Published online Aug 28, 2017. doi: 10.4254/wjh.v9.i24.1030
Peer-review started: March 31, 2017
First decision: May 9, 2017
Revised: June 26, 2017
Accepted: July 7, 2017
Article in press: July 10, 2017
Published online: August 28, 2017
Processing time: 147 Days and 8.2 Hours
To compare a novel, fully synthetic, polyurethane based glue (MAR-1) to fibrin sealant in a partial liver resection rat model.
After 50% resection of the lateral left liver lobe in male Wistar rats (n = 7/group/time point), MAR-1, Fibrin or NaCl was applied. After 14, 21 and 90 postoperative days, sealant degradation, intra-abdominal adhesions were scored, and histological examination of liver tissue was performed.
(Mean ± SEM) (MAR-1 vs Fibrin vs NaCl). Bleeding mass was significantly higher in NaCl (3.36 ± 0.51 g) compared to MAR-1 (1.44 ± 0.40 g) and Fibrin (1.16 ± 0.32 g). At 14 and 90 d, bleeding time was significantly lower in MAR-1 (6.00 ± 0.9 s; 13.57 ± 3.22 s) and Fibrin (3.00 ± 0.44 s; 22.2 ± 9.75 s) compared to NaCl (158.16 ± 11.36 s; 127.5 ± 23.3 s). ALT levels were significantly higher in MAR-1 (27.66 ± 1 U/L) compared to Fibrin (24.16 ± 0.98 U/L) and NaCl (23.85 ± 0.80 U/L). Intrabdominal adhesions were significantly lower in MAR-1 (11.22% ± 5.5%) compared to NaCl (58.57% ± 11.83%). Degradation of the glue was observed and MAR-1 showed almost no traces of glue in the abdominal cavity as compared to the Fibrin (10% ± 5% 14 d; 7% ± 3% 21 d). Survival showed no significant differences between the groups.
Compared to Fibrin, MAR-1 showed similar hemostatic properties, no adverse effects, and is biocompatible. Further studies on adhesion strength and biodegradability of synthetic sealants are warranted.
Core tip: This study evaluates the effectiveness of a novel, polyurethane based, surgical adhesive on a liver resection model. This study will further help in better sealing of wounds in a trauma model in comparison to Fibrin glue.
- Citation: Srinivasan PK, Sperber V, Afify M, Tanaka H, Fukushima K, Kögel B, Gremse F, Tolba R. Novel synthetic adhesive as an effective alternative to Fibrin based adhesives. World J Hepatol 2017; 9(24): 1030-1039
- URL: https://www.wjgnet.com/1948-5182/full/v9/i24/1030.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i24.1030
Hemorrhage due to traumatic injury is one of the leading causes of death worldwide. It is estimated that hemorrhage is responsible for more than 35% of pre-hospital mortality and 40% of mortality in the first 24 h[1]. In case of abdominal trauma, the liver is one of the most commonly injured organs; anatomical position and its delicate parenchyma make it susceptible to injury and hemorrhage[2]. Despite modern surgical techniques, management of hemorrhage after liver trauma still remains a challenge, with major liver trauma resulting in high morbidity and mortality rates[3]. Furthermore, surgeries involving liver resection are known to be of high risk; nevertheless, it is the one of the curative treatment options for hepatocellular cancer patients[4].
Management of liver injury has progressed tremendously in the last three decades[2]. Advancement in biotechnological research has resulted in a variety of hemostatic agents[5]. These hemostatic agents are either biological or synthetic in nature[5]. They are based on components including cellulose, collagen, glutaraldehyde, fibrin, and dihydroxyacetone[5-7]. Fibrin sealants (also known as fibrin adhesive or glue) are the most widely used hemostatic agents as a complimentary adjunct in various surgical procedures. Fibrin sealants comprise of two components, human-thrombin and fibrinogen, usually plasma derived[5]. During application, these two components interact to form a stable fibrin clot[5]. However, most require 2 °C-8 °C storage, extensive preparation, and, once taken out of refrigeration, have to be used within 9 h[6]. Notably, fibrin sealants are less effective in events of strong bleeding, as they can be washed away with blood or other liquids and there is a risk of re-bleeding, due to fibrin sealants’ limited sealing strength[8,9]. Due to their biological origin, fibrin sealants are associated with risk factors including immune reactions, viral transmission, and potential embolism risk[6,10,11].
In the early forties, cyanoacrylate based glues were marketed under brand names, such as, Superglue and Krazy glue. Cyanoacrylate glues are neither biocompatible nor bioabsorbable[12]. Additionally, upon degradation, cyanoacrylates form cyanoacetate and formaldehyde, which are toxic to humans[5,12]. Other options for synthetic products include urethane based polymers, such as polyurethane. Polyurethanes (PUs) are known for their tensile strength of 4-60 MPa; thus, making them highly elastic[13]. Research has shown that several factors, such as hydrolysis and enzymatic action, contribute to their degradation[14]. Because of their non-biological components, there is no risk of virus transmission or antigenic reaction like with fibrin based adhesives.
The aim of this study was to evaluate MAR-1, determining hemostatic properties, functionality, and prevention of intra-abdominal adhesions, tissue compatibility as well as biodegradation. In comparison, we tested the clinically used fibrin sealant Beriplast® P (CSL Behring GmbH, Marburg, Germany) and Sodium Chloride (NaCl) as a control solution.
MAR-1 is a polyurethane based sealant that consists of two different components: A isocyanate-functional polyester-ether pre-polymer and an amino-functional asparagine acid ester. This adhesive technology and its polyaddition reaction are well-known.
The two components were stored at 22 °C in a double chamber syringe and combined upon application (Adhesys Medical GmbH, Aachen, Germany). The Fibrin sealant used was the commercially available Beriplast® P (CSL Behring GmbH, Marburg, Germany), which consists of fibrin and thrombin mixed prior to application.
All experiments were conducted in accordance with German Federal Law regarding the protection of animals and the DIRECTIVE 2010/63/EU on the protection of animals used for scientific purposes. The Guide for the care and use of laboratory animals (8th edition, NIH Publication, 2011, United States) was also followed. The governmental care and use committee (LANUV), Recklinghausen, NRW, Germany, granted official permission. Male Wistar rats weighing between 200-260 g were used. The animals were housed in Type 2000 rat filter top cages (Tecniplast, Hohenpreisenberg, Germany) under specific pathogen free (SPF)-conditions according to Federation of European Laboratory Animal Science Associations (FELASA) guidelines (http://www.felasa.eu), in a temperature (22 °C) and humidity controlled environment (55% relative humidity) with a 12-h light/dark cycle and allowed food (standard rat diet, Ssniff-Spezial Diäten GmbH, Soest, Germany) and water ad libitum.
Sixty-three rats were randomly allocated to the following groups: MAR-1, Fibrin and NaCl. The groups were further classified into three time points: 14 d, 21 d, and 90 d. Rats received general anesthesia by inhalation of 1.5% isoflurane (Abbott GmbH and Co.KG, Wiesbaden, Germany) and administration of 0.1 mg/kg body weight Buprenorphine (Temgesic®, Essex Pharma GmbH, Munich, Germany) subcutaneously as analgesic. For perioperative anti-biotic prophylaxis, rats received 16 mg/kg bodyweight Cefuroxime s.c. (Fresenius SE and Co. KGaA, Homburg, Germany). Using a vessel loop for compression, 30% of the left lateral lobe was removed, and sealant was applied in an amount sufficient to cover the wound area. Pre-weighed gauze was placed under the liver lobe prior to resection. Post resection, the blood absorbed by the gauze was weighed and subtracted from the pre-weight of the gauze to calculate the bleeding mass. The animals were euthanized under anesthesia after 14, 21 and 90 d respectively.
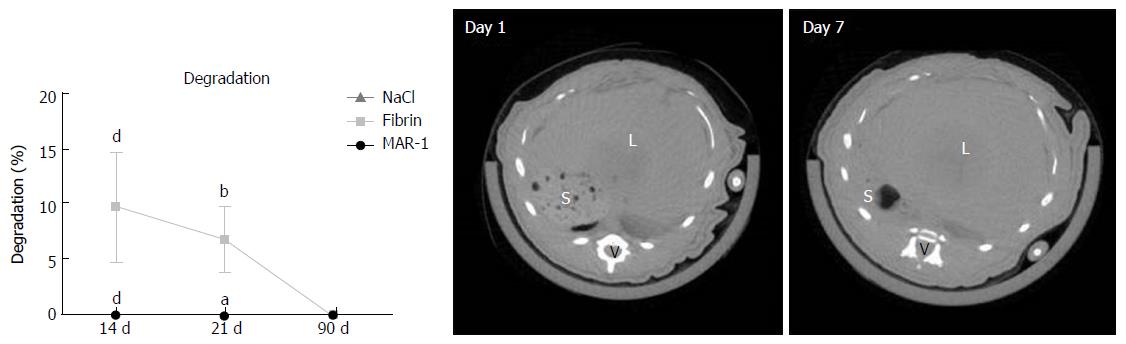
μCT to visualize the biodegradation
μCT data was measured using Tomoscope 30 s Duo (CT-Imaging GmbH, Erlangen, Germany) using a protocol (HQD-6565-90-360) that took 720 projections (1032 × 1012 Pixel) in 90 s during one rotation with radiation dose of 421 mGy[15]. Several sub-scans were taken and reconstructed using a Feldkamp algorithm with a voxelsize of 70 μm × 70 μm × 70 μm and were assembled into one volume data set. Volumetric image data was analyzed and visualized using the Imalytics Preclinical Software[16].
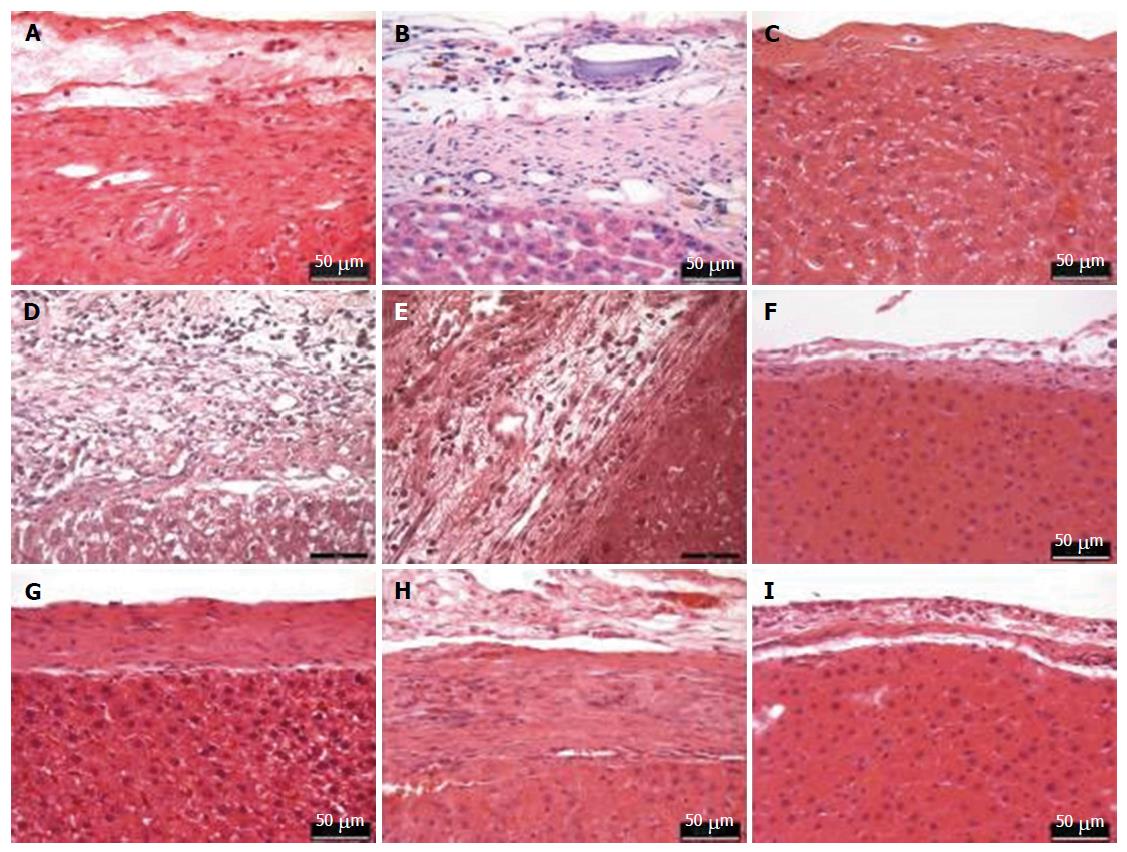
Tissue samples of the liver were collected at the time when the rats were euthanized. The samples were immediately fixed in 4% neutral buffered formalin (Roti®-Histofix 4%, Roth, Karlsruhe-Germany), and then were shaken overnight on a shaker (Lab net, International Inc., United States). The specimens were processed in grading series of alcohol and xylene, embedded in paraffin and sectioned at 4-6 μm thin slices using a microtome and were stained with hematoxylin and eosin (H and E). Paraffin-embedded liver sections were used for H and E staining and analysed using a Leica DM 2500 microscope (Leica, Bensheim, Germany).
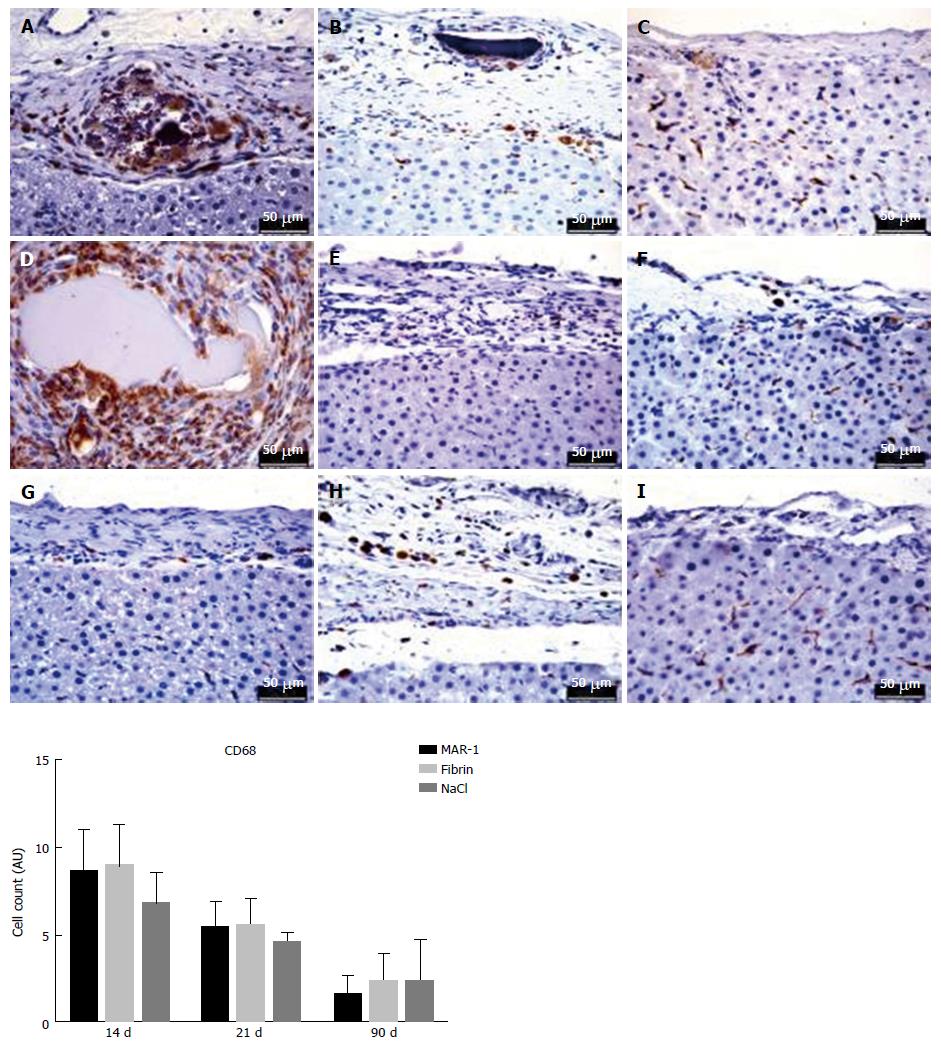
Immunohistochemistry was performed as per manufacturer’s instructions. CD68 macrophages were identified by a 1:50 mouse monoclonal antibody from Dako (Glostrup, Denmark), pre-treatment of the fixed specimen with microwave three times, citrate-buffer pH 6, and as secondary antibody rabbit anti-mouse 1:300 from Dako (Glostrup, Denmark).
Serum was withdrawn at 14, 21 and 90 d post operation and analyzed with a clinical chemistry analyzer (Ortho Clinical Diagnostics GmbH, Neckargemünd, Germany). Liver enzymes, ALT and AST were measured from serum. In addition, blood count of leukocytes (103/μL), erythrocytes (106/μL), platelets (103/μL), and hemoglobin were measured using the MEK6450K automatic cell counter (Nihon Kohden, Rosbach, Germany).
Statistical review was performed by Professor René Tolba. All results are expressed as mean ± SEM and the data was analyzed by Graph Pad Prism® Version 5 (Graph Pad, San Diego, CA, United States). Significance between different groups was measured with one-way analysis of variance (ANOVA) and posttest: Tukey-Kramer. Survival analysis was carried out by Kaplan-Meier curve and Mantel-Cox test. Values of P < 0.05 were considered statistically significant.
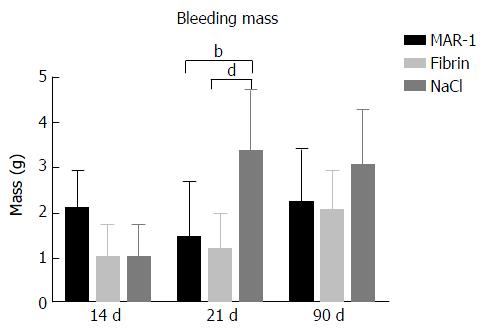
Bleeding mass (Figure 1) was assessed in order to record the amount of blood lost after liver resection. After 21 d, NaCl (3.36 ± 0.51 g) showed significantly higher levels of blood loss in comparison to MAR-1 (1.44 ± 0.40 g) and Fibrin (1.16 ± 0.32 g) treated animals. However, there were no significant differences between the animals in 14 d (MAR-1: 2.08 ± 0.30 g; Fibrin: 1.02 ± 0.29 g; NaCl: 1.02 ± 0.29 g) and 90 d (MAR-1: 2.21 ± 0.44 g; Fibrin: 2.03 ± 0.28 g; NaCl: 3.04 ± 0.50 g) group.
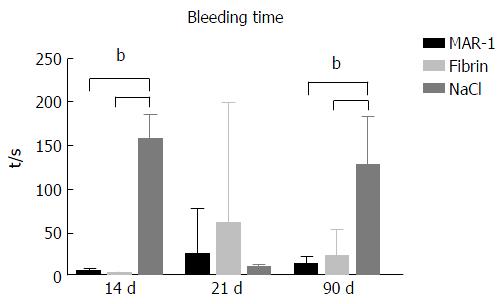
Duration of blood loss (Figure 2) was recorded to evaluate the bleeding time in different groups. NaCl (158.16 ± 11.36 s) (127.5 ± 23.3 s) showed significantly higher bleeding times on 14 and 90 d in comparison to MAR-1 (6.0 ± 0.9 s) (13.57 ± 3.22 s) and Fibrin (3.0 ± 0.44 s) (22.2 ± 9.75 s) groups respectively. However, the groups showed no significance at 21 d time point (MAR-1: 25.33 ± 17.53 s; Fibrin: 61.16 ± 56.77 s; NaCl: 10.71 ± 1.19 s).
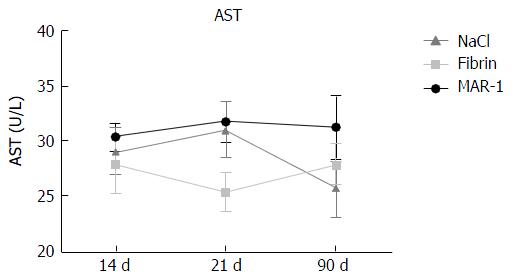
Aspartate transaminase (Figure 3) was measured as parameter for liver injury. There were no significant differences noticed in the groups at 14 (MAR-1: 30.37 ± 1.23 U/L; Fibrin: 27.83 ± 2.54 U/L; NaCl: 29.16 ± 2.12 U/L), 21 (MAR-1: 31.77 ± 1.80 U/L; Fibrin: 25.33 ± 1.70 U/L; NaCl: 31.00 ± 2.46 U/L) or 90 d (MAR-1: 31.28 ± 2.86 U/L; Fibrin: 27.90 ± 1.86 U/L; NaCl: 25.83 ± 2.71 U/L).
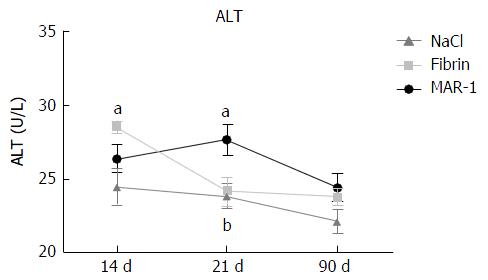
Alanine transaminase (Figure 4) release was measured as a parameter for liver parenchymal damage. Significant differences between the treatment groups were seen after 14 and 21 post-operative days. Fibrin (28.5 ± 0.42 U/L: 14 d) (24.16 ± 0.98 U/L: 21 d) showed a significantly higher release of ALT compared to NaCl (24.5 ± 1.23 U/L: 14 d) (23.85 ± 0.80 U/L: 21 d) group after 14 d. Meanwhile, MAR-1 (26.37 ± 0.92 U/L: 14 d) (27.66 ± 1 U/L: 21 d) showed significantly higher levels after 21 d in comparison to both NaCl and Fibrin treated animals.
Intra-abdominal adhesions (Figure 5) were visualized and the extent of adhesions was evaluated. After 14 d, Fibrin (13.33% ± 6.1%) treated animals showed significantly lower percentage of adhesions in comparison to NaCl (68.33% ± 14.24%). MAR-1 (11.22% ± 5.5%) showed significantly lower adhesion compared to NaCl (58.57% ± 11.83%) after 21 d. After 90 d, Fibrin group (24% ± 7.29%) showed significantly lower levels of adhesions compared to NaCl group (61.66% ± 7.03%). Whereas, there were no significant differences found between Fibrin and MAR-1 groups at any given time point.
μCT scans were performed on day 1 and day 7 to visualize the glue. Interestingly, due to its hydrogel like properties, the glue could not be distinguished from the liver tissue in the µCT images (Figure 6). Degradation of MAR-1 and Fibrin were noted and compared to NaCl treatment. MAR-1 (0% ± 0% at all time points) and NaCl (0%) were absent or negligible compared to Fibrin (10% ± 5% 14 d; 7% ± 3% 21 d; 0% 90 d). Fibrin glue levels were significantly higher compared to MAR-1 and NaCl groups after 14 and 21 d. Fibrin glue was completely metabolized after 90 d.
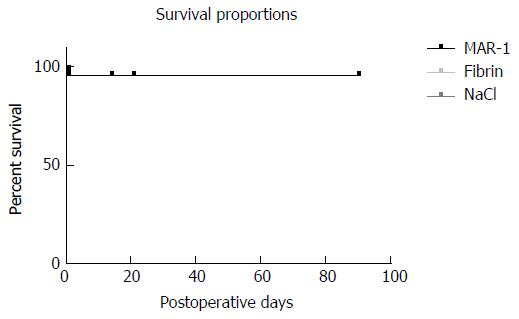
Percentage survival (Figure 7) was calculated for each treatment group. MAR-1 showed a survival percentage of 95.83% in comparison to Fibrin with 95.65% and NaCl with 95%. As per Mantel-Cox test, the P value was 0.9906 and there was no statistical significance seen between MAR-1 and other the groups.
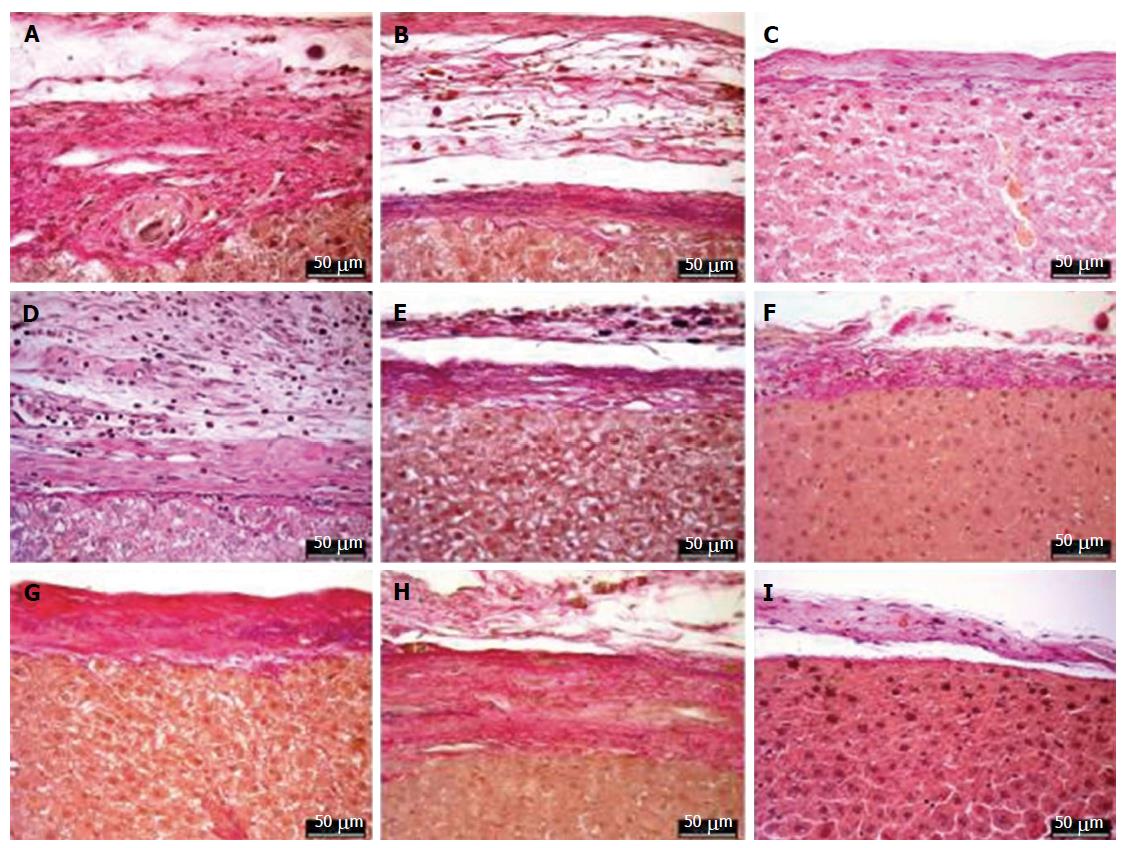
Histopathological evaluation (Figure 8) was performed on the tissue section after 90 post-operative days. There was a slight inflammation due to foreign body reaction in both MAR-1 and Fibrin groups. The reaction zone showed granulation tissue along with some collagen structures. A dense collagenous fibrotic tissue along with histiocytic inflammation was noticed. Whereas, in MAR-1 and Fibrin treated animals inflammation was noticed initially; however, the reaction was absent after 90 d. In case of NaCl treated animals, a thicker liver capsule was seen and occasional inflammation due to bleeding remnants.
Immunohistochemical staining is an ideal tool to identify the presence of CD68 positive cells (Figure 9). It specifically stains macrophages as well as Kupffer cells, Giant cells, and Monocytes. This helps in recognizing cell proliferation in tissues. The CD68 cell count at 14 d (8.6 ± 1.0, 9.0 ± 1.0 AU, 6.8 ± 0.8 AU), 21 d (5.4 ± 0.6 AU, 5.6 ± 0.67 AU, 2.4 ± 1.0 AU), and 90 d (1.6 ± 0.5 AU, 2.4 ± 0.6 AU, 2.4 ± 1.0 AU) showed no significant differences within the groups.
Elastic van Gieson staining (Figure 10) protocol specifically stains elastic fibers, which helps in differentiating between normal and pathological elastic fibers. Due to the chemical reaction in the staining process, the elastic fibers and cell nuclei are stained black, collagen fibers are stained red, and other tissue elements including cytoplasm are stained yellow. We noticed the width of the reaction zone along with the proliferative tissue reduced with time and there were no significant changes noticed in the structural integrity.
Leucocytes, Erythrocytes, Hematocrit, and Platelets were measured and the groups showed no significant differences (Table 1). However, Hemoglobin levels (Table 1) were measured in all the groups. There was a significant difference noted between Fibrin (11.69 ± 0.21 g/dL) and NaCl groups (12.48 ± 0.17 g/dL) at baseline level. Whereas, MAR-1 (12.09 ± 0.29 g/dL) showed significantly lower haemoglobin levels compared to NaCl group (13.68 ± 0.26 g/dL).
| MAR-1 | Fibrin | NaCl | P value | ||
| Leucocytes | 0 d | 7.48 ± 0.33 | 6.82 ± 0.30 | 7.26 ± 0.39 | NS |
| 14 d | 6.78 ± 0.66 | 7.18 ± 0.67 | 8.65 ± 0.75 | NS | |
| 21 d | 7.56 ± 0.75 | 7.20 ± 0.72 | 6.23 ± 0.50 | NS | |
| 90 d | 6.11 ± 0.78 | 5.76 ± 0.52 | 5.41 ± 0.32 | NS | |
| Erythrocytes | 0 d | 5.71 ± 0.09 | 5.68 ± 0.07 | 5.68 ± 0.08 | NS |
| 14 d | 6.07 ± 0.17 | 6.48 ± 0.20 | 6.03 ± 0.18 | NS | |
| 21 d | 6.33 ± 0.14 | 7.03 ± 0.10 | 6.50 ± 0.17 | NS | |
| 90 d | 7.58 ± 0.19 | 7.51 ± 0.12 | 7.72 ± 0.17 | NS | |
| Hemoglobin | 0 d | 12.29 ± 0.14 | 11.69 ± 0.21a | 12.48 ± 0.17 a | aP < 0.05 |
| 14 d | 12.09 ± 0.29b | 13.13 ± 0.43 | 13.68 ± 0.26b | bP < 0.01 | |
| 21 d | 13.08 ± 0.22 | 13.84 ± 0.28 | 14.17 ± 0.28 | NS | |
| 90 d | 14.14 ± 0.33 | 13.88 ± 0.18 | 13.90 ± 0.29 | NS | |
| Hematocrit | 0 d | 35.09 ± 0.48 | 34.59 ± 0.30 | 34.05 ± 0.42 | NS |
| 14 d | 35.66 ± 0.96 | 38.30 ± 0.94 | 35.13 ± 0.50 | NS | |
| 21 d | 35.50 ± 0.76 | 36.98 ± 0.62 | 36.31 ± 1.02 | NS | |
| 90 d | 39.69 ± 0.91 | 39.26 ± 0.55 | 39.75 ± 0.94 | NS | |
| Platelets | 0 d | 923 ± 32 | 960 ± 42 | 998 ± 30 | NS |
| 14 d | 907 ± 67 | 985 ± 63 | 1035 ± 34 | NS | |
| 21 d | 1104 ± 50 | 1024 ± 57 | 970 ± 41 | NS | |
| 90 d | 924 ± 42 | 874 ± 25 | 916 ± 48 | NS |
According to WHO 2010 database, 5.8 million deaths due to injuries were recorded worldwide[17]. A quarter of these were due to trauma and hemorrhagic shock due to injuries; thus, making it a leading cause of death across the globe[1,17]. Liver injury is most commonly observed in abdominal trauma cases[18]. Apart from trauma, liver resection in hepatocellular carcinoma patients carries a high risk of hemorrhage[19]. Hemorrhage during liver surgery is directly associated with extensive use of vascular occlusion techniques, which leads to post-operative complications and eventually hepatic failure[19]. During liver surgery, it is vital to minimize bleeding, especially from small blood vessels of liver parenchyma, in order to prevent intraoperative blood loss and to better visualize the surgical field[19].
In this study, we compared the efficacy, haemostatic properties, and biocompatibility of a novel, polyurethane based synthetic adhesive, MAR-1, with that of Fibrin, which is a clinically used medical adhesive.
Fibrin sealants mimic the coagulation cascade, which depends on various factors such as enzymes, proteins, and co-factors[20]. Polyurethane-based adhesives mainly react with the amino groups of proteins in the tissue, which enables the formation of urea linkages and eventually adhesion[12]. Polyurethanes are known to activate platelets, which enhances the blood clotting process[21]. Moreover, polyurethanes have demonstrated strong thrombogenic properties due to their hydrophobic nature, this promotes the proteins to adhere and initiate the coagulation cascade[12]. We measured the bleeding mass and time to assess the capacity of these sealants to stop bleeding after liver resection. The results showed no significant differences between the two sealants and the results were comparable. However, we noticed a significant difference between MAR-1, Fibrin and NaCl groups, this clearly showed the effectiveness of a sealant in minimizing blood loss, thereby reducing the bleeding time. On the other hand, liver parenchymal enzymes, AST and ALT, were measured and we noticed no significant changes in AST levels throughout the time course; whereas, a significant increase in ALT levels were seen in MAR-1 group after 21 d, in comparison to Fibrin and NaCl groups. AST and ALT levels are routinely measured to assess the functionality of liver and their ratio between the concentrations is of clinical relevance. AST/ALT ratio of 2:1 or more is considered as a sign of liver damage. The elevated ALT levels in the MAR-1 group was probably due to repeated manipulation of the liver lobe during the surgical procedure. Nevertheless, the values were within the physiological range and did not increase at a later time point.
Depending on the origin of thrombin in the fibrin sealants, severe immune reactions have been observed, leading to anaphylactic shock in some cases[22-24]. When extracted from human pooled blood, it carries a high risk of viral contamination[25,26]. Despite improved methods of viral inactivation[27], it still carries a risk of parvovirus infection[28]. Whereas, MAR-1, the polyurethane based adhesive, showed no adverse reaction in this study. Polyurethanes in general are considered biocompatible and biodegradable; they are polymers consisting of urethane links[13]. Research has shown that polyurethanes containing biodegradable diisocyanates degrade into non-cytotoxic decomposition products[13,29,30]. After 14 d, the quantity of MAR-1 was either negligible or absent in the abdominal cavity, suggesting the rapid and efficient degradation of the glue. These results were significant in comparison to Fibrin glue, which was present even after 21 d. Nevertheless, both the glues were efficiently degraded by the end of 90 d. Meanwhile, it was difficult to visualize MAR-1 with the help of μCT, which can be attributed to its hydrogel like properties causing low contrast to the adjacent liver tissue. Studies have suggested that degradation of polyurethanes was mainly dependent on the polyester polyol composition[13,31,32]. Polyurethanes exhibit great versatility in their polymeric properties. Rapid degradation of MAR-1 proves its biocompatibility without any adverse effects. This also supported the previously established properties of polyurethanes such as toughness, durability, elasticity, biocompatibility, which is not achieved by any other available material[33].
Intra-abdominal adhesions are commonly noticed after abdominal surgery. Their incidence is estimated at 67%-93%, which affects the final outcome of the surgery[34]. When a foreign body is introduced into the abdominal cavity it leads to fibrosis and adhesion formation[35]. Demirel et al[36] showed that fibrin sealant drastically reduced adhesions in comparison to primary suture. In general, polyurethanes have been known to exhibit strong adhesion to the tissue[37], as mentioned earlier, their interaction with the amino acids results in the adhesion of the glue to the tissue[12]. We noticed the formation of adhesions during the time course; however, there were no significant differences between MAR-1 and Fibrin treated animals. However, significantly more adhesions were noticed in NaCl group compared to MAR-1 group. These results supports our hypothesis, which is the biocompatibility and non-inferiority of MAR-1 compared to Fibrin glue, the clinical gold standard. Furthermore, the survival rate showed no significant differences between the groups. Meanwhile, the histopathological examination revealed a few structural changes, however, the tissue sections failed to show any significant differences between the groups.
In summary, MAR-1 has been shown to be non-inferior to Fibrin in terms of effective and safe sealing of a liver in a resection model. Based on the obtained results, MAR-1 is biocompatible and showed no adverse effects. We agree that further research is needed to study the chemistry and biodegradability. Nevertheless, MAR-1 is ready to be used in its current form as a topical wound sealant. Moreover, due to the fully synthetic nature, there is no risk of increased immune reactions or viral transmission like with Fibrin.
The authors thank Mr. Pascal Paschenda for his skillful technical assistance.
Despite advanced surgical techniques, hemostasis after liver trauma is a major cause for morbidity and mortality. Fibrin glue is the current gold standard for managing hemostasis; however, there are some disadvantages like production costs, allergic reactions, and storage conditions. In this study, the authors introduce a novel, polyurethane based, synthetic adhesive, which is biocompatible and controls bleeding effectively.
Hemostasis in trauma and surgery is of prime importance, their study primarily focuses on management of blood loss during surgical and trauma procedures.
Novel polyurethane based adhesive, MAR-1, helps in managing blood loss effectively in rat partial liver resection model. This study shows variety of parameters, which plays an important role during traumatic situations.
Partial liver resection model in rats is an established model for liver trauma studies. Results from this study shows the effectiveness of fully synthetic, polyurethane based novel adhesive. This model provides all the necessary information to study the application of surgical adhesives.
MAR-1: Medical adhesive revolution-1 is a novel polyurethane based adhesive; PU: Polyurethanes.
The manuscript is well-written and the data shown in the manuscript was understandable.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Chuang WL, De Ponti F, Ding MX, Savopoulos CG, Tajiri K S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60:S3-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 713] [Cited by in RCA: 831] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 2. | Ahmed N, Vernick JJ. Management of liver trauma in adults. J Emerg Trauma Shock. 2011;4:114-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Zentai C, Braunschweig T, Rossaint R, Daniels M, Czaplik M, Tolba R, Grottke O. Fibrin patch in a pig model with blunt liver injury under severe hypothermia. J Surg Res. 2014;187:616-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Dhanasekaran R, Limaye A, Cabrera R. Hepatocellular carcinoma: current trends in worldwide epidemiology, risk factors, diagnosis, and therapeutics. Hepat Med. 2012;4:19-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 163] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 5. | Achneck HE, Sileshi B, Jamiolkowski RM, Albala DM, Shapiro ML, Lawson JH. A comprehensive review of topical hemostatic agents: efficacy and recommendations for use. Ann Surg. 2010;251:217-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 317] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 6. | Burks S, Spotnitz W. Safety and usability of hemostats, sealants, and adhesives. AORN J. 2014;100:160-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Singer AJ, Perry L. A comparative study of the surgically relevant mechanical characteristics of the topical skin adhesives. Acad Emerg Med. 2012;19:1281-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Borin JF, Deane LA, Sala LG, Abdelshehid CS, White SM, Poulson AK, Khan F, Edwards RA, McDougall EM, Clayman RV. Comparison of healing after cystotomy and repair with fibrin glue and sutured closure in the porcine model. J Endourol. 2008;22:145-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Musella M, Susa A, Greco F, De Luca M, Manno E, Di Stefano C, Milone M, Bonfanti R, Segato G, Antonino A. The laparoscopic mini-gastric bypass: the Italian experience: outcomes from 974 consecutive cases in a multicenter review. Surg Endosc. 2014;28:156-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 167] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 10. | Sapala JA, Wood MH, Schuhknecht MP. Anastomotic leak prophylaxis using a vapor-heated fibrin sealant: report on 738 gastric bypass patients. Obes Surg. 2004;14:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Blondeel PN, Murphy JW, Debrosse D, Nix JC 3rd, Puls LE, Theodore N, Coulthard P. Closure of long surgical incisions with a new formulation of 2-octylcyanoacrylate tissue adhesive versus commercially available methods. Am J Surg. 2004;188:307-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Ferreira P, Silva AF, Pinto MI, Gil MH. Development of a biodegradable bioadhesive containing urethane groups. J Mater Sci Mater Med. 2008;19:111-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Shi R, Chen D, Liu Q, Wu Y, Xu X, Zhang L, Tian W. Recent advances in synthetic bioelastomers. Int J Mol Sci. 2009;10:4223-4256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Santerre JP, Woodhouse K, Laroche G, Labow RS. Understanding the biodegradation of polyurethanes: from classical implants to tissue engineering materials. Biomaterials. 2005;26:7457-7470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 434] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 15. | Gremse F, Doleschel D, Zafarnia S, Babler A, Jahnen-Dechent W, Lammers T, Lederle W, Kiessling F. Hybrid µCT-FMT imaging and image analysis. J Vis Exp. 2015;100:e52770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Gremse F, Stärk M, Ehling J, Menzel JR, Lammers T, Kiessling F. Imalytics Preclinical: Interactive Analysis of Biomedical Volume Data. Theranostics. 2016;6:328-341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 17. | WHO. Injuries and Violence: THE FACTS. 2010. . |
| 18. | Coccolini F, Montori G, Catena F, Di Saverio S, Biffl W, Moore EE, Peitzman AB, Rizoli S, Tugnoli G, Sartelli M. Liver trauma: WSES position paper. World J Emerg Surg. 2015;10:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | Romano F, Garancini M, Uggeri F, Degrate L, Nespoli L, Gianotti L, Nespoli A, Uggeri F. Bleeding in Hepatic Surgery: Sorting through Methods to Prevent It. HPB Surg. 2012;2012:169351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Spotnitz WD. Commercial fibrin sealants in surgical care. Am J Surg. 2001;182:8S-14S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 116] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Ou W, Qiu H, Chen Z, Xu K. Biodegradable block poly(ester-urethane)s based on poly(3-hydroxybutyrate-co-4-hydroxybutyrate) copolymers. Biomaterials. 2011;32:3178-3188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 22. | Orsel I, Guillaume A, Feiss P. [Anaphylactic shock caused by fibrin glue]. Ann Fr Anesth Reanim. 1997;16:292-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Oswald AM, Joly LM, Gury C, Disdet M, Leduc V, Kanny G. Fatal intraoperative anaphylaxis related to aprotinin after local application of fibrin glue. Anesthesiology. 2003;99:762-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Shirai T, Shimota H, Chida K, Sano S, Takeuchi Y, Yasueda H. Anaphylaxis to aprotinin in fibrin sealant. Intern Med. 2005;44:1088-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Hino M, Ishiko O, Honda KI, Yamane T, Ohta K, Takubo T, Tatsumi N. Transmission of symptomatic parvovirus B19 infection by fibrin sealant used during surgery. Br J Haematol. 2000;108:194-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 159] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 26. | Kawamura M, Sawafuji M, Watanabe M, Horinouchi H, Kobayashi K. Frequency of transmission of human parvovirus B19 infection by fibrin sealant used during thoracic surgery. Ann Thorac Surg. 2002;73:1098-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 118] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 27. | Jackson MR. Fibrin sealants in surgical practice: An overview. Am J Surg. 2001;182:1S-7S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 258] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 28. | Horowitz B, Busch M. Estimating the pathogen safety of manufactured human plasma products: application to fibrin sealants and to thrombin. Transfusion. 2008;48:1739-1753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 29. | Guelcher S, Srinivasan A, Hafeman A, Gallagher K, Doctor J, Khetan S, McBride S, Hollinger J. Synthesis, in vitro degradation, and mechanical properties of two-component poly(ester urethane)urea scaffolds: effects of water and polyol composition. Tissue Eng. 2007;13:2321-2333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Guelcher SA, Patel V, Gallagher KM, Connolly S, Didier JE, Doctor JS, Hollinger JO. Synthesis and in vitro biocompatibility of injectable polyurethane foam scaffolds. Tissue Eng. 2006;12:1247-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Gorna K, Gogolewski S. Preparation, degradation, and calcification of biodegradable polyurethane foams for bone graft substitutes. J Biomed Mater Res A. 2003;67:813-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 121] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 32. | Guan J, Sacks MS, Beckman EJ, Wagner WR. Biodegradable poly(ether ester urethane)urea elastomers based on poly(ether ester) triblock copolymers and putrescine: synthesis, characterization and cytocompatibility. Biomaterials. 2004;25:85-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 170] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 33. | Coury AJ, Slaikeu PC, Cahalan PT, Stokes KB, Hobot CM. Factors and interactions affecting the performance of polyurethane elastomers in medical devices. J Biomater Appl. 1988;3:130-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 60] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Ellis H, Moran BJ, Thompson JN, Parker MC, Wilson MS, Menzies D, McGuire A, Lower AM, Hawthorn RJ, O’Brien F. Adhesion-related hospital readmissions after abdominal and pelvic surgery: a retrospective cohort study. Lancet. 1999;353:1476-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 651] [Cited by in RCA: 638] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 35. | Beyene RT, Kavalukas SL, Barbul A. Intra-abdominal adhesions: Anatomy, physiology, pathophysiology, and treatment. Curr Probl Surg. 2015;52:271-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 36. | Demirel AH, Basar OT, Ongoren AU, Bayram E, Kisakurek M. Effects of primary suture and fibrin sealant on hemostasis and liver regeneration in an experimental liver injury. World J Gastroenterol. 2008;14:81-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 37. | Annabi N, Yue K, Tamayol A, Khademhosseini A. Elastic sealants for surgical applications. Eur J Pharm Biopharm. 2015;95:27-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 157] [Article Influence: 15.7] [Reference Citation Analysis (0)] |