Published online Aug 8, 2016. doi: 10.4254/wjh.v8.i22.942
Peer-review started: March 18, 2016
First decision: April 19, 2016
Revised: May 12, 2016
Accepted: July 11, 2016
Article in press: July 13, 2016
Published online: August 8, 2016
Processing time: 140 Days and 23.9 Hours
AIM: To assess the impact of model for end-stage liver disease (MELD) score on patient survival and morbidity post living donor liver transplantation (LDLT).
METHODS: A retrospective study was performed on 80 adult patients who had LDLT from 2011-2013. Nine patients were excluded and 71 patients were divided into two groups; Group 1 included 38 patients with a MELD score < 20, and Group 2 included 33 patients with a MELD score > 20. Comparison between both groups was done regarding operative time, intra-operative blood requirement, intensive care unit (ICU) and hospital stay, infection, and patient survival.
RESULTS: Eleven patients died (15.5%); 3/38 (7.9%) patients in Group 1 and 8/33 (24.2%) in Group 2 with significant difference (P = 0.02). Mean operative time, duration of hospital stay, and ICU stay were similar in both groups. Mean volume of blood transfusion and cell saver re-transfusion were 8 ± 4 units and 1668 ± 202 mL, respectively, in Group 1 in comparison to 10 ± 6 units and 1910 ± 679 mL, respectively, in Group 2 with no significant difference (P = 0.09 and 0.167, respectively). The rates of infection and systemic complications (renal, respiratory, cardiovascular and neurological complications) were similar in both groups.
CONCLUSION: A MELD score > 20 may predict mortality after LDLT.
Core tip: We assessed the impact of model for end-stage liver disease (MELD) score on patient survival and morbidity after living donor liver transplantation (LDLT). A total of 71 patients were included and divided into two groups: Group 1 had 38 patients with a MELD score < 20 and Group 2 had 33 patients with a MELD score > 20. We compared between both groups regarding operative time, intra-operative blood requirement, duration of intensive care unit and hospital stay, infection, and patient survival. We found that a MELD score > 20 could predict mortality after LDLT.
- Citation: Dabbous H, Sakr M, Abdelhakam S, Montasser I, Bahaa M, Said H, El-Meteini M. Living donor liver transplantation for high model for end-stage liver disease score: What have we learned? World J Hepatol 2016; 8(22): 942-948
- URL: https://www.wjgnet.com/1948-5182/full/v8/i22/942.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i22.942
Orthotopic liver transplantation (OLT) is now considered an established treatment option for patients with end-stage liver diseases (ESLD). However, the increasing scarcity of grafts in comparison to the number of waiting patients, as well as the high procedure cost, lead to difficult decisions about how to distribute such scarce organs[1,2]. This highlights the need to identify patients who are likely to have good outcome following liver transplantation[3,4]. The Child-Turcotte-Pugh (CTP) score was originally developed for assessing the outcome of patients with liver cirrhosis and portal hypertension and was extended to stratify patients on the waiting list for liver transplantation[5]. The use of CTP in prioritizing potential liver transplant recipients is limited by several factors. Ascites and hepatic encephalopathy are subjective variables and are affected by medical treatment; also CTP score lacks renal function assessment which strongly affects prognosis in cirrhotic patients[6]. The model for end-stage liver disease (MELD) was first described by Malinchoc et al[7] as a mathematical model for predicting postoperative three-month survival for patients who underwent transjugular intrahepatic porto-systemic shunt. The MELD score was then validated as a predictor of mortality for a wide variety of liver diseases[8], including cirrhotic patients awaiting liver transplantation[9]. Afterwards, MELD score was incorporated as a clear and objective system based on easily measurable laboratory parameters to reduce mortality among patients on the waiting list[10,11]. The ideal allocation system should allocate livers to candidates who are most likely to die without transplantation, and also to those who have a high probability of survival after OLT[12]. In February 2002, the United Network for Organ Sharing introduced a new allocation policy for cadaveric liver transplants based on the MELD score[13]. This new policy stratified patients according to the risk of death while they are on the waiting list[14]. The impact of the MELD score on postoperative mortality is still elusive.
The aim of this retrospective study was to assess the impact of the MELD score on patient survival and morbidity post living donor liver transplantation (LDLT).
Between January 2011 and January 2013, 80 adult patients with ESLD had received LDLT at the Ain Shams Center for Organ Transplant, Cairo, Egypt. Nine patients were excluded: Three had small-for-size grafts; one recipient had a combined organ (liver and kidney) transplant and 5 recipients had incomplete follow-up records. The remaining 71 transplants were included in this retrospective study. Seventy patients had LDLT with a right liver graft, and one patient had a left liver graft. The graft recipient weight ratio was between 0.8 and 1.7. The immunosuppressive regimen included cyclosporine or tacrolimus, mycophenolate mofetil (MMF), and corticosteroids in all patients except those transplanted for hepatocellular carcinoma (HCC). In patients transplanted for HCC, the regimen included calcineurin inhibitor and steroids only. Trough levels of cyclosporine were maintained between 200 and 300 ng/mL. Trough levels of tacrolimus were maintained between 8 and 12 ng/mL. Rapid withdrawal of corticosteroids within three months was routine in all patients (all transplanted for hepatitis C virus). In cases of acute rejection, the first-line therapy consisted of optimization of the maintenance level of immunosuppression. If there was no response, then MMF or rapamycin were added to the patient’s regimen, if not already being taken. In some cases, a shift from cyclosporine to tacrolimus was beneficial. A small dose of steroids was used if all other measures failed.
The seventy one patients included in this study were divided into two groups. Group 1 included 38 patients with a MELD score less than 20, and Group 2 included 33 patients with a MELD score more than 20.
The MELD score was calculated using laboratory results collected immediately before liver transplantation with no adjustments for malignancy. We calculated the MELD score using the following formula: MELD = [0.957 × ln (creatinine mg/dL) + 0.378 × ln (bilirubin mg/dL) + 1.12 × ln (INR) + 0.643 × 108]. We reported the age, sex of the recipient, diagnosis, indication for liver transplantation, modified CTP score as well as cold and warm ischemia time. The diagnosis of chronic liver disease was confirmed by histopathology of the explanted liver. The modified CTP score was calculated and each patient was categorized as A, B, or C. Operative data (including operative time and intra-operative blood transfusion) and early post-operative outcomes [including intensive care unit (ICU) stay, hospital stay, incidence of infection and other morbidities including renal impairment, cardiovascular, respiratory and neurological complications] were compared between the two groups. Overall patient survival was also compared between the two groups. Survival was calculated using the date of transplant to either 5 years post-transplant or to the end-point of this study in January 2016.
Categorical data were presented as numbers and percentages. Quantitative data were presented as the mean, standard deviations, ranges, median and interquartile ranges. For qualitative data, the comparison between the two groups was performed by using the χ2 test and Fisher exact test. For quantitative data, the comparison between the two groups was performed using an independent t-test for parametric data and a Mann-Whitney test for non-parametric data. The Kaplan-Meier survival analysis was used to assess the overall survival of both groups. The confidence interval was set to 95%, and the margin of error that was accepted was set to 5%. All data were analyzed using SPSS version 17. A P-value more than 0.05 was considered to indicate a non-significant (NS) difference between the two groups; a P-value less than 0.05 was considered to be statistically significant (S).
The statistical methods of this study were reviewed by Ahmed Mukhtar, Department of Anesthesia and Critical Care, Diploma of Medical Biostatistics, Faculty of Medicine, Cairo University, Cairo, Egypt.
This retrospective study included 71 patients classified into two groups according to their preoperative MELD score. Demographic data, Child classification, and cold and warm ischemia time were comparable between both groups (Table 1).
| Variable | MELD < 20 (n = 38) | MELD > 20 (n = 33) |
| Age (yr) (mean ± SD) | 47.8 ± 7.8 | 46.2 ± 7.9 |
| Sex | ||
| Male | 34 (89.5 | 32 (97) |
| Female | 4 (10.5) | 1 (3) |
| Diagnosis | ||
| ESLD | 27 (71.1) | 26 (78.8) |
| HCC | 3 (7.9) | 0 |
| ESLD + HCC | 8 (21) | 7 (21.2) |
| Child-Turcotte-Pugh | ||
| A | 0 | 0 |
| B | 3 (7.9) | 0 |
| C | 35 (92.1) | 33 (100) |
| Cold ischemia time (min) | 47 ± 23 | 42 ± 30 |
| Warm ischemia time (min) | 54.4 ± 20.2 | 53.7 ± 16.9 |
Overall patient survival was compared between both groups from the date of transplant to 5 years post-transplant or to the end-point of this study in January 2016. Eleven patients (15.5%) died during this study: Three patients out of 38 (7.9%) in Group 1 with a MELD less than 20 and 8 patients out of 33 (24.2%) in Group 2 with a MELD more than 20.
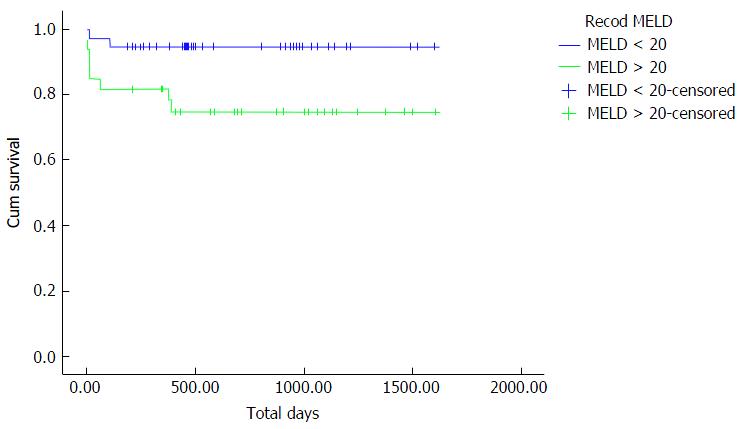
The 1, 3 and 5-year survival rates in Group 1 were 94.7%, 94.7% and 92.1% respectively, in comparison to 81.8%, 81.8% and 75.8% respectively in Group 2 with statistically significant difference between both groups (P = 0.02). Mortality occurred mainly in the early postoperative period in ICU because of respiratory failure due to weak respiratory muscles with poor weaning capability from mechanical ventilation (two patients in Group 1 and six patients in Group 2).
Figure 1 shows the Kaplan-Meier curve for overall survival of both groups, where Group 1 patients had a statistically significant higher overall survival rate compared to Group 2 patients.
In this study, there was no statistically significant difference observed among the two groups with regard to mean hospital and ICU stay. In Group 1, the mean hospital stay was 30 ± 14 d in comparison to 29 ± 18 d in Group 2 (P = 0.937). The mean ICU stay in Group 1 was 7 ± 3 d, while in Group 2, it was 9 ± 4 d (P = 0.315).
There was no statistically significant difference between the groups with respect to operative time, blood loss, and intra-operative blood transfusion (cell saver, blood product). The mean operative time in Group 1 was 11.1 ± 2 h (with a range of 7-15 h), and in Group 2, it was 10.6 ± 1.4 h (with a range of 9-14 h), (P = 0.292). The mean volume of blood transfusion and cell saver re-transfusion were 8 ± 4 units and 1668 ± 202 mL, respectively, in Group 1 in comparison to 10 ± 6 units and 1910 ± 679 mL, respectively, in Group 2 (P = 0.09 and 0.167).
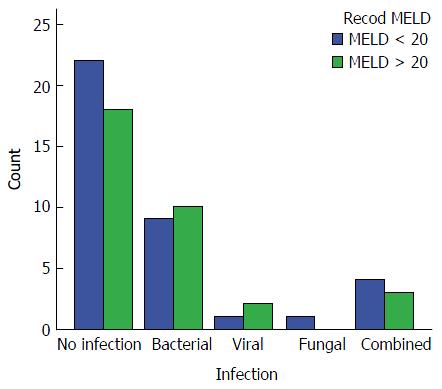
Infection: The overall incidence of infection in this study was 42.3% (30 out of 71 patients). In Group 1, the incidence of infection was 39.5% (15/38 patients). Bacterial infection was the most common representing 23.6% of the patients, while viral infection [cytomegalovirus (CMV)] was detected in 2.6%, fungal in 2.6% and combined infection in 10.5%. In Group 2, the incidence of infection was 45.5% (15/33 patients). Bacterial infection was the most common type of infection, representing 30.3%, while viral infection (CMV) was detected in 6%, fungal in 0% and combined infection in 9.1%. No statistically significant difference was detected between the groups regarding infection rates (P = 0.79) (Figure 2).
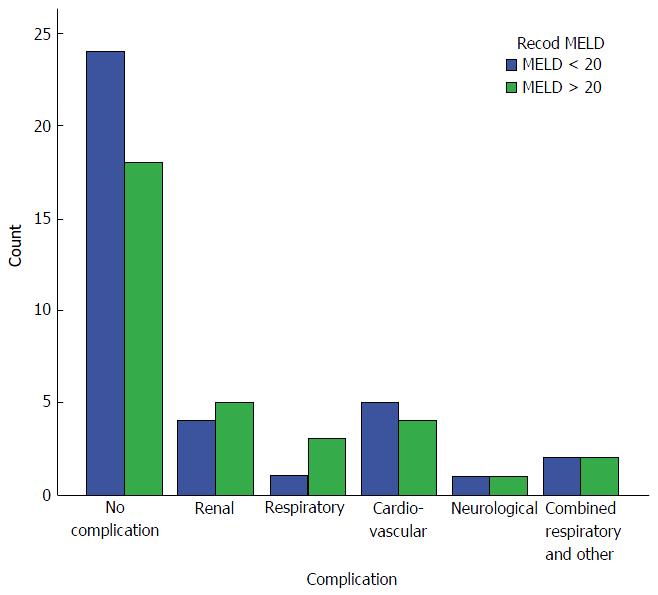
Systemic complications: There were no significant differences observed between groups with regard to the incidence of systemic complications including renal, respiratory, cardiovascular, and neurological complications (34.2% and 45.5% in Groups 1 and 2, respectively, P = 0.869).
Renal impairment was the most common complication in both group (10.5% in Group 1 and 15.2% in Group 2), followed by cardiovascular complications (13.2% in Group 1 and 12.1% in Group 2) consisting of mainly hypertension in most patients and arrhythmia in 2 patients. Neurological complications occurred in 2.6% and in 3% of the patients in Groups 1 and 2, respectively. Respiratory complications (basal atelectasis, pleural effusion, adult respiratory distress syndrome and respiratory infection) occurred in 7.9% of the patients in Group 1 compared to 15.2% in Group 2. Two patients in Group 1 (5.3%) and 2 patients in Group 2 (6.1%) had a combined respiratory and other system complications (Figure 3).
The large imbalance between patient demand and donated organs is a pressing problem in LDLT. The best solution to this problem is still a matter of debate. Unfortunately, prioritizing extremely sick patients makes it likely that patients who are not as sick will be forced to wait until getting worse and their chances for success become also diminished[15]. Patients who are very sick may have worse post-transplant outcomes than healthier patients[16]. Thus, the optimal system would offer grafts to those who are sufficiently sick to justify the transplantation but not too sick to benefit from it[17]. The urgency of need should be optimized with the likelihood of satisfactory postoperative outcomes so as to avoid ‘‘ineffective transplantation’’[18].
An accurate prognostic model could also help potential transplant recipients and their families make decisions by providing them with information on the patient’s survival probability post-transplantation[19,20]. The MELD score was achieved to help prioritizing prospective liver allograft recipients. Its accuracy to predict short-term mortality among patients with end-stage liver disease has been largely established[21]. However, an ideal selection system should incorporate predictions for survival while the patient is on the waiting list as well as following transplantation. The development of a model that may predict post-transplant outcomes based on pre-transplant variables is difficult because of variation in surgical skills and chance events that occur in the perioperative period. In addition to other factors such as graft rejection, biliary and vascular complications which are independent of pre-transplant events. Although it seems reasonable that pre-transplant variables which constitute the MELD score may influence the immediate post-transplant phase, their ability to predict long term outcome appears less likely. Recently, several investigators examined the predictive value of MELD for post-transplantation outcome, but with conflicting results and limited follow-up period; thus, a clear consensus has not emerged yet[22,23].
In a systematic review about the performance of MELD score in the setting of liver transplantation, Cholongitas et al[9] concluded that the MELD is not a good predictor for short-term mortality following liver transplantation, and further studies were needed to assess its long term performance. Additionally, Batista et al[24] demonstrated that the preoperative MELD score showed low overall accuracy for predicting survival after liver transplantation; similar to what was described in other Brazilian studies. On the other hand, worse survival rates in recipients with higher MELD scores has been reported by some authors[25-27]. The current study confirms the relation between MELD score and post liver transplantation survival. The incidence of mortality was 7.9% in patients with a MELD score less than 20 compared to 24.2% in patients with a MELD greater than 20, with significant difference between both groups (P = 0.02).
Our study shows no significant impact of MELD score on the duration of hospital and ICU stay; these findings are comparable with those of Poon et al[28], while many studies such as Foxton et al[29], demonstrated that liver transplantation of patients with higher MELD scores resulted in an increased ICU and hospital stay as well as increased need for renal replacement therapy. Additionally, Buchanan et al[30] showed that patients in the highest MELD group had a longer ICU stay than those in the lower MELD group (P = 0.008). Lee et al[31] and Massicotte et al[32] concluded that the MELD score did not predict blood loss or blood product requirements during liver transplantation, while others such as Feng et al[33] demonstrated that massive blood transfusion during liver transplantation can be predicted by preoperative MELD score. In our study, no definite relation was detected between MELD score and intra-operative blood loss or requirements of blood transfusion.
In the current study, the incidence of infection was comparable between both groups with no significant difference between a MELD score that was less or more than 20. This conclusion is the same finding of Li et al[34] in which a univariate analysis of risk factors for postoperative bacterial and fungal infections showed no statistically significant difference in regards to the MELD score. However, in the study of Selzner et al[35], high MELD score recipients had more frequent postoperative pneumonia in comparison to those with low MELD (P = 0.003), while no differences were observed in rates of biliary complications or overall infections.
In conclusion, a MELD score more than 20 can predict poor overall survival post LDLT. No significant relation was found between MELD score and intra-operative blood loss or blood requirement, hospital and ICU stay, or post LDLT morbidity.
Orthotopic liver transplantation has become an established treatment approach for patients with end-stage liver disease, but the growing scarcity of grafts compared to numbers of waiting patients, and the high cost of this procedure, make it difficult to make decisions about how to distribute such scarce organs. The impact of the model for end-stage liver disease (MELD) score on postoperative mortality and morbidity following liver transplantation is not well-established yet.
The authors assessed the impact of MELD score on patient survival and morbidity post living donor liver transplantation (LDLT) in the current retrospective study that was performed on 71 adult patients who had LDLT from 2011-2013. They were divided into two groups: Group 1 included 38 patients with a MELD score < 20, and Group 2 included 33 patients with a MELD score > 20. They found that MELD score > 20 can predict poor overall survival post LDLT. No significant relation was found between MELD score and intra-operative blood loss or blood requirement, hospital and intensive care unit stay, or post LDLT morbidity.
This is the first Egyptian study that addresses the impact of MELD score on patient survival and morbidity post living donor liver transplantation.
The findings of this study may represent a future strategy that may help prioritize prospective liver allograft recipients and predict post-transplantation outcome.
The MELD score is a mathematical model based on easily measurable laboratory tests. It is calculated immediately prior to liver transplantation through the following formula: MELD = [0.957 × ln (creatinine mg/dL) + 0.378 × ln (bilirubin mg/dL) + 1.12 × ln (INR) + 0.643 × 108].
The authors have done a retrospective study on impact of MELD score on patient survival and morbidity after living donor liver transplantation. The data may be useful for liver transplantation.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Egypt
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: He JY, Julie NL, Srivastava M S- Editor: Qiu S L- Editor: A E- Editor: Li D
| 1. | Merion RM, Schaubel DE, Dykstra DM, Freeman RB, Port FK, Wolfe RA. The survival benefit of liver transplantation. Am J Transplant. 2005;5:307-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 626] [Cited by in RCA: 617] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 2. | Biggins SW. Beyond the numbers: rational and ethical application of outcome models for organ allocation in liver transplantation. Liver Transpl. 2007;13:1080-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Schaubel DE, Sima CS, Goodrich NP, Feng S, Merion RM. The survival benefit of deceased donor liver transplantation as a function of candidate disease severity and donor quality. Am J Transplant. 2008;8:419-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 252] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 4. | Schaubel DE, Guidinger MK, Biggins SW, Kalbfleisch JD, Pomfret EA, Sharma P, Merion RM. Survival benefit-based deceased-donor liver allocation. Am J Transplant. 2009;9:970-981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 243] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 5. | Christensen E. Prognostic models including the Child-Pugh, MELD and Mayo risk scores--where are we and where should we go? J Hepatol. 2004;41:344-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 103] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 6. | Durand F, Valla D. Assessment of the prognosis of cirrhosis: Child-Pugh versus MELD. J Hepatol. 2005;42 Suppl:S100-S107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 424] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 7. | Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 2064] [Article Influence: 82.6] [Reference Citation Analysis (0)] |
| 8. | Boursier J, Cesbron E, Tropet AL, Pilette C. Comparison and improvement of MELD and Child-Pugh score accuracies for the prediction of 6-month mortality in cirrhotic patients. J Clin Gastroenterol. 2009;43:580-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Cholongitas E, Marelli L, Shusang V, Senzolo M, Rolles K, Patch D, Burroughs AK. A systematic review of the performance of the model for end-stage liver disease (MELD) in the setting of liver transplantation. Liver Transpl. 2006;12:1049-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 215] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 10. | Tenório AL, Macedo FI, Miranda LE, Fernandes JL, da Silva CM, Neto OL, Lacerda CM. Survival on waiting list for liver transplantation before and after introduction of the model for end-stage liver disease score. Transplant Proc. 2010;42:407-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Freeman RB, Wiesner RH, Edwards E, Harper A, Merion R, Wolfe R. Results of the first year of the new liver allocation plan. Liver Transpl. 2004;10:7-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 331] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 12. | Ghobrial RM, Gornbein J, Steadman R, Danino N, Markmann JF, Holt C, Anselmo D, Amersi F, Chen P, Farmer DG. Pretransplant model to predict posttransplant survival in liver transplant patients. Ann Surg. 2002;236:315-322; discussion 322-323. [PubMed] |
| 13. | Martin AP, Bartels M, Hauss J, Fangmann J. Overview of the MELD score and the UNOS adult liver allocation system. Transplant Proc. 2007;39:3169-3174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Ravaioli M, Grazi GL, Ballardini G, Cavrini G, Ercolani G, Cescon M, Zanello M, Cucchetti A, Tuci F, Del Gaudio M. Liver transplantation with the Meld system: a prospective study from a single European center. Am J Transplant. 2006;6:1572-1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | UNOS. Rationale for Objectives of Equitable Organ Allocation. [accessed. 2011;Aug 15] Available from: http//www.unos.org/resources/bioethics.asp?index = 10. |
| 16. | Neuberger J, Gimson A, Davies M, Akyol M, O’Grady J, Burroughs A, Hudson M. Selection of patients for liver transplantation and allocation of donated livers in the UK. Gut. 2008;57:252-257. [PubMed] |
| 17. | Dawwas MF, Gimson AE. Candidate selection and organ allocation in liver transplantation. Semin Liver Dis. 2009;29:40-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Zhang M, Yin F, Chen B, Li YP, Yan LN, Wen TF, Li B. Pretransplant prediction of posttransplant survival for liver recipients with benign end-stage liver diseases: a nonlinear model. PLoS One. 2012;7:e31256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Jacob M, Lewsey JD, Sharpin C, Gimson A, Rela M, van der Meulen JH. Systematic review and validation of prognostic models in liver transplantation. Liver Transpl. 2005;11:814-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Lewsey JD, Dawwas M, Copley LP, Gimson A, Van der Meulen JH. Developing a prognostic model for 90-day mortality after liver transplantation based on pretransplant recipient factors. Transplantation. 2006;82:898-907. [PubMed] |
| 21. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D’Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3462] [Cited by in RCA: 3674] [Article Influence: 153.1] [Reference Citation Analysis (0)] |
| 22. | Brown RS, Kumar KS, Russo MW, Kinkhabwala M, Rudow DL, Harren P, Lobritto S, Emond JC. Model for end-stage liver disease and Child-Turcotte-Pugh score as predictors of pretransplantation disease severity, posttransplantation outcome, and resource utilization in United Network for Organ Sharing status 2A patients. Liver Transpl. 2002;8:278-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 174] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 23. | Jacob M, Copley LP, Lewsey JD, Gimson A, Toogood GJ, Rela M, van der Meulen JH. Pretransplant MELD score and post liver transplantation survival in the UK and Ireland. Liver Transpl. 2004;10:903-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 104] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 24. | Batista TP, Sabat BD, Melo PS, Miranda LE, Fonseca-Neto OC, Amorim AG, Lacerda CM. Employment of MELD score for the prediction of survival after liver transplantation. Rev Col Bras Cir. 2012;39:105-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Brandão A, Fuchs SC, Gleisner AL, Marroni C, Zanotelli ML, Cantisani G. MELD and other predictors of survival after liver transplantation. Clin Transplant. 2009;23:220-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Monteiro F, Coria SA, Boni R, Pereira LA. Model for end-stage liver disease: impact of the new deceased donor liver allocation policy in São Paulo, Brazil. Transplant Proc. 2009;41:226-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Yoo HY, Thuluvath PJ. Short-term postliver transplant survival after the introduction of MELD scores for organ allocation in the United States. Liver Int. 2005;25:536-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Poon KS, Chen TH, Jeng LB, Yang HR, Li PC, Lee CC, Yeh CC, Lai HC, Su WP, Peng CY. A high model for end-stage liver disease score should not be considered a contraindication to living donor liver transplantation. Transplant Proc. 2012;44:316-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Foxton MR, Al-Freah MA, Portal AJ, Sizer E, Bernal W, Auzinger G, Rela M, Wendon JA, Heaton ND, O’Grady JG. Increased model for end-stage liver disease score at the time of liver transplant results in prolonged hospitalization and overall intensive care unit costs. Liver Transpl. 2010;16:668-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 30. | Buchanan P, Dzebisashvili N, Lentine KL, Axelrod DA, Schnitzler MA, Salvalaggio PR. Liver transplantation cost in the model for end-stage liver disease era: looking beyond the transplant admission. Liver Transpl. 2009;15:1270-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 31. | Lee J, Chung MY. Does the model for end-stage liver disease score predict transfusion amount, acid-base imbalance, haemodynamic and oxidative abnormalities during living donor liver transplantation? J Int Med Res. 2011;39:1773-1782. [PubMed] |
| 32. | Massicotte L, Beaulieu D, Roy JD, Marleau D, Vandenbroucke F, Dagenais M, Lapointe R, Roy A. MELD score and blood product requirements during liver transplantation: no link. Transplantation. 2009;87:1689-1694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 33. | Feng ZY, Jin XD, Chen YZ. [Predictors of massive blood transfusion in liver transplantation for patients with benign end-stage liver disease]. Zhonghua Yi Xue Za Zhi. 2008;88:3040-3044. [PubMed] |
| 34. | Li C, Wen TF, Mi K, Wang C, Yan LN, Li B. Analysis of infections in the first 3-month after living donor liver transplantation. World J Gastroenterol. 2012;18:1975-1980. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 35. | Selzner M, Kashfi A, Cattral MS, Selzner N, McGilvray ID, Greig PD, Levy GA, Renner EL, Grant DR. Live donor liver transplantation in high MELD score recipients. Ann Surg. 2010;251:153-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |