Published online May 18, 2015. doi: 10.4254/wjh.v7.i8.1125
Peer-review started: August 22, 2014
First decision: December 17, 2014
Revised: January 15, 2015
Accepted: January 30, 2015
Article in press: February 2, 2015
Published online: May 18, 2015
Processing time: 271 Days and 21.8 Hours
Chronic hepatitis C virus (HCV) infection can cause liver cirrhosis and hepatocellular carcinoma (HCC). Several studies have demonstrated that the eradication of HCV reduces the occurrence of HCC. In Japan, as many people live to an advanced age, HCV-infected patients are also getting older, and the age at HCC diagnosis has also increased. Although older HCV-infected patients have a risk of developing HCC, the treatment response to peginterferon-alpha plus ribavirin therapy is relatively poor in these patients because of drop-out or discontinuation of this treatment due to adverse events. It is established that the mechanism of action between interferon-alpha and interferon-beta is slightly different. Short-term natural interferon-beta monotherapy is effective for patients with acute hepatitis C and patients infected with HCV genotype 2 and low viral loads. Natural interferon-beta plus ribavirin for 48 wk or for 24 wk are also effective for some patients with HCV genotype 1 or HCV genotype 2. Natural interferon-beta plus ribavirin has been used for certain “difficult-to-treat” HCV-infected patients. In the era of direct-acting anti-virals, natural interferon-beta plus ribavirin may be one of the therapeutic options for special groups of HCV-infected patients. In the near future, signal transduction pathways of interferon-beta will inform further directions.
Core tip: The use of natural interferon-beta plus ribavirin can eradicate hepatitis C virus (HCV) from non-responders to peginterferon-alpha plus ribavirin treatment. Some of these patients may have anti-interferon-alpha neutralizing antibodies. In Japan, natural interferon-beta plus ribavirin has been used for certain “difficult-to-treat” HCV-infected patients such as elderly patients, patients with mental disorders and patients with lower platelet counts, before the era of interferon-free regimens. To eradicate hepatocellular carcinoma and end-stage liver diseases associated with HCV, the use of natural interferon-beta with or without ribavirin should be one of the useful treatment options for HCV-infected patients.
- Citation: Sasaki R, Kanda T, Nakamoto S, Haga Y, Nakamura M, Yasui S, Jiang X, Wu S, Arai M, Yokosuka O. Natural interferon-beta treatment for patients with chronic hepatitis C in Japan. World J Hepatol 2015; 7(8): 1125-1132
- URL: https://www.wjgnet.com/1948-5182/full/v7/i8/1125.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i8.1125
Chronic hepatitis C virus (HCV) infection is a major health problem, and causes liver cirrhosis and hepatocellular carcinoma (HCC)[1-3]. Chronic HCV infection is the leading cause of HCC in southern European countries, North America and Japan[4,5]. Despite the recent progress of treatments for HCC[6,7], the prognosis of HCV-positive HCC patients is still poor without liver transplantation[5,8]. It is known that the incubation period until HCC detection is usually shorter when the liver fibrosis is more advanced in patients infected with HCV[9]. Interferon therapy can reduce the risk for HCC[10] and improve liver fibrosis[11] if patients with chronic hepatitis C achieve a sustained virological response (SVR) to this therapy[12,13].
In the direct-acting antivirals (DAAs) against HCV era, peginterferon-alpha plus ribavirin therapy with or without DAAs, as well as interferon-free regimens, led to the higher SVR rates in patients infected with HCV[14,15]. However, these treatments also had disadvantages due to specific adverse events. We should have more additional therapeutic options for chronic hepatitis C because standard of care treatments are not always available according to the condition of the patients. In this article, we discuss the recent trends in natural interferon-beta treatment for patients with chronic hepatitis C in Japan.
There are an estimated 1 million HCV-infected patients in Japan[16,17]. In Japan, as many people live to an advanced age[18], HCV-infected patients are also growing older[16,17,19]. Peginterferon-alpha plus ribavirin may lead to a approximately 50% SVR and approximately 80% SVR, respectively, in HCV genotype 1 treated for 48-72 wk and genotype 2-infected Japanese patients treated for 24 wk[14]. In male and female HCV genotype 1-infected patients aged ≥ 65 years and treated with peginterferon-alpha plus ribavirin, an SVR was achieved in 54.8% and 29.5%, respectively[18]. In male and female HCV genotype 2-infected patients aged ≥ 65 years and treated with peginterferon-alpha plus ribavirin, an SVR was achieved in 54.5% and 66.6%, respectively[18]. However, several contraindications exist for these treatments involving peginterferon-alpha, with an increasing number of contraindication in older patients.
Interferon-alpha/-beta and related molecules are classified as type I interferons, and two other types are type II (interferon-gamma) and type III (interferon-lambda)[20]. Type I interferons signal through a ubiquitously expressed receptor composed of two chains: interferon-alpha receptor 1 and interferon-alpha receptor 2[20]. These receptors are linked to JAK-STAT pathways to induce the expression of interferon-stimulated genes[21,22]. The interferon response to infection is rapid and these cytokines serve as a first-line of defense against many pathogens and diseases[23]. Whereas the murine interferon-alpha gene subtypes have approximately 90% homology, murine interferon-beta appears to be rather more divergent, with only approximately 55% homology to a murine interferon-alpha consensus sequence[24]. It is clinically established that interferon-alpha and natural interferon-beta have different actions[25,26]. Although these mechanisms may rely on differential signaling pathways between interferon-alpha and interferon-beta, it is possible that only interferon-beta is induced directly by viral infection and that interferon-alpha induction is a consequence of this initial interferon-beta expression[24,27]. Of interest, in mice, interferon-alpha can be induced by interferon-beta, but interferon-beta cannot be induced by interferon-alpha[28].
In 1991, Omata et al[29] reported that the intravenous administration of natural interferon-beta may prevent patients with acute hepatitis C from developing chronic infection. The researchers performed a prospective controlled trial in 25 patients; 11 were treated for an average of 30 d, with a mean 52 megaunits of natural interferon-beta and 14 patients without. The follow-up at 3 years revealed that serum HCV RNA became undetectable in 10 of 11 treated subjects and in only 1 of 12 untreated controls[29]. It was also reported that a daily intravenous injection of natural interferon-beta at a dosage of 6 million units for 8 wk eradicated HCV RNA from a 29-year-old nurse with acute hepatitis C genotype 1 caused by a needle-stick injury; she had no severe adverse events[30]. These studies suggested that natural interferon-beta is a therapeutic option for patients with acute hepatitis C, instead of peginterferon-alpha[31], although there was a contrary opinion[32].
Japan’s national health insurance approves natural interferon-beta with or without ribavirin for treating patients with chronic hepatitis C or compensated liver cirrhosis, although the natural interferon-beta treatment requires intravenous injection at least three times weekly. It was reported that short-term treatment for 4 wk and low doses of natural interferon-beta has only a temporary effect on controlling the disease activity in patients with post-transfusion non-A, non-B chronic active hepatitis[33]. However, it was reported that three million units of intravenous natural interferon-beta twice daily for 2 wk reduces the HCV RNA levels by 3 log IU/mL which has a stronger effect against HCV, compared with the combination of peginterferon-alpha plus ribavirin, which reduces the HCV RNA levels by 1-2 log IU/mL[34]. Thus, it is possible that a 3-million-unit twice-daily natural interferon-beta regimen is more effective for reducing HCV RNA levels. However, the SVR rates of patients infected with HCV genotype 1b and a high viral load, who have been treated by natural interferon-beta monotherapy, are 0% to 11%[35-37].
Katamura et al[37] reported that treatment with natural interferon-beta plus ribavirin for 48 wk leads to an SVR in 27% (3/11) of Japanese patients infected with HCV genotype 1b and a high viral load; the researchers also observed that during the treatment, the platelet count increased above the baseline after week 4 in this treatment group but not in the peginterferon-plus-ribavirin group (Table 1). Among the 11 with HCV G1, 7 were re-treated patients, and in 4 of 7 a transient virological response had been observed during the first cycle[37]. By the end of the second cycle of therapy, a sustained virological response was observed in 3 cases. The study of Arase et al[38] is retrospective, and the 40 patients treated with natural interferon-beta were recruited over a period ranging from December 2004 to May 2008. They[38] reported that treatment with natural interferon-beta plus ribavirin for 48 wk led to an SVR in 38% (15/40) of patients infected with HCV genotype 1b and a high viral load; moreover, the SVR rate was 87% (13/15) in patients who were negative for HCV RNA at 8 wk after the commencement of treatment (Table 1). One patient discontinued the treatment due to exacerbation of depression, and another patient discontinued the treatment due to a skin rash[38].
| Ref. | G | No. of patients | Naïve (%) | Age (yr, mean ± SD)/gender (male) (%) | Formula of treatment | SVR rates (%) |
| Katamura et al[37] | 1 | 11 | 4 (36) | 57/(64) | Natural interferon-beta plus ribavirin for 48 wk | 27 |
| 1 | 22 | 8 (36) | 54/(64) | Peginterferon-alpha plus ribavirin for 48 wk | 41 | |
| Arase et al[38] | 1 | 40 | 12 (30) | 51.9 ± 10.0/(70) | Natural interferon-beta plus ribavirin for 48 wk | 38 |
| Arase et al[39] | 2 | 24 | 12 (50) | 55.9 ± 10.2/(46) | Natural interferon-beta plus ribavirin for 24 wk | 88 |
| Arase et al[42] | 1 | 14 | 0 (0) | 62.1 ± 4.3/(43) | Natural interferon-beta plus ribavirin for 48 wk | 38 |
| Arase et al[44] | 1 | 23 | 11 (48) | 68.1 ± 2.6/(30) | Natural interferon-beta plus reduction-dose-ribavirin for 48 wk | 39 |
| 1 | 22 | 7 (32) | 66.9 ± 3.0/(68) | Natural interferon-beta plus standard-dose-ribavirin for 48 wk | 27 | |
| Arase et al[47] | 2 | 33 | 20 (60) | 70.4 ± 3.7/(24) | Natural interferon-beta plus ribavirin for 24 wk | 75 |
| Nomura et al[48] | 1 | 21 | 21 (100) | 71.8 ± 5.1/(46) | Natural interferon-beta plus ribavirin | 29 |
| 2 | 18 | 18 (100) | Natural interferon-beta plus ribavirin | 72 | ||
| 1 | 21 | 21 (100) | 69.1 ± 3.5/(48) | Peginterferon-alpha plus ribavirin | 29 | |
| 2 | 18 | 18 (100) | Peginterferon-alpha plus ribavirin | 86 |
Arase et al[39] reported that treatment with natural interferon-beta plus ribavirin for 24 wk led to an SVR in 88% (21/24) of patients infected with HCV genotype 2 and a high viral load, and that the SVR rate was 94% (18/19) in patients who were negative for HCV RNA at 8 wk after the commencement of treatment (Table 1). No patients discontinued the treatment due to treatment-related adverse events[39]. Arase et al[40] also reported that treatment with natural interferon-beta for only 6-8 wk led to an SVR of 56% (14/25) in cirrhotic patients infected with HCV genotype 2 and a low viral load. Natural interferon-beta monotherapy for 6-8 wk should be sufficient to eradicate HCV in patients infected with HCV genotype 2 at a low viral load (< 5000 IU/mL).
Montalto et al[41] reported that natural interferon-beta is well tolerated and yield modest results in white patients with chronic hepatitis C who are non-responders to interferon-alpha. Natural interferon-beta administration was neither interrupted nor its dosage reduced due to side effects[41]. We also observed a patient infected with HCV genotype 1 who failed to respond to interferon with or without ribavirin seven times but then achieved a SVR using natural interferon-beta plus ribavirin for 48 wk[25]. Arase et al[42] observed that re-treatment with natural interferon-beta plus ribavirin for 48 wk led to an SVR in 38% (5/14) of previously treated-patients infected with HCV genotype 1b and a high viral load; moreover, the SVR rates were 100% (4/4) and 83% (5/6) in patients who were negative for HCV RNA at 12 wk and 24 wk, respectively (Table 1).
It has recently been reported that anti-interferon-alpha neutralizing antibody is associated with a non-SVR to peginterferon-alpha plus ribavirin in chronic hepatitis C patients[43]. The same researchers observed 19 non-SVR patients who were positive for anti-interferon-alpha neutralizing antibodies and found that no anti-interferon-alpha neutralizing antibodies interfered with the antiviral activity of natural interferon-beta; furthermore, the re-treatment of patients carrying anti-interferon-alpha neutralizing antibodies with natural interferon-beta plus ribavirin led to the eradication of HCV[43].
Arase et al[44] reported that the SVR rate was achieved by natural interferon-beta plus reduction-dose or standard-dose ribavirin, respectively, for 48 wk in 39% (9/23) or 27% (6/22) of patients aged 65 years or older who were infected with HCV genotype 1b and a high viral load. They stressed that natural interferon-beta plus a reduction-dose is a possible treatment for patients aged 65 years or older who are infected with HCV genotype 1b and a high viral load[44]. In this study[44], the SVR rates were 44% (15/34) and 0% (0/11) in patients with interleukin-28B (IL28B) rs8099917TT and TG, respectively. The IL28B genotype[18,45,46] is useful for predicting the treatment results of natural interferon-beta plus ribavirin. Arase et al[47] reported that the SVR rate was achieved by natural interferon-beta plus reduction-dose or standard-dose ribavirin, respectively, for 24 wk in 72% (13/18) or 80% (12/15) of patients aged 65 years or older who were infected with HCV genotype 2 and a high viral load; additionally, they found that natural interferon-beta plus a reduction-dose is an optional treatment for patients aged 65 years and older who were infected with HCV genotype 2 and a high viral load[47]. Nomura et al[48] also reported that natural interferon-beta plus ribavirin therapy is safe in elderly patients and that the SVR rate is similar to that of peginterferon-alpha plus ribavirin (Table 1), although this recent study is also a retrospective, non-randomized trial. Among 66 recruited to treatment with natural interferon-beta and ribavirin, 15 were side effect-related treatment discontinuation, 36 patients were available for final analysis according to these figures, and 15 additional patients were lost during the study[48]. However, they observed in the group of patients treated with peginterferon-alpha plus ribavirin, the rates of patients who discontinued the treatment for adverse effects is 66% (42/66). Thus, this study in elderly patients exceeds the corresponding rate of withdrawals reported in previous studies[14].
Natural interferon-beta plus ribavirin has been available for mild mental disorders such as mild depression and interferon-induced depression in Japan[37]. Katamura et al[37] used natural interferon-beta plus ribavirin for 48 wk or for 24 wk, respectively, in 5 HCV genotype 1b and 3 HCV genotype 2 patients with mental disorders. Of the 5 HCV genotype 1b patients, 3 and 2 had depression and interferon-induced depression, respectively, and of the 3 patients infected with HCV genotype 2, 2 and 1 had depression and interferon-induced depression, respectively. Only one of these patients withdrew from treatment due to the exacerbation of depression at week 32. Only one and all three patients with completed treatment achieved an SVR for HCV genotype 1b and genotype 2, respectively[37]. Careful attention should also be paid if clinicians use natural interferon-beta plus ribavirin for HCV patients with mental disorders. Consultations with the attending psychiatrist may enable achieving a more successful treatment.
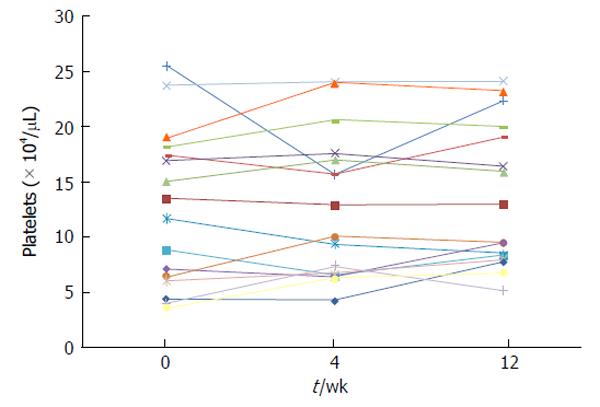
To examine the effects of natural interferon-beta on the platelet counts, the changes in platelet counts were retrospectively analyzed after beginning treatment in 16 HCV-infected patients treated with natural interferon-beta at Chiba University Hospital (Figure 1). In 3 patients with < 50000/μL platelets, no reduction in the platelet counts was observed. Katamura et al[37] also reported that the platelet count during the administration of natural interferon-beta plus ribavirin increased above the baseline after 4 wk. These results indicate that natural interferon-beta plus ribavirin is a therapeutic option for HCV-infected patients with lower platelet counts. But none of the studies regarding patients with chronic HCV related hepatitis are prospective controlled trials.
It is still controversy whether interferon-beta therapy can improve the prognosis for HCC[49,50]. Combination therapy of 5-fluorouracil and interferon-alpha or peginterferon-alpha for advanced HCC still remains challenging[51-54]. It was previously reported that interferon-beta is more potent than interferon-alpha in inhibition of human hepatoma cell growth with or without combination with anticancer drugs[55]. Further studies will be needed at this point. In present, we should treat patients infected with HCV as soon as possible, before the occurrence of HCC[56].
Natural human interferon-beta is produced by human fibroblasts, and is currently available in Japan. Recombinant human interferon-beta-1a and interferon-beta-1b are produced in mammalian cells or Escherichia coli, respectively[57]. It was reported that recombinant human interferon-beta-1a with or without ribavirin has an excellent safety profile, and after 24-wk-treatment of recombinant human interferon-beta-1a with or without ribavirin, SVR was 21.6% and 27.4% in HCV genotype 1 and genotype 2 patients, respectively[57-60]. Peginterferon-beta-1a may be beneficial for patients infected with HCV[61,62].
During the preparation of this manuscript, in Japan, since September 2014, interferon-free regimen with daclatasvir plus asunaprevir for 24 wk has been available for treatment of HCV genotype 1 patients who were ineligible, intolerant, or had not responded to prior interferon-based therapy[63]. In the near future, we might be using all-oral DAAs and interferon-free regimens for the treatment of all HCV-infected patients[15].
In summary, natural interferon-beta with or without ribavirin is a treatment option for patients infected with HCV, such as non-responders to peginterferon-alpha plus ribavirin or patients who are unable to use DAAs. In the DAA era, candidates for using natural interferon-beta might exist among special groups, such as “difficult-to-treat” HCV-infected patients including elderly patients, patients with mental disorders and in those with low platelet counts. Because these key messages are supported by current weak data, we may reconfirm this in the further clinical practice. Future studies of the interferon-β signal transduction pathways will inform further directions.
P- Reviewer: Howell J, Hernanda PY, Solinas A, Suzuki T, Tong HL
S- Editor: Gong XM L- Editor: A E- Editor: Liu SQ
| 1. | Di Bisceglie AM. Hepatitis C and hepatocellular carcinoma. Hepatology. 1997;26:34S-38S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 293] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 2. | Saito I, Miyamura T, Ohbayashi A, Harada H, Katayama T, Kikuchi S, Watanabe Y, Koi S, Onji M, Ohta Y. Hepatitis C virus infection is associated with the development of hepatocellular carcinoma. Proc Natl Acad Sci USA. 1990;87:6547-6549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 826] [Cited by in RCA: 840] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 3. | Kanda T, Yokosuka O, Omata M. Hepatitis C virus and hepatocellular carcinoma. Biology (Basel). 2013;2:304-316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Byam J, Renz J, Millis JM. Liver transplantation for hepatocellular carcinoma. Hepatobiliary Surg Nutr. 2013;2:22-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 5. | Utsunomiya T, Shimada M, Kudo M, Ichida T, Matsui O, Izumi N, Matsuyama Y, Sakamoto M, Nakashima O, Ku Y. A comparison of the surgical outcomes among patients with HBV-positive, HCV-positive, and non-B non-C hepatocellular carcinoma: a nationwide study of 11,950 patients. Ann Surg. 2015;261:513-520. [PubMed] |
| 6. | Llovet JM. Focal gains of VEGFA: candidate predictors of sorafenib response in hepatocellular carcinoma. Cancer Cell. 2014;25:560-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 7. | Shiina S, Tateishi R, Imamura M, Teratani T, Koike Y, Sato S, Obi S, Kanai F, Kato N, Yoshida H. Percutaneous ethanol injection for hepatocellular carcinoma: 20-year outcome and prognostic factors. Liver Int. 2012;32:1434-1442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Terrault NA, Shiffman ML, Lok AS, Saab S, Tong L, Brown RS, Everson GT, Reddy KR, Fair JH, Kulik LM. Outcomes in hepatitis C virus-infected recipients of living donor vs. deceased donor liver transplantation. Liver Transpl. 2007;13:122-129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 9. | Takano S, Yokosuka O, Imazeki F, Tagawa M, Omata M. Incidence of hepatocellular carcinoma in chronic hepatitis B and C: a prospective study of 251 patients. Hepatology. 1995;21:650-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 244] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 10. | Yoshida H, Shiratori Y, Moriyama M, Arakawa Y, Ide T, Sata M, Inoue O, Yano M, Tanaka M, Fujiyama S. Interferon therapy reduces the risk for hepatocellular carcinoma: national surveillance program of cirrhotic and noncirrhotic patients with chronic hepatitis C in Japan. IHIT Study Group. Inhibition of Hepatocarcinogenesis by Interferon Therapy. Ann Intern Med. 1999;131:174-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 833] [Cited by in RCA: 779] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 11. | Shiratori Y, Imazeki F, Moriyama M, Yano M, Arakawa Y, Yokosuka O, Kuroki T, Nishiguchi S, Sata M, Yamada G. Histologic improvement of fibrosis in patients with hepatitis C who have sustained response to interferon therapy. Ann Intern Med. 2000;132:517-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 547] [Cited by in RCA: 538] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 12. | Di Bisceglie AM, Shiffman ML, Everson GT, Lindsay KL, Everhart JE, Wright EC, Lee WM, Lok AS, Bonkovsky HL, Morgan TR. Prolonged therapy of advanced chronic hepatitis C with low-dose peginterferon. N Engl J Med. 2008;359:2429-2441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 377] [Cited by in RCA: 339] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 13. | Lok AS, Everhart JE, Wright EC, Di Bisceglie AM, Kim HY, Sterling RK, Everson GT, Lindsay KL, Lee WM, Bonkovsky HL. Maintenance peginterferon therapy and other factors associated with hepatocellular carcinoma in patients with advanced hepatitis C. Gastroenterology. 2011;140:840-849; quiz e12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 158] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 14. | Kanda T, Imazeki F, Yokosuka O. New antiviral therapies for chronic hepatitis C. Hepatol Int. 2010;4:548-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 15. | Kanda T, Yokosuka O, Omata M. Treatment of hepatitis C virus infection in the future. Clin Transl Med. 2013;2:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Tanaka J, Kumagai J, Katayama K, Komiya Y, Mizui M, Yamanaka R, Suzuki K, Miyakawa Y, Yoshizawa H. Sex- and age-specific carriers of hepatitis B and C viruses in Japan estimated by the prevalence in the 3,485,648 first-time blood donors during 1995-2000. Intervirology. 2004;47:32-40. [PubMed] |
| 17. | Tanaka J, Koyama T, Mizui M, Uchida S, Katayama K, Matsuo J, Akita T, Nakashima A, Miyakawa Y, Yoshizawa H. Total numbers of undiagnosed carriers of hepatitis C and B viruses in Japan estimated by age- and area-specific prevalence on the national scale. Intervirology. 2011;54:185-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 129] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 18. | Kanda T, Nakamoto S, Wu S, Yokosuka O. Role of IL28B genotype in older hepatitis C virus-infected patients. World J Immunol. 2013;3:54-61. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Osaki Y, Nishikawa H. Treatment for hepatocellular carcinoma in Japan over the last three decades: Our experience and published work review. Hepatol Res. 2015;45:59-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 20. | Zhang SY, Boisson-Dupuis S, Chapgier A, Yang K, Bustamante J, Puel A, Picard C, Abel L, Jouanguy E, Casanova JL. Inborn errors of interferon (IFN)-mediated immunity in humans: insights into the respective roles of IFN-alpha/beta, IFN-gamma, and IFN-lambda in host defense. Immunol Rev. 2008;226:29-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 248] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 21. | Basu A, Meyer K, Ray RB, Ray R. Hepatitis C virus core protein modulates the interferon-induced transacting factors of Jak/Stat signaling pathway but does not affect the activation of downstream IRF-1 or 561 gene. Virology. 2001;288:379-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Kanda T, Steele R, Ray R, Ray RB. Hepatitis C virus infection induces the beta interferon signaling pathway in immortalized human hepatocytes. J Virol. 2007;81:12375-12381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Day SL, Ramshaw IA, Ramsay AJ, Ranasinghe C. Differential effects of the type I interferons alpha4, beta, and epsilon on antiviral activity and vaccine efficacy. J Immunol. 2008;180:7158-7166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Deonarain R, Alcamí A, Alexiou M, Dallman MJ, Gewert DR, Porter AC. Impaired antiviral response and alpha/beta interferon induction in mice lacking beta interferon. J Virol. 2000;74:3404-3409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 137] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 25. | Kanda T, Nakamoto S, Arai M, Miyamura T, Wu S, Fujiwara K, Yokosuka O. Natural interferon-beta plus ribavirin therapy led to sustained virological response after seven unsuccessful courses of anti-viral treatment in a chronic hepatitis C patient. Clin J Gastroenterol. 2013;6:160-163. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Kuga C, Enomoto H, Aizawa N, Takashima T, Ikeda N, Ishii A, Sakai Y, Iwata Y, Tanaka H, Saito M. Anti-interferon-α neutralizing antibody induced telaprevir resistance under the interferon-α plus telaprevir treatment in vitro. Biochem Biophys Res Commun. 2014;454:453-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 27. | Raychoudhuri A, Shrivastava S, Steele R, Dash S, Kanda T, Ray R, Ray RB. Hepatitis C virus infection impairs IRF-7 translocation and Alpha interferon synthesis in immortalized human hepatocytes. J Virol. 2010;84:10991-10998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 28. | Asano M, Hayashi M, Yoshida E, Kawade Y, Iwakura Y. Induction of interferon-alpha by interferon-beta, but not of interferon-beta by interferon-alpha, in the mouse. Virology. 1990;176:30-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Omata M, Yokosuka O, Takano S, Kato N, Hosoda K, Imazeki F, Tada M, Ito Y, Ohto M. Resolution of acute hepatitis C after therapy with natural beta interferon. Lancet. 1991;338:914-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 126] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 30. | Kogure T, Ueno Y, Kanno N, Fukushima K, Yamagiwa Y, Nagasaki F, Kakazu E, Matsuda Y, Kido O, Nakagome Y. Sustained viral response of a case of acute hepatitis C virus infection via needle-stick injury. World J Gastroenterol. 2006;12:4757-4760. [PubMed] |
| 31. | Wiegand J, Buggisch P, Boecher W, Zeuzem S, Gelbmann CM, Berg T, Kauffmann W, Kallinowski B, Cornberg M, Jaeckel E. Early monotherapy with pegylated interferon alpha-2b for acute hepatitis C infection: the HEP-NET acute-HCV-II study. Hepatology. 2006;43:250-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 149] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 32. | Calleri G, Colombatto P, Gozzelino M, Chieppa F, Romano P, Delmastro B, Macor A, Cariti G, Brunetto MR, Grillone W. Natural beta interferon in acute type-C hepatitis patients: a randomized controlled trial. Ital J Gastroenterol Hepatol. 1998;30:181-184. [PubMed] |
| 33. | Ohnishi K, Nomura F, Iida S. Treatment of posttransfusion non-A, non-B acute and chronic hepatitis with human fibroblast beta-interferon: a preliminary report. Am J Gastroenterol. 1989;84:596-600. [PubMed] |
| 34. | Okushin H, Morii K, Uesaka K, Yuasa S. Twenty four-week peginterferon plus ribavirin after interferon-β induction for genotype 1b chronic hepatitis C. World J Hepatol. 2010;2:226-232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 35. | Kurosaki M, Enomoto N, Murakami T, Sakuma I, Asahina Y, Yamamoto C, Ikeda T, Tozuka S, Izumi N, Marumo F. Analysis of genotypes and amino acid residues 2209 to 2248 of the NS5A region of hepatitis C virus in relation to the response to interferon-beta therapy. Hepatology. 1997;25:750-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 124] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 36. | Kainuma M, Ogata N, Kogure T, Kohta K, Hattori N, Mitsuma T, Terasawa K. The efficacy of a herbal medicine (Mao-to) in combination with intravenous natural interferon-beta for patients with chronic hepatitis C, genotype 1b and high viral load: a pilot study. Phytomedicine. 2002;9:365-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 37. | Katamura Y, Suzuki F, Akuta N, Sezaki H, Yatsuji H, Nomura N, Kawamura Y, Hosaka T, Kobayashi M, Suzuki Y. Natural human interferon beta plus ribavirin combination therapy in Japanese patients infected with hepatitis C virus and a high viral load. Intern Med. 2008;47:1827-1834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 38. | Arase Y, Suzuki F, Akuta N, Sezaki H, Suzuki Y, Kawamura Y, Kobayashi M, Hosaka T, Yatsuji H, Hirakawa M. Efficacy and safety of combination therapy of natural human interferon beta and ribavirin in chronic hepatitis C patients with genotype 1b and high virus load. Intern Med. 2010;49:957-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 39. | Arase Y, Suzuki F, Akuta N, Sezaki H, Suzuki Y, Kawamura Y, Kobayashi M, Hosaka T, Yatsuji H, Hirakawa M. Efficacy and safety of combination therapy of natural human interferon beta and ribavirin in chronic hepatitis C patients with genotype 2 and high virus load. Intern Med. 2010;49:965-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 40. | Arase Y, Suzuki F, Sezaki H, Kawamura Y, Suzuki Y, Kobayashi M, Akuta N, Hosaka T, Yatsuji H, Kobayashi M. The efficacy of short-term interferon-beta therapy for type C cirrhotic patients with genotype 2a and low virus load. Intern Med. 2008;47:1085-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 41. | Montalto G, Tripi S, Cartabellotta A, Fulco M, Soresi M, Di Gaetano G, Carroccio A, Levrero M. Intravenous natural beta-interferon in white patients with chronic hepatitis C who are nonresponders to alpha-interferon. Am J Gastroenterol. 1998;93:950-953. [PubMed] |
| 42. | Arase Y, Suzuki Y, Suzuki F, Matsumoto N, Akuta N, Imai N, Seko Y, Sezaki H, Kawamura Y, Kobayashi M. Efficacy and safety of combination therapy of natural human interferon beta and ribavirin in chronic hepatitis C patients. Intern Med. 2011;50:2083-2088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 43. | Matsuda F, Torii Y, Enomoto H, Kuga C, Aizawa N, Iwata Y, Saito M, Imanishi H, Shimomura S, Nakamura H. Anti-interferon-α neutralizing antibody is associated with nonresponse to pegylated interferon-α plus ribavirin in chronic hepatitis C. J Viral Hepat. 2012;19:694-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 44. | Arase Y, Kawamura Y, Suzuki Y, Suzuki F, Akuta N, Matsumoto N, Seko Y, Sezaki H, Kobayashi M, Hosaka T. Efficacy of reduction therapy of natural human β-interferon and ribavirin in elderly patients with chronic hepatitis C, genotype 1b and high viral load. Hepatol Res. 2012;42:949-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 45. | Tanaka Y, Nishida N, Sugiyama M, Kurosaki M, Matsuura K, Sakamoto N, Nakagawa M, Korenaga M, Hino K, Hige S. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet. 2009;41:1105-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1779] [Cited by in RCA: 1775] [Article Influence: 110.9] [Reference Citation Analysis (0)] |
| 46. | Nakamoto S, Kanda T, Imazeki F, Wu S, Arai M, Fujiwara K, Yokosuka O. Simple assay based on restriction fragment length polymorphism associated with IL28B in chronic hepatitis C patients. Scand J Gastroenterol. 2011;46:955-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 47. | Arase Y, Suzuki Y, Suzuki F, Akuta N, Sezaki H, Kawamura Y, Kobayashi M, Imai N, Seko Y, Hosaka T. Efficacy of reduction therapy of natural human β-interferon and ribavirin in elderly patients with chronic hepatitis C, genotype 2 and high virus load. Hepatol Res. 2012;42:750-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 48. | Nomura H, Miyagi Y, Tanimoto H, Yamashita N. Interferon-beta plus ribavirin therapy can be safely and effectively administered to elderly patients with chronic hepatitis C. J Infect Chemother. 2014;20:489-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 49. | Colleoni M, Buzzoni R, Bajetta E, Bochicchio AM, Bartoli C, Audisio R, Bonfanti G, Nolè F. A phase II study of mitoxantrone combined with beta-interferon in unresectable hepatocellular carcinoma. Cancer. 1993;72:3196-3201. [PubMed] |
| 50. | Falkson G, Lipsitz S, Borden E, Simson I, Haller D. Hepatocellular carcinoma. An ECOG randomized phase II study of beta-interferon and menogaril. Am J Clin Oncol. 1995;18:287-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 51. | Sakon M, Nagano H, Dono K, Nakamori S, Umeshita K, Yamada A, Kawata S, Imai Y, Iijima S, Monden M. Combined intraarterial 5-fluorouracil and subcutaneous interferon-alpha therapy for advanced hepatocellular carcinoma with tumor thrombi in the major portal branches. Cancer. 2002;94:435-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 143] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 52. | Obi S, Yoshida H, Toune R, Unuma T, Kanda M, Sato S, Tateishi R, Teratani T, Shiina S, Omata M. Combination therapy of intraarterial 5-fluorouracil and systemic interferon-alpha for advanced hepatocellular carcinoma with portal venous invasion. Cancer. 2006;106:1990-1997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 203] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 53. | Monden M, Sakon M, Sakata Y, Ueda Y, Hashimura E; FAIT Research Group. 5-fluorouracil arterial infusion + interferon therapy for highly advanced hepatocellular carcinoma: A multicenter, randomized, phase II study. Hepatol Res. 2012;42:150-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 54. | Uchino K, Obi S, Tateishi R, Sato S, Kanda M, Sato T, Arano T, Enooku K, Goto E, Masuzaki R. Systemic combination therapy of intravenous continuous 5-fluorouracil and subcutaneous pegylated interferon alfa-2a for advanced hepatocellular carcinoma. J Gastroenterol. 2012;47:1152-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 55. | Damdinsuren B, Nagano H, Sakon M, Kondo M, Yamamoto T, Umeshita K, Dono K, Nakamori S, Monden M. Interferon-beta is more potent than interferon-alpha in inhibition of human hepatocellular carcinoma cell growth when used alone and in combination with anticancer drugs. Ann Surg Oncol. 2003;10:1184-1190. [PubMed] |
| 56. | Kanda T, Imazeki F, Mikami S, Kato K, Shimada N, Yonemitsu Y, Miyauchi T, Arai M, Fujiwara K, Tsubota A. Occurrence of hepatocellular carcinoma was not a rare event during and immediately after antiviral treatment in Japanese HCV-positive patients. Oncology. 2011;80:366-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 57. | Pellicano R, Craxi A, Almasio PL, Valenza M, Venezia G, Alberti A, Boccato S, Demelia L, Sorbello O, Picciotto A. Interferon beta-1a alone or in combination with ribavirin: a randomized trial to compare efficacy and safety in chronic hepatitis C. World J Gastroenterol. 2005;11:4484-4489. [PubMed] |
| 58. | Cheng PN, Marcellin P, Bacon B, Farrell G, Parsons I, Wee T, Chang TT. Racial differences in responses to interferon-beta-1a in chronic hepatitis C unresponsive to interferon-alpha: a better response in Chinese patients. J Viral Hepat. 2004;11:418-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 59. | Chan HL, Ren H, Chow WC, Wee T. Randomized trial of interferon beta-1a with or without ribavirin in Asian patients with chronic hepatitis C. Hepatology. 2007;46:315-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 60. | Han Q, Liu Z, Kang W, Li H, Zhang L, Zhang N. Interferon beta 1a versus interferon beta 1a plus ribavirin for the treatment of chronic hepatitis C in Chinese patients: a randomized, placebo-controlled trial. Dig Dis Sci. 2008;53:2238-2245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 61. | Baker DP, Lin EY, Lin K, Pellegrini M, Petter RC, Chen LL, Arduini RM, Brickelmaier M, Wen D, Hess DM. N-terminally PEGylated human interferon-beta-1a with improved pharmacokinetic properties and in vivo efficacy in a melanoma angiogenesis model. Bioconjug Chem. 2006;17:179-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 113] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 62. | Reuss R. PEGylated interferon beta-1a in the treatment of multiple sclerosis - an update. Biologics. 2013;7:131-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 63. | Kumada H, Suzuki Y, Ikeda K, Toyota J, Karino Y, Chayama K, Kawakami Y, Ido A, Yamamoto K, Takaguchi K. Daclatasvir plus asunaprevir for chronic HCV genotype 1b infection. Hepatology. 2014;59:2083-2091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 468] [Cited by in RCA: 453] [Article Influence: 41.2] [Reference Citation Analysis (0)] |