important role in the pathogenesis of immune evasion[91]. To estimate damage of this disbalance, Rosenblum et al[92] (2015) detected Th17/Treg functions at different levels of immune reaction by analyzing the cell frequencies, related cytokine secretions and key transcription factors in CE (Figure 3). Findings showed that a Th17/Treg functional disbalance in patients with chronic CE, suggesting a potential role for a Th17/Treg imbalance in the pathogenesis and immune evasion of E. granulosus. Regarding to genes associated with autoimmune diseases, the strongest associations with HLA alleles were detected[93]. But, there are no data how different HLA alleles facilitate any autoimmune disease. Bearing in mind that many HLA alleles are capable to present self-antigens in healthy and in infected subjects, it is not clear how different HLA alleles influences autoimmune diseases. It is unlikely that a disease-associated allele is especially efficient at displaying the autoantigens targeted by self-reactive T cells due to most HLA alleles are capable of presenting self-antigens also in healthy subjects. Yet, most healthy individuals have autoreactive T cells that escape thymic deletion[94,95].
Figure 3 Echinococcus granulosus Induced autoimmunity.
Ags: Antigens.
Genetic susceptibility to host autoimmunity
Knowledge of genes that are involved in induction of autoimmune diseases are discouraging, and is in lower level than those for HLA alleles. Completely different, genetic polymorphism of cytokine and cytokine receptors are well examined. Findings indicate that cytokines have been linked to many different autoimmune diseases, and IL-23 and IL-23R augmenting inflammatory capability of Th17 cells[94,95]. In that way, IL-23R have been discovered in ankylosing spondylitis, Behcet’s disease, Crohn’s disease, psoriasis, and ulcerative colitis[96]. Moreover, in all of these diseases IL-17 positive inflammatory cells have also been associated with tissue damage in all mentioned disorders. By using of monoclonal antibodies specific for either p40 (a subunit of IL-23) or IL-17A their efficacy were confirmed in almost all of these disorders[43,97]. Thus, genetic polymorphisms in IL-23R have in some cases been correlated with responses to targeted anti-cytokine therapies. Nevertheless the development of many human autoimmune diseases is result of reaction of multiple genes involved. There is opinion that gene polymorphism is responsible for the most human autoimmune diseases. Only a few examples existing in which genetic alterations in a single gene result in severe autoimmune syndroms. The two best examined monogenetic autoimmune syndroms resulting from the mutations in AIRE and FOXP3 genes are autoimmune polyendocrine syndrome (APS) and immunodysregulation polyendocrinopathy enteropathy X-linked (IPEX) syndrome[98,99]. These mutations leading to dysfunction in central (APS) and peripheral (IPEX) tolerance. Another example of autoimmune lymphoproliferative disfunction of Fas gene, Fas ligand or of in caspases downstream of Fas signaling, resulting in a defective Fas-mediated apoptotosis and chronic lymphoproliferative causing lymphadenopathy, splenomegaly, and autoimmune cytopenias[100]. Discovering of the single genes responsible for development of aforementioned autoimmune disorders has greatly contribution in our understanding of cellular and subcellular machanisms responsible for the development and follow-up of many by parasite induced autoimmune diseases.
Environmental factors in host autoimmunity
Environmental triggers are factors originating outside of the body, such as parasites, bacteria, viruses, toxins and medications. No doubt that infections could be important triggering factor for autoimmune disfunctions[101,102]. Many theories have been created to explain this connection and excessive innate/pattern recognition receptor activation in autoimmunity. Epitope spreading and antigenic complementary are few between many theories proposed. There is evidence that the presence of Epstein bar virus in postmortem brain tissue has been linked with appearance of Multiple sclerosis (MS), but not with other autoimmune inflammatory diseases[103]. Furthermore, peridontal infections and rheumatoid arthritis are also linked with autoimmunity induced by infection[104]. In contrary, ideas existing that infection could protect from some autoimmune disorders. Thus germ-free mice exposed to Bacteroides fragilis could be protected from development of experimental autoimmune encephalomyelitis (EAE), by induction of Treg cells[105]. In that way, a higher incidence of MS and type 1 diabetes in developed countries is supposed to correlate with decreased number of infections[106].
Defective regulation as the cause of autoimmunity
The peripheral tolerance to tissue antigens could be induced by the low-level of natural cell death through the tolerance of dendritic cell populations[107]. This tolerance of can influence low level of natural cell death in the tissue antigens, respectively[107]. If tolerance is the main abnormality in autoimmune processes, which kind of tolerance is processed in induction of autoimmune diseases? In SLE it is maturation of naïve B cells that can produce autoantibodies even before encountering with antigens. Findings that defects in early B cell tolerance checkpoints possible contribute development of an autoimmune disease[108]. Beside others, deletion cross influences B cell maturation of immature B cells in the bone marrow, receptor editing, and the control of mature B cells in peripheral tissues[108].
Regarding T cell-dependent autoimmunity and inflammatory autoimmune diseases, imbalance between effector and regulatory T cells play a fundamental role in initiation of human autoimmune diseases[109].
Decreased number of functional T regulatory or resistance of effector T cells play decisive role in the initiation of autoimmune diseases in human. Obtained results from patients with autoimmune disorders are often variable and inconsistent, due to the limited assesibility of tissue for examination. Even when an optimal number of cells for analysis is supplied, in vitro assays that often cannot be recapitulated, is not reliable for examination of functional capacity in vivo. The disease in autoimmunity might be explaned by self-perpetuating ability of autoimmune reactions. The self-antigen that drive autoimmune reaction remain functional and cannot be eliminated from the organism due to the emergency of a new antigenic epitopes in damaged tissue and alterations of self-proteins. This process is known as antigen epitope spreading phenomenon, and a vicious cyrcle is set-up.
Longitudinal studies of effector and Treg cells that are specific for target self-antigens in human disease remain a considerable technical challenge. Newly created antigenic epitopes activate more different lymphocytes in autoimmune reaction, leading to more tissue damage, and more novel epitopes to autoreactive lymphocytes. In that way, potentiated autoimmune reaction creates a new convenient environment for activation of multiple immune cells, cytokines and other mediators that amplify autoimmune reaction with catastrophic results to patient. In that way IFN-I production may induce SLE. Then inflammation in SLE showed is involved in the propagation of the disease[110]. The prolonged survival of E. granulosus metacestodes within the human host indicates that some mechanisms are operating to permit evasion of the host immune response. Several authors tried to describe autoimmune phenomena in patients with CE. Autoimmune hemolytic anemia due to IgM cold agglutinin with anti-I specificity was found to be induced in patients with CE. Moreover, the cleavage fragment of C3 has been detected on the erythrocyte membrane of a number of CE patients, suggesting that parasitic antigens may evoke antibodies that cross with human erythrocytes[111]. Furthermore, anti-neutrophil cytoplasmic, anti-myeloperoxidase and anti-lactoferrin antibodies have also been revealed in the sera of CE patients[112]. However, no significant correlations have been observed between CE and anti-nuclear antibodies, tissue specific autoantibodies and rheumatoid factors. In contrast, Aslan and coworkers have measured significant levels of antinuclear antibodies, anti-mitochondrial and anti-smooth muscle antibodies in patients with CE in comparison to age- and sex-matched healthy individuals[113]. Antiphospholipid antibodies, anti-cardiolipin antibodies and anti-dsDNA have also been shown to be associated with several infectious diseases and some autoimmune diseases such as systemic lupus erythematosus and anti-phospholipid syndrome. Such elevated levels of these antibodies could be explained by the antigenic mimicry between the parasite antigens and host proteins[114]. Since cardiolipin and phospholipids are abundant in most cells of multicellular organisms, the former is an important component of the inner mitochondrial membrane, where it constitutes approximately 20% of the total lipid composition, while phospholipids are a class of lipids and a major component of all cell membranes as they can form a lipid bilayer[115,116]. Moreover, autoantibodies class I and class II MHC gene products have also been demonstrated in CE patients, which may contribute to impairment of the host immune responses. Chronic and multiple infections with viruses, such as Epstein-Barr virus, cytomegalovirus and bacteria, such as H. pylori, may also be involved in the devolvement of an autoimmune disease in susceptible individuals[117].
Finaly, the parasites survive well in the human host and the host attempting to destroy them[118]. From a promotional perspective, knowledge of immune events to response on infection with a helminth parasite could be used to reduce the intensity of undesirable inflammatory reactions. But, poorly characterized cestode extracts cannot help the regulation of human immunocyte function. Yet the impact of these for treatment of autoimmune or allergic diseases is poorly understood. No doubt that helminth parasites are masters for immune evasion and regulation. A likely prerequisite for long-term survival is outwit of their host’s attempt to eradicate them[119].
CONCLUSION
E. granulosus is very complex multicellular parasite. As many pathogens is highly immunogenic for human host. Thus, the host immunity play a most important role in host-parasite relationship in human ehinococcosis. The secretory and excretory products from parasite influences immune and immune competent cells in human host and stimulate humoral and proinflammatory cell-mediated immune responses, releasing of significant antibody production, and activate T cells and other antigen-presenting cells in human host. Thus, the understanding of the immune mechanisms is of fundamental importance for revealing of a basic protective processes in human with hydatidosis. No doubt that protective antibodies are also extremely important for development of a new more effective vaccines against E. granulosus and other parasites. Knowledge of immune events as a response to infection with a helminth parasite could be used to reduce the intensity of undesired immune and autoimmune reactions such as a variety of auto-inflammatory diseases and allergy. Relevant findings is accumulating showing that inflammatory reactions that promote a variety of auto-inflammatory disease are dampened as a consequence of infection with helminth parasites via either the mobilization of anti-worm spectrum of immune reactions or direct effects of bioactive immunomodulatory molecules and chemical compounds released from the parasite. Also the cestode extracts are poorly characterized and their impact on autoimmune and allergic diseases are not fully examinated due to the mechanisms of reaction are not understood. Yet issues related to this topics regarding purification of immunomodulatory molecules, their site effects and action to parasites remains as challenges that need to be addressed.