Published online Aug 28, 2015. doi: 10.4254/wjh.v7.i18.2155
Peer-review started: April 24, 2015
First decision: July 17, 2015
Revised: July 22, 2015
Accepted: August 16, 2015
Article in press: August 17, 2015
Published online: August 28, 2015
Processing time: 127 Days and 16.1 Hours
An increase in the prevalence of obesity and diabetes mellitus has been associated with the rise in nonalcoholic fatty liver disease (NAFLD). Two-thirds of the obese and diabetic populations are estimated to develop NAFLD. Currently, NAFLD is the most common etiology for chronic liver disease globally. The clinical spectrum of NAFLD ranges from simple steatosis, an accumulation of fat greater than 5% of liver weight, to nonalcoholic steatohepatitis (NASH), a more aggressive form with necroinflammation and fibrosis. Among the patients who develop NASH, up to 20% may advance to cirrhosis and are at risk for complications of end-stage liver disease. One of the major complications observed in patients with NASH-related cirrhosis is hepatocellular carcinoma (HCC), which has emerged as the sixth most common cancer and second leading etiology of cancer-related deaths worldwide. The incidence of HCC in the United States alone has tripled over the last three decades. In addition, emerging data are suggesting that a small proportion of patients with NAFLD may be at higher risk for HCC in the absence of cirrhosis - implicating obesity and diabetes mellitus as potential risk factors for HCC.
Core tip: The worldwide rise in overweight and obesity has been associated with increasing rates of nonalcoholic fatty liver disease (NAFLD), which is now the most common etiology of chronic liver disease. The more aggressive form of NAFLD, nonalcoholic steatohepatitis (NASH), promotes the development of hepatocellular carcinoma (HCC). As NASH-related cirrhosis has emerged as the most rapidly increasing indication for HCC-related liver transplantation in the United States, new strategies for HCC surveillance and targeted therapies in this patient population are warranted.
- Citation: Khan FZ, Perumpail RB, Wong RJ, Ahmed A. Advances in hepatocellular carcinoma: Nonalcoholic steatohepatitis-related hepatocellular carcinoma. World J Hepatol 2015; 7(18): 2155-2161
- URL: https://www.wjgnet.com/1948-5182/full/v7/i18/2155.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i18.2155
Increases in the prevalence of obesity and diabetes mellitus (DM) have been associated with the rise in nonalcoholic fatty liver disease (NAFLD). Currently, NAFLD is the most common cause of chronic liver disease worldwide. The clinical spectrum of NAFLD ranges from simple steatosis, an accumulation of fat greater than 5% of liver weight, to nonalcoholic steatohepatitis (NASH), a more aggressive form with inflammation and necrosis. NAFLD afflicts an estimated 30%-40% of the adult population in the United States. Even though the majority of these patients remain stable, up to 25% of patients with NAFLD can progress to NASH. Among the patients who develop NASH, many advance to cirrhosis and are at risk for complications of end-stage liver disease[1-4]. One of the major complications observed in patients with NASH-related cirrhosis is hepatocellular carcinoma (HCC), which has emerged as the sixth most common cancer and second leading etiology of cancer-related deaths worldwide[5].
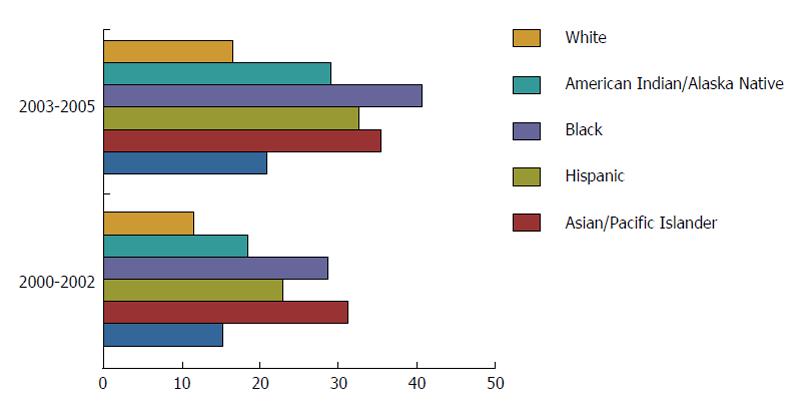
The incidence of HCC in the United States alone has tripled over the last three decades. A recent study evaluated the trends in the age-adjusted incidence rates of HCC utilizing the Surveillance, Epidemiology, and End Result (SEER) database. It revealed a rise in the incidence rate of HCC from 1.6 per 100000 in 1975 to 4.9 per 100000 in 2000[6]. A notable increase was observed among Hispanic, Black and White males (Figures 1 and 2). Historically, the leading etiologies underlying HCC have included hepatitis B virus (HBV), hepatitis C virus (HCV), alcoholic liver disease and other chronic liver diseases[7]. In the majority of cases, HCC develops in the setting of cirrhosis with the exception of HBV. A significant number of patients with HBV can develop HCC in the absence of cirrhosis because HBV itself is a known carcinogen. Emerging data are suggesting that a small proportion of patients with NAFLD may be at higher risk for HCC in the absence of cirrhosis - implicating obesity as an independent risk factor for HCC[7-10]. Globally, HBV-related liver disease is the leading etiology underlying HCC. In the United States, the increase in the incidence of HCC is mainly attributable to chronic HCV infection, particularly as the baby boomer generation ages. However, the etiology in a significant proportion of cases remains unclear, indicating that other risk factors play an important role as well. Nevertheless, as NAFLD has become the leading cause of chronic liver disease in developing and developed countries plagued by rising rates of obesity, DM and the metabolic syndrome, the prevalence and impact of NAFLD is expected to rise and drive the epidemic of HCC in the United States Two-thirds of those who suffer from obesity and DM are estimated to suffer from NAFLD as well[8,9]. NASH has a prevalence of 2%-5% in the United States, with up to 20% of patients exhibiting cirrhosis[10]. Similar to HCV patients with cirrhosis, patients with NASH-related cirrhosis are also at an increased risk of developing HCC. In a single-center study, Ascha et al[11] compared the incidence of HCV and NASH-related cirrhosis. Among 510 patients with cirrhosis, 195 had underlying NASH, while 315 had cirrhosis secondary to HCV. Median follow-up of 3.2 years revealed an annual cumulative HCC incidence of 2.6% for NASH-related cirrhosis as compared to 4% for HCV-related cirrhosis cases. Additional large population-based studies with longer durations of follow-up are needed to re-confirm that patients with NASH-related cirrhosis carry a significant risk of developing HCC and should be closely monitored.
Despite the estimated low HCC incidence rate of 2.6% in patients with NASH-related cirrhosis, the surge in the number of cases with NAFLD is projected to lead to an increase in the number of patients with NASH-related HCC. A recent study by Wong et al[12] demonstrated a nearly fourfold increase in the prevalence of NASH-related HCC cases among liver transplant recipients since implementation of the model for end-stage liver disease in 2002[12]. In a large United States population-based study utilizing the United Network for Organ Sharing database from 2002-2012, Wong et al[12] reported 10061 patients with HCC among 61868 liver transplant recipients. In order to achieve a more accurate assessment of the true prevalence of NASH, Wong et al[12] created a modified NASH category, which included patients with a formal diagnosis of NASH as well as obese patients [body mass index (BMI) > 30 kg/m2] with cryptogenic cirrhosis and obese patients with unknown etiology of HCC. The proportion of HCC patients undergoing liver transplantation increased from 3.3% in the year 2000 to 23.3% in 2012 (Table 1). Although HCV remained the leading etiology of HCC, NASH was found to be the second leading cause of HCC in patients undergoing liver transplantation and the most rapidly growing indication for liver transplantation among patients with HCC in the United States. Yet, despite increased rates of liver transplantation among patients with NASH, these patients have poorer liver transplant waitlist survival and are less likely to undergo liver transplantation than patients with HCV or alcoholic liver disease[13].
| Etiologies of HCC | HCC patients undergoing livertransplantation in the MELD era | ||
| 2002 | 2007 | 2012 | |
| HCV | 43.4% | 46.3% | 49.9% |
| NASH | 0% | 4.0% | 6.0% |
| ALD + HCV | 4.9% | 6.5% | 6.4% |
| HBV | 10.2% | 8.3% | 4.6% |
| Modified NASH | 8.3% | 10.3% | 13.5% |
The remarkable increase in the number of HCC cases in developed countries is linked to several important risk factors. Half of the new cases of HCC are attributable to HCV, whereas the etiology of cirrhosis leading to HCC in 15%-50% of new cases remains unclear[1,6]. With the growing burden of obesity and DM in developed countries leading to NAFLD and NASH, there is cumulative evidence suggesting that NASH may account for a large proportion of these cases of idiopathic or cryptogenic cirrhosis[1,14]. NASH-related HCC patients undergoing liver transplantation have significantly higher rates of DM and higher BMI[12]. Similar to the NASH population, patients with cryptogenic cirrhosis have a high prevalence of obesity and DM as well. Additionally, a significant number of liver transplant recipients with cryptogenic cirrhosis develop NAFLD (25.4%) or NASH (15.7%) within 2 years following transplant surgery[15]. This provides further support that patients with end stage or burned out NASH are potentially being misclassified with cryptogenic cirrhosis. Typically, in patients with NASH-related cirrhosis characteristic histologic features of NASH, including steatosis, lobular inflammation, balloon degeneration, necrosis and Mallory bodies in zone 3 are not evident, and may lead to misdiagnosis.
Regardless of the underlying cause of liver disease, cirrhosis is believed to be a major risk factor for the development of HCC. Yasui et al[16] performed a multicenter retrospective study in Japan to understand the pathological course of NASH progressing to HCC. These investigators identified 19 patients with histologically proven NASH who developed HCC during a median follow-up period of 3.8 years. Patients were screened and underwent surveillance using ultrasound, computed tomography, des-gamma-carboxyprothrombin testing, and AFP testing. The majority of the patients were elderly, obese (84%), diabetic (58%), and hypertensive (63%). When histopathology at the time of NASH diagnosis was compared to histopathology at the time of HCC diagnosis, the degree of steatosis remained unchanged in the majority of patients (86%), but the stage of fibrosis was significantly more advanced at the time of HCC diagnosis (P = 0.02). These findings suggest that fibrosis is a significant risk factor for the development of HCC among patients with NASH. However, there were 3 male patients (21%) of 19 who only had stage 2 fibrosis at the time of HCC diagnosis. Interestingly, in an earlier cross-sectional study performed by Yasui et al[16], 28% of NASH patients, with an older male predominance, only had stage 1 or stage 2 fibrosis at the time of HCC diagnosis. These findings indicate that HCC can occur in NASH patients regardless of the degree of fibrosis, particularly in older men. Although the association between cirrhosis secondary to NAFLD and HCC is well documented, few recent studies have pointed toward a positive correlation between non-cirrhotic fatty liver disease and HCC. The prevalence of histologically confirmed steatosis and steatohepatitis is about 54% and 15% in HCC cases without any underlying cirrhosis, respectively[17]. In another retrospective study, cirrhosis was detected in only 53% of NASH-related HCC patients, which was significantly less than in the group with non-NASH HCC (90%). Therefore, though cirrhosis is a crucial risk factor, NASH is likely a significant independent risk factor for HCC[18]. Future prospective, large population-based studies with longer durations of follow-up are needed to determine the validity of this potentially important observation.
NAFLD is considered the hepatic manifestation of the metabolic syndrome, which includes a cluster of interlinked metabolic risk factors, such as hypertension, hyperglycemia, central obesity and dyslipidemia. The majority of patients with HCC secondary to NAFLD have two or more types of metabolic disease, the most common being hyperglycemia due to insulin resistance. Based on a large United States population-based study using the SEER-Medicare database of patients over 65 years of age with histologically diagnosed HCC between 1994 and 2005, Welzel et al[19] concluded that the metabolic syndrome is an important risk factor for HCC development. Comparison with a randomly selected control group revealed that each component of the metabolic syndrome was individually associated with a statistically significant increased risk of HCC development (P < 0.0001).
Obesity is considered a significant risk factor for the development of not only HCC but also for a number of other malignancies. Welzel et al[19] reported a 1.93-fold increased risk of HCC in the obese population. In a large prospective study by Calle et al[20], out of 900000 United States adults who were enrolled in the study in 1982, 57145 died from cancer during the 16 year follow-up period. Morbidly obese (BMI > 40 kg/m2) males had a 52% higher mortality, while morbidly obese women had a 62% higher mortality compared to corresponding mortality in their normal weight counterparts. There is growing evidence supporting a positive correlation between excess body weight and HCC risk. Results from a meta-analysis of 11 cohort studies performed in 2007 showed that the liver cancer risk was 17% among the overweight and 89% among the obese as compared to those with normal weight. Furthermore, it was estimated that 28% of liver cancer cases in men and 27% of liver cancer cases in women were linked to overweight or obesity (BMI > 25 kg/m2)[21]. Even among those with normal weight, visceral fat accumulation is considered pro-oncogenic and is a potential risk factor for the recurrence of HCC. Ohki et al[22] investigated whether visceral adiposity increased the risk of cancer recurrence in patients with NASH-related HCC after undergoing curative treatment with radiofrequency ablation. Those with higher visceral fat area (> 130 cm2 in males, > 90 cm2 in females) were at increased risk of recurrent HCC (75%), compared to those with lower visceral fat area (45%).
DM, another important risk factor for HCC, is associated with 2.90 fold increased risk of HCC[19]. Insulin resistance in patients with DM leads to the increased release of free fatty acids and pro-inflammatory cytokines, including tumor necrosis factor (TNF) alpha, leptin, and interleukin-6 (IL-6), as well as reactive oxygen species, which favor fat deposition and inflammation in the liver[1,20]. Statins have been reported to have a cancer preventative effect if prescribed to patients with DM[23]. Among a large cohort of patients diagnosed with DM, a matched, nested case control study of 1303 diabetics with HCC and 5212 diabetics without HCC demonstrated that increased duration and frequency of statin prescriptions in diabetics was associated with a decreased incidence of HCC (risk reduction: 25%-40%). However, the study was limited by its retrospective design and relatively short length of follow-up (median 2.4 years). Further studies exploring this anti-inflammatory role of statins and their potentially beneficial role in preventing HCC development may have significant clinical implications.
Hepatic iron overload is also being increasingly recognized as a clinically important risk factor contributing to the development of NASH and its progression to HCC[24]. In patients with hereditary hemochromatosis, excess iron deposits drive oxidative stress, leading to the production of iron catalyzed oxyradicals. These oxyradicals contribute to the progression of fibrosis to cirrhosis and later to HCC. Lesser degrees of iron accumulation are frequently observed in patients with other forms of liver diseases, such as alcoholic liver disease, HCV-related liver cirrhosis and NAFLD. A study by Sorrentino et al[24] retrospectively assessed the hepatic iron stores in 51 patients with HCC diagnosis and NASH-related cirrhosis and 102 HCC-free controls with NASH-related cirrhosis. Conditional regression analysis revealed that histologic sinusoidal iron deposition was larger in size and more frequent in HCC patients than in controls.
In the aforementioned study by Ascha et al[11] that evaluated the annual cumulative incidence of HCC in patients with NASH-related cirrhosis, the investigators also studied and identified additional risk factors impacting HCC development in these patients. Old age (P = 0.006) and any lifetime alcohol consumption (P = 0.002) were statistically significant risk factors for progression to NASH-related cirrhosis and development of HCC. While heavy consumption of alcoholic beverages is widely considered a major risk factor for development of HCC, the study highlights that any lifetime alcohol consumption among patients with NASH was associated with a 3.6 times increased risk of HCC compared to those without lifetime alcohol consumption (P = 0.003). This suggests that alcohol consumption is a modifiable risk factor; even in quantities generally considered safe may increase the risk of HCC development in patients with NASH-related cirrhosis.
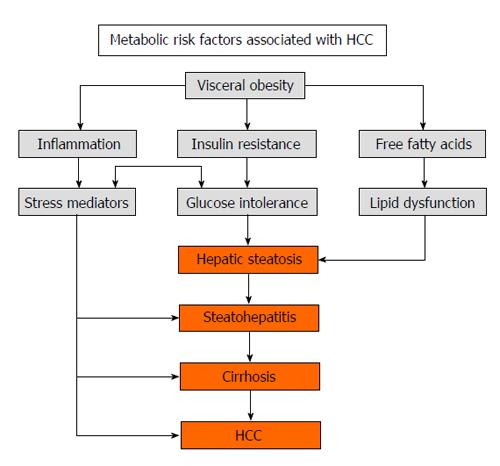
Numerous unique mechanisms underlie the pathogenesis of NASH-related HCC (Figure 3). Insulin resistance, associated with NAFLD, predisposes to the production of free fatty acids and several pro-inflammatory cytokines, including TNF-α and IL-6[1]. TNF-α promotes pro-oncogenic pathways, which specifically involve nuclear factor κB, c-Jun amino acid-terminal kinase (JNK), and mammalian target of rapamycin complex (mTOR)[25,26]. Obesity is associated with increased IL-6 levels, while weight loss reduces levels of TNF-α and IL-6, resulting in a decreased inflammatory and potentially carcinogenic response[27]. Prolonged upregulation of the IL-6/STAT3 axis results in an increased probability that hepatocytes that have already acquired oncogenic mutations from exposure to carcinogens will continue malignant transformation[28]. Insulin resistance upregulates the production of insulin-like growth factor-1 (IGF-1). IGF-1 promotes processes linked to HCC development, such as expression of proto-oncogenes c-fos and c-jun in vitro and activating mitogen activated protein kinases (MAPK)[29]. JNK, a MAPK, is activated by IR and downregulated by weight loss[30]. Histopathological analysis reveals that 70% of HCC tissue specimens stain positive for phosphorylated JNK, suggesting its role in the development of HCC[31]. Overall, several mechanisms underlying NASH-related HCC have been elucidated and pave the way for new therapeutic targets.
The majority of cases of HCC occur in patients with known cirrhosis. United States and European societies recommend regular surveillance of HCC in cirrhotic patients on a semiannual basis[32,33]. Ultrasound is regarded as the most effective tool for regular surveillance, while the role of AFP as a reliable tool for surveillance is still controversial due to high false positive and negative rates[34]. Despite evidence of improved survival among patients who underwent regular surveillance for HCC[35], Davila et al[36] suggests that the actual implementation of HCC surveillance in the cirrhotic patient population is inadequate. Davila et al[36] utilized Medicare databases to identify 1873 HCC patients with underlying cirrhosis between 1994 and 2002 and noted that among these patients only 17% received regular surveillance, while 38% received inconsistent surveillance prior to the diagnosis of HCC. Moreover, among all patients who received regular surveillance, 52% had ultrasound and AFP levels, 2% received ultrasound only, and 46% were monitored with AFP level only. Disparities were noted in surveillance, with affluent patients and patients with higher education at increased likelihood of receiving regular surveillance. Future studies would be helpful to explore whether on-time HCC surveillance in NASH patients would be effective in decreasing morbidity and mortality from the disease.
Despite several gaps in our current knowledge of NAFLD and NASH, the increasing prevalence of NASH is partly responsible for the current growth in HCC incidence. Ascha et al[11] has reported HCC incidence of 2.6% per year in patients with NASH-related cirrhosis. Currently, NASH-related cirrhosis is the most rapidly increasing indication for HCC-related liver transplantation in the United States[12]. It is essential to control the growing epidemic of obesity and DM. The role of statins in reducing the risk of HCC in patients with DM is an interesting finding which needs to be further evaluated. Timely detection of HCC is difficult due to its indolent clinical course in the initial stages and high index of clinical suspicion is crucial to early diagnosis. Patients with NASH often present at an increased age and after having developed advanced complications of end-stage liver disease, which include inoperable HCC. Efforts to devise noninvasive methods of detecting NASH and maximizing the management of common risk factors shared by NASH and HCC can lead to a potential reduction in liver disease and liver cancer burden.
P- Reviewer: Panduro A, Peluso O, Swierczynski J S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51:1820-1832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 968] [Cited by in RCA: 1014] [Article Influence: 67.6] [Reference Citation Analysis (0)] |
| 2. | Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1522] [Cited by in RCA: 1620] [Article Influence: 115.7] [Reference Citation Analysis (1)] |
| 3. | Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3655] [Cited by in RCA: 3719] [Article Influence: 161.7] [Reference Citation Analysis (2)] |
| 4. | Caldwell SH, Oelsner DH, Iezzoni JC, Hespenheide EE, Battle EH, Driscoll CJ. Cryptogenic cirrhosis: clinical characterization and risk factors for underlying disease. Hepatology. 1999;29:664-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 781] [Cited by in RCA: 751] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 5. | Ferlay JSI, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer 2013; . |
| 6. | Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1196] [Cited by in RCA: 1326] [Article Influence: 82.9] [Reference Citation Analysis (0)] |
| 7. | Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4333] [Cited by in RCA: 4507] [Article Influence: 225.4] [Reference Citation Analysis (0)] |
| 8. | Targher G, Bertolini L, Padovani R, Rodella S, Tessari R, Zenari L, Day C, Arcaro G. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2007;30:1212-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 702] [Cited by in RCA: 701] [Article Influence: 38.9] [Reference Citation Analysis (1)] |
| 9. | Leite NC, Salles GF, Araujo AL, Villela-Nogueira CA, Cardoso CR. Prevalence and associated factors of non-alcoholic fatty liver disease in patients with type-2 diabetes mellitus. Liver Int. 2009;29:113-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 368] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 10. | McCullough AJ. The clinical features, diagnosis and natural history of nonalcoholic fatty liver disease. Clin Liver Dis. 2004;8:521-533, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 397] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 11. | Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51:1972-1978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 893] [Cited by in RCA: 965] [Article Influence: 64.3] [Reference Citation Analysis (1)] |
| 12. | Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology. 2014;59:2188-2195. [PubMed] |
| 13. | Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, Ahmed A. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1211] [Cited by in RCA: 1382] [Article Influence: 138.2] [Reference Citation Analysis (1)] |
| 14. | Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, Musso A, De Paolis P, Capussotti L, Salizzoni M. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1036] [Cited by in RCA: 1018] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 15. | Ong J, Younossi ZM, Reddy V, Price LL, Gramlich T, Mayes J, Boparai N. Cryptogenic cirrhosis and posttransplantation nonalcoholic fatty liver disease. Liver Transpl. 2001;7:797-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 167] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 16. | Yasui K, Hashimoto E, Tokushige K, Koike K, Shima T, Kanbara Y, Saibara T, Uto H, Takami S, Kawanaka M. Clinical and pathological progression of non-alcoholic steatohepatitis to hepatocellular carcinoma. Hepatol Res. 2012;42:767-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Alexander J, Torbenson M, Wu TT, Yeh MM. Non-alcoholic fatty liver disease contributes to hepatocarcinogenesis in non-cirrhotic liver: a clinical and pathological study. J Gastroenterol Hepatol. 2013;28:848-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (2)] |
| 18. | Ertle J, Dechêne A, Sowa JP, Penndorf V, Herzer K, Kaiser G, Schlaak JF, Gerken G, Syn WK, Canbay A. Non-alcoholic fatty liver disease progresses to hepatocellular carcinoma in the absence of apparent cirrhosis. Int J Cancer. 2011;128:2436-2443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 383] [Article Influence: 27.4] [Reference Citation Analysis (1)] |
| 19. | Welzel TM, Graubard BI, Zeuzem S, El-Serag HB, Davila JA, McGlynn KA. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatology. 2011;54:463-471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 426] [Cited by in RCA: 423] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 20. | Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5430] [Cited by in RCA: 5283] [Article Influence: 240.1] [Reference Citation Analysis (0)] |
| 21. | Larsson SC, Wolk A. Overweight, obesity and risk of liver cancer: a meta-analysis of cohort studies. Br J Cancer. 2007;97:1005-1008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 332] [Cited by in RCA: 352] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 22. | Ohki T, Tateishi R, Shiina S, Goto E, Sato T, Nakagawa H, Masuzaki R, Goto T, Hamamura K, Kanai F. Visceral fat accumulation is an independent risk factor for hepatocellular carcinoma recurrence after curative treatment in patients with suspected NASH. Gut. 2009;58:839-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 23. | El-Serag HB, Johnson ML, Hachem C, Morgana RO. Statins are associated with a reduced risk of hepatocellular carcinoma in a large cohort of patients with diabetes. Gastroenterology. 2009;136:1601-1608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 226] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 24. | Sorrentino P, D’Angelo S, Ferbo U, Micheli P, Bracigliano A, Vecchione R. Liver iron excess in patients with hepatocellular carcinoma developed on non-alcoholic steato-hepatitis. J Hepatol. 2009;50:351-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 25. | Stickel F, Hellerbrand C. Non-alcoholic fatty liver disease as a risk factor for hepatocellular carcinoma: mechanisms and implications. Gut. 2010;59:1303-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 26. | Marra F. Leptin and liver fibrosis: a matter of fat. Gastroenterology. 2002;122:1529-1532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Bougoulia M, Triantos A, Koliakos G. Effect of weight loss with or without orlistat treatment on adipocytokines, inflammation, and oxidative markers in obese women. Hormones (Athens). 2006;5:259-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 28. | Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, Osterreicher CH, Takahashi H, Karin M. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1403] [Cited by in RCA: 1369] [Article Influence: 91.3] [Reference Citation Analysis (1)] |
| 29. | Price JA, Kovach SJ, Johnson T, Koniaris LG, Cahill PA, Sitzmann JV, McKillop IH. Insulin-like growth factor I is a comitogen for hepatocyte growth factor in a rat model of hepatocellular carcinoma. Hepatology. 2002;36:1089-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Cho H, Black SC, Looper D, Shi M, Kelly-Sullivan D, Timofeevski S, Siegel K, Yu XH, McDonnell SR, Chen P. Pharmacological characterization of a small molecule inhibitor of c-Jun kinase. Am J Physiol Endocrinol Metab. 2008;295:E1142-E1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Chang Q, Zhang Y, Beezhold KJ, Bhatia D, Zhao H, Chen J, Castranova V, Shi X, Chen F. Sustained JNK1 activation is associated with altered histone H3 methylations in human liver cancer. J Hepatol. 2009;50:323-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 32. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6573] [Article Influence: 469.5] [Reference Citation Analysis (1)] |
| 33. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3252] [Cited by in RCA: 3243] [Article Influence: 135.1] [Reference Citation Analysis (0)] |
| 34. | Forner A, Reig M, Bruix J. Alpha-fetoprotein for hepatocellular carcinoma diagnosis: the demise of a brilliant star. Gastroenterology. 2009;137:26-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 35. | Chan AC, Poon RT, Ng KK, Lo CM, Fan ST, Wong J. Changing paradigm in the management of hepatocellular carcinoma improves the survival benefit of early detection by screening. Ann Surg. 2008;247:666-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 36. | Davila JA, Morgan RO, Richardson PA, Du XL, McGlynn KA, El-Serag HB. Use of surveillance for hepatocellular carcinoma among patients with cirrhosis in the United States. Hepatology. 2010;52:132-141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 313] [Cited by in RCA: 312] [Article Influence: 20.8] [Reference Citation Analysis (0)] |