Published online Jul 28, 2015. doi: 10.4254/wjh.v7.i15.1894
Peer-review started: January 27, 2015
First decision: April 27, 2015
Revised: May 17, 2015
Accepted: June 4, 2015
Article in press: June 8, 2015
Published online: July 28, 2015
Processing time: 193 Days and 1.8 Hours
An ever-increasing number of 3.0 Tesla (T) magnets are installed worldwide. Moving from the standard of 1.5 T to higher field strength implies a number of potential advantage and drawbacks, requiring careful optimization of imaging protocols or implementation of novel hardware components. Clinical practice and literature review suggest that state-of-the-art 3.0 T is equivalent to 1.5 T in the assessment of focal liver lesions and diffuse liver disease. Therefore, further technical improvements are needed in order to fully exploit the potential of higher field strength.
Core tip: The editorial focuses on potential advantages and drawbacks related to the use of 3.0 Tesla (T) magnets in liver imaging. Current clinical applications are discussed, with special emphasis on the comparison with 1.5 T. If careful optimization is performed, state-of-the-art 3.0 T is equivalent to 1.5 T. Further technical improvements are needed in order to fully exploit the potential of higher field strength.
- Citation: Girometti R. 3.0 Tesla magnetic resonance imaging: A new standard in liver imaging? World J Hepatol 2015; 7(15): 1894-1898
- URL: https://www.wjgnet.com/1948-5182/full/v7/i15/1894.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i15.1894
Because of limited availability and costs, magnetic resonance imaging (MRI) of the liver is usually performed as a problem-solving tool after inconclusive prior ultrasound and/or computed tomography (CT). However, MRI is, per se, the imaging modality of choice for the detection and characterization of focal liver lesions[1], owing to superior contrast resolution and the “all-in-one” information provided by hepatospecific contrast agents such as gadobenate dimeglumine (Gd-BOPTA) and gadoxetic acid (Gd-EOB-DTPA). Less defined is the role of MRI in assessing diffuse liver disease, as exemplified by current, intensive research on different techniques aimed to quantify fibrosis, steatosis or iron overload[2].
1.5 Tesla (T) systems still represent the technical standard for abdominal MRI[3]. Nonetheless, the use of ultra-high field strength is a major focus in liver imaging, given the ever-increasing number of new 3.0 T magnets installed worldwide for research and clinical practice. One might wonder whether 3.0 T might become the new standard, as occurred in the past when moving from lower field strength to 1.5 T. In theory, 3.0 T magnets have the capability to provide better image quality as the base for improved diagnostic performance. This is because doubling the field strength (almost) doubles signal-to-noise ratio[4], that is the quantity of signal made available from the patient in order to build MRI images. Exceeding signal can be converted into better image detail (higher spatial resolution) and/or faster acquisition (higher temporal resolution), as well as more efficient fat suppression and better lesion conspicuity because of improved lesion-to-liver contrast after gadolinium administration[5]. Both conventional imaging and functional techniques such as diffusion-weighted imaging (DWI), dynamic contrast-enhanced MRI and spectroscopy may benefit from the above changes.
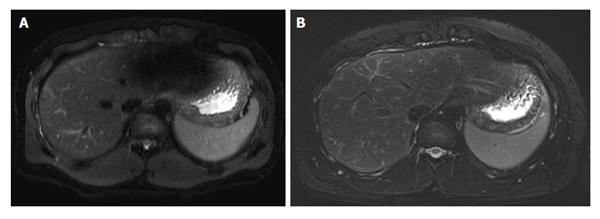
Despite theoretical promises, the available evidence shows some disappointing results when comparing 3.0 T vs 1.5 T, especially for T2-weighted imaging. For example, two studies[6,7] on patients with chronic liver disease showed that radiologists perceive equal or lower image quality at higher field strength. The explanation for such a discrepancy is that the transition from 1.5 T to 3.0 T harbours technical challenges at serious risk of impairing the gain in signal-to-noise ratio. Major concerns in liver imaging are related to three factors[5]. First, changes in tissue relaxation times affect image contrast, at a larger degree on T1-weighted Spoiled Gradient Echo images. This can make the detection of focal lesions, fibrosis or steatosis more challenging at 3.0 T[8]. Second, the radiofrequency (RF) power deposition to the patient significantly increases, especially for Turbo Spin Echo (TSE)-designed T2-weighted sequence using a large number of RF pulses to generate image contrast. RF power deposition represents the energy administered to the patient to obtain signal back, and is measured as specific absorption rate (SAR). Unfortunately, strategies to reduce 3.0 T-related increase in SAR frequently occur at the expense of the gain in signal. Third, image quality can be degraded by the so called standing wave artefact, resulting from inhomogeneous RF deposition due to interactions between RF waves and the patients’ body[5]. Standing wave artefact consists of zones of gross signal drop affecting T2-weighted images at a serious extent[9], usually in correspondence of the left liver lobe (Figure 1). Despite there is no definite correlation with body mass index or body fat content, the artefact prevails in larger patients, being characteristically exacerbated by the presence of ascites[5,8,9].
How to overcome technical limitations? In a study by von Falkenhausen et al[10], image quality at 3.0 T was found equivalent to 1.5 T using comparable acquisition parameters, emphasizing that the implementation of standard 1.5 T MRI protocols on 3.0 T magnets requires careful optimization and/or new technical solutions to exploit the potential of higher field-strength. While problems in T1 contrast and SAR are faced by implementing proper sequence design[11,12], standing wave artefacts should be more consistently prevented by intervening on the magnet hardware[13], that is by implementing more than one conventional RF source in order to independently correct phase and amplitude of the RF pulses for patient-induced B1-inhomogeneity. Studies using new-generation 3.0 T systems with dual-source parallel RF transmission[9,13,14] showed significant qualitative and quantitative image improvement for TSE-based T2-weighted imaging, which is the real “Achilles heel” of liver MRI at 3.0 T. Results with and without hardware implementation are conflicting in terms of better lesions detectability[9,14]. However, dual-source systems are reasonably the best state-of-the-art solution to minimize standing wave effect in obese individuals and/or patients with ascites, in whom lesions can be missed because of degraded image quality.
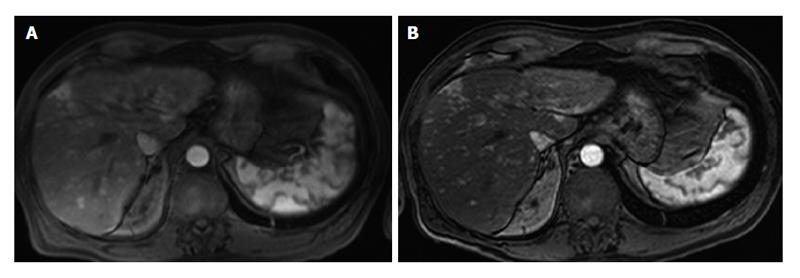
On the bright side, 3.0 T was proven to provide superior post-gadolinium image quality using 1.5 T-equivalent volumetric fat-saturated Gradient-Echo T1-weighted imaging[7,12]. This is in accordance with the experience in many centers using 3.0 T, including our Institution (Figure 2). Lee et al[15] suggested that the quality of the dynamic study is further improved when replacing conventional fat suppression technique at 3.0 T (spectrally adiabatic inversion recovery) with the Dixon approach. These results have potential diagnostic impact in terms of better detection and characterization of smaller lesions, especially in late arterial phase or hepatobiliary phase[8].
One might wonder whether superior quality of post-contrast imaging is just a matter of the sequence used or rather the type and dose of contrast medium. Indeed, the T1 relaxation time of the liver in vivo increases of about 41% at 3.0 T compared to 1.5 T[5], translating into a theoretical increase in contrast differences using an equivalent dose of gadolinium-based contrast agents[16]. A study by Kim et al[17] supports this assumption. Comparing arterial late phases acquired in same individuals with the standard dose of gadoxetic acid (0.025 mmol/kg) and half dose of gadobenate dimeglumine (0.05 mmol/kg), the Authors found higher relative enhancement of the liver at 3.0 T rather than 1.5 T, for both contrast agents (19.4% vs 11.4% and 33.4% vs 18.9%, respectively). Alternatively, one can achieve adequate image contrast at 3.0 T using less contrast medium, as shown by de Campos et al[18] with a quarter dose of gadobenate dimeglumine (0.025 mmol/kg). Potential clinical consequences are better lesions detectability and reduction of the risk of nephrogenic systemic fibrosis in selected patients. However, image contrast after the administration of gadolinium chelates is a matter of complex interactions. Not surprisingly, studies in vitro and in vivo[16] are concordant in showing comparable contrast enhancement of the liver between 1.5 T and 3.0 T at equivalent concentrations, regardless of the dose. In summary, it is difficult to quantify the impact of contrast agent properties in determining superior image quality of 3.0 T contrast-enhanced studies.
The ever-increasing diffusion of magnets for everyday clinical practice, and rise in publications of radiological studies performed with 3.0 T suggest that higher field strength is at least equivalent to 1.5 T in diagnostic terms. Unfortunately, there is paucity of prospective works comparing 1.5 T and 3.0 T on an intraindividual basis. In a study on 35 patients who underwent both 1.5 T and 3.0 T with a superparamagnetic iron oxide contrast agent, Chang et al[19] showed equivalent accuracy in assessing malignant focal liver lesions, with lower image quality at higher field strength. Only a few papers focus on hepatocellular carcinoma (HCC) and colorectal cancer metastases. In a 3.0 T standing-alone study by Lee et al[15], the Authors found an overall accuracy in the detection of HCC with gadoxetic acid similar to 1.5 T (mean AUC 0.95). Interestingly, two different studies[20,21] compared the detection of HCC between 3.0 T MRI and triple-phase multidetector CT (MDCT), showing equivalent high accuracy, though MRI was able to detect more lesions on a per-patient basis (2.7 vs 2.3)[20] and performed better for smaller HCC (≤ 1 cm in size)[21]. It is difficult to compare these results with those obtained in other studies with lower field strength, e.g., by Akai et al[22], who showed a trend to a better performance of gadoxetic-acid-enhanced 1.5 T MRI vs 64-raw MDCT. Based on the experience in my Institution, 3.0 T MRI is at least equivalent to 1.5 T, being helpful in assessing cases in which the number of lesions is crucial to plan the treatment (e.g., liver transplant), as well in the scenarios of lesion characterization and detection of recurrence. Concerning colorectal cancer metastases, 3.0 T showed excellent detection rates combining gadoxetic acid and DWI, with AUCs of 0.915-0.937 at ROC analysis[23]. Compared to MDCT, 3.0 T MRI showed better performance, though without statistical significance[24], especially in the detection of smaller lesions, having the potential to change initial management plan in about one-third of patients[25]. Due to superior contrast resolution, 3.0 T MRI more clearly outperforms MDCT in the subset of patients with fatty liver infiltration (detection rate of 97% vs 72%)[26]. Indeed, fatty infiltration may impair detection of metastases on MDCT by diminishing the contrast between an hypodense lesion and the surrounding liver tissue[27]. Despite theoretical advantages of thinner slice thickness and improved lesion-to-liver contrast in the hepatobiliary phase at 3.0 T, it is still challenging to prove any superiority in detecting metastases compared to 1.5 T.
A few studies deal with the role of 3.0 T in diffuse liver disease. Promising results have been obtained in different scenarios, including the use of DWI in staging liver fibrosis in patients with nonalcoholic fatty liver disease[28], iron quantification[29], or the assessment of liver function with relative-contrast enhancement of liver parenchyma on the hepatobiliary phase after gadoxetic acid administration[30]. Because of the increase of spectral resolution, 3.0 T has the potential to better differentiate between hepatic metabolites, thus providing robust magnetic resonance spectroscopy (MRS) for the assessment of chronic liver disease, e.g., by measuring hepatic fat content[31]. Several pilot studies[31,32] show that liver MRS is feasible. Nonetheless, this technique is still in its infancy and requires further optimization and validation. Research on diffuse liver diseases is particularly intense in the setting of DWI, a popular technique exploiting normal and pathological water Brownian motion within the liver under the form of both signal intensity and apparent diffusion coefficients (ADC)[33]. DWI is expected to benefit from increased signal-to-noise ratio in terms of better image quality and more robust estimation of the ADC. Available results are somewhat disappointing, suggesting that ADC quantification is equivalent compared to 1.5 T, thought at the expense of lower image quality[34]. Thus, optimization of DWI at 3.0 T is still a matter of research[35,36].
In conclusion, optimization of 3.0 T liver protocols is still in progress, both in terms of sequence design and hardware upgrade. Using comparable imaging technique, T2-weighted sequnce provide images of worse quality than 1.5 T, unless new magnets with dual-source RF transmission are used. On the bright side, dynamic study after contrast injection is qualitatively and quantitatively better at 3.0 T. Such an equilibrium translates into similar diagnostic accuracy compared to the reference of 1.5 T, as shown by an increasing amount of evidence from literature. Concerning clinical practice, our experience shows that liver imaging is routinely feasible at 3.0 T, unless patients show conditions such as obesity and/or ascites that may significantly degrade T2-weighted imaging. 3.0 T should be avoided in these subjects. In the remaining cases, 3.0 T is a reliable alternative to 1.5 T, especially in those patients in whom improved dynamic study is expected to provide “key” information, such as detection and characterization of hypervascular lesions (e.g., HCC).
In summary, if the new standard in liver imaging should be undoubtedly better than the older one, state-of-the-art 3.0 T is far from representing it. However, ongoing technical improvements are expected to exploit all the potential advantages inherent to higher field strength, suggesting that 3.0 T candidates for the new standard in liver imaging in the next future.
The author thanks Dr. Iliana Bednarova for her help in revising the text.
P- Reviewer: Maroni L, Sargsyants N S- Editor: Tian YL L- Editor: A E- Editor: Liu SQ
| 1. | American College of Radiology. ACR Appropriateness Criteria®, Liver. [Accessed 2015 July 18th]. Available from: http://acsearch.acr.org/docs/69472/Narrative/. |
| 2. | Van Beers BE, Daire JL, Garteiser P. New imaging techniques for liver diseases. J Hepatol. 2015;62:690-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 3. | Chang KJ, Kamel IR, Macura KJ, Bluemke DA. 3.0-T MR imaging of the abdomen: comparison with 1.5 T. Radiographics. 2008;28:1983-1998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 163] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 4. | Schindera ST, Merkle EM, Dale BM, Delong DM, Nelson RC. Abdominal magnetic resonance imaging at 3.0 T what is the ultimate gain in signal-to-noise ratio? Acad Radiol. 2006;13:1236-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Soher BJ, Dale BM, Merkle EM. A review of MR physics: 3T versus 1.5T. Magn Reson Imaging Clin N Am. 2007;15:277-290, v. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Ramalho M, Herédia V, Tsurusaki M, Altun E, Semelka RC. Quantitative and qualitative comparison of 1.5 and 3.0 Tesla MRI in patients with chronic liver diseases. J Magn Reson Imaging. 2009;29:869-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Tsurusaki M, Semelka RC, Zapparoli M, Elias J, Altun E, Pamuklar E, Sugimura K. Quantitative and qualitative comparison of 3.0T and 1.5T MR imaging of the liver in patients with diffuse parenchymal liver disease. Eur J Radiol. 2009;72:314-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Ramalho M, Altun E, Herédia V, Zapparoli M, Semelka R. Liver MR imaging: 1.5T versus 3T. Magn Reson Imaging Clin N Am. 2007;15:321-347, vi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Kukuk GM, Gieseke J, Weber S, Hadizadeh DR, Nelles M, Träber F, Schild HH, Willinek WA. Focal liver lesions at 3.0 T: lesion detectability and image quality with T2-weighted imaging by using conventional and dual-source parallel radiofrequency transmission. Radiology. 2011;259:421-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | von Falkenhausen MM, Lutterbey G, Morakkabati-Spitz N, Walter O, Gieseke J, Blömer R, Willinek WA, Schild HH, Kuhl CK. High-field-strength MR imaging of the liver at 3.0 T: intraindividual comparative study with MR imaging at 1.5 T. Radiology. 2006;241:156-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Akisik FM, Sandrasegaran K, Aisen AM, Lin C, Lall C. Abdominal MR imaging at 3.0 T. Radiographics. 2007;27:1433-1444; discussion 1462-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Zapparoli M, Semelka RC, Altun E, Tsurusaki M, Pamuklar E, Dale BM, Gasparetto EL, Elias J. 3.0-T MRI evaluation of patients with chronic liver diseases: initial observations. Magn Reson Imaging. 2008;26:650-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Willinek WA, Gieseke J, Kukuk GM, Nelles M, König R, Morakkabati-Spitz N, Träber F, Thomas D, Kuhl CK, Schild HH. Dual-source parallel radiofrequency excitation body MR imaging compared with standard MR imaging at 3.0 T: initial clinical experience. Radiology. 2010;256:966-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 117] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 14. | Hwang J, Kim YK, Park MJ, Lee MH, Kim SH, Lee WJ, Choi D. Liver MRI at 3.0 tesla: comparison of image quality and lesion detectability between single-source conventional and dual-source parallel radiofrequency transmissions. J Comput Assist Tomogr. 2012;36:546-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Lee MH, Kim YK, Park MJ, Hwang J, Kim SH, Lee WJ, Choi D. Gadoxetic acid-enhanced fat suppressed three-dimensional T1-weighted MRI using a multiecho dixon technique at 3 tesla: emphasis on image quality and hepatocellular carcinoma detection. J Magn Reson Imaging. 2013;38:401-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Ramalho M, AlObaidy M, Busireddy KK, Altun E, Liu B, Semelka RC. Quantitative and qualitative comparison of 0.025 mmol/kg gadobenate dimeglumine for abdominal MRI at 1.5T and 3T MRI in patients with low estimated glomerular filtration rate. Eur J Radiol. 2015;84:26-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Kim HJ, Kim BS, Kim MJ, Kim SH, de Campos RO, Hernandes M, Semelka RC. Enhancement of the liver and pancreas in the hepatic arterial dominant phase: comparison of hepatocyte-specific MRI contrast agents, gadoxetic acid and gadobenate dimeglumine, on 3 and 1.5 Tesla MRI in the same patient. J Magn Reson Imaging. 2013;37:903-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | de Campos RO, Heredia V, Ramalho M, De Toni MS, Lugo-Somolinos A, Fuller ER, Semelka RC. Quarter-dose (0.025 mmol/kg) gadobenate dimeglumine for abdominal MRI in patients at risk for nephrogenic systemic fibrosis: preliminary observations. AJR Am J Roentgenol. 2011;196:545-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Chang JM, Lee JM, Lee MW, Choi JY, Kim SH, Lee JY, Han JK, Choi BI. Superparamagnetic iron oxide-enhanced liver magnetic resonance imaging: comparison of 1.5 T and 3.0 T imaging for detection of focal malignant liver lesions. Invest Radiol. 2006;41:168-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Maiwald B, Lobsien D, Kahn T, Stumpp P. Is 3-Tesla Gd-EOB-DTPA-enhanced MRI with diffusion-weighted imaging superior to 64-slice contrast-enhanced CT for the diagnosis of hepatocellular carcinoma? PLoS One. 2014;9:e111935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Kim SH, Kim SH, Lee J, Kim MJ, Jeon YH, Park Y, Choi D, Lee WJ, Lim HK. Gadoxetic acid-enhanced MRI versus triple-phase MDCT for the preoperative detection of hepatocellular carcinoma. AJR Am J Roentgenol. 2009;192:1675-1681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 261] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 22. | Akai H, Kiryu S, Matsuda I, Satou J, Takao H, Tajima T, Watanabe Y, Imamura H, Kokudo N, Akahane M. Detection of hepatocellular carcinoma by Gd-EOB-DTPA-enhanced liver MRI: comparison with triple phase 64 detector row helical CT. Eur J Radiol. 2011;80:310-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 23. | Chung WS, Kim MJ, Chung YE, Kim YE, Park MS, Choi JY, Kim KW. Comparison of gadoxetic acid-enhanced dynamic imaging and diffusion-weighted imaging for the preoperative evaluation of colorectal liver metastases. J Magn Reson Imaging. 2011;34:345-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 24. | Scharitzer M, Ba-Ssalamah A, Ringl H, Kölblinger C, Grünberger T, Weber M, Schima W. Preoperative evaluation of colorectal liver metastases: comparison between gadoxetic acid-enhanced 3.0-T MRI and contrast-enhanced MDCT with histopathological correlation. Eur Radiol. 2013;23:2187-2196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 25. | Sofue K, Tsurusaki M, Murakami T, Onoe S, Tokue H, Shibamoto K, Arai Y, Sugimura K. Does Gadoxetic acid-enhanced 3.0T MRI in addition to 64-detector-row contrast-enhanced CT provide better diagnostic performance and change the therapeutic strategy for the preoperative evaluation of colorectal liver metastases? Eur Radiol. 2014;24:2532-2539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Berger-Kulemann V, Schima W, Baroud S, Koelblinger C, Kaczirek K, Gruenberger T, Schindl M, Maresch J, Weber M, Ba-Ssalamah A. Gadoxetic acid-enhanced 3.0 T MR imaging versus multidetector-row CT in the detection of colorectal metastases in fatty liver using intraoperative ultrasound and histopathology as a standard of reference. Eur J Surg Oncol. 2012;38:670-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 27. | Kulemann V, Schima W, Tamandl D, Kaczirek K, Gruenberger T, Wrba F, Weber M, Ba-Ssalamah A. Preoperative detection of colorectal liver metastases in fatty liver: MDCT or MRI? Eur J Radiol. 2011;79:e1-e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 28. | Papalavrentios L, Sinakos E, Chourmouzi D, Hytiroglou P, Drevelegas K, Constantinides M, Drevelegas A, Talwalkar J, Akriviadis E. Value of 3 Tesla diffusion-weighted magnetic resonance imaging for assessing liver fibrosis. Ann Gastroenterol. 2015;28:118-123. [PubMed] |
| 29. | Anwar M, Wood J, Manwani D, Taragin B, Oyeku SO, Peng Q. Hepatic Iron Quantification on 3 Tesla (3 T) Magnetic Resonance (MR): Technical Challenges and Solutions. Radiol Res Pract. 2013;2013:628150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Verloh N, Haimerl M, Zeman F, Schlabeck M, Barreiros A, Loss M, Schreyer AG, Stroszczynski C, Fellner C, Wiggermann P. Assessing liver function by liver enhancement during the hepatobiliary phase with Gd-EOB-DTPA-enhanced MRI at 3 Tesla. Eur Radiol. 2014;24:1013-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 112] [Article Influence: 10.2] [Reference Citation Analysis (1)] |
| 31. | Fischbach F, Schirmer T, Thormann M, Freund T, Ricke J, Bruhn H. Quantitative proton magnetic resonance spectroscopy of the normal liver and malignant hepatic lesions at 3.0 Tesla. Eur Radiol. 2008;18:2549-2558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | van Werven JR, Hoogduin JM, Nederveen AJ, van Vliet AA, Wajs E, Vandenberk P, Stroes ES, Stoker J. Reproducibility of 3.0 Tesla magnetic resonance spectroscopy for measuring hepatic fat content. J Magn Reson Imaging. 2009;30:444-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 33. | Girometti R, Esposito G, Bagatto D, Avellini C, Bazzocchi M, Zuiani C. Is water diffusion isotropic in the cirrhotic liver? a study with diffusion-weighted imaging at 3.0 Tesla. Acad Radiol. 2012;19:55-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Rosenkrantz AB, Oei M, Babb JS, Niver BE, Taouli B. Diffusion-weighted imaging of the abdomen at 3.0 Tesla: image quality and apparent diffusion coefficient reproducibility compared with 1.5 Tesla. J Magn Reson Imaging. 2011;33:128-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 186] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 35. | Choi JS, Kim MJ, Chung YE, Kim KA, Choi JY, Lim JS, Park MS, Kim KW. Comparison of breathhold, navigator-triggered, and free-breathing diffusion-weighted MRI for focal hepatic lesions. J Magn Reson Imaging. 2013;38:109-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 36. | Saremi F, Jalili M, Sefidbakht S, Channual S, Quane L, Naderi N, Schultze-Haakh H, Torrone M. Diffusion-weighted imaging of the abdomen at 3 T: image quality comparison with 1.5-T magnet using 3 different imaging sequences. J Comput Assist Tomogr. 2011;35:317-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |