Published online Jan 28, 2017. doi: 10.4254/wjh.v9.i3.155
Peer-review started: September 14, 2016
First decision: November 14, 2016
Revised: November 28, 2016
Accepted: December 13, 2016
Article in press: December 14, 2016
Published online: January 28, 2017
Processing time: 132 Days and 23 Hours
Mucosa-associated lymphoid tissue (MALT) lymphoma of the liver is a very rare condition and thus the diagnosis may be challenging. The clinical presentation is usually variable, ranging from minimal clinical symptoms to severe end stage liver disease. In this paper, we describe the clinicopathologic findings in two cases of primary hepatic MALT lymphoma. One case is an 80-year-old female with no underlying chronic liver disease and the second case is a 30-year-old female with autoimmune hepatitis complicated by MALT lymphoma. In both specimens, there was diffuse infiltration of atypical B-lymphocytes that were positive for CD20 and CD79a, but negative for CD5, CD43 and CD10. There were occasional lymphoepithelial lesions involving the hepatocytes or bile ducts. Polymerase chain reaction analysis showed monoclonal immunoglobulin heavy chain gene rearrangement in both cases. The first case was treated with surgery but developed pulmonary recurrence a year after complete resection but went into remission following treatment with rituximab. A second recurrence occurred in the right parotid gland 7 years later, which was treated with idelalisib. The second case was effectively treated with rituximab. To our knowledge, the second case is the first reported case linked to autoimmune hepatitis.
Core tip: The diagnosis and management of mucosa-associated lymphoid tissue lymphoma of the liver can be a clinical dilemma. Recognition of the clinic-pathologic pattern and its associations with underlying autoimmune disease can prevent misdiagnosis. This case report not only represents the first reported association with autoimmune hepatitis and the development of multiple recurrences of the lymphoma in the literature, but also it applies new successful treatment regimens as an alternative to current clinical practice.
- Citation: Obiorah IE, Johnson L, Ozdemirli M. Primary mucosa-associated lymphoid tissue lymphoma of the liver: A report of two cases and review of the literature. World J Hepatol 2017; 9(3): 155-160
- URL: https://www.wjgnet.com/1948-5182/full/v9/i3/155.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i3.155
Mucosa-associated lymphoid tissue (MALT) lymphoma, is a distinct subgroup of non-Hodgkin’s lymphoma (NHL) that accounts for 7%-8% of all B cell lymphomas[1]. MALT lymphomas are considered low-grade and they can occur in a variety of organs, including the stomach, orbit, conjunctiva, salivary gland, skin, thyroid, lung, stomach, intestine, dura and rarely liver. MALT lymphomas usually arise in areas that are devoid of lymphoid tissue, but are preceded by chronic inflammation, either infectious or autoimmune, which result in the accumulation of extranodal lymphoid proliferation[1]. Prolonged lymphoid proliferation can eventually result in the development of a malignant clone. The stomach is the most common site of MALT lymphoma and its association with H. pylori is well documented[2-4]. An increased occurrence of MALT lymphomas, especially in the salivary glands has been reported in patients with Sjögren’s syndrome[5-7]. Patients with Hashimoto’s thyroiditis have a 67- to 80-fold increased risk of developing primary thyroid lymphoma[8-10] and B-cell type NHL is the most common type and features of MALT lymphoma can be seen in over one-third of cases[10]. Case studies on MALT lymphoma of the liver have been rarely reported and very little is known about this disease entity. Here we present two cases with primary MALT lymphoma of the liver, one with no underlying chronic liver disorder and the other is associated with autoimmune hepatitis.
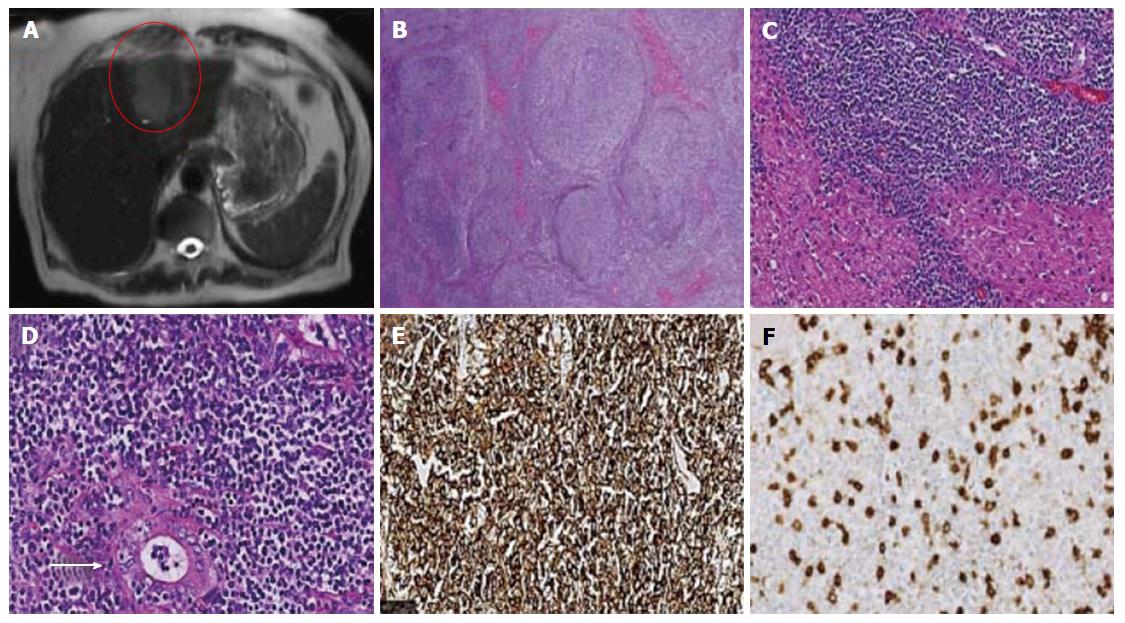
An 80-year-old Caucasian female, presents with a history of nausea, loss of appetite and a 20-pound weight loss. History was negative for any underlying infectious or autoimmune process. Physical examination was notable for weight loss. Abnormal laboratory results obtained was as follows: WBC 13.5 K/UL, aspartate aminotransferase (AST) 137 U/L and alanine aminotransferase (ALT) 166 U/L, alkaline phosphatase 53 U/L, albumin 2.5 g/dL, bilirubin total 1.1 mg/dL, Bilirubin direct 0.2 mg/dL. Abdominal computed tomography (CT) scan identified a bi-lobed mass in the left hepatic lobe (Figure 1A). No dilatation of the intra- or extra-hepatic bile ducts was seen. The spleen, pancreas, gallbladder kidneys and adrenal glands were unremarkable. Histological sections of the mass revealed a nodular infiltrate of atypical lymphocytes with small irregular nuclei and abundant clear cytoplasm surrounding occasional reactive germinal centers (Figure 1B and C). Focal fibrosis and plasmacytosis was identified at the periphery of the nodules. There were occasional lymphoepithelial lesions (Figure 1D). Immunohistochemical staining showed that the neoplastic cells were positive for CD20 (Figure 1E), CD79a and BCL-2 and negative for CD10, CD5 (Figure 1F), CD23, BCL-6, CD43, CD3, CD21, CD138 and IgD. Ki-67 was positive in approximately 30% of the cells. Polymerase chain reaction (PCR) analysis by capillary electrophoresis was clonal for immunoglobulin heavy chain (IgH) rearrangement. Bone marrow biopsy showed normocellular marrow with trilineage hematopoiesis and no evidence of lymphoma. These results supported the diagnosis of MALT lymphoma of the liver. On further follow-up, a year later, the patient developed pulmonary nodules which were proven to be MALT lymphoma on biopsy. She went into remission following treatment with rituximab for one year. Seven years later she presented with a right neck mass which was positive for MALT lymphoma of the parotid gland. The lung and parotid MALT lymphoma showed the same IgH rearrangement by PCR, which indicated that they were the same clones.
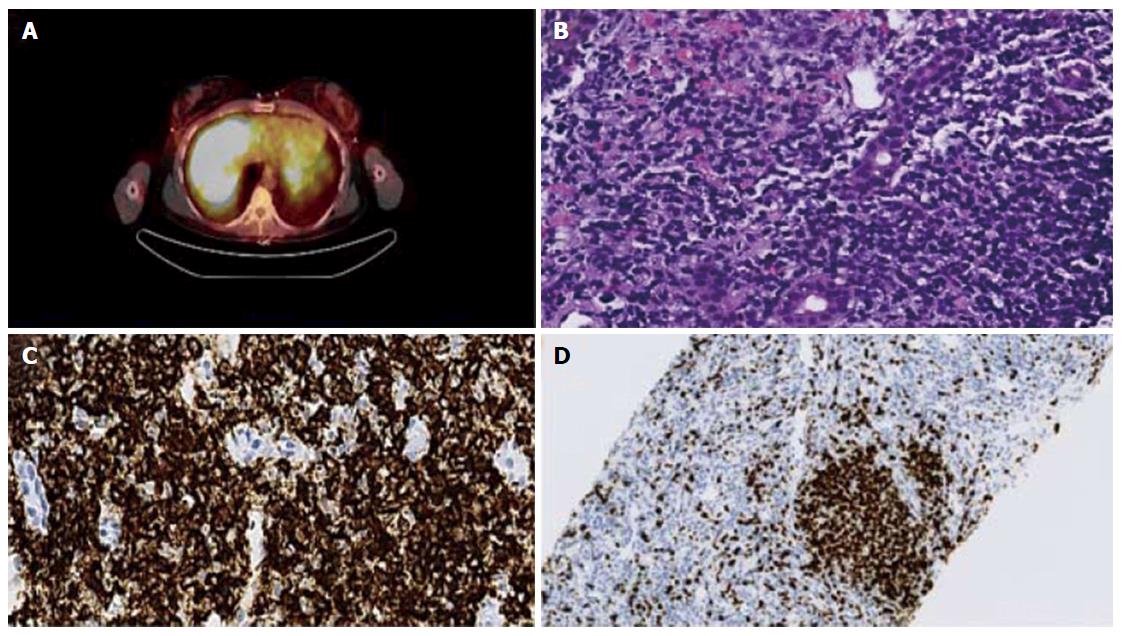
A 30-year-old lady presented to the clinic for management of a previously diagnosed autoimmune hepatitis. At 10 years of age, she was diagnosed with Hashimoto’s thyroiditis and further work up revealed autoimmune hepatitis on a liver biopsy which was managed on azathioprine. In the previous year prior to presentation, she had a flare up of the autoimmune hepatitis when her liver enzymes were found to be in the 500’s range and remained abnormal despite having been on medication. At the time of presentation, the patient was asymptomatic. The laboratory results obtained were as follows: WBC 1.5 K/UL, platelet 70 K/UL, HB 11.1 g/dL, AST 209 mg/dL, ALT 232U/L, alkaline phosphatase 239 U/L, bilirubin total 1.4 mg/dL, bilirubin direct 0.5 mg/dL. Biochemical investigation for chronic viral hepatitis was negative. Although previously positive, her current report for anti-smooth muscle antibodies was negative. Positron emission tomography/CT abdomen showed an enlarged liver (20 cm) with heterogeneity and diffuse FDG activity (Figure 2A), which was highly suspicious for malignancy. The spleen was slightly enlarged with mild portacaval and left paraaortic adenopathy. Liver biopsy identified atypical lymphoid infiltrate with focal interface activity, fibrosis and lymphoepithelial lesions on histopathological examination (Figure 2B). Immunohistochemistry analysis showed predominantly CD20 positive B-lymphocytes in the infiltrate (Figure 2C) that were negative for CD5, CD10, or CD43. Ki-67 proliferative index was low (30%) except in the reactive germinal center (Figure 2D). PCR analysis of the liver biopsy was positive for the IgH gene rearrangement, indicating monoclonal B cell proliferation. Her bone marrow biopsy showed trilineage maturation with no evidence of lymphoma. Treatment with rituximab was commenced and the patient is still in remission three years later.
The etiology of MALT lymphoma of the liver is still unclear. In most extranodal MALT lymphomas, chronic inflammation due to either an infectious process or autoimmune process has been implicated. Several disease conditions have been associated with the development of hepatic MALT lymphoma which makes initial diagnosis very difficult. Nagata et al[11] reviewed 51 cases of MALT lymphoma of the liver. They reported that 25% was not associated with any disease condition. However, hepatic MALT lymphoma was associated with carcinomas (21%), viral and drug related-hepatitis (20%), biliary cirrhosis (10%), liver cirrhosis (10%), ascariasis (4%), gastric MALT lymphoma (4%), rheumatoid arthritis (2%), multiple biliary unilocular cysts (2%) and no information was reported in 2% of the cases. Majority of the cases presented as a solitary mass and were effectively treated with surgical resection without any adjuvant therapy. Our first case had no underlying history of hepatitis, infection, cancer or autoimmune condition. The only concerning clinical sign was drastic weight loss. The abnormal liver enzymes and liver mass were incidental findings. Clinically an initial diagnosis of hepatocellular carcinoma was made and the patient underwent surgery with complete resection of the lesion, which prevented recurrence in the liver but the lymphoma recurred in the lungs. The most frequent location of recurrence of hepatic MALT lymphoma following treatment appears to be the lungs[11,12] and this occurs at a mean average of 65 mo. Of the 3 reported cases in the literature, 2 patients were treated with resection and the remaining one, with radiation. Hepatic recurrence is rare after complete resection and only one case has been reported[11]. Our patient developed pulmonary recurrence only after one year, but after treatment with rituximab the patient remained in remission for 7 years. However, the lymphoma recurred in the right parotid gland which was treated with 7 mo of idelalisib. The patient is currently in remission a year later. MALT lymphoma generally has an indolent course but recurrences can occur over many years and it tends to involve other common extranodal sites[13]. In our experience, we report for the first time multiple recurrences of MALT lymphoma following complete resection of hepatic MALT lymphoma.
The mean age of patients with MALT lymphoma of the liver is about 60 years of age[11,14], however our second case was 30 years old with an underlying confirmed diagnosis of autoimmune hepatitis which is a disorder frequently seen in young women. Wöhrer et al[15] analyzed 158 patients with MALT lymphoma and 39% had an autoimmune disease. The patients were predominantly women and significantly younger at lymphoma diagnosis. The most commonly reported autoimmune disorder associated with liver MALT lymphoma is biliary cirrhosis[14,16,17] and the usual presentation is as a solitary mass. To our knowledge, this is the first reported case associated with autoimmune hepatitis. Autoimmune hepatitis is a chronic progressive liver disease, characterized by hepatocellular inflammation and liver damage and a tendency to progress to liver cirrhosis. Most patients also have other autoimmune diseases including type 1 diabetes, thyroiditis, vitiligo and Sjögren’s syndrome. In support of this, in addition to autoimmune hepatitis, our patient had hashimoto thyroiditis. Similar to MALT lymphoma in other organs, chronic immune activation in autoimmune hepatitis may contribute to lymphomagenesis in the liver. Perhaps the prolonged history and severity of the disease in case 2 explains the diffuse liver involvement with MALT lymphoma. It is important to note that reactive lymphoid hyperplasia, also known as pseudolymphoma, is associated with autoimmune hepatitis[18]. Interestingly pseudolymphoma of the liver can cause a focal liver mass with atypical lymphoid proliferation on histology, which predominantly stain positive for CD20 and reactive germinal center formation[19]. The benign entity can be difficult to differentiate from hepatic MALT lymphoma without further molecular investigation and majority of these lesions are resected due to suspicion for a malignancy. Sato et al[20] reported transformation of a pseudolymphoma of the liver, in a background of biliary cirrhosis, into a diffuse B cell NHL. Accordingly, patients with pseudolymphoma will require close follow-up to prevent a misdiagnosis of MALT lymphoma. Indeed, further studies are needed to determine if there is an association between pseudolymphoma and subsequent transformation into low grade lymphomas such as MALT lymphoma.
Rituximab has shown promise as an effective treatment in extra-gastric MALT lymphoma[21,22] but very few cases involving treatment with rituximab have been reported in hepatic MALT lymphoma. Stable remission have been described in patients treated with rituximab following surgical resection[23] or in combination with chemotherapy[24] or radiofrequency ablation[25]. Both of our patients were treated with rituximab and our second case had no previous surgical treatment or chemotherapy. Although high remission rates are achieved by chemotherapy[26], treatment with rituximab can minimize toxicity while maintaining efficacy. Idelalisib, a selective inhibitor of the delta isoform of phosphatidylinositol 3-kinase[27], which plays an important role in B-cell development, proliferation, migration, adhesion and survival[28] has shown efficacy by inducing apoptosis in malignant B-cells in patients with relapsed follicular lymphoma or refractory chronic lymphocytic lymphoma[27,29]. To our knowledge, our study is the first reported case to use idelalisib in the treatment of recurrent MALT lymphoma of the liver and should be considered as a possible effective therapy following treatment failure with rituximab.
Primary hepatic MALT lymphoma is very rare. From our experience, we recommend that young patients with autoimmune hepatitis with sudden elevation of liver enzymes, should raise the suspicion of MALT lymphoma of the liver. Since the liver was diffusely involved, rituximab as the sole agent can be used as an effective treatment of choice. Patients with hepatic MALT lymphoma should be closely followed up for recurrence especially in common extranodal sites. Rituximab or Idelalisib can be a suitable treatment in patients with relapse. In solitary cases of MALT lymphoma of the liver, complete resection of the lesion with adjuvant rituximab therapy should be considered to prevent recurrence.
Mucosa-associated lymphoid tissue (MALT) lymphoma of the liver is a very rare condition, which can be misdiagnosed.
Hepatic MALT lymphoma is associated with various inflammatory and autoimmune diseases, and one of the authors’ patients had autoimmune hepatitis. The disease may also occur in the absence of any underlying disorder.
Hepatocellular carcinoma (HCC), hepatic reactive lymphoid hyperplasia, cholangiocarcinoma, metastatic carcinoma to the liver.
The liver enzymes were elevated in both cases of MALT lymphoma of the liver.
Lesions of hepatic MALT lymphoma may resemble HCC, cholangiocarcinoma or hepatic metastasis on computed tomography or position emission tomography scan, often leading to misdiagnosis.
MALT lymphoma of the liver often presents as infiltration of the liver with atypical lymphocytes, forming lymphoepithelial lesions on histologic sections and monoclonal immunoglobulin heavy chain gene rearrangement on PCR analysis.
Complete surgical excision of lesion or rituximab in localized disease and the use of rituximab or idelalisib in recurrent or advanced disease.
Although MALT lymphoma of the liver is considered a low grade lymphoma, patients should be closely followed up for recurrence especially in common extranodal sites. Recurrences or advanced disease can be treated with biological targeted therapy to achieve remission while avoiding the toxic effects of chemotherapy.
MALT lymphoma of the liver is associated with an inflammatory or autoimmune condition such as, primary biliary cirrhosis, hashimoto disease or like in our patient, autoimmune hepatitis. In some cases, there may be no underlying associations.
MALT lymphoma of the liver should be considered when imaging studies show a hepatic lesion with an underlying autoimmune condition or when a solitary liver mass is identified in a patient with no clear risk factors for HCC and cholangiocarcinoma.
Well written and excellent case report on two patients with MALT lymphoma of the liver.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Lo ZJ, Zielinski J S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Project TN-HsLC. A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. The Non-Hodgkin’s Lymphoma Classification Project. Blood. 1997;89:3909-3918. [PubMed] |
| 2. | Wotherspoon AC, Doglioni C, Diss TC, Pan L, Moschini A, de Boni M, Isaacson PG. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet. 1993;342:575-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1564] [Cited by in RCA: 1383] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 3. | Morgner A, Bayerdörffer E, Neubauer A, Stolte M. Gastric MALT lymphoma and its relationship to Helicobacter pylori infection: management and pathogenesis of the disease. Microsc Res Tech. 2000;48:349-356. [PubMed] |
| 4. | Neubauer A, Thiede C, Morgner A, Alpen B, Ritter M, Neubauer B, Wündisch T, Ehninger G, Stolte M, Bayerdörffer E. Cure of Helicobacter pylori infection and duration of remission of low-grade gastric mucosa-associated lymphoid tissue lymphoma. J Natl Cancer Inst. 1997;89:1350-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 181] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 5. | Theander E, Henriksson G, Ljungberg O, Mandl T, Manthorpe R, Jacobsson LT. Lymphoma and other malignancies in primary Sjögren’s syndrome: a cohort study on cancer incidence and lymphoma predictors. Ann Rheum Dis. 2006;65:796-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 366] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 6. | Royer B, Cazals-Hatem D, Sibilia J, Agbalika F, Cayuela JM, Soussi T, Maloisel F, Clauvel JP, Brouet JC, Mariette X. Lymphomas in patients with Sjogren’s syndrome are marginal zone B-cell neoplasms, arise in diverse extranodal and nodal sites, and are not associated with viruses. Blood. 1997;90:766-775. [PubMed] |
| 7. | Voulgarelis M, Dafni UG, Isenberg DA, Moutsopoulos HM. Malignant lymphoma in primary Sjögren’s syndrome: a multicenter, retrospective, clinical study by the European Concerted Action on Sjögren’s Syndrome. Arthritis Rheum. 1999;42:1765-1772. [PubMed] [DOI] [Full Text] |
| 8. | Holm LE, Blomgren H, Löwhagen T. Cancer risks in patients with chronic lymphocytic thyroiditis. N Engl J Med. 1985;312:601-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 326] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 9. | Watanabe N, Noh JY, Narimatsu H, Takeuchi K, Yamaguchi T, Kameyama K, Kobayashi K, Kami M, Kubo A, Kunii Y. Clinicopathological features of 171 cases of primary thyroid lymphoma: a long-term study involving 24553 patients with Hashimoto’s disease. Br J Haematol. 2011;153:236-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 10. | Skacel M, Ross CW, Hsi ED. A reassessment of primary thyroid lymphoma: high-grade MALT-type lymphoma as a distinct subtype of diffuse large B-cell lymphoma. Histopathology. 2000;37:10-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Nagata S, Harimoto N, Kajiyama K. Primary hepatic mucosa-associated lymphoid tissue lymphoma: a case report and literature review. Surg Case Rep. 2015;1:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 12. | Chen F, Ike O, Wada H, Hitomi S. Pulmonary mucosa-associated lymphoid tissue lymphoma 8 years after resection of the same type of lymphoma of the liver. Jpn J Thorac Cardiovasc Surg. 2000;48:233-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Raderer M, Streubel B, Woehrer S, Puespoek A, Jaeger U, Formanek M, Chott A. High relapse rate in patients with MALT lymphoma warrants lifelong follow-up. Clin Cancer Res. 2005;11:3349-3352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 127] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 14. | Doi H, Horiike N, Hiraoka A, Koizumi Y, Yamamoto Y, Hasebe A, Ichikawa S, Yano M, Miyamoto Y, Ninomiya T. Primary hepatic marginal zone B cell lymphoma of mucosa-associated lymphoid tissue type: case report and review of the literature. Int J Hematol. 2008;88:418-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Wöhrer S, Troch M, Streubel B, Zwerina J, Skrabs C, Formanek M, Hauff W, Hoffmann M, Müllauer L, Chott A. MALT lymphoma in patients with autoimmune diseases: a comparative analysis of characteristics and clinical course. Leukemia. 2007;21:1812-1818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Ye MQ, Suriawinata A, Black C, Min AD, Strauchen J, Thung SN. Primary hepatic marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue type in a patient with primary biliary cirrhosis. Arch Pathol Lab Med. 2000;124:604-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 17. | Prabhu RM, Medeiros LJ, Kumar D, Drachenberg CI, Papadimitriou JC, Appelman HD, Johnson LB, Laurin J, Heyman M, Abruzzo LV. Primary hepatic low-grade B-cell lymphoma of mucosa-associated lymphoid tissue (MALT) associated with primary biliary cirrhosis. Mod Pathol. 1998;11:404-410. [PubMed] |
| 18. | Kwon YK, Jha RC, Etesami K, Fishbein TM, Ozdemirli M, Desai CS. Pseudolymphoma (reactive lymphoid hyperplasia) of the liver: A clinical challenge. World J Hepatol. 2015;7:2696-2702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Yuan L, Zhang Y, Wang Y, Cong W, Wu M. Reactive lymphoid hyperplasia of the liver: a clinicopathological study of 7 cases. HPB Surg. 2012;2012:357694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Sato S, Masuda T, Oikawa H, Satoh T, Suzuki Y, Takikawa Y, Yamazaki K, Suzuki K, Sato S. Primary hepatic lymphoma associated with primary biliary cirrhosis. Am J Gastroenterol. 1999;94:1669-1673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Conconi A, Martinelli G, Thiéblemont C, Ferreri AJ, Devizzi L, Peccatori F, Ponzoni M, Pedrinis E, Dell’Oro S, Pruneri G. Clinical activity of rituximab in extranodal marginal zone B-cell lymphoma of MALT type. Blood. 2003;102:2741-2745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 282] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 22. | Lossos IS, Morgensztern D, Blaya M, Alencar A, Pereira D, Rosenblatt J. Rituximab for treatment of chemoimmunotherapy naive marginal zone lymphoma. Leuk Lymphoma. 2007;48:1630-1632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Gockel HR, Heidemann J, Lugering A, Mesters RM, Parwaresch R, Domschke W, Lugering N. Stable remission after administration of rituximab in a patient with primary hepatic marginal zone B-cell lymphoma. Eur J Haematol. 2005;74:445-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Mariko Tanaka NF, Yamasaki F, Ohshima K, Sueoka E, and Kimura S. Primary hepatic extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue type is associated with chronic inflammatory process. Open J Hematol. 2010;1:1-5. |
| 25. | Hamada M, Tanaka Y, Kobayashi Y, Takeshita E, Joko K. [A case of MALT lymphoma of the liver treated by RFA and Rituximab]. Nihon Shokakibyo Gakkai Zasshi. 2006;103:655-660. [PubMed] |
| 26. | Raderer M, Kiesewetter B, Ferreri AJ. Clinicopathologic characteristics and treatment of marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma). CA Cancer J Clin. 2016;66:153-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 170] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 27. | Barrientos JC. Idelalisib for the treatment of indolent non-Hodgkin lymphoma: a review of its clinical potential. Onco Targets Ther. 2016;9:2945-2953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 28. | Pauls SD, Lafarge ST, Landego I, Zhang T, Marshall AJ. The phosphoinositide 3-kinase signaling pathway in normal and malignant B cells: activation mechanisms, regulation and impact on cellular functions. Front Immunol. 2012;3:224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 29. | Lannutti BJ, Meadows SA, Herman SE, Kashishian A, Steiner B, Johnson AJ, Byrd JC, Tyner JW, Loriaux MM, Deininger M. CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood. 2011;117:591-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 573] [Cited by in RCA: 606] [Article Influence: 43.3] [Reference Citation Analysis (0)] |