Peer-review started: May 10, 2016
First decision: June 18, 2016
Revised: July 29, 2016
Accepted: September 21, 2016
Article in press: September 22, 2016
Published online: January 8, 2017
Processing time: 243 Days and 16.2 Hours
To evaluate the long-term treatment outcomes of tenofovir therapy in patients in a real world Australian tertiary care setting.
We performed a retrospective analysis of treatment outcomes among treatment-naïve and treatment-experienced patients receiving a minimum 3 mo tenofovir therapy through St Vincent’s Hospital Melbourne, Australia. We included patients receiving tenofovir [tenofovir disoproxil fumarate (TDF)] monotherapy, as well as patients treated with TDF in combination with a second antiviral agent. Patients were excluded if they demonstrated human immune-deficiency virus/hepatitis C virus/hepatitis delta virus coinfection or were less than 18 years of age. We considered virological and biochemical response, as well as safety outcomes. Virological response was determined by measurement of hepatitis B virus (HBV) DNA using sensitive assays; biochemical response was determined via serum liver function tests; histological response was determined from liver biopsy and fibroscan; safety analysis focused on glomerular renal function and bone mineral density. The primary efficacy endpoint was complete virological suppression over time, defined by HBV DNA < 20 IU/mL. Secondary efficacy endpoints included rates of biochemical response, and HB e antigen (HBeAg)/HB surface antigen loss and seroconversion over time.
Ninety-two patients were identified who fulfilled the enrolment criteria. Median follow-up was 26 mo (range 3-114). Mean age was 46 (24-78) years, 64 (70%) were male and 77 (84%) were of Asian origin. 55 (60%) patients were treatment-naïve and 62 patients (67%) were HBeAg-negative. Complete virological suppression was achieved by 45/65 (71%) patients at 12 mo, 37/46 (80%) at 24 mo and 25/28 (89%) at 36 mo. Partial virological response (HBV DNA 20-2000 IU/mL) was achieved by 89/92 (96.7%) of patients. Multivariate analysis showed a significant relationship between virological suppression at end of follow-up and baseline HBV DNA level (OR = 0.897, 95%CI: 0.833-0.967, P = 0.0046) and HBeAg positive status (OR = 0.373, 95%CI: 0.183-0.762, P = 0.0069). There was no difference in response comparing treatment-naïve and treatment-experienced patients. Three episodes of virological breakthrough occurred in the setting of non-compliance. Tenofovir therapy was well tolerated.
Tenofovir is an efficacious, safe and well-tolerated treatment in an Australian real-world tertiary care setting. Our data are similar to the reported experience from registration trials.
Core tip: Clinical trials have demonstrated that tenofovir is a safe and efficacious treatment for patients with chronic hepatitis B, with high rates of sustained virological suppression. There are limited data evaluating the safety and efficacy of tenofovir in real-world settings. The aim of this study was to evaluate the long-term treatment outcomes of tenofovir therapy in patients in an Australian tertiary care setting. We performed a retrospective analysis of treatment outcomes among treatment-naïve and treatment-experienced patients.
- Citation: Lovett GC, Nguyen T, Iser DM, Holmes JA, Chen R, Demediuk B, Shaw G, Bell SJ, Desmond PV, Thompson AJ. Efficacy and safety of tenofovir in chronic hepatitis B: Australian real world experience. World J Hepatol 2017; 9(1): 48-56
- URL: https://www.wjgnet.com/1948-5182/full/v9/i1/48.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i1.48
Chronic hepatitis B (CHB) affects 240-400 million people around the world[1]. It is estimated that 218000 people in Australia live with CHB, a population prevalence of approximately 1%[2]. CHB is associated with the long-term complications of cirrhosis, liver failure and hepatocellular carcinoma (HCC), in 15%-40% of patients. CHB is one of the most common causes of HCC, the most rapidly rising cause of cancer deaths in Australia[3-5].
The goal of treatment for CHB is to improve survival by preventing disease progression to cirrhosis, liver failure and HCC[6]. This can be achieved by long-term suppression of hepatitis B virus (HBV) DNA levels[7-10]. In long-term follow-up, sustained virological suppression has been associated with histological improvement and regression of cirrhosis, as well as reduced risk of hepatic decompensation and HCC[6,11-14]. Surrogate endpoints used in clinical trials include rates of biochemical [serum alanine aminotransferase (ALT) < upper limit of normal (ULN)], virological (undetectable HBV DNA level), serological [HB e antigen (HBeAg)/HB surface antigen (HBsAg) loss ± seroconversion] and histological (improvements in necro-inflammatory grade and fibrosis stage) response[13]. Current therapies approved for CHB include peginterferon-alpha, lamivudine (LMV), adefovir (ADV), telbivudine, entecavir (ETV) and tenofovir (TDF).
Tenofovir is a nucleotide analogue (NA) recommended as first-line treatment for CHB. Tenofovir was first developed as an antiviral for the treatment of human immune-deficiency virus (HIV). The safety and efficacy of TDF for the treatment of chronic HBV infection was confirmed in two phase-III clinical trials, enrolling patients with HBeAg-positive and HBeAg-negative CHB respectively. Rates of virological suppression were 76% and 93% at week 48 in HBeAg-positive and HBeAg-negative patients respectively[13], and > 98% overall at week 240[15]. Among HBeAg-positive patients, rates of HBeAg seroconversion were 21% and 40%, and rates of HBsAg seroconversion were 3% and 7%, at weeks 48 and 240, respectively[15]. Genotypic resistance to TDF has not been described. TDF is effective for the treatment of both treatment-naïve and treatment-experienced patients. TDF has a reported good safety profile. Reversible renal toxicity has been reported in < 2% of patients in registration/post-registration studies[16]. Decreased bone mineral density has been reported in HIV-infected patients treated with TDF, but the effect in HBV-mono-infected patients remains unclear[17,18].
There are limited data that describe the safety and efficacy of TDF in the “real world”. The few studies that have been published describe populations in Europe and North America[19-21]. There have been no reports of the experience with TDF in Australia. Such data are important. Australia is a multi-cultural country, and the CHB population is unique for the diversity of HBV genotypes, reflecting immigration patterns from Southern Europe, South-East Asia and Sub-Saharan Africa[2]. The rates of TDF response and resistance in Australia are unknown.
The aim of this study was to evaluate the efficacy and safety of long term TDF therapy in an Australian single-centre real-world cohort of CHB patients.
Data were collected retrospectively from a comprehensive clinical database of CHB patients receiving TDF through liver clinics at St Vincent’s Hospital Melbourne (Australia) between 7 March 2006 and 18 February 2014.
All patients receiving TDF 300 mg daily therapy for HBV mono-infection through St Vincent’s Hospital Melbourne were considered for analysis. Inclusion criteria included age > 18 years and treatment duration > 3 mo. Patients could be treatment-naïve or treatment-experienced. We included patients receiving TDF monotherapy, as well as patients treated with TDF in combination with a second antiviral agent. Patients were excluded in the setting of HIV, hepatitis C or hepatitis D co-infection.
TDF was prescribed in accordance with the Australian Pharmaceutical Benefit Scheme. Patients were required to satisfy the following criteria: Non-cirrhotic patients must demonstrate documented chronic liver injury confirmed via liver function tests or liver biopsy and must demonstrate appropriate HBV DNA levels according to HBeAg status (HBeAg positive patients HBV DNA > 20000 IU/mL; HBeAg negative patients HBV DNA > 2000 IU/mL). Patients with cirrhosis are required to demonstrate detectable HBV DNA. Patients may be NA naïve or experienced (having failed previous therapy).
Prior to 2010, HBV DNA levels were measured using the versant HBV DNA 3.0 assay (bDNA) (Siemens Healthcare Diagnostics, Tarrytown, NY) with a lower limit of detection (LLD) of 351 IU/mL. From 2010, HBV DNA levels were measured using the Cobas Taqman assay (LLD = 20 IU/mL, Roche Molecular Systems, Pleasanton, CA, United States).
Complete virological suppression was defined as plasma HBV DNA level < 20 IU/mL. Partial virological suppression was defined as plasma HBV DNA level of ≥ 20 IU/mL and < 2000 IU/mL. Virological breakthrough (VBT) was defined as an increase in viral load > 1 log10 from nadir, or by a detectable HBV DNA level on two serial measures in a patient who had previously achieved an undetectable HBV DNA level. Biochemical response was defined as the normalisation of serum ALT to < 45 IU/L. Serological response was defined as the loss of detectable HBeAg and/or HBsAg from serum (HBeAg/HBsAg loss) ± the development of antibodies against these antigens (HBeAg/HBsAg seroconversion).
The primary efficacy endpoint was complete virological suppression over time, defined by HBV DNA < 20 IU/mL. Secondary efficacy endpoints included rates of biochemical response, and HBeAg/HBsAg loss and seroconversion over time. We also measured rates of VBT and the occurrence of clinical events including hepatic decompensation and HCC. The assessment of safety was specifically focussed on renal function and, where available, bone mineral density.
All statistical analyses were performed using SAS 9.4. For descriptive statistics, continuous variables were summarised as median (25th-75th centile). Categorical variables were described as frequency and percentage. Comparisons between groups for demographic, clinical and virological data were performed using the Wilcoxon signed pair test for continuous data and Fisher’s exact test for categorical data. Significance was defined at P-value < 0.05. Kaplan Meier analysis was used to determine influences on the time to virological suppression. The associations between baseline HBeAg status, baseline HBV DNA, treatment experience, age, gender, baseline ALT, fibrosis stage and end of follow-up virological suppression were tested using Cox proportional hazards regression analysis and direct multivariate analysis. Ninty-two patients were included in the analysis of demographics and on-treatment safety and efficacy. Patients who had undetectable HBV DNA levels at time of commencement of TDF were excluded from the multivariable analysis (n = 18).
This study was approved by the Human Research Ethics Committee at St Vincent’s Hospital Melbourne (QA: 009/14).
A total of 92 patients were identified. Patient characteristics are summarised in Table 1. The majority of patients were male (70%), of Asian ethnicity (84%) and had HBeAg-negative disease (69%). Fifty-five (60%) were treatment-naive at the time TDF was commenced. Thirty-seven (40%) patients had been previously treated with NA therapy. Compared to treatment-naïve patients, treatment-experienced patients were more likely to have a lower serum HBV DNA level, and a normal serum ALT at the time TDF therapy was commenced. Seventeen treatment-experienced patients had a baseline HBV DNA level less than the lower limit of detection, and had directly switched to TDF for convenience. Nity-seven percent of patients received TDF monotherapy. Median duration of follow-up was 24 mo (6-42 mo).
| Baseline demographics | Total population (n = 92) | Treatment naïve (n = 55) | Treatment experienced, viraemic (n = 20) | Treatment experienced, non-viraemic (n = 17) |
| Age (yr) | ||||
| Mean (IQR) | 46 (36-54) | 42 (32-53) | 48 (41-57) | 55 (44-60) |
| Gender n (%) | ||||
| Male | 64 (69.6) | 39 (70.9) | 11 (55) | 14 (82.4) |
| Female | 28 (30.4) | 16 (29.1) | 9 (45) | 3 (17.6) |
| Ethnic origin n (%) | ||||
| African | 4 (4.3) | 3 (5.5) | 0 | 1 (5.9) |
| Asian | 77 (83.7) | 48 (87.3) | 16 (80) | 13 (76.5) |
| Caucasian | 5 (5.4) | 3 (5.5) | 0 | 2 (11.8) |
| Mediterranean | 2 (2.2) | 1 (1.8) | 1 (5) | 0 |
| Middle Eastern | 1 (1.1) | 0 | 1 (5) | 0 |
| Duration of therapy (mo) | ||||
| Median (IQR) | 24 (6-42) | 24 (12-36) | 24 (6-54) | 24 (12-42) |
| HBe antigen status n (%) | ||||
| HBeAg positive | 30 (32.6) | 19 (34.5) | 10 (50) | 1 (5.9) |
| HBeAg negative | 62 (67.4) | 36 (65.5) | 10 (50) | 16 (94.1) |
| Treatment history n (%) | ||||
| Experienced | 37 (40.2) | 0 | 20 (100) | 17 (100) |
| Adefovir | 10 (27) | 4 (20) | 6 (35.3) | |
| Adefovir/lamivudine | 13 (35.1) | 6 (30) | 7 (41.2) | |
| Lamivudine | 6 (16.2) | 4 (20) | 2 (11.8) | |
| Lamivudine/entecavir | 1 (2.7) | 1 (5) | 0 | |
| Entecavir | 5 (13.5) | 4 (20) | 1 (5.9) | |
| Entecavir/adefovir | 2 (5.4) | 1 (5) | 1 (5.9) | |
| Naïve | 55 (59.8) | 55 (100) | 0 | 0 |
| HBV DNA load (IU/mL) n (%) | ||||
| < 20 | 17 | - | - | 17 (100) |
| 20-2000 | 29 (31.5) | 6 (10.9) | 6 (30) | 0 |
| 2000-100000 | 11 (12) | 8 (14.5) | 3 (15) | 0 |
| > 100000 | 52 (56.5) | 41 (74.5) | 11 (55) | 0 |
| Median (IQR) | 1.8 × 105 (302-1.6 × 107) | 9.4 × 105 (9.7 × 104-3.7 × 107) | 1.8 × 105 (790-4.1 × 106) | N/A |
| ALT (U/L) n (%) | ||||
| 0-20 | 11 (12) | 2 (3.6) | 4 (20) | 5 (29.4) |
| 20-40 | 26 (28.3) | 10 (18.2) | 8 (40) | 8 (47.1) |
| 40-400 | 51 (55.4) | 39 (70.9) | 8 (40) | 4 (23.5) |
| > 400 | 4 (4.3) | 4 (7.3) | 0 | 0 |
| Median (IQR) | 30 (22-41.8) | 73 (41-140) | 34 (22.3-62.3) | 24 (19-44) |
| Serum creatinine (IU/mL) | ||||
| Median (IQR) | 70 (60-81.5) | 66.5 (50.8-71.5) | 71 (63.5-84.5) | 83 (69-93) |
| Pre-treatment biopsy n (%) | 69 (75) | 40 (72.7) | 16 (80) | 14 (82.4) |
| Fibrosis score n (%) | ||||
| 0 | 9 (9.8) | 5 (9.1) | 3 (15) | 3 (17.6) |
| 1 | 31 (33.7) | 21 (38.2) | 6 (30) | 4 (23.5) |
| 2 | 13 (14.1) | 8 (14.5) | 3 (15) | 2 (11.8) |
| 3 | 7 (7.6) | 1 (1.8) | 2 (10) | 4 (23.5) |
| 4 | 9 (9.8) | 5 (9.1) | 2 (10) | 2 (11.8) |
| Genotype n (%) | 35 (38) | 6 (10.9) | 9 (45) | 12 (70.6) |
| A | 3 (3.3) | 2 (3.6) | 0 | 1 (5.9) |
| B | 7 (7.6) | 2 (3.6) | 1 (5) | 4 (23.5) |
| C | 13 (14.1) | 2 (3.6) | 7 (35) | 4 (23.5) |
| D | 3 (3.3) | 0 | 0 | 2 (11.8) |
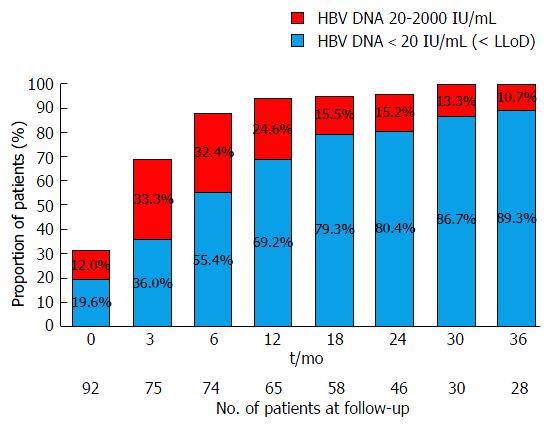
Virological response to TDF is detailed in Table 2 and Figure 1. Overall, 77 (83.7%) patients achieved complete virological suppression by the end-of-follow-up, with a median time to suppression of 6 mo (IQR = 3-12 mo). The rates of complete virological suppression were 71% (45/65) at 12 mo, 80% (37/46) at 24 mo and 89% (25/28) at 36 mo. Eighty-nine/ninety-two (96.7%) achieved a partial virological response (HBV DNA 20-2000 IU/mL).
| Follow-up (mo) | 0 | 6 | 12 | 18 | 24 | 30 | 36 |
| Patients with viral load n (%) | 92 (100) | 74 (80.4) | 65 (70.7) | 58 (63) | 46 (50) | 30 (32.6) | 28 (30.4) |
| Virological suppression n (%) | 18 (19.6) | 41 (55.4) | 45 (69.2) | 46 (79.3) | 37 (80.4) | 26 (86.7) | 25 (89.3) |
Treatment-naïve individuals: Complete virological suppression was achieved in 43/55 (78%) of patients with a median time to suppression of 6 mo (IQR = 3-12 mo). Rates of complete virological suppression were 70% (29/44) at 12 mo, 87% (26/30) at 24 mo and 100% at 36 mo (18/18). This was maintained by 50/55 (91%) of patients throughout follow-up. While a total of five patients failed to maintain complete virological suppression, only three patients experienced VBT. This was associated with reported non-compliance. In the first patient, HBV DNA levels rose from undetectable viral load at 12 mo to 55 IU/mL at 18 mo and 23 IU/mL at 24 mo. In the second patient, HBV DNA levels rose from undetectable at 18 mo to 1940 IU/mL at 24 mo and 578 IU/mL at 30 mo. In the final patient, HBV DNA levels increased from undetectable at 24 mo to 46300 IU/mL at 42 mo and 29 IU/mL at 48 mo (results for the intervening 12 mo were unavailable). A transient low level viraemia not meeting the definition for VBT (single HBV DNA level of 23 IU/mL and 21 IU/mL, respectively, following achievement of complete virological suppression) was observed in two additional patients before returning to undetectable levels.
Treatment-experienced individuals: Viraemia was seen in 54% (20/37) of treatment-experienced individuals at the time TDF therapy was commenced. Complete virological suppression was achieved among 85% (17/20) of viraemic patients with a median time to suppression of 6 mo (IQR = 3-18 mo). Rates of complete virological suppression were 64% (9/14) at 12 mo, 58% (7/12) at 24 mo and 63% (5/8) at 36 mo. While 3 patients showed persistent viraemia at 36 mo, all had an HBV DNA level < 2000 IU/mL and subsequently achieved complete virological suppression by 60 mo. This was maintained in 17/20 (85%) patients throughout follow-up. No patient met the strict definition for virological breakthrough. Two patients demonstrated a single instance of HBV DNA > 20 IU/mL (28 IU/mL and 27 IU/mL) before returning to undetectable levels, but did not meet the criteria for VBT. Among patients with an undetectable plasma HBV DNA level at baseline, 16/17 patients (94%) maintained complete virological suppression throughout follow-up. One patient experienced a single HBV DNA level of 40 IU/mL.
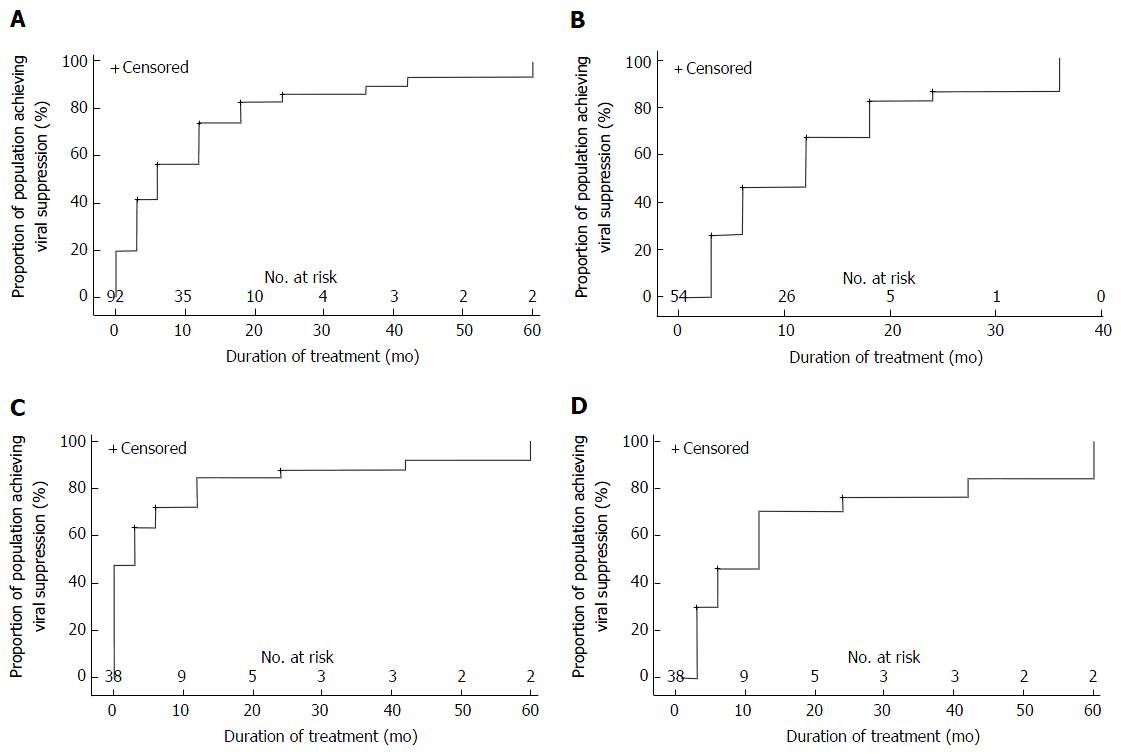
Survival analysis of the influence of treatment experience on complete virological suppression is presented in Figure 2. Cox proportional hazards analysis was carried out on viraemic patients at baseline with the final model including baseline HBV DNA, HBeAg status treatment experience, age and baseline ALT (Table 3). Multivariate analysis showed a significant relationship between virological suppression at end of follow-up and baseline HBV DNA (OR = 0.897, 95%CI: 0.833-0.967, P = 0.0046) and HBeAg status (HR = 0.373, 95%CI: 0.183-0.762, P = 0.0069).
| Covariates | Multivariable | |
| Hazard ratio (95%CI) | P value | |
| Baseline HBV DNA (log10 IU/mL) | 0.897 (0.833-0.967) | 0.0046 |
| HBeAg status (HBeAg pos vs neg) | 0.373 (0.183-0.762) | 0.0069 |
| Treatment experience (Naïve vs experienced) | 1.189 (0.598-2.364) | 0.6207 |
| Age (yr) | 1.018 (0.992-1.044) | 0.1760 |
| ALT (log10 IU/mL) | 1.093 (0.816-1.465) | 0.5505 |
HBeAg loss/seroconversion: Among 30 HBeAg-positive patients at baseline, 5 (16.7%) underwent HBeAg loss and seroconversion. Median time to seroconversion was 30 mo (9-60 mo). Mean age was 38 years (24-48 mo) and median baseline HBV DNA was 1.7 × 107 IU/mL. There was no significant difference in HBeAg seroconversion rates between treatment-naïve and treatment-experienced patients (P = 0.87). Two patients showed documented HBeAg seroreversion while on TDF treatment. One patient who was HBeAg-negative at baseline underwent HBeAg seroreversion at 24 mo of TDF therapy. HBeAg seroconversion then reoccurred at 36 mo and was sustained for the remainder of follow-up. The second case showed HBeAg loss without seroconversion at 12 mo, followed by seroreversion at 36 mo of treatment. This patient had only been on therapy for 36 mo at end of follow-up.
HBsAg: One treatment-naïve male underwent HBsAg loss and seroconversion following 12 mo of TDF therapy. This individual was 28 years of age and HBeAg-negative at the time TDF was started.
Mean ALT at baseline was 134 ± 340 U/mL and 33 ± 13 U/mL at end of follow-up, with a mean change of -101.3 ± 340.4 U/mL. Treatment experienced patients had a lower mean baseline ALT than treatment naïve patients (52 ± 70.2 U/mL vs 190 ± 431.1 U/mL, P = 0.02). They consequently had a lower mean change in ALT at the end of follow-up (-21 ± 68 U/mL vs -155 ± 432 U/mL, P = 0.28). Baseline serum ALT levels were within the normal range in 42/92 (45.7%) patients. By the end of treatment, 76/92 (83%) patients were within the normal range. Of the 50 patients who were above the ULN at baseline, 38 (76%) achieved ALT normalisation by the end of follow-up.
Hepatocellular cancer was diagnosed in two patients within 12 mo of starting TDF treatment. Both patients were diagnosed with cirrhosis prior to commencing TDF and one patient died as a result of their malignancy. A third patient was diagnosed with HCC 12 mo after ceasing TDF. No episodes of hepatic decompensation were recorded in the study population.
Treatment was discontinued at the discretion of individual clinicians in 11 patients (12%). Treatment was discontinued as a result of a rise in serum creatinine levels in 3 patients (3%). All 3 patients had a peak serum creatinine < 1.5 × ULN. Two patients had only been taking TDF for 3 mo, and both had previously been treated with long-term LMV plus ADV therapy. Creatinine returned to the normal range on switch to ETV in one patient and LMV plus ADV in the other. The clinical decision to return the latter patient to LMV plus ADV was determined by the treating physician and is not a standard treatment recommendation. One treatment-naïve patient was noted to have a rising serum creatinine at month 42 of treatment (peak creatinine = 118 μmol/L, ULN = 104). Serum phosphate levels were normal. Treatment was switched to ETV and creatinine returned to the normal range. Bone mineral density measurements were not routine and were only performed in a minority. There were 4 patients who were noted to have osteopenia or osteoporosis after treatment durations of 18-42 mo. Two of these patients were treatment naïve at the time TDF was started, one patient had previously been treated with adefovir for 5 years, and one patient had previously received LMV for 4 years. None of the 4 patients had a baseline bone mineral density measure available for comparison. Tenofovir was discontinued in another 4 patients after reports of non-specific adverse events including nausea, dizziness, fatigue, weight loss and myalgia.
Tenofovir is a potent antiviral therapy for CHB. It has been associated with high rates of virological suppression in clinical trials and virological resistance is yet to be described in clinical practice[13]. Post-registration real-world studies provide confirmation of therapy efficacy outside of the selected clinical trial situation, and monitor for rare adverse events. This is the first real-life study of TDF in an Australian setting. It validates the efficacy and safety of TDF in NA-naïve and experienced patients with CHB.
Similarly to registration trials and real-life studies, the study population was predominantly male and HBeAg negative, with 75% over the age of 40. However, while registration trials studied predominantly Caucasian populations, this population was mostly of Asian origin. Other ethnic minorities were also represented, reflecting Australian migration patterns. HBV genotype data were available for a minority of patients (35/92). Genotypes C and B were the most common genotypes, with A and D also represented. Studies from Europe and Asia are dominated by genotype A/D (Europe) and genotype B/C (Asia), limiting cross genotype comparisons. The tenofovir registration studies included mainly Western genotype A/D individuals, as have most of the real world data[13,22,23]. While this study’s patient size may be limited, the population studied here are unique for the breadth of ethnicity and HBV genotypes and comprise the first dataset described in an Australian population. Liver fibrosis ranged predominately between stages 1 and 2, with 10% of patients diagnosed with cirrhosis at baseline. Forty percent of the population were NA treatment-experienced.
The efficacy of TDF therapy in our cohort largely reflects the clinical trial experience. A daily dose of 300 mg of TDF was found to achieve at least partial virological suppression in 97% of patients and complete virological suppression in 84% of patients, demonstrating robust efficacy. Complete virological suppression was sustained by 94% of patients over time. Patients with persistent viraemia had HBV DNA levels < 2080 IU/mL, except for two patients who had a viral load 1.2-2.6 × 105 IU/mL after 3 mo on therapy. Virological breakthrough was only observed in one patient with documented non-compliance. The clinical variables that were independently associated with time to suppression were high HBV DNA level at baseline, and HBeAg seropositivity. Previous NA therapy was not associated with reduced response rate. HBeAg seroconversion was achieved in 17% of HBeAg positive patients, with median duration of follow-up of 24 mo. One patient underwent HBsAg loss and seroconversion after 12 mo of treatment. The efficacy data are therefore broadly consistent with the experience in the registration studies[13,22,24].
Our findings are also in keeping with “real life” international studies. Pol et al[23] reviewed safety and efficacy data from two real-life cohorts in the United Kingdom and Europe. The cohorts had a combined sample size of 362 NA-naïve patients with a median follow-up of 9-28 mo. Virological suppression was achieved in 80%-89% of patients with breakthrough identified in 2% of patients, without any corresponding resistance mutations. HBeAg seroconversion occurred in 7%-18% of patients and HBsAg loss occurred in 2% of the European cohort. Eighty-seven percent of patients achieved ALT normalisation by 30 wk[5]. Pan et al[21] analysed the real-life safety and efficacy of TDF in 90 Asian-American patients over 48 wk. Ten percent of the population had a history of prior treatment with lamivudine or adefovir. Virological suppression was achieved in 82% of patients, 12% of patients underwent HBeAg seroconversion and 66% of patients showed ALT normalisation by the end of follow-up. No resistance to TDF was detected and the treatment was considered well-tolerated with few related adverse events. While our results reflect those of other “real life” data, few studies have included treatment-experienced patients and if so they compose only a small minority. This is an area for future focus considering clinical practice of switching patients over to TDF from older less effective NAs.
Therapy was ceased in 12% of patients at the discretion of individual clinicians due to concern about renal (3%) and bone impairment. Tenofovir was self-ceased by 4% of patients due to non-specific adverse events. It was not possible to establish causality for these events; all possible renal events were mild and reversible with discontinuation. No confirmed cases of proximal tubular dysfunction were observed. Isolated cases of osteomalacia and osteopenia concurrent with TDF therapy have also been reported in the HBV literature[25]; there are more numerous reports in the HIV literature. In our cohort, although cases of osteopaenia and osteoporosis were noted, the absence of baseline bone mineral density scans meant that causality could not be speculated. Chronic liver disease itself is a risk factor for osteoporosis. We now perform routine monitoring of renal function and fasting serum phosphate levels every six months, as well as bone mineral densitometry at baseline and every 3 years to screen for osteoporosis. This approach needs prospective validation.
In conclusion, our Australian experience shows TDF to be an effective and safe therapy for patients with CHB. Rates of sustained virological suppression were very high. Elevated baseline HBV DNA level and HBeAg-positive disease were associated with slower time to suppression, but TDF resistance was not observed, and most patients achieved complete virological suppression with continued therapy. Tenofovir was generally well tolerated. This study supports the findings of other real-life experience into the efficacy and safety of TDF in the treatment of CHB.
Chronic hepatitis B (CHB) affects 240-400 million people around the world. It is estimated that 218000 people in Australia live with CHB, a population prevalence of approximately 1%. CHB is associated with the long-term complications of cirrhosis, liver failure and hepatocellular carcinoma (HCC), in 15%-40% of patients. CHB is one of the most common causes of HCC, the fastest increasing cause of cancer death in Australia.
Tenofovir is a nucleotide analogue recommended as first-line treatment for CHB. The safety and efficacy of tenofovir disoproxil fumarate (TDF) for the treatment of chronic HBV infection has been confirmed in two phase-III clinical trials. There are limited data that describe the safety and efficacy of TDF in the “real world”. The few studies that have been published describe populations in Europe and North America. There have been no reports of the experience with TDF in Australia.
Out of 92 patients, 89 (96.7%) achieved partial virological response and 77 (83.7%) achieved complete virological suppression by the end-of-follow-up. Predictors of virological suppression included lower baseline HBV DNA and HBeAg negative disease.
The authors’ Australian experience shows that TDF is an effective and safe therapy for patients with CHB. Rates of sustained virological suppression were very high and most patients achieved complete virological suppression with continued therapy. TDF resistance was not observed and treatment was generally well tolerated. This study supports the findings of other real-life experience into the efficacy and safety of TDF in the treatment of CHB.
The hepatitis B virus (HBV) is transmitted vertically, parenterally or via mucosal exposure to infected blood or bodily fluids. CHB is associated with long-term complications of cirrhosis, liver failure and hepatocellular carcinoma. They carry high rates of morbidity and mortality and affect 15%-40% of patients at some point in their life. Tenofovir disoproxil fumarate is a nucleotide analogue used in the treatment of CHB. Prior to its role in CHB, TDF was used in the treatment of HIV type 1 infection.
This is a well-designed and well-written real life data study of tenofovir treatment for hepatitis B.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Australia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kadayifci A, Pai CG, Silva LD S- Editor: Qiu S L- Editor: A E- Editor: Li D
| 1. | Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30:2212-2219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1217] [Cited by in RCA: 1328] [Article Influence: 102.2] [Reference Citation Analysis (0)] |
| 2. | MacLachlan JH, Allard N, Towell V, Cowie BC. The burden of chronic hepatitis B virus infection in Australia, 2011. Aust N Z J Public Health. 2013;37:416-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 3. | MacLachlan JH, Cowie BC. Liver cancer is the fastest increasing cause of cancer death in Australians. Med J Aust. 2012;197:492-493. [PubMed] |
| 4. | Lok AS. Chronic hepatitis B. N Engl J Med. 2002;346:1682-1683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 337] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 5. | Liver EAFTSOT. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2323] [Cited by in RCA: 2401] [Article Influence: 184.7] [Reference Citation Analysis (0)] |
| 6. | Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2125] [Cited by in RCA: 2171] [Article Influence: 135.7] [Reference Citation Analysis (0)] |
| 7. | Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1739] [Cited by in RCA: 1740] [Article Influence: 82.9] [Reference Citation Analysis (0)] |
| 8. | Realdi G, Fattovich G, Hadziyannis S, Schalm SW, Almasio P, Sanchez-Tapias J, Christensen E, Giustina G, Noventa F. Survival and prognostic factors in 366 patients with compensated cirrhosis type B: a multicenter study. The Investigators of the European Concerted Action on Viral Hepatitis (EUROHEP). J Hepatol. 1994;21:656-666. [PubMed] |
| 9. | Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, Iloeje UH. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2309] [Cited by in RCA: 2365] [Article Influence: 124.5] [Reference Citation Analysis (0)] |
| 10. | Iloeje UH, Yang HI, Su J, Jen CL, You SL, Chen CJ. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology. 2006;130:678-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1164] [Cited by in RCA: 1174] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 11. | Chen LP, Zhao J, Du Y, Han YF, Su T, Zhang HW, Cao GW. Antiviral treatment to prevent chronic hepatitis B or C-related hepatocellular carcinoma. World J Virol. 2012;1:174-183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Liaw YF, Chu CM. Hepatitis B virus infection. Lancet. 2009;373:582-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 929] [Cited by in RCA: 993] [Article Influence: 62.1] [Reference Citation Analysis (1)] |
| 13. | Marcellin P, Heathcote EJ, Buti M, Gane E, de Man RA, Krastev Z, Germanidis G, Lee SS, Flisiak R, Kaita K. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med. 2008;359:2442-2455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 890] [Cited by in RCA: 910] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 14. | Lee JM, Ahn SH, Kim HS, Park H, Chang HY, Kim DY, Hwang SG, Rim KS, Chon CY, Han KH. Quantitative hepatitis B surface antigen and hepatitis B e antigen titers in prediction of treatment response to entecavir. Hepatology. 2011;53:1486-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 15. | Gordon SC, Krastev Z, Horban A, Petersen J, Sperl J, Dinh P, Martins EB, Yee LJ, Flaherty JF, Kitrinos KM. Efficacy of tenofovir disoproxil fumarate at 240 weeks in patients with chronic hepatitis B with high baseline viral load. Hepatology. 2013;58:505-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 16. | Pipili C, Cholongitas E, Papatheodoridis G. Review article: nucleos(t)ide analogues in patients with chronic hepatitis B virus infection and chronic kidney disease. Aliment Pharmacol Ther. 2014;39:35-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Carr A, Hoy J. Low bone mineral density with tenofovir: does statistically significant mean clinically significant? Clin Infect Dis. 2010;51:973-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Grund B, Peng G, Gibert CL, Hoy JF, Isaksson RL, Shlay JC, Martinez E, Reiss P, Visnegarwala F, Carr AD. Continuous antiretroviral therapy decreases bone mineral density. AIDS. 2009;23:1519-1529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 175] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 19. | Carey I, Nguyen DorothyJoe H, Al-Freah M, Knighton S, Bruce M, Suddle A, Harrison PM, Agarwal K. P47 De-novo antiviral therapy with nucleos(t)ide analogues in ‘real-life’ patients with chronic hepatitis B infection: comparison of virological responses between lamivudine adefovir vs entecavir vs tenofovir therapy. Gut. 2011;60:A22-A23. [DOI] [Full Text] |
| 20. | Lampertico P, Soffredini R, Viganò M, Yurdaydin C, Idilman R, Papatheodoris G, Margheriti K, Buti M, Esteban R, Zaltron S. Oc.07.1 2-Year Effectiveness and Safety of Tenofovir in 302 Nuc-Naive Patients with Chronic Hepatitis B: A Multicenter European Study in Clinical Practice. Digestive and Liver Disease. 2012;44:S70. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Pan CQ, Trinh H, Yao A, Bae H, Lou L, Chan S. Efficacy and safety of tenofovir disoproxil fumarate in Asian-Americans with chronic hepatitis B in community settings. PLoS One. 2014;9:e89789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Heathcote EJ, Marcellin P, Buti M, Gane E, De Man RA, Krastev Z, Germanidis G, Lee SS, Flisiak R, Kaita K. Three-year efficacy and safety of tenofovir disoproxil fumarate treatment for chronic hepatitis B. Gastroenterology. 2011;140:132-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 364] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 23. | Pol S, Lampertico P. First-line treatment of chronic hepatitis B with entecavir or tenofovir in ‘real-life’ settings: from clinical trials to clinical practice. J Viral Hepat. 2012;19:377-386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 24. | Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, Washington MK, Germanidis G, Flaherty JF, Aguilar Schall R. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1228] [Cited by in RCA: 1369] [Article Influence: 114.1] [Reference Citation Analysis (0)] |
| 25. | Parsonage MJ, Wilkins EG, Snowden N, Issa BG, Savage MW. The development of hypophosphataemic osteomalacia with myopathy in two patients with HIV infection receiving tenofovir therapy. HIV Med. 2005;6:341-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |