Published online Jul 18, 2016. doi: 10.4254/wjh.v8.i20.838
Peer-review started: February 22, 2016
First decision: March 25, 2016
Revised: March 28, 2016
Accepted: June 14, 2016
Article in press: June 16, 2016
Published online: July 18, 2016
Processing time: 144 Days and 16.4 Hours
AIM: To determine whether hepatocyte lipogenesis, in an in vitro cell culture model, is modulated by adjusting culture media monosaccharide content and concentration.
METHODS: Hepatocytes (Huh7), demonstrating glucose and fructose uptake and lipid biosynthesis, were incubated in culture media containing either glucose alone (0.65-0.72 mmol/L) or isosmolar monosaccharide (0.72 mmol/L) comprising fructose:glucose (F:G) molar ratios ranging from 0.58-0.67. Following a 24-h incubation, cells were harvested and analyzed for total protein, triglyceride (TG) and cholesterol (C) content. Significant differences (P < 0.05) among groups were determined using analysis of variance followed by Dunnett’s test for multiple comparisons.
RESULTS: After a 24 h incubation period, Huh7 cell mass and viability among all experimental groups were not different. Hepatocytes cultured with increasing concentrations of glucose alone did not demonstrate a significant change either in C or in TG content. However, when the culture media contained increasing F:G molar ratios, at a constant total monosaccharide concentration, synthesis both of C and of TG increased significantly [F:G ratio = 0.58, C/protein (μg/μg) = 0.13; F:G = 0.67, C/protein = 0.18, P < 0.01; F:G ratio = 0.58, TG/protein (μg/μg) = 0.06; F:G ratio = 0.67, TG/protein = 0.11, P < 0.01].
CONCLUSION: In an in vitro hepatocyte model, glucose or fructose plus glucose support total cell mass and lipogenic activity. Increasing the fructose:glucose molar ratio (but not glucose alone) enhances triglyceride and cholesterol synthesis. These investigations demonstrate fructose promotes hepatocellular lipogenesis, and they provide evidence supporting future, in vivo studies of fructose’s role in the development of hepatic steatosis and non-alcoholic fatty liver disease.
Core tip: Employing an in vitro hepatocyte culture model, these data demonstrate fructose promotes intracellular synthesis both of cholesterol and of triglyceride. The results support the requirement for future, in vivo investigations to determine whether diets high in fructose are risk factors for hepatic steatosis and development of non-alcoholic fatty liver disease.
- Citation: Windemuller F, Xu J, Rabinowitz SS, Hussain MM, Schwarz SM. Lipogenesis in Huh7 cells is promoted by increasing the fructose: Glucose molar ratio. World J Hepatol 2016; 8(20): 838-843
- URL: https://www.wjgnet.com/1948-5182/full/v8/i20/838.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i20.838
Fructose, a five-carbon monosaccharide, comprises an increasing component of the Western diet, particularly in the form of high fructose corn syrup (HFCS). In the United States, this sweetener is both readily available from domestically grown corn and inexpensive, when compared to imported, granulated sugar. It was introduced in the 1960’s, with subsequent expansion into a vast array of foods and beverages. HFCS is made from corn starch, processed by glucose isomerase to convert a portion of its glucose fraction into fructose. HFCS preparations contain approximately 25% water, fructose (up to 55% of the water-free fraction), glucose and 0%-5% unprocessed glucose oligomers. HFCS’s use as a commercial sweetener has doubled in the last decade and, as a consequence, fructose intake in developed countries has increased five-fold[1].
In several human studies to date, increased fructose intake has been linked both to abnormal plasma lipid profiles and to the development of non-alcoholic fatty liver disease (NAFLD)[2-4]. However, because the effects of fructose, per se, are difficult to distinguish from the influences of other dietary carbohydrates and fats, current clinical evidence does not establish the precise contribution of fructose-containing food and beverage products to the etiology and/or the exacerbation of specific biochemical, clinical and histopathologic abnormalities. Further, since the causes of dietary carbohydrate and lipid-related problems (e.g., hyperlipidemia, type II diabetes, NAFLD, metabolic syndrome) are multifactorial and also related to age, gender and lifestyle, additional investigations are required to determine the relative contributions of dietary and other co-factors.
In attempting to identify the importance of fructose in hepatocellular lipid metabolism, available in vitro studies indicate fructose may preferentially promote de novo lipogenesis[5]. Since fructose bypasses the glycolytic rate-limiting enzyme phosphofructokinase, it is metabolized efficiently and provides a readily available substrate for hepatic lipid synthesis[6]. In addition, fructose has been shown to inhibit peroxisome proliferator-activated receptor (PPAR)-alpha mediated hepatocellular fatty acid beta-oxidation and lipid clearance[7].
The potential lipogenic role of fructose also may be related to its relationship to endogenous insulin activity. Insulin insensitivity has been implicated in the pathogenesis and progression of NAFLD[8]. Impaired insulin responsiveness to circulating carbohydrate is associated both with increased adipocyte lipolysis and with increased levels of circulating free fatty acids (FFA). Thus, insulin resistance promotes lipolysis, particularly in intra-abdominal, white adipose tissue. This phenomenon occurs as a consequence of dysregulation of lipid regulatory transcription factors (e.g., PPAR-gamma), instability of adipocyte lipid and impaired lipogenesis. Released FFA lead to upregulation of inflammatory cytokines and chemokines[8,9]. Subsequently, liberated adipocyte FFA are taken-up by the liver and re-esterified to triglyceride. As fructose does not promote insulin secretion, it may therefore exacerbate the effects of insulin resistance on hepatic lipid synthesis, steatosis and the development of NAFLD[10]. Further evidence of fructose’ role in the development of NAFLD derives from a recently reported rat model of hepatic steatosis. A high fructose-containing diet promoted not only lipogenesis leading to steatosis, but also increased expression of lipocalin-2, a ubiquitous glycoprotein involved in the response to inflammation and oxidative stress[11].
Despite evidence linking fructose to the development of altered lipid dynamics, hepatic steatosis and NAFLD, a recent meta-analysis concluded that studies examining the hepatocellular effects of fructose were confounded by the co-stimulation of lipogenesis resulting from increased total energy intake[12]. This observation suggests the deleterious effects of HFCS are merely a reflection of overall calorie excess in the Western diet.
In light of the above clinical and experimental data, the present study seeks to establish the influence of fructose on hepatocyte lipogenesis and provide a basis for future, translational investigations of fructose-mediated lipid biosynthesis. These experiments employ an established, immortal and metabolically active human hepatocellular carcinoma cell line, Huh7, used extensively in studies of hepatocyte metabolism[13-15]. Since facilitated uptake of glucose and fructose by the transmembrane GLUT2 transporter is demonstrated in Huh7 cells[16], these cells provide an excellent model for studies of carbohydrate-induced lipogenesis. Accordingly, the studies herein were carried out to determine whether hepatocyte lipogenesis, in an in vitro cell culture model, is modulated by adjusting culture media monosaccharide content and concentration.
Huh7 cells (American Type Culture Collection, Manassas, VA) were maintained in Dulbecco’s Modified Eagle Medium (DMEM) containing 10% Fetal Bovine Serum (FBS), 1% penicillin-streptomycin and 1% L-glutamine, in a 37 °C, 5% CO2 cell culture incubator on 100 mm × 200 mm tissue culture dishes (BD Falcon Durham, NC). Once 80% confluence was achieved (cell count - 1 × 105), cells were incubated for 24 h in culture media (DMEM) with 10% FBS containing either glucose alone (0.65, 0.68 and 0.72 mmol/L) or isosmolar monosaccharide (0.72 mmol/L) comprising fructose:glucose (F:G) molar ratios of 0.58, 0.61, and 0.67. For all experiments, total media osmolality was 400 mOsm/L. All incubations were performed in triplicate. Following the 24 h incubation, as described above, culture media was removed, plates were washed with phosphate buffered saline, and cells were lysed and collected in 750 microliters isopropanol, as previously described[17]. The cell lysates were kept at 4 °C for 12 h. Each sample was then centrifuged at 4 °C for 10 min at 10000 rpm. Supernatants were removed for lipid studies and the remaining cellular precipitate was re-suspended in 0.1N NaOH for protein quantification, employing a previously validated method[17].
Cholesterol and triglyceride were measured utilizing samples from the isopropanol supernatant[18]. The samples were placed in a 96 well plate for lipid quantification, using established spectrophotometric methods[19,20]. Spectrophotometric absorbancies of each cell lysate sample were compared to known lipid standards, and the concentrations of cholesterol and triglyceride were calculated from the derived measurements[19,20]. Protein was quantified in triplicate utilizing samples from the NaOH suspension, employing standard spectrophotometric methods[21].
For these cell culture studies, estimates of cell mass in all experimental groups were made using total protein measurements in Huh7 lysates[22]. All single cell-line incubations were performed concurrently, under the same environmental conditions, thereby synchronizing cell cycles among experimental groups. Accordingly, as previously described, calculation of total cell protein content was employed to estimate relative cell biomass among incubations[23].
Differences among all experimental groups were assessed by analysis of variance, followed by Dunnett’s test for multiple comparisons. A P-value of < 0.05 was considered significant.
Cultured Huh7 cells incubated in glucose-only supplemented media (0.65, 0.68, and 0.72 mmol/L) and in media containing varying F:G molar ratios (total monosaccharide concentration 0.72 mmol/L), did not show any statistically significant differences in protein content among all study groups. These data indicate total cell mass was not affected by varying the monosaccharide concentration or distribution (Table 1).
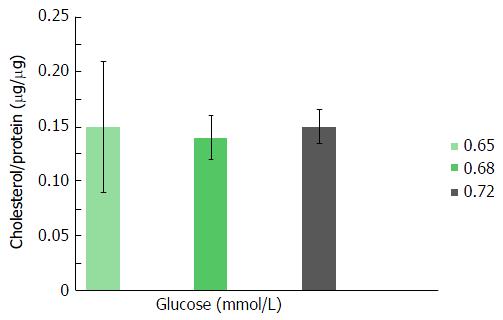
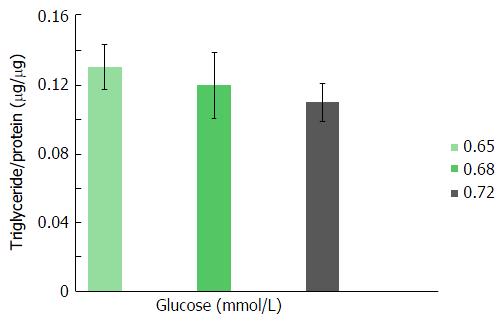
As shown in Figures 1 and 2, triglyceride and cholesterol content (μg/mg cell protein) did not differ significantly among Huh7 cells incubated for 24 h in media containing 0.65, 0.68 or 0.72 mmol/L glucose per plate. Further increases (> 0.72 mmol/L) in glucose molar concentration did not result in any additional enhancement in cellular lipid content (data not shown).
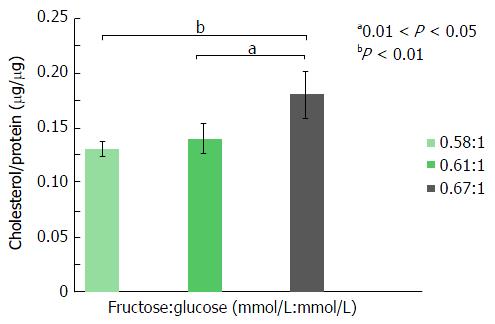
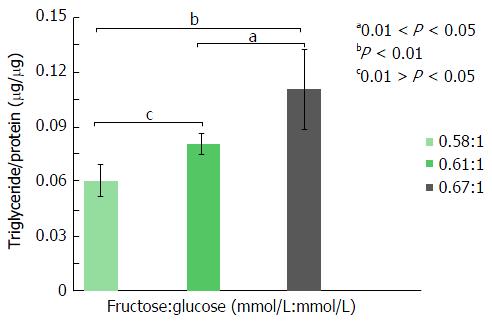
For these experiments, all cells were incubated in media containing 0.72 mmol/L monosaccharide (total media osmolality = 400 mOsm/L), the maximum sugar concentration employed in the “glucose-only” experiments, described above. To determine the effects of fructose on lipogenesis, cells were incubated in the presence of increasing molar ratios of F:G (0.58, 0.61 and 0.67). Following a 24 h incubation, Huh7 cell cholesterol content (μg/μg cell protein) increased significantly at a F:G molar ratio of 0.67:1 (Figure 3), compared both to a 0.61:1 ratio (0.18 μg/μg vs 0.14 μg/μg protein, P < 0.05) and to a 0.58:1 ratio (0.18 μg/μg vs 0.13 μg/μg protein, P < 0.01). Triglyceride analyses (Figure 4) demonstrated fructose-mediated Huh7 triglyceride synthesis also increased significantly in step-wise fashion. Thus, increased triglyceride content was noted at a F:G ratio of 0.61:1 (compared to the ratio of 0.58:1, 0.08 μg/μg vs 0.06 μg/μg protein, P < 0.05) and at a ratio of 0.67:1 (compared to the ratio of 0.61:1, 0.11 μg/μg vs 0.08 μg/μg protein, P < 0.05; to 0.67:1, 0.11 μg/μg vs 0.06 μg/μg protein, P < 0.01).
The results presented in this report demonstrate Huh7 cells, an immortal hepatocellular carcinoma cell line, grown in standard culture media containing increasing glucose concentrations (up to 0.72 mmol/L), exhibit no differences in relative cellular TG and C, as a consequence of increased media glucose content. However, when the nutrient monosaccharides comprise fructose plus glucose (at increasing F:G molar ratios), significant promotion of lipogenesis is demonstrated by increased hepatocellular TG and C concentrations. For these studies, the cell culture media monosaccharide content of 0.72 mmol/L (glucose alone or glucose plus fructose) was found to maximize hepatocellular lipogenesis. This molar amount was determined following a series of experiments, employing a step-wise increase in sugar content and based on previous human studies showing serum total monosaccharide concentrations of approximately 0.50 mmol/L following a fructose-rich meal[24]. Higher amounts of monosaccharide (> 0.72 mmol/L) in the Huh7 incubating media did not yield statistically significant increases either in cellular TG or in cellular C content, while further increases (> 400 mmol/L) in media osmolality resulted in decreased cell viability.
These results are consistent with a prior clinical report, suggesting hepatic fat (estimated by magnetic resonance imaging) in subjects fed fructose, in addition to a specific weight maintenance diet, was increased compared to a study group fed the same diet supplemented with glucose alone[4]. Conversely, another report failed to demonstrate any promotion of lipogenesis following four weeks of a fructose supplemented diet. As stated previously, a recent meta-analysis concluded the potential association between fructose and NAFLD was confounded by the concurrent consumption of hypercaloric diets[12]. The influence of fructose on lipogenesis, as a consequence of excess energy intake, may be mediated by this monosaccharide’s direct attenuation of post-prandial ghrelin suppression[24]. On the other hand, another study examining the effects of dietary sucrose vs HFCS on endogenous hormone levels, failed to demonstrate any significant differences in serum insulin, leptin and ghrelin levels[25]. These combined in vivo studies therefore suggest the amount of carbohydrate taken up by hepatocytes is increased (thus leading to enhanced lipogenesis) as a consequence of total calorie intake, rather than resulting directly from the lipogenic effects of any specific dietary substrate.
In contrast to available clinical data examining fructose mediated steatosis (often derived from imprecise imaging techniques), considerable biochemical evidence supports the role of fructose in promoting de novo lipogenesis. Since fructose is more efficiently utilized intracellularly, as compared with glucose, it may provide a more readily available substrate for lipid synthesis[26]. Interestingly, one recent study failed to demonstrate any effects of fructose on regulating hepatocellular lipogenic genes, thus providing further, indirect evidence linking the lipogenic role of this monosaccharide to its ability to provide increased amounts of carbon fragments for lipid synthesis[27]. The present results extend this observation by demonstrating that lipogenesis, in cultured hepatocytes, is not solely related to the provision of carbon precursors, since all incubations contained similar total monosaccharide concentrations. Thus, fructose, in this experimental model, appears to exert a direct effect on promotion of lipogenesis, and this effect is independent of carbon supply.
Because fructose metabolism is insulin-independent and does not stimulate pancreatic insulin secretion[28], this monosaccharide was previously thought to be a superior dietary substrate, as compared with other sugars[29]. However, more recent experimental data, while confirming these metabolic characteristics, also demonstrate fructose promotion of insulin resistance[12]. Fructose stimulates Jun-N-terminal kinase-1 (JNK-1), an intracellular mitogen activated protein kinase. JNK-1 in turn phosphorylates insulin receptor substrate-1 resulting in suppression of cellular glucose uptake, increased blood glucose levels and increased insulin secretion[30-32]. These findings, therefore, suggest another potential pathway for fructose-mediated steatosis.
Despite results presented in this report and published previously, the precise mechanisms by which fructose increases hepatic TG are not completely understood. Carbohydrate responsive element binding protein (ChREBP) and sterol regulatory element binding protein 1c (SREBP1c) are two transcription regulators of hepatic lipogenesis induced by glucose and insulin, respectively. Animal knockdown studies of peroxisome proliferator-activated receptor gamma co-activator-1 beta, a transcriptional co-activator of SREBP1c, have demonstrated improved hepatic lipid profiles in fructose fed rats. In a separate study[7], fructose fed rats showed increased expression of ChREBP hepatic mRNA, compared with a glucose fed group. Because recent data shows fetal bovine serum (FBS) contains factors that promote cellular lipogenesis, independent of insulin[33], and because FBS concentrations were held constant in our studies, stimulation of either SREBP1c or ChREBP represents an unlikely contributor to the observation of fructose-mediated de novo lipogenesis.
Our preliminary study certainly has several important limitations. These observations, in an in vitro cell culture model, cannot accurately predict the metabolic fate of fructose, in terms of its ability to enter lipogenic pathways. While the data clearly suggest direct effects of fructose on hepatocellular synthesis of triglyceride and cholesterol, they do not provide further elucidation of the underlying mechanisms leading to these findings. Nevertheless, the observation that fructose exerts a promoting influence on lipid synthesis, confirms prior studies suggesting the significant role of this monosaccharide in the development and/or exacerbation of hepatic steatosis. These data, therefore, warrant further investigations into the mechanisms and extent of fructose-mediated, de novo hepatic lipogenesis.
Available studies, both in vitro and in clinical trials, indicate the dietary monosaccharide, fructose, may be an important substrate for hepatic lipid synthesis, and may promote the development of hepatic steatosis. However, whether fructose-associated lipogenesis is related to dietary intake of fructose, per se, or merely reflects excess total energy consumption, remains unclear. The present study seeks to establish the effects of fructose on hepatocyte lipogenesis and provide a basis for future, translational investigations of fructose-mediated lipid biosynthesis. These experiments employ an established, immortal and metabolically active human hepatocellular carcinoma cell line, Huh7, used extensively in studies of hepatocyte metabolism. The studies herein were carried out to determine whether hepatocyte lipogenesis, in an in vitro cell culture model, is modulated by adjusting culture media monosaccharide content and concentration.
The results of these experiments clearly demonstrate, in a stable, in vitro hepatocyte culture model, at constant monosaccharide concentrations (glucose ± fructose), by increasing the culture medium fructose to glucose molar ratio, but not by increasing glucose alone, significant enhancement of lipogenesis.
The observation that fructose exerts a promoting influence on lipid synthesis, confirms prior studies suggesting the significant role of this monosaccharide in the development and/or exacerbation of hepatic steatosis. These studies, therefore, support the need for further investigations into the mechanisms and extent of fructose-mediated, de novo hepatic lipogenesis. Most important, these results provide a basis for future, clinical studies of fructose’s role in development of hepatic steatosis and non-alcoholic liver disease.
Huh7 cells, an immortal, stable hepatocyte line, derived from human hepatocellular carcinoma. These cells take-up both glucose and fructose, and have been used extensively in studies of hepatic metabolism.
This is an interesting study that reveals fructose is linked to lipogenesis in a concentration dependent way.
Manuscript source: Invited manuscript
P- Reviewer: de F Higuera-de la Tijera M, Galvao FHF, Peltec A, Ratnasari N S- Editor: Qiu S L- Editor: A E- Editor: Li D
| 1. | Lustig RH. Fructose: metabolic, hedonic, and societal parallels with ethanol. J Am Diet Assoc. 2010;110:1307-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Ouyang X, Cirillo P, Sautin Y, McCall S, Bruchette JL, Diehl AM, Johnson RJ, Abdelmalek MF. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J Hepatol. 2008;48:993-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 3. | Silbernagel G, Machann J, Unmuth S, Schick F, Stefan N, Häring HU, Fritsche A. Effects of 4-week very-high-fructose/glucose diets on insulin sensitivity, visceral fat and intrahepatic lipids: an exploratory trial. Br J Nutr. 2011;106:79-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 123] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 4. | Lecoultre V, Egli L, Carrel G, Theytaz F, Kreis R, Schneiter P, Boss A, Zwygart K, Lê KA, Bortolotti M. Effects of fructose and glucose overfeeding on hepatic insulin sensitivity and intrahepatic lipids in healthy humans. Obesity (Silver Spring). 2013;21:782-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 5. | Spence JT, Pitot HC. Induction of lipogenic enzymes in primary cultures of rat hepatocytes. Relationship between lipogenesis and carbohydrate metabolism. Eur J Biochem. 1982;128:15-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Basciano H, Federico L, Adeli K. Fructose, insulin resistance, and metabolic dyslipidemia. Nutr Metab (Lond). 2005;2:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 569] [Cited by in RCA: 554] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 7. | Nagai Y, Yonemitsu S, Erion DM, Iwasaki T, Stark R, Weismann D, Dong J, Zhang D, Jurczak MJ, Löffler MG. The role of peroxisome proliferator-activated receptor gamma coactivator-1 beta in the pathogenesis of fructose-induced insulin resistance. Cell Metab. 2009;9:252-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 163] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 8. | Delarue J, Magnan C. Free fatty acids and insulin resistance. Curr Opin Clin Nutr Metab Care. 2007;10:142-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 335] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 9. | Shah A, Mehta N, Reilly MP. Adipose inflammation, insulin resistance, and cardiovascular disease. JPEN J Parenter Enteral Nutr. 2008;32:638-644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 150] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 10. | Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, McCullough AJ, Natale S, Forlani G, Melchionda N. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844-1850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1725] [Cited by in RCA: 1745] [Article Influence: 72.7] [Reference Citation Analysis (0)] |
| 11. | Alwahsh SM, Xu M, Seyhan HA, Ahmad S, Mihm S, Ramadori G, Schultze FC. Diet high in fructose leads to an overexpression of lipocalin-2 in rat fatty liver. World J Gastroenterol. 2014;20:1807-1821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 64] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 12. | Bremer AA, Mietus-Snyder M, Lustig RH. Toward a unifying hypothesis of metabolic syndrome. Pediatrics. 2012;129:557-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 121] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 13. | Chung M, Ma J, Patel K, Berger S, Lau J, Lichtenstein AH. Fructose, high-fructose corn syrup, sucrose, and nonalcoholic fatty liver disease or indexes of liver health: a systematic review and meta-analysis. Am J Clin Nutr. 2014;100:833-849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 164] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 14. | Goto D, Okimoto T, Ono M, Shimotsu H, Abe K, Tsujita Y, Kuwano M. Upregulation of low density lipoprotein receptor by gemfibrozil, a hypolipidemic agent, in human hepatoma cells through stabilization of mRNA transcripts. Arterioscler Thromb Vasc Biol. 1997;17:2707-2712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Yao H, Ye J. Long chain acyl-CoA synthetase 3-mediated phosphatidylcholine synthesis is required for assembly of very low density lipoproteins in human hepatoma Huh7 cells. J Biol Chem. 2008;283:849-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 16. | Zhao Y, Chen YQ, Bonacci TM, Bredt DS, Li S, Bensch WR, Moller DE, Kowala M, Konrad RJ, Cao G. Identification and characterization of a major liver lysophosphatidylcholine acyltransferase. J Biol Chem. 2008;283:8258-8265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 154] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 17. | Tuch BE, Szymanska B, Yao M, Tabiin MT, Gross DJ, Holman S, Swan MA, Humphrey RK, Marshall GM, Simpson AM. Function of a genetically modified human liver cell line that stores, processes and secretes insulin. Gene Ther. 2003;10:490-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Egusa G, Brady DW, Grundy SM, Howard BV. Isopropanol precipitation method for the determination of apolipoprotein B specific activity and plasma concentrations during metabolic studies of very low density lipoprotein and low density lipoprotein apolipoprotein B. J Lipid Res. 1983;24:1261-1267. [PubMed] |
| 19. | Cramp DG. Lipid methodology. J Clin Pathol Suppl (Assoc Clin Pathol). 1973;5:17-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Roeschlau P, Bernt E, Gruber W. Enzymatic determination of total cholesterol in serum. Z Klin Chem Klin Biochem. 1974;12:226. [PubMed] |
| 21. | Fossati P, Prencipe L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin Chem. 1982;28:2077-2080. [PubMed] |
| 22. | Spector T. Refinement of the coomassie blue method of protein quantitation. A simple and linear spectrophotometric assay for less than or equal to 0.5 to 50 microgram of protein. Anal Biochem. 1978;86:142-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1223] [Cited by in RCA: 1190] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 23. | Butler M, Spearman M, Braasch K. Monitoring cell growth, viability, and apoptosis. Methods Mol Biol. 2014;1104:169-192. [PubMed] |
| 24. | Teff KL, Grudziak J, Townsend RR, Dunn TN, Grant RW, Adams SH, Keim NL, Cummings BP, Stanhope KL, Havel PJ. Endocrine and metabolic effects of consuming fructose- and glucose-sweetened beverages with meals in obese men and women: influence of insulin resistance on plasma triglyceride responses. J Clin Endocrinol Metab. 2009;94:1562-1569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 210] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 25. | Melanson KJ, Zukley L, Lowndes J, Nguyen V, Angelopoulos TJ, Rippe JM. Effects of high-fructose corn syrup and sucrose consumption on circulating glucose, insulin, leptin, and ghrelin and on appetite in normal-weight women. Nutrition. 2007;23:103-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 26. | Basaranoglu M, Basaranoglu G, Sabuncu T, Sentürk H. Fructose as a key player in the development of fatty liver disease. World J Gastroenterol. 2013;19:1166-1172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 156] [Cited by in RCA: 164] [Article Influence: 13.7] [Reference Citation Analysis (3)] |
| 27. | Hirahatake KM, Meissen JK, Fiehn O, Adams SH. Comparative effects of fructose and glucose on lipogenic gene expression and intermediary metabolism in HepG2 liver cells. PLoS One. 2011;6:e26583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Curry DL. Effects of mannose and fructose on the synthesis and secretion of insulin. Pancreas. 1989;4:2-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 69] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Schultz A, Neil D, Aguila MB, Mandarim-de-Lacerda CA. Hepatic adverse effects of fructose consumption independent of overweight/obesity. Int J Mol Sci. 2013;14:21873-21886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 30. | Hirosumi J, Tuncman G, Chang L, Görgün CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2445] [Cited by in RCA: 2457] [Article Influence: 106.8] [Reference Citation Analysis (0)] |
| 31. | Lim JS, Mietus-Snyder M, Valente A, Schwarz JM, Lustig RH. The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat Rev Gastroenterol Hepatol. 2010;7:251-264. [PubMed] |
| 32. | Havel PJ. Dietary fructose: implications for dysregulation of energy homeostasis and lipid/carbohydrate metabolism. Nutr Rev. 2005;63:133-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 387] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 33. | Berenguer M, Martinez L, Giorgetti-Peraldi S, Le Marchand-Brustel Y, Govers R. A serum factor induces insulin-independent translocation of GLUT4 to the cell surface which is maintained in insulin resistance. PLoS One. 2010;5:e15560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |