Published online Nov 18, 2015. doi: 10.4254/wjh.v7.i26.2631
Peer-review started: June 1, 2015
First decision: July 27, 2015
Revised: October 12, 2015
Accepted: November 3, 2015
Article in press: November 4, 2015
Published online: November 18, 2015
Processing time: 174 Days and 4.6 Hours
The molecular basis of the carcinogenesis of hepatocellular carcinoma (HCC) has not been adequately clarified, which negatively impacts the development of targeted therapy protocols for this overwhelming neoplasia. The aberrant activation of signaling in the HCC is primarily due to the deregulated expression of the components of the Wnt-/-β-catenin. This leads to the activation of β-catenin/T-cell factor-dependent target genes that control cell proliferation, cell cycle, apoptosis, and cell motility. The deregulation of the Wnt pathway is an early event in hepatocarcinogenesis. An aggressive phenotype was associated with HCC, since this pathway is implicated in the proliferation, migration, and invasiveness of cancer cells, regarding the cell’s own survival. The disruption of the signaling cascade Wnt-/-β-catenin has shown anticancer properties in HCC’s clinical evaluations of therapeutic molecules targeted for blocking the Wnt signaling pathway for the treatment of HCC, and it represents a promising perspective. The key to bringing this strategy in to clinical practice is to identify new molecules that would be effective only in tumor cells with aberrant signaling β-catenin.
Core tip: The Wnt signaling pathway is decisive in the rule of mechanisms of proliferation and survival, as well as the differentiation of liver cells during hepatic embryogenesis and morphogenesis. The atypical initiation of signaling in the hepatocellular carcinoma (HCC) is primarily because of deregulated expressions of the components of the Wnt-/-β-catenin. The mechanisms that are considered more functional and that sustain aberrant activation of signaling pathways act via alterations in the β-catenin gene or the AXIN1/2-gene’s encoding axin, a protein necessary for the degradation of β-catenin. The development of targeted therapeutic molecules for the blockade of the Wnt-signaling pathway for the treatment of HCC depends on the identification of molecules that would be effective only in tumor cells that carry an aberrant signaling β-catenin.
- Citation: Waisberg J, Saba GT. Wnt-/-β-catenin pathway signaling in human hepatocellular carcinoma. World J Hepatol 2015; 7(26): 2631-2635
- URL: https://www.wjgnet.com/1948-5182/full/v7/i26/2631.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i26.2631
Hepatocellular carcinoma (HCC) is distributed globally. It is the second most important cause of cancer deaths and caused approximately 750000 deaths in 2012[1].
Management options for HCC are restricted, and this neoplasm can just be cured by radical treatments, such as hepatectomy or liver transplantation, when the diagnosis is made while the tumor still has small proportions. However, the diagnosis is often made at a late stage in the development of HCC, when the cancer has already grown too much and/or is widespread. Moreover, malignant tumors also have significant resistance to multidisciplinary treatment protocols[2].
The increasing incidence of HCC triggered an intense phase of research to clarify the main molecular, genetic, and cellular mechanisms involved in its pathogenesis, which could encourage the development of more effective treatments for this neoplasia[3,4]. However, the molecular basis of the carcinogenesis of HCC still has not been adequately clarified[5,6], which impairs the development of targeted therapy protocols for this overwhelming neoplasia[2].
Complex processes are involved in several steps within the molecular pathogenesis of HCC. The normal hepatocytes can have their phenotype transformed by an accumulation of aberrant genetic mutations or epigenetic nature, as well as by signaling the pathway’s activation of growth factors[7-13].
In chronic hepatic disease, it is decisive to recognize the mitogenic signaling pathways that do not participate in liver regeneration. Also, the mitogenic signaling pathways are essential to trigger the emergence of a clonal expansion that promotes tumor growth. This identification can prevent the occurrence of adverse side effects on the eventual use of a target therapy for the steps involved in the carcinogenesis of HCC. However, non-transformed hepatocytes can show higher molecular redundancy and continue to proliferate, though some signaling pathways are inhibited. Moreover, due to the fact that the altered hepatocytes might provide incomplete terminated molecular mechanisms, they may block specific pathways that control cell growth and survival[5].
The signaling of those specific anomalies and inherited and epigenetic mechanisms related to risk features are specific cellular changes and are considered the center of initiation and development of HCC[5]. Due to the complex genetic heterogeneity of HCC, the investigation targets the molecular signaling pathways and their shared molecular mechanisms[4].
The main determinants’ mechanisms of liver carcinogenesis are related to cirrhosis renewal subsequent to liver injury caused by hepatitis, contaminants, or metabolites and mutations in oncogenes (sole or multiple) or in neoplasm suppressor genes. These mechanisms are associated with the main changes in cell signaling pathways with interest from the therapeutic point of view because its lock can reverse, delay, or prevent hepatocarcinogenesis[14].
The atypical initiation of signaling in HCC is primarily related to a deregulated expression of the components of the Wnt-/-β-catenin. These activate β-catenin/TCF-dependent target genes that monitor cell proliferation, cell phase, apoptosis, and cell motility. The Wnt pathway is the input constituent of the physiological processes implicated in the embryonic progress and homeostasis of human tissues[3].
Although it is inactive in adult livers, the Wnt pathway participates in liver pathobiology[15]. This route is markedly decisive in the active surroundings of hepatic development and controls the progressions of proliferation, survival and the differentiation of hepatocytes. Abnormal initiation of this pathway has also been recognized in hepatoblastoma as well as in HCC[5].
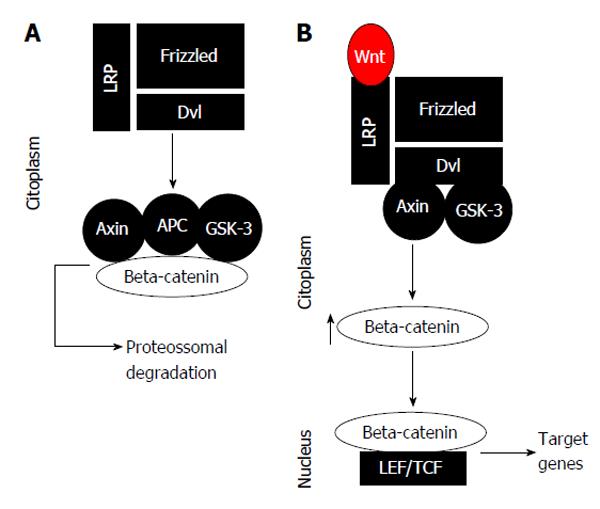
Under normal conditions, the β-catenin level of a hepatocyte is lowered, because the complex activity destruction of the β-catenin protein, involving the APC, axin, and GSK-3 proteins, and the fact that it connects itself to β-catenin molecules, phosphorylating it, with consecutive deprivation in the proteasome[4]. Stimulation of the non-canonical Wnt-/-β-catenin pathway is started by the connecting of extracellular ligands of the transmembrane receptor’s Wnt-/-FZD-related protein and low-density lipoprotein receptor, which afterward liquefies the complex configuration destruction of β-catenin proteins, which results in the increase of β-catenin in the cytoplasm[4]. The β-catenin proteins are able to displace to the nucleus and forming a binding complex with the transcription factor LEF-/-TCF proteins. This binding complex promotes an activation of target genes that regulate cell proliferation, migration, invasion, cell cycle progress, and metastasis propagation[4] (Figure 1). For that reason, the constitutive start of this pathway could possibly be significant for establishing and maintaining the malignant liver phenotype[4].
In liver carcinogenesis, early deregulation of the Wnt pathway occurs. An aggressive phenotype was associated with HCC, since this pathway is implicated in the proliferation, migration, and invasiveness of cancer cells, within the course of the cell’s survival[2].
The start of this pathway happens when a ligand connects to a Wnt receptor Frizzled (FZD) on the cell membrane. The routes identified in the Wnt signaling pathway are the non-canonical pathway and the canonical pathway, where β-catenin protein is involved[3].
The Wnt signaling pathway mediated by the β-catenin protein involves the binding of 1 or more of the 19 Wnt ligands to 1 or more of the FZD transmembrane cell surface receptors of the tumor cells. This stimulates the activation of the associated β-catenin canonical pathway and the non-canonical pathway in which the participation of c-Jun NH2-terminal kinase (Jnk) plus protein kinase C occurs, both of which are primarily active during embryogenesis and adult tissue homeostasis[3].
The mechanisms considered more functionally aberrant and the sustained initiation of the signaling pathway happen via the β-catenin mutations in the genes or AXIN1-/-2 axin genes encoding a protein essential for the degradation of β-catenin[5].
Kan et al[6] described the complete genome sequencing of 88 HCC, 81 of which are positive for the hepatitis B virus (HBV). The author identified genes with genetic modifications and signaling pathways involved in HCC related to HBV. They found the β-catenin gene (15.9%) and TP53 (35.2%), the oncogene and tumor suppressor gene, respectively, to be the most often mutated. The signaling pathway Wnt-/-β-catenin and Janus kinase protein (JAK)-/-STAT were changed from 62.5% to 45.5% of the patients, respectively, and were considered probable to perform as the two main oncogenic conductors in HCC. The mutation/activation of JAK 1 was found in 9.1% of patients[6].
The deregulated signaling cascade Wnt-/-β-catenin was observed in 95% of HCC[16]. Moreover, this route can likewise be initiated by deletions or mutations in the β-catenin gene, thus making non-degradable proteins by destruction complex. This event facilitates the increase of β-catenin protein in the cytoplasm. In turn, such a molecule translocates to the nucleus and activates genes related to cell growing[9]. Most mutations/deletions are located in the N-terminus of beta-catenin, thus leading to a change in beta-catenin protein turnover after failure of phosphorylation (GSK3, CK1). Thus, the overexpression of FZD Wnt ligands and receptors leads the activation of these ligands and receptors as the primary mechanism causing the increase of β-catenin protein in the cytoplasm and the displacement of β-catenin to the cell nucleus[4].
An important ligand that is involved is the Wnt3, which is normally overexpressed in CHC. After attaching to the FZD7, it triggers the canonical signaling triggered by hepatitis B and hepatitis C virus[17,18]. In this context, the interruption of the interaction involving the ligands and Wnt-/-FZD receptors was suggested as a mechanism of inhibition of Wnt signaling/β-catenin to reduce the migration and invasiveness of tumor cells of HCC[18].
Wnt ligands focus on the cell surface for linking with heparan sulfate proteoglycans; then, the Wnt ligand is unrestricted and able to interact with the FZD receptors to start the signaling pathway of β-catenin[4].
The mechanism further clarified that the mutations in the β-catenin gene or CTNNB1, which were observed in about 20% to 40% of all cases of HCC[19,20] concerned the activation of β-catenin in HCC. The mutations have also been reported in the constituents of the complex of β-catenin degradation, including AXIN1 gene mutation, which was observed in 3% to 16% of all cases of HCC[19,20] and the AXIN2 gene and in approximately 3% of all cases of HCC[21]. Interestingly, HCC occurs in HCV patients, up to 40% of whom show an incidence of CTNNB1 gene mutations[22]. Further, the HCV patients led to an increased expression of the gene Wnt1 in HCC cells due to mechanisms not yet completely understood[23]. Studies of HCC occurring in patients with HBV have implicated protein X of the HBV to stimulate the activation of β-catenin, representing an independent CTNNB1 gene mutation[24]. Interestingly, the majority of the functionally important mutations that cause the activation of Wnt signaling are those that affect the CTNNB1 gene and correlate significantly with the concentration of the nuclear β-catenin protein. The ultimate consequence of continued signaling pathway Wnt-/-β-catenin brings about an amplified expression of genes’β-catenin dependents, which influence the whole tumor[25,26].
Mutations of the β-catenin gene mostly have been described as late events in HCC[27], while other authors reported that these mutations are early events[28,29]. The tumors with the mutated β-catenin gene have been reported as having less vascular invasion[30] and higher grades of cell differentiation[30,31]. These mutations have been associated with better prognoses for patients with HCC. However, other authors observed a higher nuclear and cytoplasmic concentration of the β-catenin protein in HCC with the most micro- and macro-vascular invasion[32,33], increased neoplastic cell proliferation, and poorly differentiated tumors[29].
Moreover, it is interesting that a diminutive but important number of patients with HCV developed HCC with no confirmation of fibrosis[34]. The HCV has a preference in the use of the Wnt pathway as an HCC development mechanism[35]. Similarly, it is worth noting that the diminutive number of hepatic adenomas that develop into HCC often have mutations in the gene for β-catenin[36]. The neoplastic conversion of hepatic adenomas in HCC usually takes place in the healthy liver without confirmation of fibrosis. Thus, this finding indicates the involvement of the mutation of the β-catenin gene, regardless of the presence of liver fibrosis[5].
The findings of the Wnt signaling pathway activity in HCC suggest that the activation of β-catenin is sometimes found in up to 90% of HCC. However, in 40% to 60% of HCC patients, mutation of the CTNNB1 or AXIN1/AXIN2 genes was not observed. Actually, this finding may reflect the significant participation of Wnt-/-β-catenin signaling pathway in the maintenance of the normal function of hepatocytes in liver parenchyma, even without the presence of neoplastic cells in its interior[5].
The interruption of the signaling cascade Wnt-/-β-catenin has shown antineoplastic activity in HCC, although therapeutic molecules are not currently blocking the Wnt signaling pathway for the treatment of HCC[2]. Still, proteins that are part of the Wnt signaling pathway are considered potential targets for pharmacological therapy[3,37]. However, the complexity of transcription - dependent mechanisms of β-catenin becomes the challenge of ambitious drug therapy. Moreover, such medicaments may have important side-effects in organs, such as the intestine, where the Wnt-/-β-catenin is significant for the regeneration of tissues. The key toward this strategy coming into clinical practice is to identify new molecules that would be effective only in tumor cells that carry an aberration that signals β-catenin.
P- Reviewer: Odenthal M, Schmelzer E S- Editor: Tian YL L- Editor: A E- Editor: Liu SQ
| 1. | Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer 2013; Available from: http://globocan.iarc.fr. |
| 2. | Allegretta M, Filmus J. Therapeutic potential of targeting glypican-3 in hepatocellular carcinoma. Anticancer Agents Med Chem. 2011;11:543-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Pez F, Lopez A, Kim M, Wands JR, Caron de Fromentel C, Merle P. Wnt signaling and hepatocarcinogenesis: molecular targets for the development of innovative anticancer drugs. J Hepatol. 2013;59:1107-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 214] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 4. | Wands JR, Kim M. WNT/β-catenin signaling and hepatocellular carcinoma. Hepatology. 2014;60:452-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 5. | Nejak-Bowen KN, Monga SP. Beta-catenin signaling, liver regeneration and hepatocellular cancer: sorting the good from the bad. Semin Cancer Biol. 2011;21:44-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 210] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 6. | Kan Z, Zheng H, Liu X, Li S, Barber TD, Gong Z, Gao H, Hao K, Willard MD, Xu J. Whole-genome sequencing identifies recurrent mutations in hepatocellular carcinoma. Genome Res. 2013;23:1422-1433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 355] [Cited by in RCA: 412] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 7. | Calvisi DF, Ladu S, Gorden A, Farina M, Lee JS, Conner EA, Schroeder I, Factor VM, Thorgeirsson SS. Mechanistic and prognostic significance of aberrant methylation in the molecular pathogenesis of human hepatocellular carcinoma. J Clin Invest. 2007;117:2713-2722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Hoshida Y, Villanueva A, Kobayashi M, Peix J, Chiang DY, Camargo A, Gupta S, Moore J, Wrobel MJ, Lerner J. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med. 2008;359:1995-2004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1044] [Cited by in RCA: 1005] [Article Influence: 59.1] [Reference Citation Analysis (0)] |
| 9. | Laurent-Puig P, Zucman-Rossi J. Genetics of hepatocellular tumors. Oncogene. 2006;25:3778-3786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 254] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 10. | Lee HC, Kim M, Wands JR. Wnt/Frizzled signaling in hepatocellular carcinoma. Front Biosci. 2006;11:1901-1915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 121] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 11. | Revill K, Wang T, Lachenmayer A, Kojima K, Harrington A, Li J, Hoshida Y, Llovet JM, Powers S. Genome-wide methylation analysis and epigenetic unmasking identify tumor suppressor genes in hepatocellular carcinoma. Gastroenterology. 2013;145:1424-1435.e1-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 174] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 12. | Ye QH, Qin LX, Forgues M, He P, Kim JW, Peng AC, Simon R, Li Y, Robles AI, Chen Y. Predicting hepatitis B virus-positive metastatic hepatocellular carcinomas using gene expression profiling and supervised machine learning. Nat Med. 2003;9:416-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 642] [Cited by in RCA: 643] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 13. | Zender L, Xue W, Zuber J, Semighini CP, Krasnitz A, Ma B, Zender P, Kubicka S, Luk JM, Schirmacher P. An oncogenomics-based in vivo RNAi screen identifies tumor suppressors in liver cancer. Cell. 2008;135:852-864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 363] [Cited by in RCA: 364] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 14. | Whittaker S, Marais R, Zhu AX. The role of signaling pathways in the development and treatment of hepatocellular carcinoma. Oncogene. 2010;29:4989-5005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 608] [Cited by in RCA: 680] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 15. | Thompson MD, Monga SP. WNT/beta-catenin signaling in liver health and disease. Hepatology. 2007;45:1298-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 393] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 16. | Bengochea A, de Souza MM, Lefrançois L, Le Roux E, Galy O, Chemin I, Kim M, Wands JR, Trepo C, Hainaut P. Common dysregulation of Wnt/Frizzled receptor elements in human hepatocellular carcinoma. Br J Cancer. 2008;99:143-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 175] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 17. | Kim M, Lee HC, Tsedensodnom O, Hartley R, Lim YS, Yu E, Merle P, Wands JR. Functional interaction between Wnt3 and Frizzled-7 leads to activation of the Wnt/beta-catenin signaling pathway in hepatocellular carcinoma cells. J Hepatol. 2008;48:780-791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 148] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 18. | Nambotin SB, Tomimaru Y, Merle P, Wands JR, Kim M. Functional consequences of WNT3/Frizzled7-mediated signaling in non-transformed hepatic cells. Oncogenesis. 2012;1:e31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Zucman-Rossi J, Benhamouche S, Godard C, Boyault S, Grimber G, Balabaud C, Cunha AS, Bioulac-Sage P, Perret C. Differential effects of inactivated Axin1 and activated beta-catenin mutations in human hepatocellular carcinomas. Oncogene. 2007;26:774-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 185] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 20. | Kim YD, Park CH, Kim HS, Choi SK, Rew JS, Kim DY, Koh YS, Jeung KW, Lee KH, Lee JS. Genetic alterations of Wnt signaling pathway-associated genes in hepatocellular carcinoma. J Gastroenterol Hepatol. 2008;23:110-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Taniguchi K, Roberts LR, Aderca IN, Dong X, Qian C, Murphy LM, Nagorney DM, Burgart LJ, Roche PC, Smith DI. Mutational spectrum of beta-catenin, AXIN1, and AXIN2 in hepatocellular carcinomas and hepatoblastomas. Oncogene. 2002;21:4863-4871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 351] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 22. | Wong CM, Fan ST, Ng IO. beta-Catenin mutation and overexpression in hepatocellular carcinoma: clinicopathologic and prognostic significance. Cancer. 2001;92:136-145. [PubMed] |
| 23. | Fukutomi T, Zhou Y, Kawai S, Eguchi H, Wands JR, Li J. Hepatitis C virus core protein stimulates hepatocyte growth: correlation with upregulation of wnt-1 expression. Hepatology. 2005;41:1096-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 115] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 24. | Lian Z, Liu J, Li L, Li X, Clayton M, Wu MC, Wang HY, Arbuthnot P, Kew M, Fan D. Enhanced cell survival of Hep3B cells by the hepatitis B x antigen effector, URG11, is associated with upregulation of beta-catenin. Hepatology. 2006;43:415-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837-1851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1290] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 26. | Paul S, Dey A. Wnt signaling and cancer development: therapeutic implication. Neoplasma. 2008;55:165-176. [PubMed] |
| 27. | Park JY, Park WS, Nam SW, Kim SY, Lee SH, Yoo NJ, Lee JY, Park CK. Mutations of beta-catenin and AXIN I genes are a late event in human hepatocellular carcinogenesis. Liver Int. 2005;25:70-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 28. | Fujie H, Moriya K, Shintani Y, Tsutsumi T, Takayama T, Makuuchi M, Kimura S, Koike K. Frequent beta-catenin aberration in human hepatocellular carcinoma. Hepatol Res. 2001;20:39-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Suzuki T, Yano H, Nakashima Y, Nakashima O, Kojiro M. Beta-catenin expression in hepatocellular carcinoma: a possible participation of beta-catenin in the dedifferentiation process. J Gastroenterol Hepatol. 2002;17:994-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 30. | Yuan RH, Jeng YM, Chen HL, Hsieh FJ, Yang CY, Lee PH, Hsu HC. Opposite roles of human pancreatitis-associated protein and REG1A expression in hepatocellular carcinoma: association of pancreatitis-associated protein expression with low-stage hepatocellular carcinoma, beta-catenin mutation, and favorable prognosis. Clin Cancer Res. 2005;11:2568-2575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Boyault S, Rickman DS, de Reyniès A, Balabaud C, Rebouissou S, Jeannot E, Hérault A, Saric J, Belghiti J, Franco D. Transcriptome classification of HCC is related to gene alterations and to new therapeutic targets. Hepatology. 2007;45:42-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 827] [Cited by in RCA: 926] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 32. | Cieply B, Zeng G, Proverbs-Singh T, Geller DA, Monga SP. Unique phenotype of hepatocellular cancers with exon-3 mutations in beta-catenin gene. Hepatology. 2009;49:821-831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 125] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 33. | Dal Bello B, Rosa L, Campanini N, Tinelli C, Torello Viera F, D’Ambrosio G, Rossi S, Silini EM. Glutamine synthetase immunostaining correlates with pathologic features of hepatocellular carcinoma and better survival after radiofrequency thermal ablation. Clin Cancer Res. 2010;16:2157-2166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 34. | Lok AS, Seeff LB, Morgan TR, di Bisceglie AM, Sterling RK, Curto TM, Everson GT, Lindsay KL, Lee WM, Bonkovsky HL. Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology. 2009;136:138-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 452] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 35. | Huang H, Fujii H, Sankila A, Mahler-Araujo BM, Matsuda M, Cathomas G, Ohgaki H. Beta-catenin mutations are frequent in human hepatocellular carcinomas associated with hepatitis C virus infection. Am J Pathol. 1999;155:1795-1801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 205] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 36. | Zucman-Rossi J, Jeannot E, Nhieu JT, Scoazec JY, Guettier C, Rebouissou S, Bacq Y, Leteurtre E, Paradis V, Michalak S. Genotype-phenotype correlation in hepatocellular adenoma: new classification and relationship with HCC. Hepatology. 2006;43:515-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 606] [Cited by in RCA: 535] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 37. | Dahmani R, Just PA, Perret C. The Wnt/β-catenin pathway as a therapeutic target in human hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2011;35:709-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |