Published online May 28, 2016. doi: 10.4254/wjh.v8.i15.659
Peer-review started: January 25, 2016
First decision: February 29, 2016
Revised: March 30, 2016
Accepted: May 7, 2016
Article in press: May 9, 2016
Published online: May 28, 2016
Processing time: 115 Days and 10.7 Hours
Non-alcoholic fatty liver disease (NAFLD) is a recognized problem in patients after orthotopic liver transplantation and may lead to recurrent graft injury. As the increased demand for liver allografts fail to match the available supply of donor organs, split liver transplantation (SLT) has emerged as an important technique to increase the supply of liver grafts. SLT allows two transplants to occur from one donor organ, and provides a unique model for observing the pathogenesis of NAFLD with respect to the role of recipient environmental and genetic factors. Here we report on two recipients of a SLT from the same deceased donor where only one developed non-alcoholic steatohepatitis (NASH), suggesting that host factors are critical for the development of NASH.
Core tip: Split liver transplantation provides a unique model of the pathogenesis of non-alcoholic fatty liver disease with respect to the role of recipient environmental risk factors and genetic background because the same donor graft is shared by two distinct recipients. Here we present two recipients of a split liver transplantation from same deceased donor, with one developing nonalcoholic steatohepatitis and the other without any evidence of hepatic steatosis three years after they were transplanted. These cases provide a unique natural experiment to explore host factors that contributed to the development of nonalcoholic steatohepatitis after liver transplantation.
- Citation: Boga S, Munoz-Abraham AS, Rodriguez-Davalos MI, Emre SH, Jain D, Schilsky ML. Host factors are dominant in the development of post-liver transplant non-alcoholic steatohepatitis. World J Hepatol 2016; 8(15): 659-664
- URL: https://www.wjgnet.com/1948-5182/full/v8/i15/659.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i15.659
Non-alcoholic fatty liver disease (NAFLD) affects around one third of the western population with an incidence that continues to grow in other parts of the world[1]. Histopathological findings of NAFLD in the liver range from simple steatosis to non-alcoholic steatohepatitis (NASH), and can eventually progress to cirrhosis and liver cancer[2]. NAFLD is recognized as a potential complication following LT and studies are being conducted to determine the prevalence and risk factors for development of NAFLD in LT recipients[3,4].
Split liver transplantation (SLT) has emerged as an important strategy to increase the supply of liver grafts by allowing two transplants to occur from one donor organ, and provide a unique opportunity to observe the role of host factors in the development of NAFLD. The technique of SLT is continuously evolving with reduced ischemia times and reduced vascular and biliary complications,and when performed in situ, SLT has yielded excellent outcomes[5]. However, SLT still involves significant complexity and short and long term complications of the split grafts need to be continually analyzed. Here we present data on the clinical course and outcomes of two recipients of a SLT from the same deceased donor where only one developed NASH, suggesting that extrahepatic host factors are critical for the development of NASH.
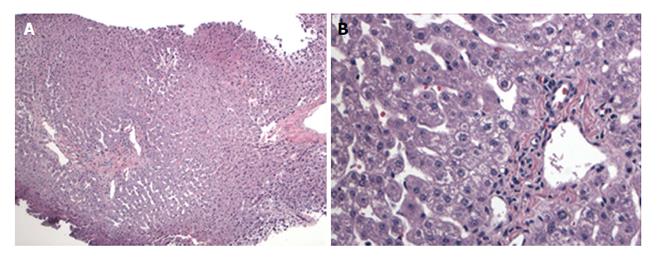
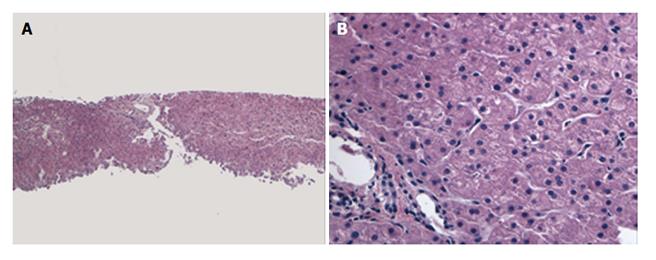
A female infant with a diagnosis of Crigler Najjar syndrome underwent a SLT at age 15 mo old, receiving a left lateral liver segment from a deceased donor who was exitus because of head trauma at age 16 years without any history of obesity, diabetes, hyperlipidemia or hypertension. The explanted liver did not reveal any significant histopathological abnormality and the donor pre-reperfusion biopsy was also negative for any significant pathologic findings, including inflammation, fibrosis, necrosis and steatosis (Figure 1). During the first year following transplantation, several episodes of liver test elevations were noted. Histology of the liver biopsy performed 19 mo post-SLT was not consistent with acute cellular rejection but showed minimal lobular inflammation, mild periportal edema and mild fibrosis (stage 1-2/4) without significant ductular reaction. No steatosis was present. A second biopsy performed 25 mo post-transplant due to continued liver test abnormalities revealed no histologic evidence of steatosis or progression of fibrosis (Figure 2). At this time the liver biopsy showed minimal portal fibrosis and no evidence of rejection, duct injury or duct loss. Subsequently magnetic resonance cholangiopancreatography was performed and an anatomic biliary stricture and dilated intrahepatic biliary ducts were identified. The patient underwent biliary reconstruction and a Roux-en-Y hepatojejunostomy and biliary stenting with internal-external drain placement at age 4 years. Three years following transplantation and two months after the biliary repair, liver tests improved [alanine aminotransferase (ALT): 29 U/L, aspartate aminotransferase (AST): 39 U/L, T/D Bil: 0.34/0.10 mg/dL, international normalized ratio (INR): 0.93]. Growth was in the normal range with a body mass index (BMI): 17.58 kg/m2. She was maintained on tacrolimus, mycophenolate mofetil and ursodeoxycholic acid treatment with routine biliary drain checks and close follow-up.
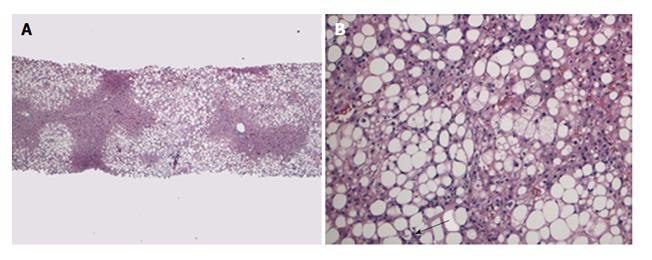
A 69-year-old male patient with a history of heterozygocity for genetic hemochromatosis (single copy of C282Y for the HFE gene) and alcoholic cirrhosis complicated by development of hepatocellular carcinoma (HCC) within Milan criteria (a 2.6 cm in segment III, and 1.5 and 1.1 cm lesions in segment VI). He was treated with chemoembolization and 8 mo later underwent an extended right lobe LT (segments I-IV-VIII) from the same deceased donor. The explanted liver revealed cirrhosis with residual viable nodules of moderately differentiated hepatocellular carcinoma without any vascular invasion, mild steatosis with steatohepatitis and increased hepatocellular siderosis. His past medical history was remarkable for hypertension, hyperlipidemia and diabetes mellitus. Initial immunosuppression was with steroid and tacrolimus, and he was changed to sirolimus and a lower dosage of tacrolimus at eight weeks post-transplant to try to reduce the risk of recurrent HCC. Although his postoperative course was uncomplicated but he remained on insulin for glycemic control. Twenty-four months after SLT, he had HCC recurrence with a solitary 1.5 cm nodule in segment VIII found on surveillance imaging. The tumor was treated by selective chemoembolization and he is without recurrence on follow up magnetic resonance images; the most recent being 34 mo post-transplant. Three years after transplantation, his liver function tests were found to be elevated on routine testing, and increased cholesterol and triglyceride levels were noted (ALT: 154 U/L, AST: 125 U/L, T/D Bil: 0.85/0.20 mg/dL, INR: 1.1, total cholesterol: 218 mg/dL, HDL cholesterol: 34 mg/dL, triglyceride: 501 mg/dL). At the same time, his weight had increased by 12% (had BMI increased from 30.5 kg/m2 to 34 kg/m2). He denied drinking alcohol. A liver biopsy was performed and showed no evidence of acute or chronic cellular rejection but was notable for marked macrovesicular steatosis involving about 70% of liver parenchyma, steatohepatitis and perisinusoidal fibrosis (grade 1 of 3, stage 1 of 4, Brunt system) (Figure 3).
LT is the accepted treatment of end-stage liver disease. The establishment of standard transplantation techniques, development of better immunosuppressive medications and accumulated experience in their safe use, improvement of intensive care and anesthesia all have played a major role in improving current 1-year survival after LT to 90%. Long-term outcomes, however, are still compromised by recurrent liver disease, increased risk of cancer, adverse effects of immunosuppressive drugs and possible metabolic complications[6,7]. One of the possible metabolic complications is the development of NASH/NAFLD.
SLT has developed as an alternative to increase the donor pool of organ for LT. The concept of splitting a liver allograft between two recipients was reported almost simultaneously by Pichlmayr et al[8] and Bismuth et al[9]. Recipients of SLT in the mid 1990s[10,11] were primarily one child who received the left-lateral segments and one adult who received the extended right lobe. SLT provides a unique model of the pathogenesis of NAFLD with respect to the role of recipient environmental risk factors and genetic background because the same donor graft is shared by two distinct recipients. Here we present two recipients of a SLT from same deceased donor, with one developing NASH and the other without any evidence of hepatic steatosis three years after they were transplanted. These cases provide a unique natural experiment to explore host factors that contributed to the development of NASH after LT.
LT recipients have several risk factors that put them at risk for NAFLD. Age and rapid weight gain causing obesity and long-term exposure to immunosuppressive medications can in part be responsible for NAFLD. Hyperlipidemia ocurrs frequently following solid-organ transplantation. Between 16% and 43% of adult LT recipients can have increased plasma cholesterol levels[7,12,13]. Furthermore corticosteroids and calcineurin inhibitors promote hypertension and hyper-cholesterolemia, prednisone, tacrolimus, and cyclosporine A are diabetogenic, and sirolimus induces hyperlipidemia. Although both of our recipients were placed on tacrolimus, the older patient was also treated with sirolimus. In our adult recipient, use of tacrolimus and the aberrant gain in weight likely increased his already present insulin resistance and contributed to further deterioration of glucose regulation, causing an increase in hepatic fatty infiltration and inflammation that ended with steatohepatitis.
In the first few months after liver LT, weight gain may be regarded as one of the positive effects of transplantation, especially in patients with advanced liver disease and pre-transplant cachexia. However, within two years of transplantation, an excess body weight is recorded in up to 60% to 70% of patients and 20% of previously non-obese transplant recipients become obese[14,15]. The recipient with steatohepatitis showed an increase in BMI from 30.5 kg/m2 at time of transplant to 34 kg/m2, corresponding to a 12% increase in weight over 3 years time. NAFLD is strongly linked to obesity, (BMI > 30 kg/m2) with a reported prevalence as high as 80% in obese patients and only 16% in individuals with a normal BMI[16]. Although the exact mechanisms leading to excessive weight gain in post-LT patients are uncertain, a major role is attributed to the development of post-LT insulin resistance, diabetes mellitus, arterial hypertension, hyperlipidemia and the metabolic effects of immunosuppressive medications (corticosteroids, mTOR inhibitors and calcineurin inhibitors).
There are other factors in our steatohepatitic recipient that may have increased hepatic fatty infiltration. The patient had a history of alcoholic cirrhosis, and some patients transplanted for alcoholic disease have a significantly higher risk of post-LT NAFLD even in the absence of recurrent alcoholic intoxication. Kim et al[4], reported that even though in 156 patients who had stopped drinking or had a limited amount of alcohol after LT, pre-LT alcoholic liver cirrhosis was a significant factor for their development of post-LT NAFLD. Similarly Dumortier et al[17] suggested that many patients with post-LT NAFLD have an unrecognized combination of alcoholic and non-alcoholic steatohepatitis that put them at risk of secondary liver failure because of persistent metabolic abnormalities. Recently Hejlova et al[18] examined 2360 post-transplant biopsies of 548 LT recipients to identify risk factors for the development of significant steatosis and found alcohol induced cirrhosis as a pre-transplant factor that is associated with significant post-transplant steatosis. It is likely patients with this combination of alcoholic and non-alcoholic steatohepatitis pre-transplanthave an increased risk of persistence of metabolic abnormalities post-LT due to newly de-novo or aggravated insulin resistance.
Though the adult recipient had a pre-transplant diagnosis of iron overload disorder, genetic hemochromatosis is cured by liver transplantation[19]. There are instances where recipients received organs from patients with hemochromatosis and iron accumulation has occured[20]. In the adult recipient, we did not find iron accumulation in his liver biopsy, suggesting iron did not play any additional role in the genesis of his steatohepatitis. Of the factors mentioned above; age, genetic background and even pretransplant history of alcoholic cirrhosis may be considered as unchangeable host factors where as post-transplant life style changes, diet, glycemic control by anti-diabetic medications, control of weight gain, hyperlipidemia therapy and immunesuppressive medications are changeable host factors that can affect the presence and progression of post-LT NASH.
In conclusion, this SLT provided a unique opportunity to observe the pathogenesis of NAFLD in the post-transplant setting. Although we can not exclude an interaction of donor and host factors, our data suggest host factors may be dominant for the development of post-LT NASH.
Because we can not change the genetic background of the donor organ, careful attention to potentially alterable host factors or treatments like diet, life style changes, hyperlipidemia therapy and immunesuppressive medications can result in improved long term outcomes for recipients.
A female infant with a diagnosis of Crigler Najjar syndrome and a 69-year-old male patient with cirrhosis complicated by development of hepatocellular carcinoma (HCC) underwent split liver transplantation (SLT).
Infant had jaundice and the elderly patient had signs of cirrhosis such as jaundice, ascites and spider angioma.
Inherited disorders of bilirubin metabolism for the first patient and primary and metastatic malignities of the liver for the second patient.
The first patient had elevated bilirubin levels (total bilirubin: 21 mg/dL, direct bilirubin: 0.25 mg/dL) and the second patient had an alpha fetoprotein of 109 ng/mL.
The adult patient had a 2.6 cm HCC lesion in segment III, and 1.5 and 1.1 cm HCC lesions in segment VI on magnetic resonance scan.
While the explanted liver did not reveal any significant histopathological abnormality in the first patient and revealed cirrhosis with residual viable nodules of HCC in the second patient; post-transplant liver biopsies showed minimal portal fibrosis and no histologic evidence of steatosis in the first patient and showed macrovesicular steatosis, steatohepatitis and perisinusoidal fibrosis in the second patient.
First patient underwent biliary reconstruction, a Roux-en-Y hepatojejunostomy and biliary stenting with internal-external drain placement and was maintained on tacrolimus, mycophenolate mofetil and ursodeoxycholic acid treatment and the second patient had an initial immunosuppression with steroid and tacrolimus, and then was changed to sirolimus and a lower dosage of tacrolimus at eight weeks post-transplant and had selective chemoembolization for recurrent HCC.
Even though emerging literature puts non-alcoholic fatty liver disease (NAFLD) forward as a potential complication following liver transplantation, SLT presented in this report provided a unique model of the pathogenesis of NAFLD with respect to the role of recipient environmental risk factors and genetic background because the same donor graft was shared by two distinct recipients.
Crigler-Najjar syndrome is a rare hereditary disorder of bilirubin metabolism characterized by unconjugated hyperbilirubinemia due to deficiency of the enzymatic activity of glucuronosyltransferase.
This SLT provided a unique opportunity to observe the pathogenesis of NAFLD in the post-transplant setting. The data suggest host factors may be dominant for the development of post-LT non-alcoholic steatohepatitis (NASH). The authors recommend paying careful attention to potentially alterable host factors or treatments like diet, life style changes, hyperlipidemia therapy and immunesuppressive medications to improve the long term outcomes for recipients.
This is an interesting case report about post transplant NASH comparing two different scenarios (two different hosts) for a unique donor from a split liver transplant.
P- Reviewer: Inoue K, Ikura Y, Komatsu H, Villela-Nogueira CA S- Editor: Gong XM L- Editor: A E- Editor: Liu SQ
| 1. | Milić S, Stimac D. Nonalcoholic fatty liver disease/steatohepatitis: epidemiology, pathogenesis, clinical presentation and treatment. Dig Dis. 2012;30:158-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 149] [Article Influence: 11.5] [Reference Citation Analysis (1)] |
| 2. | Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, Musso A, De Paolis P, Capussotti L, Salizzoni M. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134-140. [PubMed] |
| 3. | Seo S, Maganti K, Khehra M, Ramsamooj R, Tsodikov A, Bowlus C, McVicar J, Zern M, Torok N. De novo nonalcoholic fatty liver disease after liver transplantation. Liver Transpl. 2007;13:844-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 124] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 4. | Kim H, Lee K, Lee KW, Yi NJ, Lee HW, Hong G, Choi Y, You T, Suh SW, Jang JJ. Histologically proven non-alcoholic fatty liver disease and clinically related factors in recipients after liver transplantation. Clin Transplant. 2014;28:521-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Emre S, Umman V. Split liver transplantation: an overview. Transplant Proc. 2011;43:884-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Reuben A. Long-term management of the liver transplant patient: diabetes, hyperlipidemia, and obesity. Liver Transpl. 2001;7:S13-S21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Sheiner PA, Magliocca JF, Bodian CA, Kim-Schluger L, Altaca G, Guarrera JV, Emre S, Fishbein TM, Guy SR, Schwartz ME. Long-term medical complications in patients surviving > or = 5 years after liver transplant. Transplantation. 2000;69:781-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 194] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 8. | Pichlmayr R, Ringe B, Gubernatis G, Hauss J, Bunzendahl H. [Transplantation of a donor liver to 2 recipients (splitting transplantation)--a new method in the further development of segmental liver transplantation]. Langenbecks Arch Chir. 1988;373:127-130. [PubMed] |
| 9. | Bismuth H, Morino M, Castaing D, Gillon MC, Descorps Declere A, Saliba F, Samuel D. Emergency orthotopic liver transplantation in two patients using one donor liver. Br J Surg. 1989;76:722-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 182] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Azoulay D, Astarcioglu I, Bismuth H, Castaing D, Majno P, Adam R, Johann M. Split-liver transplantation. The Paul Brousse policy. Ann Surg. 1996;224:737-746; discussion 746-748. [PubMed] |
| 11. | Rogiers X, Malagó M, Gawad K, Jauch KW, Olausson M, Knoefel WT, Gundlach M, Bassas A, Fischer L, Sterneck M. In situ splitting of cadaveric livers. The ultimate expansion of a limited donor pool. Ann Surg. 1996;224:331-339; discussion 339-341. [PubMed] |
| 12. | Imagawa DK, Dawson S, Holt CD, Kirk PS, Kaldas FM, Shackleton CR, Seu P, Rudich SM, Kinkhabwala MM, Martin P. Hyperlipidemia after liver transplantation: natural history and treatment with the hydroxy-methylglutaryl-coenzyme A reductase inhibitor pravastatin. Transplantation. 1996;62:934-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 58] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Gisbert C, Prieto M, Berenguer M, Bretó M, Carrasco D, de Juan M, Mir J, Berenguer J. Hyperlipidemia in liver transplant recipients: prevalence and risk factors. Liver Transpl Surg. 1997;3:416-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 65] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Everhart JE, Lombardero M, Lake JR, Wiesner RH, Zetterman RK, Hoofnagle JH. Weight change and obesity after liver transplantation: incidence and risk factors. Liver Transpl Surg. 1998;4:285-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 142] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Palmer M, Schaffner F, Thung SN. Excessive weight gain after liver transplantation. Transplantation. 1991;51:797-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1522] [Cited by in RCA: 1619] [Article Influence: 115.6] [Reference Citation Analysis (1)] |
| 17. | Dumortier J, Giostra E, Belbouab S, Morard I, Guillaud O, Spahr L, Boillot O, Rubbia-Brandt L, Scoazec JY, Hadengue A. Non-alcoholic fatty liver disease in liver transplant recipients: another story of “seed and soil”. Am J Gastroenterol. 2010;105:613-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 163] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 18. | Hejlova I, Honsova E, Sticova E, Lanska V, Hucl T, Spicak J, Jirsa M, Trunecka P. Prevalence and risk factors of steatosis after liver transplantation and patient outcomes. Liver Transpl. 2016;22:644-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 19. | Moini M, Mistry P, Schilsky ML. Liver transplantation for inherited metabolic disorders of the liver. Curr Opin Organ Transplant. 2010;15:269-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Dwyer JP, Sarwar S, Egan B, Nolan N, Hegarty J. Hepatic iron overload following liver transplantation of a C282y homozygous allograft: a case report and literature review. Liver Int. 2011;31:1589-1592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |