Published online Jul 8, 2016. doi: 10.4254/wjh.v8.i19.790
Peer-review started: March 25, 2016
First decision: May 17, 2016
Revised: May 19, 2016
Accepted: June 14, 2016
Article in press: June 16, 2016
Published online: July 8, 2016
Processing time: 103 Days and 6.3 Hours
Cirrhotic patients with recurrent variceal bleeds who have failed prior medical and endoscopic therapies and are not transjugular intrahepatic portosystemic shunt candidates face a grim prognosis with limited options. We propose that mesocaval shunting be offered to this group of patients as it has the potential to decrease portal pressures and thus decrease the risk of recurrent variceal bleeding. Mesocaval shunts are stent grafts placed by interventional radiologists between the mesenteric system, most often the superior mesenteric vein, and the inferior vena cava. This allows flow to bypass the congested hepatic system, reducing portal pressures. This technique avoids the general anesthesia and morbidity associated with surgical shunt placement and has been successful in several case reports. In this paper we review the technique, candidate selection, potential pitfalls and benefits of mesocaval shunt placement.
Core tip: Cirrhotic patients who have recurrent variceal hemorrhage despite medical and endoscopic therapy have limited options if they are not transjugular intrahepatic portosystemic shunting candidates. One promising new method to decrease portal pressures while avoiding surgical shunt placement is mesocaval shunt placement with fluoroscopic guidance. In this paper we review the technique, candidate selection, potential pitfalls and benefits of mesocaval shunt placement.
- Citation: Davis J, Chun AK, Borum ML. Could there be light at the end of the tunnel? Mesocaval shunting for refractory esophageal varices in patients with contraindications to transjugular intrahepatic portosystemic shunt. World J Hepatol 2016; 8(19): 790-795
- URL: https://www.wjgnet.com/1948-5182/full/v8/i19/790.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i19.790
Patients with cirrhosis and recurrent variceal bleeding face a high mortality, 20% in the first year vs 5.4% for compensated patients[1]. Current standard of care for variceal bleeding includes three primary modalities: Medical therapy with beta blockade, endoscopic therapy with ligation of varices and shunt therapy with transjugular intrahepatic portosystemic shunting (TIPS). Each of these have been shown to improve rebleeding rates and mortality[2-4].
For an unfortunate cohort of patients with varices who fail medical and endoscopic therapy and are not TIPS candidates, there are limited options in the face of a grim prognosis. Historically, these patients have been offered surgical shunt approaches, however, mortality of surgical shunt placements is high - 20%-50% if emergent - and many patients may not be suitable surgical candidates[5]. First described in 1996 by Nyman et al[6], mesocaval shunting may provide an alternate route to alleviate portal hypertension in these challenging patients. This paper will review the technique, candidate selection, potential pitfalls and benefits of mesocaval shunting. While there are not enough data to comment on a mortality benefit, we believe that mesocaval shunting is a feasible procedure for the prevention of variceal bleeding. It will likely be most useful for patients whose anatomy prohibits TIPS to provide a bridge to transplant.
Mesocaval shunting involves the creation of a shunt from the mesenteric vasculature, typically the superior mesenteric vein (SMV), into the inferior vena cava (IVC). Similar to TIPS, this provides relief of portal pressures by allowing blood to bypass the congested hepatic vasculature. Shunt placement is performed by interventional radiologists. There have been both femoral and transabdominal approaches reported (Table 1)[6-9].
| Ref. | Case history | Details and outcomes | |
| Nyman et al[6] 1996 | 37-year-old male with history of recurrent massive variceal bleeds attributed to congenital PVT and failed prior surgical shunt attempt | Visualization | CT angiography |
| Approach | Transcolonic | ||
| Duration of follow-up | 5, 12 and 14 mo | ||
| Thrombosis | Yes1 | ||
| Recurrent bleeding | No | ||
| Hepatic encephalopathy | NR | ||
| Moriarty et al[9] 2012 | 57-year-old male with history of metastatic CRC and extrahepatic PVT who failed prior TIPs and was thought not to be surgical candidate | Visualization | CT and fluoroscopy |
| Approach | Transgastric | ||
| Duration of follow-up | 3 mo | ||
| Thrombosis | Yes2 | ||
| Recurrent bleeding | Yes2 | ||
| Hepatic encephalopathy | NR | ||
| Bercu et al[8] 2015 | 58-year-old female with history of HCV cirrhosis, PVT with recurrent ascites who failed prior TIPs attempt and was a poor surgical candidate | Visualization | Fluoroscopy |
| Approach | Transabdominal | ||
| Duration of follow-up | 3 and 6 mo | ||
| Thrombosis | No | ||
| Recurrent bleeding | No | ||
| Hepatic encephalopathy | Yes3 | ||
| Hong et al[7] 2012 | 16-year-old female with history of chronic PVT and hematemesis who was felt to have high surgical risk | Visualization | Fluoroscopy and IVUS |
| Approach | Endovascular | ||
| Duration of follow-up | 1 mo | ||
| Thrombosis | No | ||
| Recurrent bleeding | No | ||
| Hepatic encephalopathy | NR | ||
| Hong et al[7] 2012 | 60-year-old female with history of HBV, HCV, HCC with thrombus obliterating PV | Visualization | Fluoroscopy and IVUS |
| Approach | Endovascular | ||
| Duration of follow-up | 2 and 10 mo | ||
| Thrombosis | No | ||
| Recurrent bleeding | No | ||
| Hepatic encephalopathy | NR | ||
| Hong et al[7] 2012 | 53-year-old male with history of pancreatic teratoma treated with Whipple with clot at SMV and splenic veins | Visualization | Fluoroscopy and IVUS |
| Approach | Endovascular | ||
| Duration of follow-up | 1 and 3 mo | ||
| Thrombosis | No | ||
| Recurrent bleeding | No | ||
| Hepatic encephalopathy | NR | ||
Fluoroscopy from a recent case of refractory variceal bleeding in a patient with a portal vein thrombus (PVT) from our institution will be used to graphically illustrate the basic technique (Figure 1). Our patient had cirrhosis and prior medical and endoscopic attempts to control her varices were limited by significant chest pain attributed to her banding procedures that required inpatient admission. Her PVT prohibited TIPS placement and she consented to undergo endovascular mesocaval shunt placement.
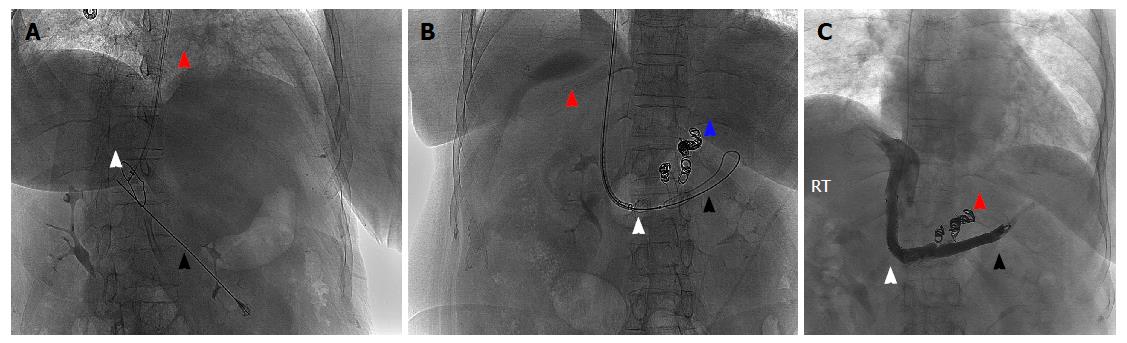
In our patient, and, in general, first, a needle is directed, in our case transabdominally, through the SMV, or, in this instance, a portal vein remnant, at a target placed via internal jugular (IJ) access (Figure 2A). Then, a wire is threaded from this needle through the IVC and out the IJ access so that, when the needle is removed, the distal tip of the the wire is in the splenic vein and its proximal end functions as a guidewire exiting the IJ access (Figure 2B). Finally, a stent graft, in our case a covered VIATORR stent, is passed over the guidewire via IJ access using Seldinger technique and placement is confirmed with fluoroscopic guidance (Figure 2C).
In the initial case report[6], contrast-enhanced computed tomography (CT) was first performed to define cross-sectional anatomy. The patient underwent bowel preparation pre-procedurally and was given prophylactic antibiotics as a transcolonic approach was anticipated. Using CT and fluoroscopic guidance, a needle was inserted through and through the transverse colon and SMV into the IVC to a retrieval basket. The retrieval basket had been placed in the IVC via the right internal jugular vein. A guide wire was then passed from the abdominal access through the SMV to IVC and jugular access. A stent was placed under angiographic guidance from the internal jugular access across the IVC to SMV and the wire was removed.
Another case report, by Moriarty et al[9], used similar methodology but opted for a transgastric rather than transcolonic approach to reduce the risk of infection. Interestingly, the case reported by Moriarty et al[9] required a cardiac transseptal needle to puncture the IVC as attempts made with a Rosch-Uchida TIPS needle were unsuccessful. The final published percutaneous approach to date was remarkable for the ability to avoid luminal puncture; Bercu et al[8] were able to approach transabdominally without perforating the bowel and relied on fluoroscopic rather than CT-guidance for visualization of the patient’s anatomy during the procedure.
Hong et al[7] reported an interesting series of three cases in which they were able to place mesocaval shunts but avoid a transabdominal approach. Using techniques similar to direct intra-hepatic portosystemic shunt (DIPS) placement, they describe a series of cases in which they relied on intravascular ultrasound to avoid transabdominal puncture to access the SMV. The stent itself is extra-hepatic (and thus distinct from DIPS) and possible in patients who are not candidates for TIPS or DIPS given portal vein thrombi. In short, sheaths were placed both femorally and in the internal jugular vein. A guide wire was used to couple the jugular and femoral sheaths. Following guide wire placement, a longitudinal side-firing intravascular ultrasound (IVUS), akin to those used in placement of DIPS, was introduced through the femoral sheath so that the SMV could be cannulated using a needle introduced at the jugular access. In this way, they were able to avoid a percutaneous transabdominal approach altogether. It should be noted that the third patient included in this series was not a cirrhotic patient but rather had portal and SMV clots due to a pancreatic tumor; we chose to include this patient in our review to demonstrate the feasibility of the procedure but appreciate that his underlying pathophysiology may be different from the others presented.
Candidates likely to benefit from mesocaval shunting include those with recurrent variceal bleeds who have failed prior medical and endoscopic therapies. Traditionally, TIPS has been employed in these patients to alleviate portal pressures. We propose that mesocaval shunting be offered to patients who are not TIPS candidates, particularly the group awaiting transplant, as there are not yet mortality data for mesocaval shunting and the mortality benefit of other portosystemic shunts, including TIPs, has been questioned[10].
As in our illustrative case, PVT, for example, are known to make TIPS more difficult and result in lower success rates, ranging from 40%-75%[11]. In some cases, when PVT is chronic, TIPS is not only difficult but actually technically impossible as in order to re-establish flow, there must be functional vessels surrounding the planned recanalized clot segment. This intact vasculature is often absent in those with chronic PVT as intrahepatic vessels may have atrophied while extrahepatic vessels form collaterals at high risk of bleeding[6]. Given the relatively high prevalence of PVT in cirrhotics, up to 5% to 16% of patients at the time of liver transplantation, mesocaval shunting has the potential to offer a therapy to a large group of patients who were previously thought to be without options, particularly in those patients whose PVT prohibits them from receiving a liver transplant[12].
Prior to endovascular placement of mesocaval shunts, the other option for patients in this scenario was surgical shunt placement. Historically, surgically placed portosystemic shunts have had high mortality[5]. While experienced centers are reporting improved operative mortality[13,14], the ability to replicate these lower mortality rates at smaller, less experienced centers remains to be seen. Furthermore, several of the patients in published percutaneous mesocaval shunt cases to date were thought to be poor surgical shunt candidates due to a history of prior abdominal surgeries and/or anatomy of their PVT[6,8,9].
If a patient is felt to be appropriate for consideration of mesocaval shunt placement, assessment of cross-sectional anatomy should be undertaken with computed tomography or magnetic resonance imaging of the abdomen to assist in procedural planning. For successful shunt placement, the IVC and SMV should be aligned and proximal in an anatomic window without any significant vasculature or viscera interposed between the two vessels[8]. The IVC and SMV (or a large collateral) must be patent for shunt placement.
There are a few potential pitfalls we consider with placement of a mesocaval shunt. First, similar to the surgical expertise required for safe surgical shunt placement, institutional interventional radiologic expertise will be required to safely recommend this procedure and this may not be available at all centers. This procedure, unlike TIPS, has not been reported to be performed in the setting of active variceal bleeding and thus there are no data to support its safety in that setting.
The most serious risk is that of procedure-related hemorrhage due to puncture of proximal vasculature. As noted by Hong et al[7] both the SMV and infrahepatic IVC lack any surrounding solid organs that could provide tamponade during shunt placement, creating a risk of major hemorrhage. Of the published cases to date, one noted intraabdominal hemorrhage-multiple small bowel hematomas - which was successfully treated conservatively with intravenous fluid, transfusion support and discontinuation of anticoagulation[6]. If the cannulated vesels require predilation prior to shunt placement, this risk of bleeding is likely increased[7]. Finally, while they did not experience intrabdominal hemorrhage despite use of an uncovered stent, Moriarty et al[9] note that use of an uncovered stent certainly increases risk of bleeding and recommend using covered stents and/or balloons to minimize this risk. In addition to procedural technique, we anticipate that, similar to other invasive procedures in cirrhotic patients, platelet counts influence the risk of hemorrhage. The cases reviewed here unfortunately do not provide patient platelet counts or other measures of clotting function.
In addition to the potential for vessel perforation, depending on each individual patient’s anatomy, there are risks of perforation of different structures. If an intestinal perforation is created, risk of sepsis, hemorrhage and/or abscess formation will certainly be increased[8]. In two reported cases, the transabdominal approach necessitated intestinal puncture[6,9]. In one case the track was transcolonic while the other approach was transgastric. Bowel preparation and antibiotic prophylaxis were administered in the transcolonic case and neither case resulted in sepsis. Although there were no reported infectious complications in the cases we reviewed, this risk should be underscored as it is likely not negligible in cirrhotic patients with impaired immunity. In addition to the risk of intestinal perforation, other nearby viscera are at risk of puncture as well. If the pancreas is punctured, both hemorrhage and pancreatitis are potential risks[8]. One published case to date notes pancreatic bisection and reports that serum amylase levels were within the normal range for at least five days post-operatively[7].
In the six cases reviewed, two cases reported subsequent shunt occlusion and need for further revision, a rate of 33% in our, appreciably, small series. Shunt thrombosis is an important outcome as it presumably puts the patient at risk for further portal hypertension, variceal formation and variceal bleeding. In the first of the two cases complicated by occlusion, lack of flow through the shunt was noted on both Doppler and CT on POD #2[6]. The patient underwent repeat angiography and had ballooning and directed thrombolysis of his stent with subsequent patency on 5 mo follow-up angiogram. The stent was again noted to be occluded on 12 mo follow-up angiography but he had no further gastrointestinal bleeding and no attempt to revise the shunt further was made. In the second case complicated by shunt occlusion, lack of flow through the shunt was noted on POD #2 on CT[9]. This was attributed to the severe angle of the initial shunt placement and its proximity to the wall of the IVC. This patient experienced upper gastrointestinal bleeding on POD #3 and underwent repeat angiography with stent replacement and had a patent stent and no further bleeding at 3 mo follow-up.
Finally, as with any form of portosystemic shunting, we anticipate that these patients will have higher rates of hepatic encephalopathy (HE) than patients without portosystemic shunting. Given the limited numbers of patients who have undergone percutaneous or endovascular mesocaval shunt placement, there are no data to evaluate rates of HE with these shunts vs TIPS or surgical shunt creation but presumably the rate is similar, around 30%[15,16]. In the cases reviewed above, only one case reported on the presence or absence of encephalopathy. In that case, the patient was noted to have no encephalopathy during index hospitalization or at 3 mo follow-up but was noted to be encephalopathic 6 mo post-operatively when her lactulose and rifaximin were held at an outside hospital for partial small bowel obstruction[8]. Her encephalopathy reportedly resolved with resumption of these medications.
As above, mesocaval shunting offers a treatment for bleeding varices for patients who otherwise face a high mortality with virtually no options. It can be offered to patients with PVT who cannot undergo TIPS and may be best utilized as a bridge to transplant. Furthermore, if an IVUS is utilized, vessels are directly visualized, avoiding the blind puncture method used in TIPS[7]. As with other similar endovascular procedures, we anticipate a lower mortality with this less invasive approach vs surgical shunt placement. Regardless, the majority of the published patients to date were not felt to be surgical candidates[6,7,9].
In the six adult cases published to date, two stents thrombosed within two days post-operatively while the remaining four remained patent[6,9]. Of the two patients with shunt thrombosis, one had a recurrent upper gastrointestinal bleed. In this case, it was postulated that the severe angle of the initial stent placement may have contributed to turbulence and subsequent thrombosis[9]. In both cases, subsequent shunt revision was performed and the revised shunts remained open during five and three months follow-up respectively. One shunt ultimately lacked patency at 12 mo follow-up but the patient had no further bleeding up to 14 mo follow-up. In summary, all reported cases have follow-up ranging from 1 to 14 mo in which, with the exception of the post-operative day 2 bleed noted above, there were no further variceal bleeding episodes. These are promising results in light of the known 60% 1-year risk of rebleeding and 33% 1-year mortality in patients who survive an episode of variceal hemorrhage[17].
In addition to offering a rescue therapy for a group of patients with minimal options, mesocaval shunting has an advantage compared to local variceal therapy, it will result in lower portal pressures and thus will reduce recurrent ascites as well reducing the risk of variceal bleeding. As noted by Garcia-Tsao and Bosch in a recent review, judgment of treatment success of varices should include mindfulness about the impact of variceal treatment on other complications of portal hypertension-ascites, jaundice, encephalopathy - rather than artificially isolating the treatment’s impact on variceal bleeding alone[17]. Finally, given that the presence of portal vein thrombosis is no longer thought to be an absolute contraindication to transplant[18], for those patients that are transplant eligible, placement of a mesocaval shunt may enable survival to the operating room table for transplant, a pressing concern given that our most recent national statistics are dire. In 2014, 1821 patients died while awaiting transplant and an additional 1290 were removed from the waiting list as they were felt to be “too sick” for transplant[19].
As reviewed above, endovascular mesocaval shunting is a feasible procedure that offers a promising intervention to a patient population with few options and one-year mortality as high as 20%[1]. TIPS has been shown to be an effective intervention to prevent recurrent variceal bleeding[2] and mesocaval shunting provides similar physiologic relief of portal pressure in patients who are not TIPS candidates. Like TIPS, mesocaval shunting avoids major surgery and may require less anesthesia than a surgical shunt approach. Furthermore, it can be offered to patients who are not surgical candidates. Mesocaval shunting alleviates portal hypertension, a key component of reducing the rate of variceal bleeding, and one that will potentially reduce recurrent ascites as well. The patient who stands to gain the most from this procedure has recurrent variceal bleeds, has failed endoscopic and medical therapies, cannot undergo TIPS due to anatomy and needs a bridge to transplant to minimize the chance of further decompensating while awaiting an organ. In order to have successful shunt placement, these patients must have alignment between IVC and SMV or SMV collaterals. Potential procedural complications include perforation of nearby vessels or viscera which could result in hemorrhage, sepsis, pancreatitis or abscess formation as well as stent thrombosis. Placement of a portosystemic shunt will also increase the risk of hepatic encephalopathy although there is little data to compare mesocaval shunts to surgical shunts or TIPS. To date, several approaches and imaging techniques have been utilized by reporting groups, notably including one approach that avoids transabdominal puncture[7]. In the cases reported, all have prevented rebleeding for the post-procedural monitoring period after initial shunt or initial shunt revision[6,8,9]. Further research should be performed to better assess outcomes - variceal bleeding, hepatic encephalopathy rates and mortality - in these patients compared to standard-of-care controls so that the benefits of this promising technique may be maximized.
Authors Jessica Davis, Albert K Chun and Marie L Borum have all contributed to this manuscript and endorse the data and conclusions within.
Manuscript source: Invited manuscript
P- Reviewer: Contini S, Hoff DAL S- Editor: Qi Y L- Editor: A E- Editor: Li D
| 1. | Zipprich A, Garcia-Tsao G, Rogowski S, Fleig WE, Seufferlein T, Dollinger MM. Prognostic indicators of survival in patients with compensated and decompensated cirrhosis. Liver Int. 2012;32:1407-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 197] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 2. | Rössle M, Haag K, Ochs A, Sellinger M, Nöldge G, Perarnau JM, Berger E, Blum U, Gabelmann A, Hauenstein K. The transjugular intrahepatic portosystemic stent-shunt procedure for variceal bleeding. N Engl J Med. 1994;330:165-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 475] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 3. | Hayes PC, Davis JM, Lewis JA, Bouchier IA. Meta-analysis of value of propranolol in prevention of variceal haemorrhage. Lancet. 1990;336:153-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 153] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Laine L, Cook D. Endoscopic ligation compared with sclerotherapy for treatment of esophageal variceal bleeding. A meta-analysis. Ann Intern Med. 1995;123:280-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 417] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 5. | Villeneuve JP, Pomier-Layrargues G, Duguay L, Lapointe R, Tanguay S, Marleau D, Willems B, Huet PM, Infante-Rivard C, Lavoie P. Emergency portacaval shunt for variceal hemorrhage. A prospective study. Ann Surg. 1987;206:48-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Nyman UR, Semba CP, Chang H, Hoffman C, Dake MD. Percutaneous creation of a mesocaval shunt. J Vasc Interv Radiol. 1996;7:769-773. [PubMed] |
| 7. | Hong R, Dhanani RS, Louie JD, Sze DY. Intravascular ultrasound-guided mesocaval shunt creation in patients with portal or mesenteric venous occlusion. J Vasc Interv Radiol. 2012;23:136-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Bercu ZL, Sheth SB, Noor A, Lookstein RA, Fischman AM, Nowakowski FS, Kim E, Patel RS. Percutaneous Mesocaval Shunt Creation in a Patient with Chronic Portal and Superior Mesenteric Vein Thrombosis. Cardiovasc Intervent Radiol. 2015;38:1316-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Moriarty JM, Kokabi N, Kee ST. Transvenous creation of a mesocaval shunt: report of use in the management of extrahepatic portal vein occlusion. J Vasc Interv Radiol. 2012;23:565-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Khan S, Tudur Smith C, Williamson P, Sutton R. Portosystemic shunts versus endoscopic therapy for variceal rebleeding in patients with cirrhosis. Cochrane Database Syst Rev. 2006;CD000553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Wils A, van der Linden E, van Hoek B, Pattynama PM. Transjugular intrahepatic portosystemic shunt in patients with chronic portal vein occlusion and cavernous transformation. J Clin Gastroenterol. 2009;43:982-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Stine JG, Shah NL, Argo CK, Pelletier SJ, Caldwell SH, Northup PG. Increased risk of portal vein thrombosis in patients with cirrhosis due to nonalcoholic steatohepatitis. Liver Transpl. 2015;21:1016-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 113] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 13. | Kokudo T, Bonard E, Gillet M, Kokudo N, Halkic N. Reappraisal of shunt surgery for extrahepatic portal vein obstruction in adults: Report of a single-center case series. Hepatol Res. 2015;45:1307-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Orloff MJ. Fifty-three years’ experience with randomized clinical trials of emergency portacaval shunt for bleeding esophageal varices in Cirrhosis: 1958-2011. JAMA Surg. 2014;149:155-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Sanyal AJ, Freedman AM, Shiffman ML, Purdum PP, Luketic VA, Cheatham AK. Portosystemic encephalopathy after transjugular intrahepatic portosystemic shunt: results of a prospective controlled study. Hepatology. 1994;20:46-55. [PubMed] |
| 16. | Riggio O, Merlli M, Pedretti G, Servi R, Meddi P, Lionetti R, Rossi P, Bezzi M, Salvatori F, Ugolotti U. Hepatic encephalopathy after transjugular intrahepatic portosystemic shunt. Incidence and risk factors. Dig Dis Sci. 1996;41:578-584. [PubMed] |
| 17. | Garcia-Tsao G, Bosch J. Varices and Variceal Hemorrhage in Cirrhosis: A New View of an Old Problem. Clin Gastroenterol Hepatol. 2015;13:2109-2117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Jamieson NV. Changing perspectives in portal vein thrombosis and liver transplantation. Transplantation. 2000;69:1772-1774. [PubMed] |
| 19. | Kim WR, Lake JR, Smith JM, Skeans MA, Schladt DP, Edwards EB, Harper AM, Wainright JL, Snyder JJ, Israni AK. Liver. Am J Transplant. 2016;16 Suppl 2:69-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |