Published online Jul 18, 2016. doi: 10.4254/wjh.v8.i20.858
Peer-review started: February 29, 2016
First decision: April 15, 2016
Revised: June 1, 2016
Accepted: June 27, 2016
Article in press: June 29, 2016
Published online: July 18, 2016
Processing time: 135 Days and 17.9 Hours
The United States Food and Drug Administration recently warned that the direct acting antiviral (DAA) combination hepatitis C virus (HCV) treatment of Paritaprevir, Ombitasvir, Dasabuvir, Ritonavir, and Ribavirin (PODr + R) can cause severe liver injury in patients with advanced liver disease. Drug induced liver injury was observed in a small number of patients with decompensated cirrhosis treated with other DAAs, but has not been reported in patients with compensated cirrhosis. We report a case of a 74-year-old woman with chronic HCV and Child-Pugh class A cirrhosis (compensated cirrhosis) treated with PODr + R. The patient presented on day 14 of PODr + R therapy with jaundice and new-onset ascites. Her total bilirubin level increased to 23 mg/dL and international normalized ratio rose to 1.65, while aminotransferase levels remained relatively stable. Hepatitis C treatment was discontinued on day 24 and she gradually recovered. Follow-up testing showed that she achieved a sustained virologic response. In conclusion, hepatic decompensation developed within two weeks of starting treatment with PODr + R in a patient with Child-Pugh class A cirrhosis and was characterized by jaundice and ascites with stable aminotransferase levels. Careful monitoring is warranted in patients with HCV-related cirrhosis treated with PODr + R.
Core tip: To the best of our knowledge, this is the first report of hepatic decompensation in a hepatitis C patient with Child Pugh class A cirrhosis due to treatment with Paritaprevir, Ombitasvir, Dasabuvir, Ritonavir and Ribavirin (PODr + R). Liver aminotransferase levels did not increase prior to decompensation, depriving us of our usual alarm signs heralding hepatic decompensation. The patient achieved sustained virologic response despite very early discontinuation of therapy (day 24).
- Citation: Hasin Y, Shteingart S, Dahari H, Gafanovich I, Floru S, Braun M, Shlomai A, Verstandig A, Dery I, Uprichard SL, Cotler SJ, Lurie Y. Hepatitis C virus cures after direct acting antiviral-related drug-induced liver injury: Case report. World J Hepatol 2016; 8(20): 858-862
- URL: https://www.wjgnet.com/1948-5182/full/v8/i20/858.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i20.858
Globally, it was estimated that in 2005, more than 185 million people were anti-hepatitis C virus (HCV) seropositive (prevalence 2.8%)[1]. End-stage liver disease due to HCV is a leading indication for liver transplantation and accounts for almost 500000 deaths annually[2]. The growing proportion of patients with chronic HCV infection and cirrhosis has the highest priority for treatment, but are at risk for complications of liver disease[3].
The interferon-free, 12-wk Paritaprevir, Ombitasvir, Dasabuvir, Ritonavir and Ribavirin (PODr + R) regimen is approved for the treatment of patients with Child’s-Pugh class A cirrhosis and was shown to achieve a sustained virologic response (SVR) rate exceeding 90% in such patients[4]. However, on 10/22/15, an Food and Drug Administration (FDA) Drug Safety Communication warned that PODr + R can cause severe drug-induced liver injury (DILI) in patients with advanced liver disease[5]. Since December 2014, 26 cases of severe liver injury in patients with advanced liver disease were reported to the FDA Adverse Event Reporting System database that were considered probably or possibly related to PODr + R. Ten patients developed liver failure resulting in death or liver transplantation. The pattern of liver injury consisted of an acute increase in bilirubin level without alanine aminotransferase (ALT) elevations. The FDA Drug Safety Communication emphasized that PODr + R is contraindicated in patients with Child’s class B and C cirrhosis. More recently, four cases of severe DILI were identified in patients who had decompensated cirrhosis at pre-treatment baseline who were treated with sofosbuvir and a NS5A inhibitor, with or without Ribavirin[6-8]. Three of the patients were human immunodeficiency virus-co-infected and were receiving antiretroviral therapy. To date, severe DILI has not been reported in patients with compensated cirrhosis who receive direct acting antiviral (DAA).
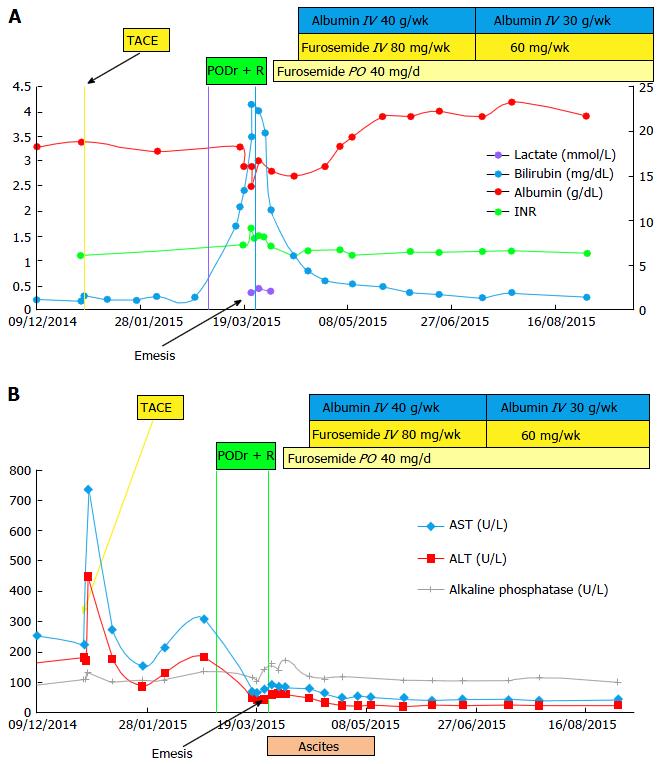
A 74-year-old woman presented to Shaare Zedek Medical Center in Jerusalem on 12/30/14 for management of HCV genotype 1b infection with compensated cirrhosis (Child’s Pugh class A-score 6, MELD score 7), and a 5.6 cm × 6.8 cm right lobe liver lesion with diagnostic features of hepatocellular carcinoma (HCC) on ultrasonography and contrast-enhanced computed tomography. Her baseline laboratory data are shown in Figure 1.
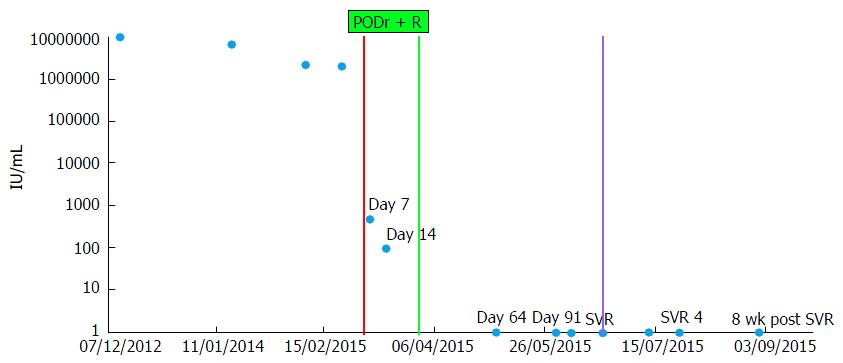
On 1/1/15 she underwent transcatheter arterial chemoembolization (TACE) as treatment for HCC. Her ALT and aspartate transaminase levels increased transiently and then declined to baseline (Figure 1B). She showed no signs of hepatic decompensation before or after the procedure. Treatment with Ombitasvir, Paritaprevir, Ritonavir (2 tablets daily), Dasabuvir (250 mg twice daily, and Ribavirin (400 mg, twice daily) was initiated on 3/1/15, two months after TACE. The HCV RNA level declined from a pre-treatment baseline of 2000000 IU/mL to 474 IU/mL in 7 d (Figure 2). However, on day 14 of treatment, she presented with nausea and increased abdominal girth. Her medications were limited to PODr + R and Ramipril for hypertension, which she had taken for four months. She reported taking no other prescription, over the counter, or alternative medications during the four months prior to the onset of liver injury. She did not drink alcohol. On physical examination, she had icteric sclera and moderate ascites. She developed vomiting and anorexia. Serial laboratories showed a rising total bilirubin (maximum 23 mg/dL) and international normalized ratio (maximum 1.65) levels and her albumin level decreased to 2.5 g/dL (Figure 1A). Of note, her aminotransferase levels remained stable (Figure 1B). An abdominal ultrasound with Doppler study showed flow in the portal and hepatic veins and no new mass lesions in the liver.
Antiviral therapy was discontinued on treatment day 24 (3/24/15) for suspected DILI. The patient was hospitalized on 4/1/15 for management of ascites. After hospital discharge, she was treated with weekly intravenous albumin and Furosemide until November 2015. Her ascites slowly improved and her liver biochemistries normalized (Figure 1). Seven months after discontinuation of antiviral therapy, she has recovered and her HCV RNA remains undetectable (Figure 2). She has no evidence of viable HCC by magnetic resonance imaging.
The time to reach cure, or SVR, was previously defined as the time to reach less than one hepatitis C virion in the extracellular fluid volume (approximately 13.5-15 L)[9-12]. Thus a value of approximately 3 × 10-5 IU/mL is the threshold for viral clearance (termed here cure boundary). The fact that the patient achieved SVR despite a very short course of therapy (24 d) was striking since her viral load level 10 d before DAA therapy stopped (3/15/15) was 97 IU/mL, which is several log IU/mL higher than the cure boundary. To estimate, retrospectively, when the patient reached cure we used the standard biphasic HCV treatment model[11,12] and the multiscale HCV treatment model[13,14]. Both these models predicted that cure occurred 3 to 6 wk after therapy was stopped (not shown).
Herein we detail hepatic decompensation in a patient treated with PODr + R. The patient had Child’s-Pugh class A cirrhosis prior to initiation of treatment and presented with jaundice and ascites 14 d after DAA therapy was started. Treatment was discontinued on day 24. She had a prolonged recovery and gradually returned to her baseline condition. She achieved SVR despite a truncated course of therapy. No causes of liver injury other than DAA were identified in the present case and the available data are consistent with the minimal elements for reporting DILI[15].
The patient’s presentation is consistent with the pattern of PODr + R-related liver injury reported by the FDA with an early onset of hyperbilirubinemia without a rise in ALT level[5]. Similarly, two cases of severe liver injury in patients with decompensated cirrhosis treated with sofosbuvir and a NS5A inhibitor were characterized by a rising bilirubin level with stable ALT levels[6]. Aminotransferase levels were not reported in the other two sofosbuvir/NS5A inhibitor DILI cases[7,8]. It is critical that clinicians be aware that aminotransferase levels do not tend to rise prior to, or in parallel with bilirubin levels in patients with DAA-related DILI, as failure to recognize this pattern could delay recognition of severe liver injury and discontinuation of therapy.
The most novel and important feature of the present case is that the patient had well compensated liver disease before starting treatment with a Child Pugh score of 6 and a MELD score of 7. In contrast, the FDA Drug Safety Communication emphasized that PODr + R is contraindicated in patients with Child’s Pugh class B and C cirrhosis. The current case provides evidence that patients with Child’s Pugh class A cirrhosis are at risk for severe DILI with PODr + R.
Of interest, HCV was eradicated with only 24 d of treatment. Both the standard biphasic and the multiscale models of HCV kinetics during therapy suggest that cure might have occurred between 3 to 6 wk after therapy was stopped. While it is not feasible that the drugs had a prolonged direct antiviral effect, in theory the ongoing liver injury (i.e., cell loss) and/or resulting inflammation might have exerted an immune mediated antiviral effect. Alternatively, cure might have occurred by the end of DAA therapy if the DAAs affected the ratio between non-infectious and infectious viral particles (i.e., viral infectivity) as previously observed during HCV DAA treatment in cell culture[16]. That is, DAAs may reduce the infectivity of the virus particles produced such that only a small fraction of the viral RNA detected 10 d before drug cessation was infectious. This alternative explanation is also consistent with reports in which some patients treated with DAAs were documented to achieve SVR despite having detectable HCV RNA at end of treatment[17,18]. Further experimental and modeling efforts are needed to provide insights into the biological and/or immunological aspects that gave rise to HCV cure after such short durations of DAA therapy[19].
Our study has several limitations. Blood samples during therapy are not available for further measurements to test for superimposed acute viral infections such as hepatitis A or B or to measure paritaprevir concentration at the time of the rise in bilirubin. However, the temporal relation between PODr + R therapy and hepatic decompensation and recovery following discontinuation of therapy provides strong evidence for DILI.
Careful laboratory and clinical monitoring, beginning early in the course of therapy, is prudent in patients with compensated cirrhosis treated with PODr + R.
Patient suffered from jaundice, nausea and vomiting 14 d following treatment with Paritaprevir, Ombitasvir, Dasabuvir, Ritonavir, and Ribavirin (PODr + R).
Aminotransferase levels did not increase. Despite that, Nausea, Vomiting, Ascites, Jaundice, hypoalbuminemia and coagulopathy occurred.
Ribavirin induced hemolysis, possible drug induced liver injury, recurrence of hepatocellular carcinoma (HCC), or vascular causes for hepatic failure. The authors performed liver ultrasonography to exclude Recurrence of HCC and vascular causes. Blood tests to exclude hemolysis as cause for jaundice were performed.
The authors witnessed increase in bilirubin levels, decrease in albumin levels and impaired coagulation tests with no increase in aminotransferase levels. Despite the above, eradication of hepatitis C virus was achieved (Figures 1 and 2).
The authors performed liver ultrasonography to rule out recurrence of HCC or vascular causes as the cause of decompensation. Eventually magnetic resonance imaging scan was also performed as follow-up on HCC.
The main treatment in this case was discontinuation of PODr + R treatment. Albumin infusions and IV furosemide were administered.
All acronyms are explained in the main text.
The new direct acting antiviral have an excellent safety record. Still, careful clinical and laboratory monitoring, beginning early in the course of therapy, is warranted especially in compensated cirrhotic patients treated with PODr + R because decompensation can occur without aminotransferase flareup.
The author presents an interesting and valuable case report.
Manuscript source: Unsolicited manuscript
P- Reviewer: Jiang W, Preda CM, Sirin G, Silva LD S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1770] [Cited by in RCA: 1847] [Article Influence: 153.9] [Reference Citation Analysis (3)] |
| 2. | Holtzman D. Hepatitis C 2016. CDC Health Information for International Travel. [updated 2015 Jul 10]. Available from: http://wwwnc.cdc.gov/travel/yellowbook/2016/infectious-diseases-related-to-travel/hepatitis-c#4627. |
| 3. | Panel AIHG. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62:932-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 912] [Cited by in RCA: 992] [Article Influence: 99.2] [Reference Citation Analysis (0)] |
| 4. | Poordad F, Hezode C, Trinh R, Kowdley KV, Zeuzem S, Agarwal K, Shiffman ML, Wedemeyer H, Berg T, Yoshida EM. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med. 2014;370:1973-1982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 698] [Cited by in RCA: 683] [Article Influence: 62.1] [Reference Citation Analysis (0)] |
| 5. | FDA Drug Safety Communication. FDA warns of serious liver injury risk with hepatitis C treatments Viekira Pak and Technivie. 2015. Available from: http://www.fda.gov/Drugs/DrugSafety/ucm468634.htm. |
| 6. | Dyson JK, Hutchinson J, Harrison L, Rotimi O, Tiniakos D, Foster GR, Aldersley MA, McPherson S. Liver toxicity associated with sofosbuvir, an NS5A inhibitor and ribavirin use. J Hepatol. 2016;64:234-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 7. | Dyson JK, McPherson S. Reply to “Liver failure in human immunodeficiency virus - Hepatitis C virus coinfection treated with sofosbuvir, ledipasvir and antiretroviral therapy”. J Hepatol. 2016;64:753-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Marchan-Lopez A, Dominguez-Dominguez L, Kessler-Saiz P, Jarrin-Estupiñan ME. Liver failure in human immunodeficiency virus - Hepatitis C virus coinfection treated with sofosbuvir, ledipasvir and antiretroviral therapy. J Hepatol. 2016;64:752-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Snoeck E, Chanu P, Lavielle M, Jacqmin P, Jonsson EN, Jorga K, Goggin T, Grippo J, Jumbe NL, Frey N. A comprehensive hepatitis C viral kinetic model explaining cure. Clin Pharmacol Ther. 2010;87:706-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Guedj J, Perelson AS. Second-phase hepatitis C virus RNA decline during telaprevir-based therapy increases with drug effectiveness: implications for treatment duration. Hepatology. 2011;53:1801-1808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 11. | Dahari H, Shteingart S, Gafanovich I, Cotler SJ, D’Amato M, Pohl RT, Weiss G, Ashkenazi YJ, Tichler T, Goldin E. Sustained virological response with intravenous silibinin: individualized IFN-free therapy via real-time modelling of HCV kinetics. Liver Int. 2015;35:289-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Dahari H, Canini L, Graw F, Uprichard SL, Araújo ES, Penaranda G, Coquet E, Chiche L, Riso A, Renou C. HCV kinetic and modeling analyses indicate similar time to cure among sofosbuvir combination regimens with daclatasvir, simeprevir or ledipasvir. J Hepatol. 2016;64:1232-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 13. | Rong L, Guedj J, Dahari H, Coffield DJ, Levi M, Smith P, Perelson AS. Analysis of hepatitis C virus decline during treatment with the protease inhibitor danoprevir using a multiscale model. PLoS Comput Biol. 2013;9:e1002959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 14. | Guedj J, Dahari H, Rong L, Sansone ND, Nettles RE, Cotler SJ, Layden TJ, Uprichard SL, Perelson AS. Modeling shows that the NS5A inhibitor daclatasvir has two modes of action and yields a shorter estimate of the hepatitis C virus half-life. Proc Natl Acad Sci USA. 2013;110:3991-3996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 257] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 15. | Barritt AS, Lee J, Hayashi PH. Detective work in drug-induced liver injury: sometimes it is all about interviewing the right witness. Clin Gastroenterol Hepatol. 2010;8:635-637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Sansone N, Dahari H, Subramanya G, Perelson AS, Uprichard SL. Modeling HCVcc infection reveals new insights into the dynamics that maintain the in vitro HCV steady state and the mechanisms of action of the NS5A inhibitor daclatasvir. Hepatology. 2014;60:4 (Suppl): 1165A. |
| 17. | Kohli A, Osinusi A, Sims Z, Nelson A, Meissner EG, Barrett LL, Bon D, Marti MM, Silk R, Kotb C. Virological response after 6 week triple-drug regimens for hepatitis C: a proof-of-concept phase 2A cohort study. Lancet. 2015;385:1107-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 125] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 18. | Harrington PR, Deming DJ, Komatsu TE, Naeger LK. Hepatitis C Virus RNA Levels During Interferon-Free Combination Direct-Acting Antiviral Treatment in Registrational Trials. Clin Infect Dis. 2015;61:666-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Meissner EG, Nelson A, Marti M, Masur H, Osinusi A, Kottilil S. Sustained Virologic Response for Chronic Hepatitis C Infection after 27 Days of Treatment with Sofosbuvir and Ribavirin. Open Forum Infect Dis. 2014;1:013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |