Published online Apr 28, 2017. doi: 10.4254/wjh.v9.i12.603
Peer-review started: October 27, 2016
First decision: December 29, 2016
Revised: January 26, 2017
Accepted: March 12, 2017
Article in press: March 13, 2017
Published online: April 28, 2017
Processing time: 182 Days and 2.5 Hours
To assess for passive expansion of sub-maximally dilated transjugular intrahepatic portosystemic shunts (TIPS) and compare outcomes with maximally dilated TIPS.
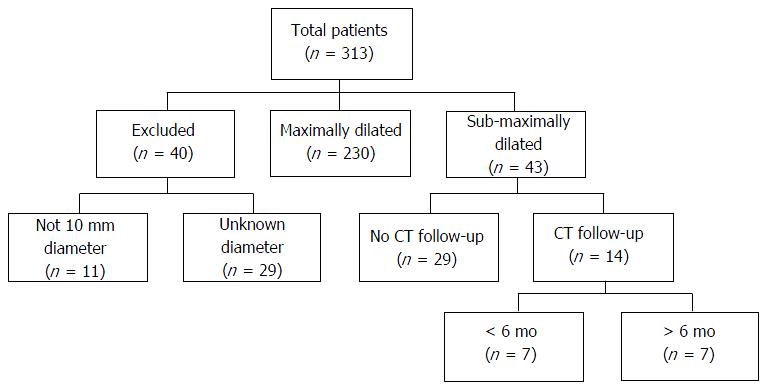
Polytetrafluoroethylene covered TIPS (Viatorr) from July 2002 to December 2013 were retrospectively reviewed at two hospitals in a single institution. Two hundred and thirty patients had TIPS maximally dilated to 10 mm (mTIPS), while 43 patients who were at increased risk for hepatic encephalopathy (HE), based on clinical evaluation or low pre-TIPS portosystemic gradient (PSG), had 10 mm TIPS sub-maximally dilated to 8 mm (smTIPS). Group characteristics (age, gender, Model for End-Stage Liver Disease score, post-TIPS PSG and clinical outcomes were compared between groups, including clinical success (ascites or varices), primary patency, primary assisted patency, and severe post-TIPS HE. A subset of fourteen patients with smTIPS underwent follow-up computed tomography imaging after TIPS creation, and were grouped based on time of imaging (< 6 mo and > 6 mo). Change in diameter and cross-sectional area were measured with 3D imaging software to evaluate for passive expansion.
Patient characteristics were similar between the smTIPS and mTIPS groups, except for pre-TIPS portosystemic gradient, which was lower in the smTIPS group (19.4 mmHg ± 6.8 vs 22.4 mmHg ± 7.1, P = 0.01). Primary patency and primary assisted patency between smTIPS and mTIPS was not significantly different (P = 0.64 and 0.55, respectively). Four of the 55 patients (7%) with smTIPS required TIPS reduction for severe refractory HE, while this occurred in 6 of the 218 patients (3%) with mTIPS (P = 0.12). For the 14 patients with follow-up computed tomography (CT) imaging, the median imaging follow-up was 373 d. There was an increase in median TIPS diameter, median percent diameter change, median area, and median percent area change in patients with CT follow-up greater than 6 mo after TIPS placement compared to follow-up within 6 mo (8.45 mm, 5.58%, 56.04 mm2, and 11.48%, respectively, P = 0.01).
Passive expansion of smTIPS does occur but clinical outcomes of smTIPS and mTIPS were similar. Sub-maximal dilation can prevent complications related to over-shunting in select patients.
Core tip: Sub-maximal dilation of transjugular intrahepatic portosystemic shunts (TIPS) is a method to reduce the risk of over-shunting and hepatic encephalopathy. The current study is a retrospective review to compare clinical outcomes of sub-maximally dilated TIPS (smTIPS) with maximally dilated TIPS (mTIPS) and assess for passive expansion of smTIPS. The study demonstrated that passive expansion of smTIPS does occur, however shunts may not fully expand and expansion may occur even after 6 mo. Clinical outcomes of smTIPS and mTIPS were similar, suggesting sub-maximal dilation may be an acceptable method to prevent complications related to over-shunting in select patients.
- Citation: Hsu MC, Weber CN, Stavropoulos SW, Clark TW, Trerotola SO, Shlansky-Goldberg RD, Soulen MC, Nadolski GJ. Passive expansion of sub-maximally dilated transjugular intrahepatic portosystemic shunts and assessment of clinical outcomes. World J Hepatol 2017; 9(12): 603-612
- URL: https://www.wjgnet.com/1948-5182/full/v9/i12/603.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i12.603
Transjugular intrahepatic portosystemic shunt (TIPS) is an established treatment for the sequelae of portal hypertension, particularly variceal hemorrhage and refractory ascites. Two major complications can arise following TIPS placement: Shunt dysfunction and hepatic encephalopathy (HE)[1-3]. Shunt dysfunction occurs from stenosis and the consequent rise in portosystemic gradient (PSG) resulting in relapse of clinical manifestations of portal hypertension[1,4-6]. In the era of bare metal stents, TIPS dysfunction was a major problem that led to relatively low primary patency rates, typically less than 50% at one year[1,5,7]. However, expanded polytetrafluoroethylene (PTFE) covered TIPS have improved patency rates and clinical outcomes compared to bare metal TIPS[1-4,8-10]. Primary patency rates at two years have now been shown to range from 62%-89%[7,10-14].
Despite these advances, HE remains a pertinent post-procedural complication as portosystemic shunt physiology can trigger or worsen HE[1,2,8]. New or progressive post-TIPS HE of any severity has been shown to occur in 5%-35% of patients, while severe post-TIPS HE that does not respond to medical management and requires TIPS reduction or occlusion, occurs in up to 7% of patients[3,10,15,16].
Given the potential conflicting relationship between portal decompression and HE, efforts have been made to develop techniques to balance the desired therapeutic effect while minimizing over-shunting[3,17]. One such technique is to sub-maximally dilate a 10 mm TIPS[18,19]. Sub-maximal dilation theoretically allows for further dilation of the TIPS in the event that the initial portal decompression is insufficient while avoiding over-shunting[6,16,18]. However, this technique would only be effective if the sub-maximally dilated TIPS do not expand significantly over time. Published data suggest the continued outward radial force of the TIPS stent may lead to passive expansion to its nominal diameter, limiting the value of initial gradient calibration[6,19,20]. The current study is a retrospective review to compare clinical outcomes of sub-maximally dilated TIPS (smTIPS) with maximally dilated TIPS (mTIPS) at a single large academic institution and assess for passive expansion of smTIPS in a sub-set of patients with follow-up cross-sectional imaging.
Approval from the Institutional Review Board was obtained for this retrospective study, which was carried out in full compliance with the Health Information Portability and Accountability Act. An interventional radiology database (Hi-IQ, Conexsys, Lincoln, RI) was used to identify all TIPS placed using an expanded PTFE-covered stent graft (Viatorr) between July 2002 and December 2013 at two hospitals in a single institution (n = 313). The electronic medical record was used to obtain patient characteristics, including age, gender, pre-TIPS Model for End-Stage Liver Disease (MELD) score, and pre-TIPS PSG, and retrospectively reviewed to assess for measurements of clinical outcomes, including post-TIPS PSG, clinical success, primary patency, primary assisted patency, and severe post-TIPS HE.
All TIPS creation was performed as previously described[12]. In patients who were considered vulnerable to post-TIPS HE based on (1) past medical history of HE on clinical evaluation by the referring hepatologist or interventional radiology service; or (2) low pre-TIPS PSG that could result in over-shunting post-TIPS as determined by the performing interventional radiologist, a modified TIPS creation procedure was performed. The modified TIPS creation involved initial placement of a nominal 10 mm TIPS stent that was sub-maximally dilated to 8 mm (smTIPS). Following initial dilation with the 8 mm balloon, the PSG was measured and post-TIPS portography was repeated at the same injection rate as the initial portogram (8-10 mL/s for 2 s for all cases). If the PSG normalized (≤ 12 mmHg) and there was no venographic evidence of elevated gradient (i.e., persistently filling varices), then the procedure was ended. Otherwise, the smTIPS stent was further dilated with a 10 mm balloon, PSG measured, and portography repeated (mTIPS). Coil embolization of persistently filling varices following TIPS creation with normalized PSG was performed in patients who initially presented with variceal hemorrhage.
Decision for angioplasty, thrombectomy, or stent placement during TIPS revision was based on venographic findings and PSG measurements. All patients received HE prophylaxis with lactulose[21]. In cases of severe post-TIPS HE refractory to medical management (protein restriction, lactulose, and/or rifaximin), TIPS reduction was performed with coaxial deployment of a FLAIR stent within the existing TIPS, or with a stent graft with parallel balloon-expandable stent as previously described[15]. All patients were instructed to maintain a protein-restricted diet. Patients with ascites were instructed to follow a fluid-restricted, low sodium diet.
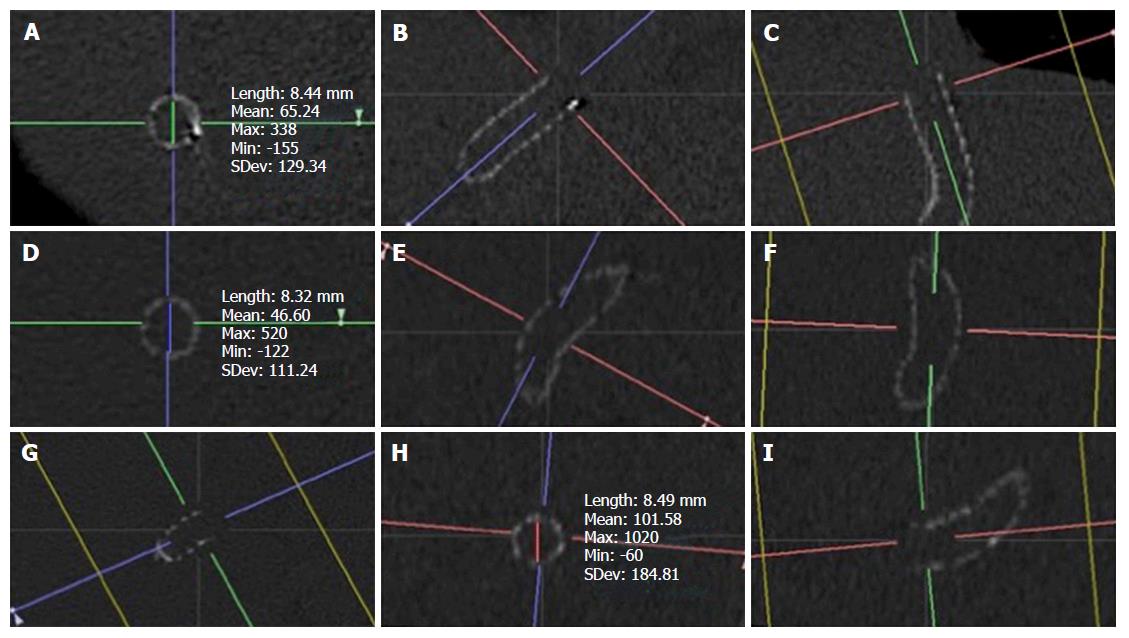
Inclusion criteria were patients with maximally dilated 10 mm PTFE-covered TIPS or 10 mm PTFE-covered TIPS sub-maximally dilated to 8 mm, as confirmed in the medical record (Figure 1). Of the 313 patients who underwent TIPS creation during the study period, forty patients were excluded due to placement of PTFE-covered TIPS of other nominal sizes (n = 11) or patients with post-TIPS stent deployment angioplasty diameters that were not confirmed in the medical record (n = 29). The remaining 273 patients had confirmed TIPS created with 10 mm nominal diameter stent, of which 230 patients had mTIPS created and 43 patients underwent creation of smTIPS. In the group of patients with smTIPS, any computed tomography (CT) imaging follow-up was identified from the medical record (n = 14) and reviewed with TeraRecon (TeraRecon, Foster City, CA), which is an advanced 3D imaging processing software. Using this imaging software, two orthogonal planes were obtained before measuring the diameter of the TIPS stent at the hepatic venous end, mid-stent, and the portal venous end (Figure 2). These values were then averaged to obtain a composite measure of TIPS diameter.
For the purposes of the current study, clinical success was defined based on the indication for TIPS placement. In patients who had TIPS placed for varices, clinical success was defined as absence of further episodes of variceal hemorrhage or development of varices requiring intervention. Patients in the varices group with less than one month of follow-up were excluded from the clinical success analysis (n = 6 for smTIPS; n = 21 for mTIPS). For patients with refractory ascites requiring TIPS placement, clinical success was categorized as complete response (absence of large-volume paracentesis within six months post-TIPS creation) or partial response (greater than 50% decrease in frequency of large-volume paracentesis). Patients in the ascites group with less than six months of follow-up were excluded from the clinical success analysis (n = 17 for smTIPS; n = 55 for mTIPS). Primary patency was defined as the time from TIPS creation until revision for identified stenosis, elevated PSG (> 12 mmHg), or recurrent symptoms. Primary assisted patency was defined as the time from TIPS creation until shunt occlusion requiring recanalization. Severe post-TIPS HE was defined as encephalopathy refractory to conservative medical management requiring TIPS reduction.
Statistical calculations were performed with GraphPad Prism software (version 6.05; GraphPad Software; La Jolla, CA). Unless otherwise indicated, all data were reported as mean ± SD. Categorical variables were compared using Fisher’s exact test. Continuous variables were compared using unpaired two-tailed Student’s t-test and Mann-Whitney test for data with a parametric and non-parametric distribution, respectively. Primary and primary assisted patency rates were estimated with the Kaplan-Meier method. Patients were censored at the time of death or liver transplantation. Patency rates between smTIPS and mTIPS groups were compared with the log-rank test. Severe post-TIPS HE was analyzed on an intention-to-treat basis resulting in 12 patients from the mTIPS group, originally dilated to 8 mm but subsequently maximally dilated to normalize the post-TIPS PSG, being included in the smTIPS group. A P-value less than 0.05 was considered significant for all analyses.
Patient characteristics and post-TIPS PSG are presented in Table 1. There were 150 males and 80 females who underwent mTIPS creation with a mean age of 54.5 years ± 0.7 (range, 20-81). Of the 43 patients that had smTIPS created, 23 were male with a mean age of 56.5 years ± 2.3 (range 10-83). There was no statistically significant difference between the two patient populations based on gender or age (P = 0.17 and 0.29, respectively). The mean pre-TIPS MELD score in patients with mTIPS was 13.5 ± 0.3 (range, 6-28) while it was 13.6 ± 0.6 (range, 6-25) for patients with smTIPS, which was not significantly different (P = 0.82). The mean pre-TIPS PSG was higher for patients with mTIPS (22.4 mmHg ± 7.1; range, 9-73) compared to those with smTIPS (19.4 mmHg ± 6.8; range, 8-45), which was statistically significant (P = 0.01). Following TIPS placement, the median PSG was 8 mmHg for both mTIPS and smTIPS (range, 2-20 and 1-13, respectively) with a mean percent decrease in PSG of 61.0% ± 12.4 (range, 0-89) and 59.1% ± 15.9 (range, 0-95), respectively. These were not statistically different (P = 0.13 and 0.53, respectively). The patients with post-TIPS PSG above the goal of 12 mmHg had a mean pre-TIPS PSG of 33 ± 13.2 (range 20-73) and experienced a mean percent decrease in PSG following TIPS creation of 48.3% ± 13.1 (range, 30.8-82.2) compared to those patients with post-TIPS PSG at or below the goal of 12 mmHg who had a mean pre-TIPS PSG of 21.1 mmHg ± 5.7 (range, 8-53) and mean percent decrease in PSG of 61.6% ± 12.6 (range, 0-95) (P < 0.01 and < 0.01) (Table 2).
| Sub-maximally dilated | Maximally dilated | P value | |
| Total patients | 43 | 230 | NA |
| Male | 23 | 150 | 0.17 |
| Female | 20 | 80 | |
| Mean age (yr) | 56.5 ± 2.3 (range 10-83) | 54.5 ± 0.7 (range 20-81) | 0.29 |
| Mean MELD | 13.6 ± 0.6 (range 6-25) | 13.5 ± 0.3 (range 6-28) | 0.82 |
| Mean pre-TIPS PSG (mmHg) | 19.4 ± 6.8 (range 8-45) | 22.4 ± 7.1 (range 9-73) | 0.01 |
| Median post-TIPS PSG (mmHg) | 8 (range 1-13) | 8 (range 2-20) | 0.13 |
| Mean percent change in PSG (%) | 59.1 ± 15.9 (range 0-95) | 61.0 ± 12.4 (range 0-89) | 0.53 |
| > 12 mmHg | ≤ 12 mmHg | P value | |
| Mean pre-TIPS PSG (mmHg) | 33 ± 13.2 (range 20-73) | 21.1 ± 5.7 (range 8-53) | < 0.01 |
| Mean percent change in PSG (%) | 48.3 ± 13.1 (range 30.8-82.2) | 61.6 ± 12.6 (range 0-95) | < 0.01 |
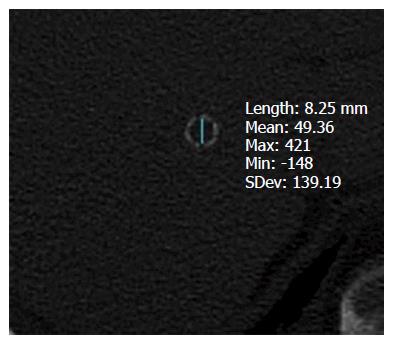
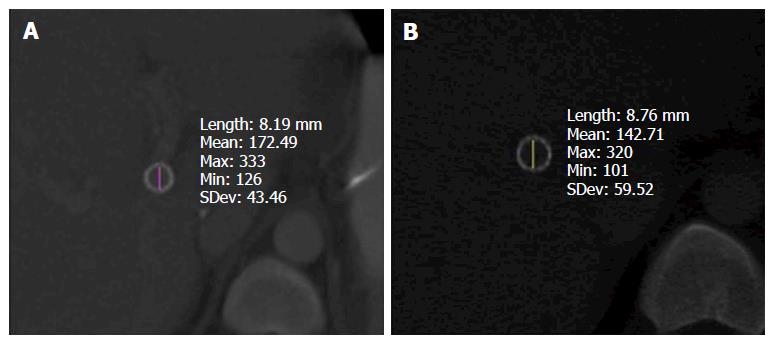
Of the 43 patients with smTIPS, there were 14 patients who had CT imaging follow-up (Table 3). Median time to last imaging follow-up was 373 d. The diameter and cross-sectional area of initial TIPS placement was assumed to be 8 mm and 50.27 mm2, corresponding to the diameter and area of the balloon used for dilation. Seven patients had last CT imaging follow-up within 6 mo (range, 4-172 d) and 7 patients had last CT imaging follow-up after 6 mo (range, 573-2131 d). The 7 patients with imaging follow-up within 6 mo had a median diameter, percent diameter change, area, and percent area change of 8.05 mm (range, 7.84-8.43 mm), 0.67%, 50.94 mm2, and 1.34%, respectively. The patients that had last imaging follow-up after 6 mo had a median diameter, percent diameter change, area, and percent area change of 8.45 mm (range, 8.23-8.72 mm), 5.58%, 56.04 mm2, and 11.48%, respectively. When comparing these two subgroups, there was a statistically significant increase in diameter, percent diameter change, area, and percent area change (P = 0.01) (Figures 3 and 4).
| Median diameter (mm) | Median percent diameter change | Median area (mm2) | Median percent area change | |
| < 6 mo (n = 7) | 8.05 (range 7.84-8.43) | 0.67% | 50.94 | 1.34% |
| > 6 mo (n = 7) | 8.45 (range 8.23-8.72) | 5.58% | 56.04 | 11.48% |
| P-value | 0.01 | 0.01% | 0.01 | 0.01% |
Post-TIPS clinical success is summarized in Table 4. Nine of 14 patients (64%) who had smTIPS placed for refractory ascites experienced complete clinical success and 11 of 14 patients (79%) experienced at least partial clinical success. Similarly, 63 of the 98 patients (64%) who underwent mTIPS placement for refractory ascites experienced complete clinical success and 89 of 98 patients (91%) had at least partial clinical success. There was no statistically significant difference in complete or partial clinical success between patients with smTIPS or mTIPS (P = 1 and P = 0.17, respectively). For variceal bleeding, 7 of 9 patients (78%) with smTIPS and 64 of 75 patients (85%) with mTIPS experienced clinical success, which was not significantly different (P = 0.62).
| Sub-maximally dilated | Maximally dilated | P value | |
| Complete Clinical Success of TIPS for Ascites | |||
| Yes | 9 (64) | 63 (64) | 1 |
| No | 5 (36) | 35 (36) | |
| Partial Clinical Success of TIPS for Ascites | |||
| Yes | 11 (79) | 89 (91) | 0.17 |
| No | 3 (21) | 9 (9) | |
| Clinical Success of TIPS for Varices | |||
| Yes | 7 (78) | 64 (85) | 0.62 |
| No | 2 (22) | 11 (15) | |
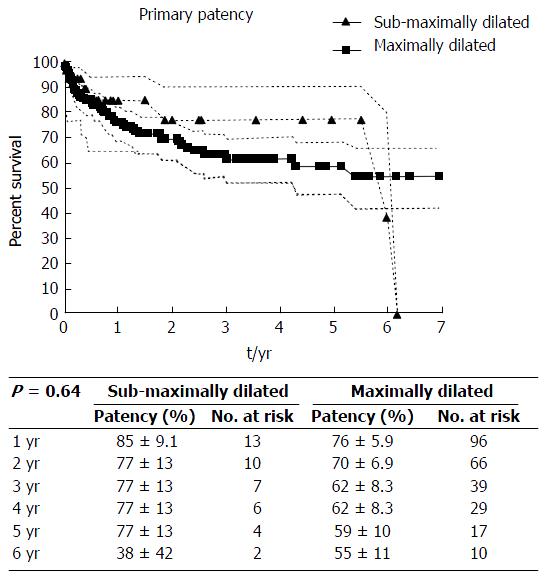
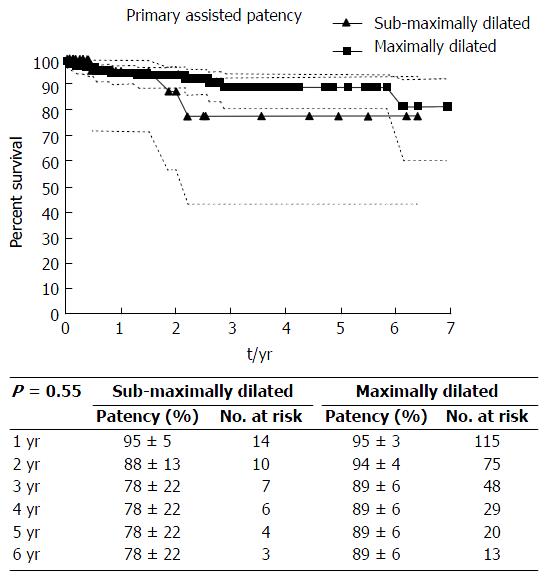
Kaplan-Meier survival curves depicting primary and primary assisted patency rates for smTIPS and mTIPS are shown in Figures 5 and 6, respectively. Primary patency for smTIPS and mTIPS was 85% ± 9.1% and 76% ± 5.9%, respectively, at one year, and 77% ± 13 and 70% ± 6.9%, respectively, after two years. Primary assisted patency for smTIPS and mTIPS was 95% ± 5% and 95% ± 3%, respectively, at one year, and 88% ± 13% and 94% ± 4%, respectively, after two years. There was no statistically significant difference between primary or primary assisted patency between the two groups (P = 0.64 and 0.55, respectively). Four of the 55 patients (7%) with smTIPS required TIPS reduction for severe refractory HE, while this occurred in 6 of the 218 patients with mTIPS (3%) using an intention-to-treat analysis, although not statistically significant (P = 0.12) (Table 5). In both smTIPS and mTIPS, the MELD scores and post-TIPS PSG were not significantly different between patients who experienced severe post-TIPS HE and those who did not (Table 6).
| Severe post-TIPS HE | Sub-maximally dilated | Maximally dilated | P value |
| Yes | 4 (7) | 6 (3) | 0.12 |
| No | 51 (93) | 212 (97) |
| Mean MELD with HE | Mean MELD without HE | P value | Median post-TIPS PSG with HE (mmHg) | Median post-TIPS PSG without HE (mmHg) | P value | |
| Sub-maximally dilated | 13.3 ± 2.9 (range 11-17) | 13.7 ± 4.3 (range 6-25) | 0.85 | 7.5 (range 6-8) | 8 (range 1-13) | 0.67 |
| Maximally dilated | 15.8 ± 4.3 (range 12-24) | 13.4 ± 4.1 (range 6-28) | 0.16 | 10 (range 4-11) | 8 (range 2-20) | 0.36 |
Despite improved patency rates and reduced need for shunt revision with PTFE-covered TIPS, HE remains a problem following TIPS placement with some speculation that improved patency rates may increase the incidence of HE[1-4,8,9]. HE arises when compounds derived from the intestine that require hepatic detoxification bypass the hepatic vascular bed in the setting of a portosystemic shunt, and subsequently enter systemic circulation. These compounds, typically nitrogenous in composition, travel to the central nervous system and disturb neurotransmission, which leads to eventual alterations in consciousness and behavior that manifest as HE[22]. This pathogenesis is further supported with the evidence that HE occurs with spontaneous portosystemic shunts, even in the absence of hepatic dysfunction or TIPS[23,24]. Prior investigations have also shown that an increased volume of shunted blood, decreased portal hepatic perfusion, and a lower PSG following TIPS placement correlate with higher rates of HE[5,17,18,25].
With knowledge of the pathogenesis of HE, different techniques have been studied in an effort to balance the desired therapeutic effect while minimizing over-shunting and increased risk of HE, such as smaller diameter TIPS or altering the goal in PSG reduction for patients with HE[3,17]. Another technique is sub-maximal dilation of TIPS, which allows for further staged dilation, if necessary, and theoretically minimizes over-shunting[6,16,18,26]. However, passive expansion of the TIPS may limit the effectiveness of this technique with prior evidence, in both peripheral circulation and TIPS, that suggests this phenomenon should be taken into consideration. Late expansion of bare metal nitinol stents was demonstrated after 6 mo in peripheral arteries of an animal model[27]. Haskal et al[20] showed that after immediate recoil of Wallstent TIPS stents after placement, passive expansion to nominal diameter occurred at follow-up venography three to six months later. Pieper et al[19] studied 29 patients with Viatorr TIPS sub-maximally dilated to a mean of 64% of their nominal area, and found passive expansion to 88% during follow-up, with significant expansion occurring within 6 mo. Finally, Gaba et al[28] evaluated 41 patients with 10 mm nominal Viatorr TIPS sub-maximally dilated to 8 mm, and demonstrated passive expansion with follow-up CT median stent diameter of 9.8 mm at a median of 76 d post TIPS creation without difference in incidence of post-TIPS HE in smTIPS vs mTIPS.
In the current study, continued passive expansion of smTIPS was observed in the subgroup of patients with cross-sectional imaging follow-up. Additionally, a significant difference in the increase in median diameter and area was observed when comparing the patients who had last imaging follow-up after 6 mo vs those within 6 mo. No patients in this subpopulation suffered severe refractory post-TIPS HE. While this change was statistically significant, the magnitude of expansion was not to the same degree as suggested by prior studies, and it also occurred over a longer time period (> 6 mo)[19,28]. The delayed and less extensive passive expansion observed in this study, although difficult to explain, may be secondary to dilation of the portosystemic tract with an 8 mm balloon prior to placement of the TIPS stent-graft. While the diameter of the balloon used to create the TIPS tract is not always described in prior investigations, a 10 mm balloon has been used previously[4]. It is hypothesized that dilating the tract to only 8 mm may lead to a greater initial counterforce on the stent from the elasticity of the surrounding liver parenchyma and new TIPS tract with minimal potential space, which leads to both slower and less passive expansion. In comparison, dilating the tract to 10 mm may hypothetically allow for a larger initial potential space for more immediate passive expansion of a TIPS sub-maximally dilated to 8 mm. Moreover, it is conceivable that more fibrotic livers with decreased compliance may differentially limit the extent of passive expansion, although this analysis was beyond the scope of this study.
In order to better understand whether or not passive expansion of the TIPS over time is clinically relevant, we compared a variety of outcomes in patients with smTIPS and mTIPS. The post-TIPS PSG demonstrated adequate portal decompression with a median PSG of 8 mmHg in both groups (P = 0.13) and no significant difference in mean percent change in PSG (P = 0.53). Overall, the observed rate of severe post-TIPS HE was low (4%), and not significantly different between mTIPS and smTIPS (P = 0.12), suggesting the step-wise approach to TIPS creation by assessing PSG following sub-maximal dilation may be effective in minimizing unnecessary over-dilation and thus, over-shunting. These findings are similar to prior reports[28]. The lack of an observable difference between the groups may be due to passive expansion allowing for an equilibrium to gradually develop as increasing amounts of blood are shunted through the liver, thus, minimizing severe refractory HE[26]. Additionally, there was no significant difference in median post-TIPS PSG or mean MELD between patients who suffered severe post-TIPS HE and those who did not for patients with smTIPS or mTIPS. Finally, no significant difference in primary and primary assisted patency or clinical success for both ascites and varices occurred between the two groups.
These results are somewhat contradictory to a prior study comparing nominal 8 mm and 10 mm TIPS which found increased rates of recurrent portal hypertensive complications in the 8 mm group, leading to early termination of the study[3]. A possible explanation for the conflicting results may be related to the small, but not insignificant amount of passive expansion demonstrated with smTIPS. Based on Poiseuille’s Law, volumetric flow rate is proportional to change in diameter to the fourth power, as well as change in pressure. It is postulated that despite a decrease in the change in pressure across the TIPS stent from passive expansion, the 5.6% increase in diameter observed in patients with CT imaging > 6 mo would disproportionately cause an increase in volumetric flow rate. As such, gradual passive expansion may slowly increase the amount of shunted blood and decrease the recurrence of portal hypertensive complications, yielding similar clinical success between the two groups obtained in the present study. Furthermore, the nominal 8 mm TIPS group in the same study had a higher incidence of shunt dysfunction, a majority without angiographically evident stenosis, than the smTIPS group in the current study, suggesting that a fixed, smaller diameter TIPS may provide insufficient portosystemic decompression and that passive expansion may be more efficacious in patients deemed to be at risk of post-TIPS HE[3]. Previously, the only mechanism to improve TIPS shunting in patients with nominal 8 mm TIPS was to place a parallel TIPS, as no further expansion was possible. The current study highlights a technique that would allow for further TIPS dilation in patients that show signs of inadequate portal decompression following initial creation of smTIPS, potentially obviating the need for a second parallel TIPS.
This study has several important limitations, including its retrospective design and data collection from a single center. The small size of the smTIPS group (n = 43) relative to the mTIPS group raises the possibility of a Type I error. As a tertiary center, identification of undocumented TIPS intervention or clinical follow-up at outside institutions is limited. There was more severe refractory post-TIPS HE in the smTIPS group vs the mTIPS group (7% vs 3%), although not statistically significant (P = 0.12). While this finding was not expected, it reflects selection bias between the two groups. Patients who underwent creation of smTIPS had a statistically significant lower mean pre-TIPS PSG compared to mTIPS (P = 0.01). This was not surprising given that patients deemed to be higher risk for HE following TIPS creation, which included a low pre-TIPS PSG, were preferentially selected to have smTIPS created to reduce the risk of over-shunting, as determined by the operating physician. Furthermore, even though shunt physiology is a known contributing factor for HE, the pathophysiology of HE is multifactorial and includes other precipitating factors such as hepatic decompensation, noncompliance with dietary restrictions, sepsis, and medications. Additional independent risk factors include older age, elevated serum creatinine, low serum sodium and low albumin; however, these clinical data were difficult to corroborate from a retrospective review spanning 10 years[2]. Only a minority (33%) of the patients with smTIPS had subsequent CT exams during the follow-up period. It is conceivable that this may not be representative of the entire subgroup. Additionally, patients did not undergo repeat angiographic TIPS evaluation following CT evidence of passive expansion, which would allow for repeat PSG measurement to determine the true hemodynamic consequences of passive expansion.
In conclusion, in patients with smTIPS there was passive expansion of 10 mm Viatorr TIPS stent-grafts even after 6 mo, however, not all reached their nominal diameter. The clinical outcomes, including incidence of severe post-TIPS HE, between sub-maximally and maximally dilated 10 mm Viatorr TIPS were similar. These findings suggest sub-maximal dilation may be an acceptable method to prevent complications related to over-shunting in select patients.
Transjugular intrahepatic portosystemic shunt (TIPS) is an established treatment for the sequelae of portal hypertension, particularly variceal hemorrhage and refractory ascites. Despite improved patency rates and reduced need for shunt revision with polytetrafluoroethylene-covered TIPS, hepatic encephalopathy (HE) remains a problem following TIPS placement with some speculation that improved patency rates may increase the incidence of HE. HE arises when compounds derived from the intestine that require hepatic detoxification bypass the hepatic vascular bed in the setting of a portosystemic shunt, and subsequently enter systemic circulation. One technique to balance portal decompression while minimizing over-shunting is sub-maximal dilation of TIPS. While sub-maximal dilation theoretically allows for further dilation of the TIPS in the event that the initial portal decompression is insufficient while avoiding over-shunting, published data suggest the continued outward radial force of the TIPS stent may lead to passive expansion to its nominal diameter and limit the value of initial gradient calibration.
As sub-maximal dilation of TIPS has gained increased clinical use, there have been more studies investigating the presence and effect of passive expansion in both peripheral circulation and TIPS. Late expansion of bare metal nitinol stents was demonstrated after 6 mo in peripheral arteries of an animal model. Haskal et al showed that after immediate recoil of Wallstent TIPS stents after placement, passive expansion to nominal diameter occurred at follow-up venography three to six months later. Pieper et al studied 29 patients with Viatorr TIPS sub-maximally dilated to a mean of 64% of their nominal area, and found passive expansion to 88% during follow-up, with significant expansion occurring within 6 mo. Finally, Gaba et al evaluated 41 patients with 10 mm nominal Viatorr TIPS sub-maximally dilated to 8 mm, and demonstrated passive expansion with follow-up computed tomography median stent diameter of 9.8 mm at a median of 76 d post TIPS creation without difference in incidence of post-TIPS HE in smTIPS vs mTIPS.
While the aforementioned studies focused on establishing the presence of passive expansion, there is a lack of published data investigating the clinical outcomes of sub-maximally dilated TIPS with maximally dilated TIPS in addition to the presence of passive expansion. While the study showed passive expansion does occur, not all shunts fully expanded to nominal diameter and expansion even occurred after 6 mo, unlike prior studies. More importantly, the comparison of clinical outcomes of smTIPS vs mTIPS showed no significant difference in primary patency, primary assisted patency, clinical success, or post-TIPS HE.
In patients who are at high risk for post-TIPS hepatic encephalopathy, based on pre-TIPS encephalopathy or low pre-TIPS portosystemic gradient, sub-maximal dilation may be an effective method to balance adequate portal decompression with the risk of over-shunting and hepatic encephalopathy with the knowledge that passive expansion following placement does not appear to affect clinical outcomes.
Sub-maximally dilated TIPS - TIPS stent grafts that are not fully dilated to nominal diameter following deployment.
The study is to compare clinical outcomes of smTIPS with mTIPS. The results suggest the method may be of significance.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chen JL, Fouad YM, Garbuzenko DV S- Editor: Qi Y L- Editor: A E- Editor: Li D
| 1. | Sommer CM, Gockner TL, Stampfl U, Bellemann N, Sauer P, Ganten T, Weitz J, Kauczor HU, Radeleff BA. Technical and clinical outcome of transjugular intrahepatic portosystemic stent shunt: bare metal stents (BMS) versus viatorr stent-grafts (VSG). Eur J Radiol. 2012;81:2273-2280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Riggio O, Angeloni S, Salvatori FM, De Santis A, Cerini F, Farcomeni A, Attili AF, Merli M. Incidence, natural history, and risk factors of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt with polytetrafluoroethylene-covered stent grafts. Am J Gastroenterol. 2008;103:2738-2746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 203] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 3. | Riggio O, Ridola L, Angeloni S, Cerini F, Pasquale C, Attili AF, Fanelli F, Merli M, Salvatori FM. Clinical efficacy of transjugular intrahepatic portosystemic shunt created with covered stents with different diameters: results of a randomized controlled trial. J Hepatol. 2010;53:267-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 110] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 4. | Barrio J, Ripoll C, Bañares R, Echenagusia A, Catalina MV, Camúñez F, Simó G, Santos L. Comparison of transjugular intrahepatic portosystemic shunt dysfunction in PTFE-covered stent-grafts versus bare stents. Eur J Radiol. 2005;55:120-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 85] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Casado M, Bosch J, García-Pagán JC, Bru C, Bañares R, Bandi JC, Escorsell A, Rodríguez-Láiz JM, Gilabert R, Feu F. Clinical events after transjugular intrahepatic portosystemic shunt: correlation with hemodynamic findings. Gastroenterology. 1998;114:1296-1303. [PubMed] |
| 6. | Hausegger KA, Karnel F, Georgieva B, Tauss J, Portugaller H, Deutschmann H, Berghold A. Transjugular intrahepatic portosystemic shunt creation with the Viatorr expanded polytetrafluoroethylene-covered stent-graft. J Vasc Interv Radiol. 2004;15:239-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 102] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 7. | Tripathi D, Ferguson J, Barkell H, Macbeth K, Ireland H, Redhead DN, Hayes PC. Improved clinical outcome with transjugular intrahepatic portosystemic stent-shunt utilizing polytetrafluoroethylene-covered stents. Eur J Gastroenterol Hepatol. 2006;18:225-232. [PubMed] |
| 8. | Angeloni S, Merli M, Salvatori FM, De Santis A, Fanelli F, Pepino D, Attili AF, Rossi P, Riggio O. Polytetrafluoroethylene-covered stent grafts for TIPS procedure: 1-year patency and clinical results. Am J Gastroenterol. 2004;99:280-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Boyer TD, Haskal ZJ. The Role of Transjugular Intrahepatic Portosystemic Shunt (TIPS) in the Management of Portal Hypertension: update 2009. Hepatology. 2010;51:306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 404] [Article Influence: 26.9] [Reference Citation Analysis (1)] |
| 10. | Weber CN, Nadolski GJ, White SB, Clark TW, Mondschein JI, Stavropoulos SW, Shlansky-Goldberg RD, Trerotola SO, Soulen MC. Long-Term Patency and Clinical Analysis of Expanded Polytetrafluoroethylene-Covered Transjugular Intrahepatic Portosystemic Shunt Stent Grafts. J Vasc Interv Radiol. 2015;26:1257-1265; quiz 1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 11. | Gaba RC, Omene BO, Podczerwinski ES, Knuttinen MG, Cotler SJ, Kallwitz ER, Berkes JL, Walzer NM, Bui JT, Owens CA. TIPS for treatment of variceal hemorrhage: clinical outcomes in 128 patients at a single institution over a 12-year period. J Vasc Interv Radiol. 2012;23:227-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Luca A, Miraglia R, Caruso S, Milazzo M, Sapere C, Maruzzelli L, Vizzini G, Tuzzolino F, Gridelli B, Bosch J. Short- and long-term effects of the transjugular intrahepatic portosystemic shunt on portal vein thrombosis in patients with cirrhosis. Gut. 2011;60:846-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 197] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 13. | Rössle M, Siegerstetter V, Euringer W, Olschewski M, Kromeier J, Kurz K, Langer M. The use of a polytetrafluoroethylene-covered stent graft for transjugular intrahepatic portosystemic shunt (TIPS): Long-term follow-up of 100 patients. Acta Radiol. 2006;47:660-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Saad WE, Darwish WM, Davies MG, Waldman DL. Stent-grafts for transjugular intrahepatic portosystemic shunt creation: specialized TIPS stent-graft versus generic stent-graft/bare stent combination. J Vasc Interv Radiol. 2010;21:1512-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Sze DY, Hwang GL, Kao JS, Frisoli JK, Kee ST, Razavi MK, Ahmed A. Bidirectionally adjustable TIPS reduction by parallel stent and stent-graft deployment. J Vasc Interv Radiol. 2008;19:1653-1658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Thalheimer U, Leandro G, Samonakis DN, Triantos CK, Senzolo M, Fung K, Davies N, Patch D, Burroughs AK. TIPS for refractory ascites: a single-centre experience. J Gastroenterol. 2009;44:1089-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Rössle M, Siegerstetter V, Olschewski M, Ochs A, Berger E, Haag K. How much reduction in portal pressure is necessary to prevent variceal rebleeding? A longitudinal study in 225 patients with transjugular intrahepatic portosystemic shunts. Am J Gastroenterol. 2001;96:3379-3383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Rossi P, Salvatori FM, Fanelli F, Bezzi M, Rossi M, Marcelli G, Pepino D, Riggio O, Passariello R. Polytetrafluoroethylene-covered nitinol stent-graft for transjugular intrahepatic portosystemic shunt creation: 3-year experience. Radiology. 2004;231:820-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 110] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 19. | Pieper CC, Sprinkart AM, Nadal J, Hippe V, Meyer C, Schild HH, Thomas D. Postinterventional passive expansion of partially dilated transjugular intrahepatic portosystemic shunt stents. J Vasc Interv Radiol. 2015;26:388-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Haskal ZJ, Pentecost MJ, Soulen MC, Shlansky-Goldberg RD, Baum RA, Cope C. Transjugular intrahepatic portosystemic shunt stenosis and revision: early and midterm results. AJR Am J Roentgenol. 1994;163:439-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 144] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 21. | Sharma P, Sharma BC, Agrawal A, Sarin SK. Primary prophylaxis of overt hepatic encephalopathy in patients with cirrhosis: an open labeled randomized controlled trial of lactulose versus no lactulose. J Gastroenterol Hepatol. 2012;27:1329-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 22. | Madoff DC, Wallace MJ, Ahrar K, Saxon RR. TIPS-related hepatic encephalopathy: management options with novel endovascular techniques. Radiographics. 2004;24:21-36; discussion 36-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Riggio O, Efrati C, Catalano C, Pediconi F, Mecarelli O, Accornero N, Nicolao F, Angeloni S, Masini A, Ridola L. High prevalence of spontaneous portal-systemic shunts in persistent hepatic encephalopathy: a case-control study. Hepatology. 2005;42:1158-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 132] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 24. | Watanabe A. Portal-systemic encephalopathy in non-cirrhotic patients: classification of clinical types, diagnosis and treatment. J Gastroenterol Hepatol. 2000;15:969-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 108] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Sarfeh IJ, Rypins EB. Partial versus total portacaval shunt in alcoholic cirrhosis. Results of a prospective, randomized clinical trial. Ann Surg. 1994;219:353-361. [PubMed] |
| 26. | Wróblewski T, Rowiński O, Ziarkiewicz-Wróblewska B, Górnicka B, Albrecht J, Jones EA, Krawczyk M. Two-stage transjugular intrahepatic porta-systemic shunt for patients with cirrhosis and a high risk of portal-systemic encephalopathy patients as a bridge to orthotopic liver transplantation: a preliminary report. Transplant Proc. 2006;38:204-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Zhao HQ, Nikanorov A, Virmani R, Jones R, Pacheco E, Schwartz LB. Late stent expansion and neointimal proliferation of oversized Nitinol stents in peripheral arteries. Cardiovasc Intervent Radiol. 2009;32:720-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 28. | Gaba RC, Parvinian A, Minocha J, Casadaban LC, Knuttinen MG, Ray CE, Bui JT. Should transjugular intrahepatic portosystemic shunt stent grafts be underdilated? J Vasc Interv Radiol. 2015;26:382-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |