Published online Feb 27, 2015. doi: 10.4254/wjh.v7.i2.177
Peer-review started: August 27, 2014
First decision: November 27, 2014
Revised: December 12, 2014
Accepted: December 29, 2014
Article in press: December 31, 2014
Published online: February 27, 2015
Processing time: 170 Days and 9.8 Hours
Elevated iron stores as indicated by hyperferritinemia with normal or mildly elevated transferrin saturation and mostly mild hepatic iron deposition are a characteristic finding in subjects with non-alcoholic fatty liver disease (NAFLD). Excess iron is observed in approximately one third of NAFLD patients and is commonly referred to as the “dysmetabolic iron overload syndrome”. Clinical evidence suggests that elevated body iron stores aggravate the clinical course of NAFLD with regard to liver-related and extrahepatic disease complications which relates to the fact that excess iron catalyses the formation of toxic hydroxyl-radicals subsequently resulting in cellular damage. Iron removal improves insulin sensitivity, delays the onset of type 2 diabetes mellitus, improves pathologic liver function tests and likewise ameliorates NAFLD histology. Several mechanisms contribute to pathologic iron accumulation in NAFLD. These include impaired iron export from hepatocytes and mesenchymal Kupffer cells as a consequence of imbalances in the concentrations of iron regulatory factors, such as hepcidin, cytokines, copper or other dietary factors. This review summarizes the knowledge about iron homeostasis in NAFLD and the rationale for its therapeutic implications.
Core tip: Hyperferritinemia with normal transferrin saturation and mostly mild hepatic iron deposition is a frequent finding in subjects with non-alcoholic fatty liver disease. Excess iron in non-alcoholic fatty liver disease (NAFLD) patients is referred to as the “dysmetabolic iron overload syndrome”. Clinical evidence suggests that elevated body iron stores aggravate the clinical course of NAFLD with regard to liver-related and extrahepatic disease complications. Iron removal improves insulin sensitivity, delays the onset of type 2 diabetes mellitus, improves pathologic liver function tests and ameliorates NAFLD histology The mechanisms contributing to iron excess in fatty liver include impaired iron export from hepatocytes and mesenchymal Kupffer.
- Citation: Aigner E, Weiss G, Datz C. Dysregulation of iron and copper homeostasis in nonalcoholic fatty liver. World J Hepatol 2015; 7(2): 177-188
- URL: https://www.wjgnet.com/1948-5182/full/v7/i2/177.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i2.177
Iron is essential for life of mammalian organisms due to its paradigmatic role in oxygen transport and also in being a central component of many enzymes and proteins involved in mitochondrial respiration, DNA biosynthesis and the citric acid cycle, among others. However, excess iron is detrimental and may lead to severe organ damage as it facilitates the formation of reactive oxygen species (ROS) via the Fenton reaction. On the other hand, iron deficiency can lead to anemia and fatigue which are among the most common disorders in the world. In order to provide enough iron for biological function and at the same time avoid iron overload and toxicity, iron trafficking and storage are diligently balanced by a mechanisms involving bone marrow, intestine, liver and the reticuloendothelial system (RES)[1,2].
Many aspects of iron metabolism have been unravelled in recent years. Dietary iron is taken up as Fe2+ in the duodenum by the cation transporter divalent metal transporter 1[3,4]. After transfer through the duodenal baso-lateral membrane via the iron exporter ferroportin (FPN)[5,6], iron is oxidized by the copper containing ferroxidase hephaestin and loaded onto transferrin for systemic distribution[7]. Most cells facilitae iron uptake by transferrin bound Fe3+via the transferrin-receptor (TfR1). Most iron is required for erythropoiesis and the biosynthesis of other heme enzymes like cytochromes, and excess iron is stored in hepatocytes[5,8]. Most iron for physiological requirements, mainly erythropoiesis, is obtained from re-utilisation of senescent erythrocytes which are taken up and degraded in splenic macrophages. Only approximately 1-2 mg of daily body iron requirements which are used for compensation of iron losses via bleeding, enteric and cutaneous cell desquamation are replenished via duodenal iron absorption. Iron export is facilitated by FPN from hepatocytes, macrophages and all other cells[9].
Systemic iron homeostasis is equilibrated by the peptide hepcidin (hepatic bactericidal protein) mainly derived from hepatocytes and regulated by iron status, hypoxia, anemia and inflammation[10-12]. Hepcidin impacts on iron trafficking by attaching to FPN which leads to the degradation of FPN and thereby to down-regulation of iron export inducing a decline in serum iron concentrations[13]. Quantitatively hepatocytes are the most important source for hepcidin, however, expression has also been reported in adipose tissue, pancreatic islets, macrophages, and even cardiac myocytes. Hence, iron homeostasis via FPN mediated iron export may be regulated in an autocrine fashion in these cells[14-16].
Perturbations of iron homeostasis are frequently observed in patients suffering from non-alcoholic fatty liver disease (NAFLD)[17,18]. As the prevalence of obesity rises, NAFLD with or without associated metabolic syndrome (MetS), has become the most frequent cause of hyperferritinemia. The first report of non-hemochromatotic iron overload linked to metabolic characteristics such as insulin resistance and overweight in a French study subsequently stimulated extensive research on the potential mechanisms underlying iron accumulation in NAFLD[19]. The dysmetabolic iron overload syndrome (DIOS) commonly refers to the characteristic association of fatty liver with moderate histological iron deposition (hemosiderosis) and increased serum ferritin[17,20].
An increase in ferritin concentrations is the key feature of iron dysregulation in subjects with NAFLD. It is found in one third to half of patients with NAFLD and ranges from mild elevations to rarely 1000-1500 ng/mL[17]. Serum ferritin concentrations increase with the number of features of the MetS[21]. Transferrin saturation (TfS) is typically in the upper range of normal or mildly elevated (45%-50%) which is distinct from hereditary hemochromatosis, where hyperferritinemia is accompanied by markedly elevated TfS and usually TfS is elevated before the development of hyperferritinemia in early stages of hemochromatosis[22].
Iron deposits in NAFLD are found in Kupffer cells which are the resident liver macrophages as well as in hepatocytes[20]. Mesenchymal iron deposition is more frequent than hepatocellular iron accumulation but mostly both compartments are affected[23]. This is different from tissue iron deposition in primary genetic iron overload, hemochromatosis, where the metal is almost exclusively found in the hepatocellular compartment (with the exception of ferroportin disease) and macrophages are iron deficient as a result of uninhibited iron export from these cells[24,25]. The extent of hyperferritinemia in subjects with NAFLD and/or the MetS overestimates the degree of iron overload compared to hemochromatosis. Phlebotomy studies demonstrated that in DIOS patients the amount of iron need to be removed for normalisation of circulating iron parameters is usually significantly less than in hemochromatosis, indicating only mild body iron excess[26,27]. Few studies have performed liver iron quantification in NAFLD subjects and these results confirm the mild degree of tissue iron excess compared to genetic iron overload disorders[19,28]. The mild degree of body iron excess compared to markedly raised serum ferritin concentrations suggests that iron overload in NAFLD subjects results from a combination of alimentary and inflammatory driven iron loading and retention[20,29,30]. This is in line with the current evidence that NAFLD is both a metabolic and an inflammatory disease[31].
In 1981 Sullivan[32] suggested that the postponed occurrence of cardiovascular diseases in women compared to men and the subsequent postmenopausal increase could be caused by low premenopausal iron stores. This report likely is the first report of an impact of iron stores in non-hemochromatotic metabolic disorders. An association of iron stores with type 2 diabetes mellitus (T2DM) and various manifestations of IR has been repeatedly confirmed and a detailed discussion thereof is beyond the scope of this review[33]. However, glucose metabolism and iron homeostasis appear to be functionally interconnected, due to the fact that gluconeogenetic signals regulate iron homeostasis via hepcidin[34] while iron loading or deficiency directly affect circulating glucose concentrations in mammals most likely via its effects on citric acid cycle enzyme activities[35,36], thereby also affecting lipid profiles[37]. Ferritin concentrations were associated with an increased rate of diabetes and gestational diabetes[38-43], with BMI[44], visceral fat mass[45], serum glucose levels and insulin sensitivity[46], blood pressure[47], the MetS[21,48], the polycystic ovary syndrome (PCOS)[49] and cholesterol[50]. Higher parameters of iron storage clustered with metabolic risk markers in a study of obese[51] and healthy lean adolescents[52]. These observations are epidemiologically important as patients with IR have a higher risk of developing cerebrovascular or cardiovascular disease[53,54]. However, the most convincing argument for causative involvement of iron in obesity-related conditions is derived from iron removal studies mentioned in detail below. In summary, available studies convincingly suggest a direct impact of body iron on manifestations of IR or the MetS.
NAFLD has been firmly established as the hepatic manifestation of the MetS/IR[55]. The disease spectrum of NAFLD ranges from simple steatosis which is generally considered benign to steatosis with various stages of inflammation, hepatocellular ballooning and fibrosis called non-alcoholic steatohepatitis (NASH). NASH is the potentially progressive manifestation leading to cirrhosis, end-stage liver disease and hepatocellular carcinoma in a minority of patients[56]. To our knowledge, there is no data available suggesting that excess iron is linked to the extent of hepatic steatosis. Although multiple associations between iron homeostasis and lipid metabolism have been reported[57], no characteristic lipid phenotype has been documented to distinguish NAFLD with iron overload from NAFLD without iron. Underlying NAFLD may explain the link between MetS features and ferritin on the population level[58].
Several studies provide evidence that iron may contribute to more advanced fibrosis and thus to progression of NAFLD[18,59-63], however, this association was not confirmed in all studies[64-66]. The to date largest study reported that iron in NAFLD liver biopsies, particularly in Kupffer cells, was linked to more fibrosis and disease severity[67]. Iron deposition particularly in the Kupffer cell compartment was associated with higher markers of hepatocellular apoptosis and oxidative stress[68]. Some studies also suggested that an increased rate of HFE mutations could account for more progressed stages of NAFLD, but this was not reported in all studies[65,69-72]. Additionally the beta-globin trait[73], TMPRSS6[74], and the alpha-1-antitrypsin genotype[75] may modify the iron phenotype of NAFLD. It appears reasonable to conclude that the contribution of the genetic background may vary according to the geographic region. Data evaluating causality of iron in disease progression is limited by the feasibility of a prospective study with serial liver biopsies in enough patients to adjust for known co-factors of disease progression[64,76]. Retrospective studies demonstrated that, hyperferritinemia was linked to mortality of patients on the transplantation waiting list and it also had an impact on post-transplant mortality[77,78]. It is important to note that particularly sinusoidal iron deposition may be linked to the development of HCC in NASH[79].
In summary, the prevailing body of evidence suggests that excess iron is a contributing factor for the progression of steatosis to NASH, liver cirrhosis and also hepatocellular carcinoma. It remains to be established to what extent different patterns of iron deposition affect outcomes such as cirrhosis, HCC or cardiovascular diseases. The data mentioned above suggest that the pattern of iron deposition may have distinct effects.
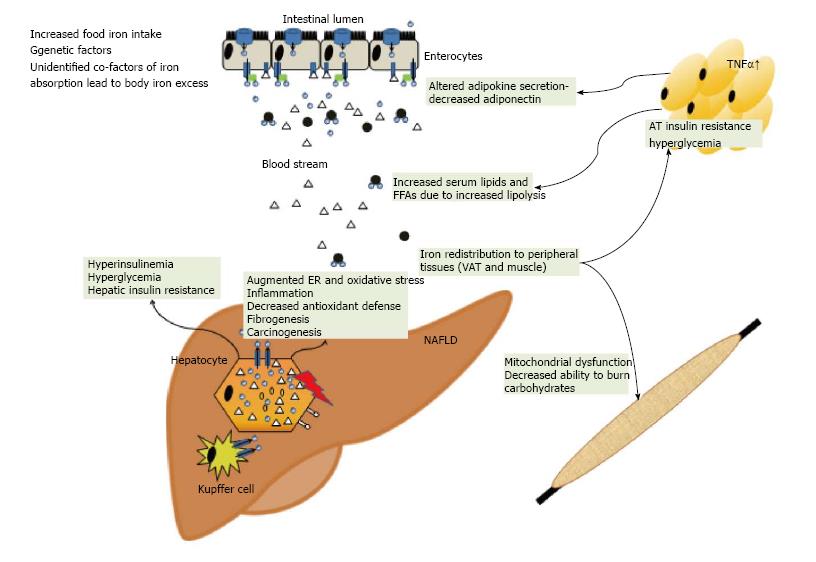
It has been well recognized that iron overload leads to diabetes in patients with hemochromatosis where IR increases and insulin secretion decreases with the rise of body iron stores[25,80-82]. Hepatic insulin sensitivity and insulin secretion are re-established in the majority once iron is removed[83,84]. However, the prediabetic stage in hemochromatotic mice and humans displays impaired β-cell function along with increased insulin sensitivity, whereas dietary iron overload similar to the prediabetic state in humans are characterized by peripheral IR[85]. Hence, lessons drawn from hemochromatosis models are likely not fully applicable to the role of iron in human IR and NAFLD.
Iron is well-recognized as a catalyst for the production of reactive oxygen intermediates via the Fenton reaction, and it is generally held that an increase of oxidative stress is a central mechanism for IR although direct proof for this hypothesis has not been obtained so far. Oxidative stress is a central pathogenic factor in NAFLD, T2DM and obesity[86-88] and markers of oxidative stress were increased in NAFLD with iron loading as compared to NAFLD without iron excess[68,89,90]. Generation of ROS may induce lipid peroxidation and cellular damage which may contribute to the progression of NAFLD. Importantly, oxidative stress induced molecules such as malonyldialdehyd and 4-hydroxynonenal may induce the formation of de-novo antigens with subsequent activation of T-lymphocytes and development of immunoglobulin G reactive against these antigens. This response was further enhanced by previous immunization against these antigens with a stimulated M1 macrophage response[91]. Although no studies have been performed, iron may contribute to this process by further augmentation of oxidative stress.
In cell culture, iron chelation re-established insulin receptor signalling and iron inhibited insulin receptor activity[92]. Desferoxamine increased the phosphorylation of Akt/protein kinase B (Akt/PKB), forkhead transcription factor O1 (FoxO1) and glycogen synthase kinase 3β (GSK3β) reflecting insulin effects on gluconeogenesis and glycogen synthesis. Likewise, genes playing a role in glucose utilization such as GLUT1 or hypoxia-inducible factor 1α (HIF1α) were up-regulated in hepatoma cells resulting in enhanced glucose removal[92]. In summary, these molecular observations indicate that iron affects IR by modulating insulin receptor signalling as has been recently reviewed[93].
Importantly, dietary iron intake may impact on glucose metabolism by affecting circadian rhythm via heme mediated effects on RevErb-α. Disruption of circadian rhythms, e.g., through night-shift work is an established risk factor for metabolic and cardiovascular diseases[94,95].
In cultured fat cells, iron favored an IR, characterised by impaired glucose uptake and suppression of lipolysis in response to insulin[96,97]. Ferritin was inversely associated with adiponectin concentrations in insulin resistant and sensitive patients[98,99]. Knockout of FPN1 in adipocytes increased intracellular iron and subsequently reduced adiponectin biosynthesis, thus establishing a molecular link between adipocyte iron concentration and insulin resistance[100]. Furthermore, excess iron the diet may be routed to visceral adipose tissue and change the expression of adipokines, as demonstrated for resistin[101] Adipokines represent a diverse group of hormones which mediate the metabolic effects of diseased adipose tissue to organs and tissues. Associations have been observed between retinol-binding protein 4 (RBP4) and visfatin serum concentrations and parameters of iron metabolism[102,103]. However, these reports may reflect the co-incidence of elevated iron stores with surrogate markers of IR and do not prove causality[93].
Liver macrophages named Kupffer cells, which are an important site of iron storage in NAFLD, are tightly involved in the initiation of the hepatic inflammatory cascade in response to the uptake of oxidized lipoproteins[104] or oxidized phosphatidylcholines[105]. It is well known that macrophage iron status affects their inflammatory response pattern and polarization towards a pro-inflammatory phenotype[106], however, the particular role of these potential interactions have to our knowledge not been investigated in NAFLD.
Thus, the potential mechanisms of iron-induced NAFLD disease progression are complex and involve protean effects of iron in extrahepatic tissues as well direct liver damage.
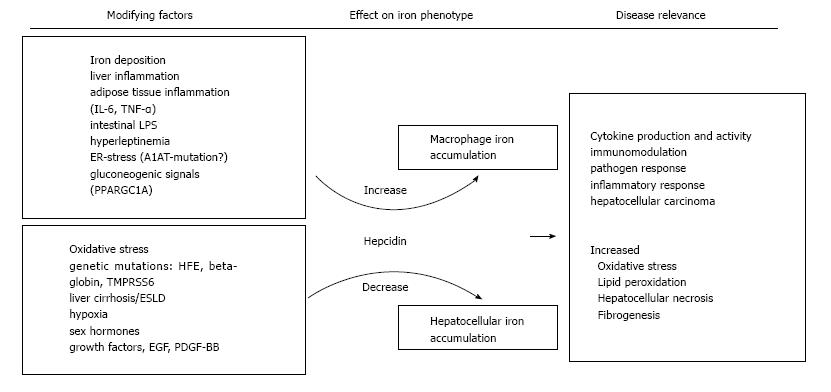
Hepcidin is the key regulator of systemic iron homeostasis and plays a role for the hemochromatotic and the inflammatory driven misdistribution of iron. Whereas the lack of hepcidin in hemochromatosis leads to uncoordinated duodenal iron absorption and iron accumulation in parenchymal tissues such as the liver[107]. the inflammation driven iron retention occurs mainly in monocytes/macrophages as a consequence of increased iron accumulation and reduced FPN mediated iron export from these cells, the latter being due to increased circulating hepcidin levels along with negative effects of certain cytokines on FPN expression[108]. The histological hallmarks of hemochromatosis, i.e., hepatocellular iron, and also the inflammatory phenotype iron deposition in macrophages are both observed concurrently, suggesting that iron dysregulation is multifaceted in NAFLD. Several stimuli of hepcidin regulation have been reported which may be of particular relevance in NAFLD and also be related to different iron phenotypes. These stimuli and their relation to NAFLD iron accumulation are summarized in Figure 1. For several of these, like sex hormones, growth factors and hypoxia-induced circulating factors, the contribution to the dys-regulation of iron homeostasis in NAFLD has not been directly demonstrated but is physiologically plausible and these have therefore been included in the summary figure[109-112]. Additionally, alcohol consumption may decrease hepcidin expression and thus modify iron accumulation in NAFLD subjects[113], and although relevant alcohol consumption should be excluded in NAFLD subjects both conditions frequently co-exist. Thus, in NAFLD multiple, potentially counteracting signals impacting on hepcidin expression may be present at the same time. It is likely that the net balance of these signals finally determines the pattern of iron accumulation in the fatty liver of the individual patient.
Hepcidin levels in urin, serum and liver were elevated in NAFLD patients with iron excess compared to healthy subjects, hemochromatosis patients and NAFLD subjects without excess iron[28,114-116]. Hepcidin expression correlated directly with liver iron indicating an intact physiological response of hepcidin biosynthesis to iron in the liver[28,114]. Additionally, hepcidin is expressed in adipocytes of morbidly obese subjects[15]. Moreover, obesity is characterised by a chronic subclinical inflammation and in humans hepcidin concentrations and TNF-α were directly related, suggesting that both iron and inflammation contribute to hepcidin biosynthesis in NAFLD[28]. Furthermore, hepcidin and cytokines may be derived from both, the inflamed adipose and the liver[117,118]. Activation of gluconeogenesis via starvation, namely activation of peroxisome proliferator activated receptor gamma co-activator-1 α (PGC1α) increased hepcidin expression in a mouse model[34]. Likewise, iron fortification decreased gluconeogenesis via PGC1α in a murine model[119]. Hence, although PGC1α offers an intriguing cellular link between glucose and iron homeostasis, its relevance to human NAFLD remains to be elucidated. Leptin, was demonstrated to up-regulate hepcidin in hepatocytes in vitro by activation of the JAK2/STAT3 pathway. Hence, hyperleptinemia may directly contribute to higher hepcidin and thereby to iron deposition in NAFLD[120,121].
In NAFLD with iron overload the iron exporter FPN is lower than in controls and hemochromatosis patients in the liver and in the duodenum[28,114,122,123]. In NAFLD without liver iron accumulation, FPN levels were comparable to control subjects, but were significantly lower in NAFLD with hepatic iron on histology[28]. Along the same line of the observations, duodenal iron absorption was decreased in DIOS patients[124]. Obesity also represents a risk factor for an inadequate dietary iron fortification, linked to high hepcidin and low FPN expression[125]. Along this line mice feed a high fat diet presented with significantly reduced iron absorption which could be traced back diminished intestinal iron uptake. Mechanistically, the impaired iron absorption was independent of hepcidin but resulted from reduced metal uptake into the mucosa and transfer of iron across enterocyte membranes as a consequence of dietary induced discordant membrane-bound oxidoreductase expression[126].
An additional mechanism may be the phagocytosis of fragile erythrocytes by liver Kupffer cells. This was documented in rabbits on a high-fat diet and the phagocytosis of fragile erythrocytes was observed in vitro. Accumulation of erythrocytes was microscopically detected in inflamed regions in human NAFLD[127] suggesting that uptake of heme-iron via erythrophagocytosis may contribute to NAFLD iron accumulation, then promoting oxidative stress and inflammation.
Although cellular iron uptake via TfR1 is the most important route of iron uptake under physiological circumstances TfR1 appears not to be involved in excess iron uptake in NAFLD[128,129]. Hepatic TfR1 expression in NAFLD patients with low iron was increased compared to NAFLD and iron accumulation or patients with hemochromatosis suggesting physiologically intact TfR1 expression in response to iron stimuli[28] (Figure 2).
Similar to iron, an adequate supply of copper is essential for proper biological function. Chronic copper deficiency can elicit anemia, leucopenia, myelopathy or skin abnormalities and excess copper may also facilitate the formation of ROS.
There are several ways in which inadequate copper supply may be involved in the pathogenesis of NAFLD. Epidemiological studies found that copper deficiency is linked to atherogenic dyslipidemia and dietary copper supplementation improved cardiovascular risk markers in healthy adults[130] Investigations in rodent models demonstrated that dietary copper restriction induces hypertension or cardiac dysfunction, hypertriglyceridemia, hypercholesterolemia and modifies LDL and VLDL composition[131,132]. We recently reported low intrahepatic copper concentrations in human NAFLD compared to other liver diseases and that rats on a copper depleted diet developed IR and liver steatosis[133]. Increased oxidative stress is considered a key trigger in the pathogenesis of human NAFLD and one of the enzymes counteracting oxidative stress, Cu/Zn superoxide dismutase (SOD) depends on adequate copper availability, suggesting a potential link between copper availability and impaired antioxidant defense in NAFLD[134]. Sprague-Dawley rats exhibited an increased activity of the pro-inflammatory protein cyclo-oxygenase-2, when fed a diet with a low copper content[135]. Systemic copper deficiency causes mitochondrial dysfunction in mice and similar morphological and functional alterations have also been described in human NAFLD[136]. Recently, a detailed examination revealed an interaction of a high-fructose diet (which is also a culprit in the rise of obesity-related conditions) with low copper intake in triggering liver steatosis and damage as well as iron overload. Fructose acts as an inhibitor of duodenal copper absorption thereby leading to impaired oxidant defense and augmented lipid peroxidation[137]. As dietary copper content of the Western diet is rather low whereas iron and fructose are consumed in excess, this model offers attractive data to speculate that a dysbalance in micronutrient intake may have a significant role in NAFLD beyond calorie excess. Hence, animal and human data suggest that the therapeutic effect of dietary copper supplementation should be investigated as a subset of patients may potentially benefit.
Copper modulates iron homeostasis and is also linked to the iron perturbations of NAFLD. Hephaestin ferroxidase activity in duodenal enterocytes is critically dependent on copper as it oxidizes ferrous to ferric iron which is subsequently loaded onto Tf[7]. Similarly, copper is necessary for ceruloplasmin function to export iron from the liver or the RES and also for FPN expression[138]. Expression of a membrane-bound form of ceruloplasmin is mandatory for stable FPN expression[139,140]. Accordingly, a lack of ceruloplasmin as found in the heritable disease aceruloplasminemia leads to tissue iron accumulation and damage most notably in the brain[141].
Low liver and serum copper concentrations were reported in iron overloaded NAFLD and were linked to decreased ferroxidase activity of ceruloplasmin[122]. The expression of FPN was found to be decreased in livers of rats on a copper deficient diet. These observations provide evidence that in addition to decreased FPN expression due to low-grade systemic inflammation, low copper bioavailability contributes to iron retention in NAFLD.
Elimination of iron may confer a beneficial effect on IR-associated conditions. Removal of iron using phlebotomies is usually well tolerated, with the caution that DIOS patients frequently show a fast decline in TfS[142]. These clinical observations are expected due to the underlying molecular mechanisms of impaired iron export. The incidence of diabetes, postprandial serum insulin and pancreatic insulin sensitivity, i.e., beta cell function were al improved in subjects with previous phlebotomy treatment[143]. Iron removal also improved coronary vascular dysfunction in patients with T2DM[144] and endothelial function in patients with known coronary artery disease and in subjects with primary iron overload[145,146]. Blood donations were linked to insulin sensitivity even in healthy subjects[46]. Studies on iron depletion in NAFLD in humans have demonstrated benefits regarding systemic or hepatic insulin resistance and pancreatic insulin sensitivity[142,147,148]. A randomized trial demonstrated improved HbA1c, insulin sensitivity and secretion subjects who received phlebotomy treatment[149]. The effects of iron depletion were additive to successful lifestyle modifications[150]. Similar observations were reported the effect of iron depletion on other cardiovascular risk factors[151] and iron removal may prevent development and progression of malignancies[152].
As far as practical treatment of iron excess in NAFLD patients with elevated ferritin is concerned, available data suggest that iron removal may thus be beneficial in addition to weight loss, diet and lifestyle modification or antidiabetic medication as indicated in an individual patient. We have adopted the practice to perform biweekly phlebotomies in these subjects until serum ferritin concentrations are between 50 and 100 ng/L, however, no evidence-based recommendation for this is currently available. In contrast to hemochromatosis patients, NAFLD subjects have impaired iron mobilisation from storage sites and may therefore develop anemia in response to phlebotomy treatment. We therefore recommend close monitoring of serum ferritin, TfS and hemoglobin at each visit for the period of time while these patients are on phlebotomy treatment[26,153].
Elevated serum ferritin concentrations are a frequent finding in NAFLD. Excess iron is linked to IR, accelerated disease progression and adverse outcomes. Removing excess iron via phlebotomies is safe and has clinical benefits. We suggest that on the basis of available evidence it can be offered to NAFLD patients as it is linked to improvement of IR and inflammation. The mechanisms underlying iron accumulation in NAFLD are tightly linked to impaired iron export from liver cells as a consequence of low expression of the iron export molecule FPN and elevated hepcidin concentrations. Inflammation of adipose tissue as indicated by TNF-α and IL-6 and altered adipokine secretion (leptin, resistin) or hepcidin represent potent signals from diseased adipose tissue to dysregulate iron as well as glucose or lipid homeostasis.
P- Reviewer: Higuera-de la Tijera MF, Sutti S S- Editor: Tian YL L- Editor: A E- Editor: Lu YJ
| 1. | Hentze MW, Muckenthaler MU, Galy B, Camaschella C. Two to tango: regulation of Mammalian iron metabolism. Cell. 2010;142:24-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1714] [Cited by in RCA: 1523] [Article Influence: 101.5] [Reference Citation Analysis (0)] |
| 2. | Wang J, Pantopoulos K. Regulation of cellular iron metabolism. Biochem J. 2011;434:365-381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 613] [Cited by in RCA: 699] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 3. | Fleming MD, Trenor CC, Su MA, Foernzler D, Beier DR, Dietrich WF, Andrews NC. Microcytic anaemia mice have a mutation in Nramp2, a candidate iron transporter gene. Nat Genet. 1997;16:383-386. [PubMed] |
| 4. | Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388:482-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2383] [Cited by in RCA: 2301] [Article Influence: 82.2] [Reference Citation Analysis (0)] |
| 5. | McKie AT, Marciani P, Rolfs A, Brennan K, Wehr K, Barrow D, Miret S, Bomford A, Peters TJ, Farzaneh F. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol Cell. 2000;5:299-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1054] [Cited by in RCA: 1029] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 6. | Donovan A, Brownlie A, Zhou Y, Shepard J, Pratt SJ, Moynihan J, Paw BH, Drejer A, Barut B, Zapata A. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature. 2000;403:776-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1226] [Cited by in RCA: 1211] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 7. | Vulpe CD, Kuo YM, Murphy TL, Cowley L, Askwith C, Libina N, Gitschier J, Anderson GJ. Hephaestin, a ceruloplasmin homologue implicated in intestinal iron transport, is defective in the sla mouse. Nat Genet. 1999;21:195-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 775] [Cited by in RCA: 746] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 8. | Wrighting DM, Andrews NC. Iron homeostasis and erythropoiesis. Curr Top Dev Biol. 2008;82:141-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Abboud S, Haile DJ. A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J Biol Chem. 2000;275:19906-19912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 943] [Cited by in RCA: 928] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 10. | De Domenico I, McVey Ward D, Kaplan J. Regulation of iron acquisition and storage: consequences for iron-linked disorders. Nat Rev Mol Cell Biol. 2008;9:72-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 333] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 11. | Ganz T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood. 2003;102:783-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1016] [Cited by in RCA: 984] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 12. | Nicolas G, Chauvet C, Viatte L, Danan JL, Bigard X, Devaux I, Beaumont C, Kahn A, Vaulont S. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest. 2002;110:1037-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 994] [Cited by in RCA: 957] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 13. | Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090-2093. [PubMed] |
| 14. | Nguyen NB, Callaghan KD, Ghio AJ, Haile DJ, Yang F. Hepcidin expression and iron transport in alveolar macrophages. Am J Physiol Lung Cell Mol Physiol. 2006;291:L417-L425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Bekri S, Gual P, Anty R, Luciani N, Dahman M, Ramesh B, Iannelli A, Staccini-Myx A, Casanova D, Ben Amor I. Increased adipose tissue expression of hepcidin in severe obesity is independent from diabetes and NASH. Gastroenterology. 2006;131:788-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 349] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 16. | Theurl I, Theurl M, Seifert M, Mair S, Nairz M, Rumpold H, Zoller H, Bellmann-Weiler R, Niederegger H, Talasz H. Autocrine formation of hepcidin induces iron retention in human monocytes. Blood. 2008;111:2392-2399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 216] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 17. | Mendler MH, Turlin B, Moirand R, Jouanolle AM, Sapey T, Guyader D, Le Gall JY, Brissot P, David V, Deugnier Y. Insulin resistance-associated hepatic iron overload. Gastroenterology. 1999;117:1155-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 342] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 18. | Valenti L, Dongiovanni P, Fracanzani AL, Santorelli G, Fatta E, Bertelli C, Taioli E, Fiorelli G, Fargion S. Increased susceptibility to nonalcoholic fatty liver disease in heterozygotes for the mutation responsible for hereditary hemochromatosis. Dig Liver Dis. 2003;35:172-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Moirand R, Mortaji AM, Loréal O, Paillard F, Brissot P, Deugnier Y. A new syndrome of liver iron overload with normal transferrin saturation. Lancet. 1997;349:95-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 229] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 20. | Turlin B, Mendler MH, Moirand R, Guyader D, Guillygomarc’h A, Deugnier Y. Histologic features of the liver in insulin resistance-associated iron overload. A study of 139 patients. Am J Clin Pathol. 2001;116:263-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 92] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Wrede CE, Buettner R, Bollheimer LC, Schölmerich J, Palitzsch KD, Hellerbrand C. Association between serum ferritin and the insulin resistance syndrome in a representative population. Eur J Endocrinol. 2006;154:333-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 106] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Nairz M, Weiss G. Molecular and clinical aspects of iron homeostasis: From anemia to hemochromatosis. Wien Klin Wochenschr. 2006;118:442-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Nelson JE, Wilson L, Brunt EM, Yeh MM, Kleiner DE, Unalp-Arida A, Kowdley KV. Relationship between the pattern of hepatic iron deposition and histological severity in nonalcoholic fatty liver disease. Hepatology. 2011;53:448-457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 269] [Cited by in RCA: 253] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 24. | Weiss G. Genetic mechanisms and modifying factors in hereditary hemochromatosis. Nat Rev Gastroenterol Hepatol. 2010;7:50-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Pietrangelo A. Hereditary hemochromatosis--a new look at an old disease. N Engl J Med. 2004;350:2383-2397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 593] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 26. | Valenti L, Fracanzani AL, Dongiovanni P, Rovida S, Rametta R, Fatta E, Pulixi EA, Maggioni M, Fargion S. A randomized trial of iron depletion in patients with nonalcoholic fatty liver disease and hyperferritinemia. World J Gastroenterol. 2014;20:3002-3010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 71] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 27. | Valenti L, Moscatiello S, Vanni E, Fracanzani AL, Bugianesi E, Fargion S, Marchesini G. Venesection for non-alcoholic fatty liver disease unresponsive to lifestyle counselling--a propensity score-adjusted observational study. QJM. 2011;104:141-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 28. | Aigner E, Theurl I, Theurl M, Lederer D, Haufe H, Dietze O, Strasser M, Datz C, Weiss G. Pathways underlying iron accumulation in human nonalcoholic fatty liver disease. Am J Clin Nutr. 2008;87:1374-1383. [PubMed] |
| 29. | Diehl AM, Li ZP, Lin HZ, Yang SQ. Cytokines and the pathogenesis of non-alcoholic steatohepatitis. Gut. 2005;54:303-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 163] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 30. | Chen J, Wildman RP, Hamm LL, Muntner P, Reynolds K, Whelton PK, He J. Association between inflammation and insulin resistance in U.S. nondiabetic adults: results from the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27:2960-2965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 102] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 31. | Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836-1846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1543] [Cited by in RCA: 1819] [Article Influence: 121.3] [Reference Citation Analysis (0)] |
| 32. | Sullivan JL. Iron and the sex difference in heart disease risk. Lancet. 1981;1:1293-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 560] [Cited by in RCA: 560] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 33. | Wood RJ. The iron-heart disease connection: is it dead or just hiding? Ageing Res Rev. 2004;3:355-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 34. | Vecchi C, Montosi G, Garuti C, Corradini E, Sabelli M, Canali S, Pietrangelo A. Gluconeogenic signals regulate iron homeostasis via hepcidin in mice. Gastroenterology. 2014;146:1060-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 35. | Choi JS, Koh IU, Lee HJ, Kim WH, Song J. Effects of excess dietary iron and fat on glucose and lipid metabolism. J Nutr Biochem. 2013;24:1634-1644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 36. | Davis MR, Rendina E, Peterson SK, Lucas EA, Smith BJ, Clarke SL. Enhanced expression of lipogenic genes may contribute to hyperglycemia and alterations in plasma lipids in response to dietary iron deficiency. Genes Nutr. 2012;7:415-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 37. | Oexle H, Gnaiger E, Weiss G. Iron-dependent changes in cellular energy metabolism: influence on citric acid cycle and oxidative phosphorylation. Biochim Biophys Acta. 1999;1413:99-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 222] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 38. | Ford ES, Cogswell ME. Diabetes and serum ferritin concentration among U.S. adults. Diabetes Care. 1999;22:1978-1983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 293] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 39. | Forouhi NG, Harding AH, Allison M, Sandhu MS, Welch A, Luben R, Bingham S, Khaw KT, Wareham NJ. Elevated serum ferritin levels predict new-onset type 2 diabetes: results from the EPIC-Norfolk prospective study. Diabetologia. 2007;50:949-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 184] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 40. | Lao TT, Tam KF. Maternal serum ferritin and gestational impaired glucose tolerance. Diabetes Care. 1997;20:1368-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 41. | Lao TT, Chan PL, Tam KF. Gestational diabetes mellitus in the last trimester - a feature of maternal iron excess? Diabet Med. 2001;18:218-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 92] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 42. | Chen X, Scholl TO, Stein TP. Association of elevated serum ferritin levels and the risk of gestational diabetes mellitus in pregnant women: The Camden study. Diabetes Care. 2006;29:1077-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 93] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 43. | Jiang R, Manson JE, Meigs JB, Ma J, Rifai N, Hu FB. Body iron stores in relation to risk of type 2 diabetes in apparently healthy women. JAMA. 2004;291:711-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 435] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 44. | Gillum RF. Association of serum ferritin and indices of body fat distribution and obesity in Mexican American men--the Third National Health and Nutrition Examination Survey. Int J Obes Relat Metab Disord. 2001;25:639-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 128] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 45. | Iwasaki T, Nakajima A, Yoneda M, Yamada Y, Mukasa K, Fujita K, Fujisawa N, Wada K, Terauchi Y. Serum ferritin is associated with visceral fat area and subcutaneous fat area. Diabetes Care. 2005;28:2486-2491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 112] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 46. | Fernández-Real JM, López-Bermejo A, Ricart W. Iron stores, blood donation, and insulin sensitivity and secretion. Clin Chem. 2005;51:1201-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 106] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 47. | Piperno A, Trombini P, Gelosa M, Mauri V, Pecci V, Vergani A, Salvioni A, Mariani R, Mancia G. Increased serum ferritin is common in men with essential hypertension. J Hypertens. 2002;20:1513-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 133] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 48. | Jehn M, Clark JM, Guallar E. Serum ferritin and risk of the metabolic syndrome in U.S. adults. Diabetes Care. 2004;27:2422-2428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 314] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 49. | Martínez-García MA, Luque-Ramírez M, San-Millán JL, Escobar-Morreale HF. Body iron stores and glucose intolerance in premenopausal women: role of hyperandrogenism, insulin resistance, and genomic variants related to inflammation, oxidative stress, and iron metabolism. Diabetes Care. 2009;32:1525-1530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 50. | Galan P, Noisette N, Estaquio C, Czernichow S, Mennen L, Renversez JC, Briançon S, Favier A, Hercberg S. Serum ferritin, cardiovascular risk factors and ischaemic heart diseases: a prospective analysis in the SU.VI.MAX (SUpplementation en VItamines et Minéraux AntioXydants) cohort. Public Health Nutr. 2006;9:70-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 51. | Dubern B, Girardet JP, Tounian P. Insulin resistance and ferritin as major determinants of abnormal serum aminotransferase in severely obese children. Int J Pediatr Obes. 2006;1:77-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 52. | Aigner E, Hinz C, Steiner K, Rossmann B, Pfleger J, Hohla F, Steger B, Stadlmayr A, Patsch W, Datz C. Iron stores, liver transaminase levels and metabolic risk in healthy teenagers. Eur J Clin Invest. 2010;40:155-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 53. | van der A DL, Grobbee DE, Roest M, Marx JJ, Voorbij HA, van der Schouw YT. Serum ferritin is a risk factor for stroke in postmenopausal women. Stroke. 2005;36:1637-1641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 77] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 54. | Ramakrishnan U, Kuklina E, Stein AD. Iron stores and cardiovascular disease risk factors in women of reproductive age in the United States. Am J Clin Nutr. 2002;76:1256-1260. [PubMed] |
| 55. | Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, McCullough AJ, Natale S, Forlani G, Melchionda N. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844-1850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1725] [Cited by in RCA: 1745] [Article Influence: 72.7] [Reference Citation Analysis (0)] |
| 56. | Dyson JK, Anstee QM, McPherson S. Non-alcoholic fatty liver disease: a practical approach to diagnosis and staging. Frontline Gastroenterol. 2014;5:211-218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 240] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 57. | Ahmed U, Latham PS, Oates PS. Interactions between hepatic iron and lipid metabolism with possible relevance to steatohepatitis. World J Gastroenterol. 2012;18:4651-4658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 74] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (2)] |
| 58. | Zelber-Sagi S, Nitzan-Kaluski D, Halpern Z, Oren R. NAFLD and hyperinsulinemia are major determinants of serum ferritin levels. J Hepatol. 2007;46:700-707. [PubMed] |
| 59. | Fargion S, Mattioli M, Fracanzani AL, Sampietro M, Tavazzi D, Fociani P, Taioli E, Valenti L, Fiorelli G. Hyperferritinemia, iron overload, and multiple metabolic alterations identify patients at risk for nonalcoholic steatohepatitis. Am J Gastroenterol. 2001;96:2448-2455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 163] [Article Influence: 6.8] [Reference Citation Analysis (1)] |
| 60. | Fargion S, Dongiovanni P, Guzzo A, Colombo S, Valenti L, Fracanzani AL. Iron and insulin resistance. Aliment Pharmacol Ther. 2005;22 Suppl 2:61-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 61. | George DK, Goldwurm S, MacDonald GA, Cowley LL, Walker NI, Ward PJ, Jazwinska EC, Powell LW. Increased hepatic iron concentration in nonalcoholic steatohepatitis is associated with increased fibrosis. Gastroenterology. 1998;114:311-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 447] [Article Influence: 16.6] [Reference Citation Analysis (1)] |
| 62. | Fierbinţeanu-Braticevici C, Bengus A, Neamţu M, Usvat R. The risk factors of fibrosis in nonalcoholic steatohepatitis. Rom J Intern Med. 2002;40:81-88. [PubMed] |
| 63. | Bhattacharya R, Kowdley KV. Iron and HFE mutations in nonalcoholic steatohepatitis: innocent bystanders or accessories to the crime? Gastroenterology. 2003;125:615-666; discussion 617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 64. | Chitturi S, Weltman M, Farrell GC, McDonald D, Kench J, Liddle C, Samarasinghe D, Lin R, Abeygunasekera S, George J. HFE mutations, hepatic iron, and fibrosis: ethnic-specific association of NASH with C282Y but not with fibrotic severity. Hepatology. 2002;36:142-149. [PubMed] |
| 65. | Bugianesi E, Manzini P, D’Antico S, Vanni E, Longo F, Leone N, Massarenti P, Piga A, Marchesini G, Rizzetto M. Relative contribution of iron burden, HFE mutations, and insulin resistance to fibrosis in nonalcoholic fatty liver. Hepatology. 2004;39:179-187. [PubMed] |
| 66. | Younossi ZM, Gramlich T, Bacon BR, Matteoni CA, Boparai N, O’Neill R, McCullough AJ. Hepatic iron and nonalcoholic fatty liver disease. Hepatology. 1999;30:847-850. [PubMed] |
| 67. | Kowdley KV, Belt P, Wilson LA, Yeh MM, Neuschwander-Tetri BA, Chalasani N, Sanyal AJ, Nelson JE. Serum ferritin is an independent predictor of histologic severity and advanced fibrosis in patients with nonalcoholic fatty liver disease. Hepatology. 2012;55:77-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 401] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 68. | Maliken BD, Nelson JE, Klintworth HM, Beauchamp M, Yeh MM, Kowdley KV. Hepatic reticuloendothelial system cell iron deposition is associated with increased apoptosis in nonalcoholic fatty liver disease. Hepatology. 2013;57:1806-1813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 69. | Nelson JE, Brunt EM, Kowdley KV. Lower serum hepcidin and greater parenchymal iron in nonalcoholic fatty liver disease patients with C282Y HFE mutations. Hepatology. 2012;56:1730-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 70. | Raszeja-Wyszomirska J, Kurzawski G, Lawniczak M, Miezynska-Kurtycz J, Lubinski J. Nonalcoholic fatty liver disease and HFE gene mutations: a Polish study. World J Gastroenterol. 2010;16:2531-2536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 71. | Valenti L, Dongiovanni P, Fracanzani AL, Fargion S. HFE mutations in nonalcoholic fatty liver disease. Hepatology. 2008;47:1794-1795; author reply 1795-1796. [PubMed] |
| 72. | Valenti L, Fracanzani AL, Bugianesi E, Dongiovanni P, Galmozzi E, Vanni E, Canavesi E, Lattuada E, Roviaro G, Marchesini G. HFE genotype, parenchymal iron accumulation, and liver fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. 2010;138:905-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 214] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 73. | Valenti L, Canavesi E, Galmozzi E, Dongiovanni P, Rametta R, Maggioni P, Maggioni M, Fracanzani AL, Fargion S. Beta-globin mutations are associated with parenchymal siderosis and fibrosis in patients with non-alcoholic fatty liver disease. J Hepatol. 2010;53:927-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 74. | Valenti L, Rametta R, Dongiovanni P, Motta BM, Canavesi E, Pelusi S, Pulixi EA, Fracanzani AL, Fargion S. The A736V TMPRSS6 polymorphism influences hepatic iron overload in nonalcoholic fatty liver disease. PLoS One. 2012;7:e48804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 75. | Valenti L, Dongiovanni P, Piperno A, Fracanzani AL, Maggioni M, Rametta R, Loria P, Casiraghi MA, Suigo E, Ceriani R. Alpha 1-antitrypsin mutations in NAFLD: high prevalence and association with altered iron metabolism but not with liver damage. Hepatology. 2006;44:857-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 76. | Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2092] [Cited by in RCA: 2128] [Article Influence: 106.4] [Reference Citation Analysis (0)] |
| 77. | Weismüller TJ, Kirchner GI, Scherer MN, Negm AA, Schnitzbauer AA, Lehner F, Klempnauer J, Schlitt HJ, Manns MP, Strassburg CP. Serum ferritin concentration and transferrin saturation before liver transplantation predict decreased long-term recipient survival. Hepatology. 2011;54:2114-2124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 78. | Walker NM, Stuart KA, Ryan RJ, Desai S, Saab S, Nicol JA, Fletcher LM, Crawford DH. Serum ferritin concentration predicts mortality in patients awaiting liver transplantation. Hepatology. 2010;51:1683-1691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 79. | Sorrentino P, D’Angelo S, Ferbo U, Micheli P, Bracigliano A, Vecchione R. Liver iron excess in patients with hepatocellular carcinoma developed on non-alcoholic steato-hepatitis. J Hepatol. 2009;50:351-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 80. | Dmochowski K, Finegood DT, Francombe W, Tyler B, Zinman B. Factors determining glucose tolerance in patients with thalassemia major. J Clin Endocrinol Metab. 1993;77:478-483. [PubMed] |
| 81. | Frayling T, Ellard S, Grove J, Walker M, Hattersley AT. C282Y mutation in HFE (haemochromatosis) gene and type 2 diabetes. Lancet. 1998;351:1933-1934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 82. | Merkel PA, Simonson DC, Amiel SA, Plewe G, Sherwin RS, Pearson HA, Tamborlane WV. Insulin resistance and hyperinsulinemia in patients with thalassemia major treated by hypertransfusion. N Engl J Med. 1988;318:809-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 147] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 83. | Abraham D, Rogers J, Gault P, Kushner JP, McClain DA. Increased insulin secretory capacity but decreased insulin sensitivity after correction of iron overload by phlebotomy in hereditary haemochromatosis. Diabetologia. 2006;49:2546-2551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 84. | McClain DA, Abraham D, Rogers J, Brady R, Gault P, Ajioka R, Kushner JP. High prevalence of abnormal glucose homeostasis secondary to decreased insulin secretion in individuals with hereditary haemochromatosis. Diabetologia. 2006;49:1661-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 126] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 85. | Huang J, Gabrielsen JS, Cooksey RC, Luo B, Boros LG, Jones DL, Jouihan HA, Soesanto Y, Knecht L, Hazel MW. Increased glucose disposal and AMP-dependent kinase signaling in a mouse model of hemochromatosis. J Biol Chem. 2007;282:37501-37507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 86. | Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3370] [Cited by in RCA: 3836] [Article Influence: 191.8] [Reference Citation Analysis (0)] |
| 87. | Urakawa H, Katsuki A, Sumida Y, Gabazza EC, Murashima S, Morioka K, Maruyama N, Kitagawa N, Tanaka T, Hori Y. Oxidative stress is associated with adiposity and insulin resistance in men. J Clin Endocrinol Metab. 2003;88:4673-4676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 297] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 88. | Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1770] [Cited by in RCA: 1894] [Article Influence: 99.7] [Reference Citation Analysis (0)] |
| 89. | Tan TC, Crawford DH, Jaskowski LA, Subramaniam VN, Clouston AD, Crane DI, Bridle KR, Anderson GJ, Fletcher LM. Excess iron modulates endoplasmic reticulum stress-associated pathways in a mouse model of alcohol and high-fat diet-induced liver injury. Lab Invest. 2013;93:1295-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 90. | van de Wier B, Balk JM, Haenen GR, Giamouridis D, Bakker JA, Bast BC, den Hartog GJ, Koek GH, Bast A. Elevated citrate levels in non-alcoholic fatty liver disease: the potential of citrate to promote radical production. FEBS Lett. 2013;587:2461-2466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 91. | Sutti S, Jindal A, Locatelli I, Vacchiano M, Gigliotti L, Bozzola C, Albano E. Adaptive immune responses triggered by oxidative stress contribute to hepatic inflammation in NASH. Hepatology. 2014;59:886-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 207] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 92. | Dongiovanni P, Valenti L, Ludovica Fracanzani A, Gatti S, Cairo G, Fargion S. Iron depletion by deferoxamine up-regulates glucose uptake and insulin signaling in hepatoma cells and in rat liver. Am J Pathol. 2008;172:738-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 137] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 93. | Datz C, Felder TK, Niederseer D, Aigner E. Iron homeostasis in the metabolic syndrome. Eur J Clin Invest. 2013;43:215-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 124] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 94. | Carter R, Mouralidarane A, Soeda J, Ray S, Pombo J, Saraswati R, Novelli M, Fusai G, Rappa F, Saracino C. Non-alcoholic fatty pancreas disease pathogenesis: a role for developmental programming and altered circadian rhythms. PLoS One. 2014;9:e89505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 95. | Cho H, Zhao X, Hatori M, Yu RT, Barish GD, Lam MT, Chong LW, DiTacchio L, Atkins AR, Glass CK. Regulation of circadian behaviour and metabolism by REV-ERB-α and REV-ERB-β. Nature. 2012;485:123-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 829] [Cited by in RCA: 835] [Article Influence: 64.2] [Reference Citation Analysis (0)] |
| 96. | Green A, Basile R, Rumberger JM. Transferrin and iron induce insulin resistance of glucose transport in adipocytes. Metabolism. 2006;55:1042-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 97. | Rumberger JM, Peters T, Burrington C, Green A. Transferrin and iron contribute to the lipolytic effect of serum in isolated adipocytes. Diabetes. 2004;53:2535-2541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 98. | Aso Y, Takebayashi K, Wakabayashi S, Momobayashi A, Sugawara N, Terasawa T, Naruse R, Hara K, Suetsugu M, Morita K. Relation between serum high molecular weight adiponectin and serum ferritin or prohepcidin in patients with type 2 diabetes. Diabetes Res Clin Pract. 2010;90:250-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 99. | Ku BJ, Kim SY, Lee TY, Park KS. Serum ferritin is inversely correlated with serum adiponectin level: population-based cross-sectional study. Dis Markers. 2009;27:303-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 100. | Gabrielsen JS, Gao Y, Simcox JA, Huang J, Thorup D, Jones D, Cooksey RC, Gabrielsen D, Adams TD, Hunt SC. Adipocyte iron regulates adiponectin and insulin sensitivity. J Clin Invest. 2012;122:3529-3540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 244] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 101. | Dongiovanni P, Fracanzani AL, Fargion S, Valenti L. Iron in fatty liver and in the metabolic syndrome: a promising therapeutic target. J Hepatol. 2011;55:920-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 267] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 102. | Fernández-Real JM, Moreno JM, Chico B, López-Bermejo A, Ricart W. Circulating visfatin is associated with parameters of iron metabolism in subjects with altered glucose tolerance. Diabetes Care. 2007;30:616-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 103. | Fernández-Real JM, Moreno JM, Ricart W. Circulating retinol-binding protein-4 concentration might reflect insulin resistance-associated iron overload. Diabetes. 2008;57:1918-1925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 104. | Wouters K, van Gorp PJ, Bieghs V, Gijbels MJ, Duimel H, Lütjohann D, Kerksiek A, van Kruchten R, Maeda N, Staels B. Dietary cholesterol, rather than liver steatosis, leads to hepatic inflammation in hyperlipidemic mouse models of nonalcoholic steatohepatitis. Hepatology. 2008;48:474-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 381] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 105. | Ikura Y, Ohsawa M, Suekane T, Fukushima H, Itabe H, Jomura H, Nishiguchi S, Inoue T, Naruko T, Ehara S. Localization of oxidized phosphatidylcholine in nonalcoholic fatty liver disease: impact on disease progression. Hepatology. 2006;43:506-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 105] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 106. | Nairz M, Schroll A, Demetz E, Tancevski I, Theurl I, Weiss G. ‘Ride on the ferrous wheel’ - The cycle of iron in macrophages in health and disease. Immunobiology. 2015;220:280-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 107. | Pietrangelo A. Hemochromatosis: an endocrine liver disease. Hepatology. 2007;46:1291-1301. [PubMed] |
| 108. | Weiss G, Schett G. Anaemia in inflammatory rheumatic diseases. Nat Rev Rheumatol. 2013;9:205-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (1)] |
| 109. | Sonnweber T, Nachbaur D, Schroll A, Nairz M, Seifert M, Demetz E, Haschka D, Mitterstiller AM, Kleinsasser A, Burtscher M. Hypoxia induced downregulation of hepcidin is mediated by platelet derived growth factor BB. Gut. 2014;63:1951-1959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 111] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 110. | Hou Y, Zhang S, Wang L, Li J, Qu G, He J, Rong H, Ji H, Liu S. Estrogen regulates iron homeostasis through governing hepatic hepcidin expression via an estrogen response element. Gene. 2012;511:398-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 142] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 111. | Goodnough JB, Ramos E, Nemeth E, Ganz T. Inhibition of hepcidin transcription by growth factors. Hepatology. 2012;56:291-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 112. | Latour C, Kautz L, Besson-Fournier C, Island ML, Canonne-Hergaux F, Loréal O, Ganz T, Coppin H, Roth MP. Testosterone perturbs systemic iron balance through activation of epidermal growth factor receptor signaling in the liver and repression of hepcidin. Hepatology. 2014;59:683-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 113. | Harrison-Findik DD, Schafer D, Klein E, Timchenko NA, Kulaksiz H, Clemens D, Fein E, Andriopoulos B, Pantopoulos K, Gollan J. Alcohol metabolism-mediated oxidative stress down-regulates hepcidin transcription and leads to increased duodenal iron transporter expression. J Biol Chem. 2006;281:22974-22982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 222] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 114. | Barisani D, Pelucchi S, Mariani R, Galimberti S, Trombini P, Fumagalli D, Meneveri R, Nemeth E, Ganz T, Piperno A. Hepcidin and iron-related gene expression in subjects with Dysmetabolic Hepatic Iron Overload. J Hepatol. 2008;49:123-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 115. | Détivaud L, Nemeth E, Boudjema K, Turlin B, Troadec MB, Leroyer P, Ropert M, Jacquelinet S, Courselaud B, Ganz T. Hepcidin levels in humans are correlated with hepatic iron stores, hemoglobin levels, and hepatic function. Blood. 2005;106:746-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 132] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 116. | Lainé F, Deugnier Y. Increased expression of hepcidin in obese patients: impact on phenotypic expression of hemochromatosis and pathophysiology of dysmetabolic iron overload syndrome. Gastroenterology. 2006;131:2028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 117. | Dallalio G, Law E, Means RT. Hepcidin inhibits in vitro erythroid colony formation at reduced erythropoietin concentrations. Blood. 2006;107:2702-2704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 106] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 118. | Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2133] [Cited by in RCA: 2165] [Article Influence: 108.3] [Reference Citation Analysis (0)] |
| 119. | Huang J, Simcox J, Mitchell TC, Jones D, Cox J, Luo B, Cooksey RC, Boros LG, McClain DA. Iron regulates glucose homeostasis in liver and muscle via AMP-activated protein kinase in mice. FASEB J. 2013;27:2845-2854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 120. | Chung B, Matak P, McKie AT, Sharp P. Leptin increases the expression of the iron regulatory hormone hepcidin in HuH7 human hepatoma cells. J Nutr. 2007;137:2366-2370. [PubMed] |
| 121. | Amato A, Santoro N, Calabrò P, Grandone A, Swinkels DW, Perrone L, del Giudice EM. Effect of body mass index reduction on serum hepcidin levels and iron status in obese children. Int J Obes (Lond). 2010;34:1772-1774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 122. | Aigner E, Theurl I, Haufe H, Seifert M, Hohla F, Scharinger L, Stickel F, Mourlane F, Weiss G, Datz C. Copper availability contributes to iron perturbations in human nonalcoholic fatty liver disease. Gastroenterology. 2008;135:680-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 124] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 123. | Zoller H, Koch RO, Theurl I, Obrist P, Pietrangelo A, Montosi G, Haile DJ, Vogel W, Weiss G. Expression of the duodenal iron transporters divalent-metal transporter 1 and ferroportin 1 in iron deficiency and iron overload. Gastroenterology. 2001;120:1412-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 215] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 124. | Ruivard M, Lainé F, Ganz T, Olbina G, Westerman M, Nemeth E, Rambeau M, Mazur A, Gerbaud L, Tournilhac V. Iron absorption in dysmetabolic iron overload syndrome is decreased and correlates with increased plasma hepcidin. J Hepatol. 2009;50:1219-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 125. | Zimmermann MB, Zeder C, Muthayya S, Winichagoon P, Chaouki N, Aeberli I, Hurrell RF. Adiposity in women and children from transition countries predicts decreased iron absorption, iron deficiency and a reduced response to iron fortification. Int J Obes (Lond). 2008;32:1098-1104. [PubMed] |
| 126. | Sonnweber T, Ress C, Nairz M, Theurl I, Schroll A, Murphy AT, Wroblewski V, Witcher DR, Moser P, Ebenbichler CF. High-fat diet causes iron deficiency via hepcidin-independent reduction of duodenal iron absorption. J Nutr Biochem. 2012;23:1600-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 127. | Otogawa K, Kinoshita K, Fujii H, Sakabe M, Shiga R, Nakatani K, Ikeda K, Nakajima Y, Ikura Y, Ueda M. Erythrophagocytosis by liver macrophages (Kupffer cells) promotes oxidative stress, inflammation, and fibrosis in a rabbit model of steatohepatitis: implications for the pathogenesis of human nonalcoholic steatohepatitis. Am J Pathol. 2007;170:967-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 118] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 128. | Davis RJ, Corvera S, Czech MP. Insulin stimulates cellular iron uptake and causes the redistribution of intracellular transferrin receptors to the plasma membrane. J Biol Chem. 1986;261:8708-8711. [PubMed] |
| 129. | Yokomori N, Iwasa Y, Aida K, Inoue M, Tawata M, Onaya T. Transcriptional regulation of ferritin messenger ribonucleic acid levels by insulin in cultured rat glioma cells. Endocrinology. 1991;128:1474-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 130. | Klevay LM. Is the Western diet adequate in copper? J Trace Elem Med Biol. 2011;25:204-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 131. | al-Othman AA, Rosenstein F, Lei KY. Copper deficiency alters plasma pool size, percent composition and concentration of lipoprotein components in rats. J Nutr. 1992;122:1199-1204. [PubMed] |
| 132. | al-Othman AA, Rosenstein F, Lei KY. Copper deficiency increases in vivo hepatic synthesis of fatty acids, triacylglycerols, and phospholipids in rats. Proc Soc Exp Biol Med. 1993;204:97-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 133. | Aigner E, Strasser M, Haufe H, Sonnweber T, Hohla F, Stadlmayr A, Solioz M, Tilg H, Patsch W, Weiss G. A role for low hepatic copper concentrations in nonalcoholic Fatty liver disease. Am J Gastroenterol. 2010;105:1978-1985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 169] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 134. | Prohaska JR, Geissler J, Brokate B, Broderius M. Copper, zinc-superoxide dismutase protein but not mRNA is lower in copper-deficient mice and mice lacking the copper chaperone for superoxide dismutase. Exp Biol Med (Maywood). 2003;228:959-966. [PubMed] |
| 135. | Schuschke DA, Adeagbo AS, Patibandla PK, Egbuhuzo U, Fernandez-Botran R, Johnson WT. Cyclooxygenase-2 is upregulated in copper-deficient rats. Inflammation. 2009;32:333-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 136. | Wei YZ, Rector RS, Thyfault JP, Ibdah JA. Nonalcoholic fatty liver disease and mitochondrial dysfunction. World J Gastroenterol. 2008;14:193-199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 240] [Cited by in RCA: 260] [Article Influence: 15.3] [Reference Citation Analysis (1)] |
| 137. | Song M, Schuschke DA, Zhou Z, Chen T, Pierce WM, Wang R, Johnson WT, McClain CJ. High fructose feeding induces copper deficiency in Sprague-Dawley rats: a novel mechanism for obesity related fatty liver. J Hepatol. 2012;56:433-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 138. | Chung J, Haile DJ, Wessling-Resnick M. Copper-induced ferroportin-1 expression in J774 macrophages is associated with increased iron efflux. Proc Natl Acad Sci USA. 2004;101:2700-2705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 139. | De Domenico I, Ward DM, di Patti MC, Jeong SY, David S, Musci G, Kaplan J. Ferroxidase activity is required for the stability of cell surface ferroportin in cells expressing GPI-ceruloplasmin. EMBO J. 2007;26:2823-2831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 295] [Cited by in RCA: 265] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 140. | Musci G, Polticelli F, Bonaccorsi di Patti MC. Ceruloplasmin-ferroportin system of iron traffic in vertebrates. World J Biol Chem. 2014;5:204-215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 47] [Reference Citation Analysis (0)] |
| 141. | Levi S, Finazzi D. Neurodegeneration with brain iron accumulation: update on pathogenic mechanisms. Front Pharmacol. 2014;5:99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 125] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 142. | Piperno A, Vergani A, Salvioni A, Trombini P, Viganò M, Riva A, Zoppo A, Boari G, Mancia G. Effects of venesections and restricted diet in patients with the insulin-resistance hepatic iron overload syndrome. Liver Int. 2004;24:471-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 143. | Facchini FS. Effect of phlebotomy on plasma glucose and insulin concentrations. Diabetes Care. 1998;21:2190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 144. | Nitenberg A, Ledoux S, Valensi P, Sachs R, Antony I. Coronary microvascular adaptation to myocardial metabolic demand can be restored by inhibition of iron-catalyzed formation of oxygen free radicals in type 2 diabetic patients. Diabetes. 2002;51:813-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 145. | Duffy SJ, Biegelsen ES, Holbrook M, Russell JD, Gokce N, Keaney JF, Vita JA. Iron chelation improves endothelial function in patients with coronary artery disease. Circulation. 2001;103:2799-2804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 178] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 146. | Gaenzer H, Marschang P, Sturm W, Neumayr G, Vogel W, Patsch J, Weiss G. Association between increased iron stores and impaired endothelial function in patients with hereditary hemochromatosis. J Am Coll Cardiol. 2002;40:2189-2194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 114] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 147. | Guillygomarc’h A, Mendler MH, Moirand R, Lainé F, Quentin V, David V, Brissot P, Deugnier Y. Venesection therapy of insulin resistance-associated hepatic iron overload. J Hepatol. 2001;35:344-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 67] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 148. | Valenti L, Fracanzani AL, Fargion S. Effect of iron depletion in patients with nonalcoholic fatty liver disease without carbohydrate intolerance. Gastroenterology. 2003;124:866; author reply 866-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 149. | Fernández-Real JM, Peñarroja G, Castro A, García-Bragado F, Hernández-Aguado I, Ricart W. Blood letting in high-ferritin type 2 diabetes: effects on insulin sensitivity and beta-cell function. Diabetes. 2002;51:1000-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 245] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 150. | Valenti L, Fracanzani AL, Dongiovanni P, Bugianesi E, Marchesini G, Manzini P, Vanni E, Fargion S. Iron depletion by phlebotomy improves insulin resistance in patients with nonalcoholic fatty liver disease and hyperferritinemia: evidence from a case-control study. Am J Gastroenterol. 2007;102:1251-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 216] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 151. | Facchini FS, Saylor KL. Effect of iron depletion on cardiovascular risk factors: studies in carbohydrate-intolerant patients. Ann N Y Acad Sci. 2002;967:342-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 152. | Zacharski LR, Chow BK, Howes PS, Shamayeva G, Baron JA, Dalman RL, Malenka DJ, Ozaki CK, Lavori PW. Decreased cancer risk after iron reduction in patients with peripheral arterial disease: results from a randomized trial. J Natl Cancer Inst. 2008;100:996-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 233] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 153. | Beaton MD, Chakrabarti S, Levstik M, Speechley M, Marotta P, Adams P. Phase II clinical trial of phlebotomy for non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2013;37:720-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |