Published online Jun 28, 2015. doi: 10.4254/wjh.v7.i12.1718
Peer-review started: March 22, 2015
First decision: April 10, 2015
Revised: May 1, 2015
Accepted: May 27, 2015
Article in press: May 28, 2015
Published online: June 28, 2015
Processing time: 99 Days and 18.7 Hours
Mild to moderate autoimmune thrombocytopenia (AITP) is a common finding in patients receiving interferon-based antiviral treatment, due to bone marrow suppression. Here we report the case of a patient with chronic genotype 1b hepatitis C virus (HCV) infection treated with pegylated-interferon alpha-2a, ribavirin and telaprevir for 24 wk; the patient developed severe AITP three weeks after treatment withdrawal. We performed a systematic literature search in order to review all published cases of AITP related to HCV antiviral treatment. To our knowledge, this is the second case of AITP observed after antiviral treatment withdrawal. In most published cases AITP occurred during treatment; in fact, among 24 cases of AITP related to interferon-based antiviral treatment, only one occurred after discontinuation. Early diagnosis of AITP is a key factor in order to achieve an early interferon discontinuation; in the era of new direct antiviral agents those patients have to be considered for interferon-free treatment regimens. Prompt prescription of immuno-suppressant treatment (i.e., corticosteroids, immunoglobulin infusion and even rituximab for unresponsive cases) leads to favourable prognosis in most of cases. Physicians using interferon-based treatments should be aware that AITP can occur both during and after treatment, specially in the new era of interferon-free antiviral treatment. Finally, in the case of suspected AITP, presence of anti-platelet antibodies should be checked not only during treatment but also after discontinuation.
Core tip: This is the second case report of autoimmune thrombocytopenia (AITP) occurred after peg-interferon/ribavirin treatment completion: generally, AITP was observed in course of interferon treatment. To our knowledge, among 24 interferon-related AITP cases reported in literature, in 23 cases the side effect occurred during treatment while in only one after treatment completion. Physicians using interferon-based antiviral therapy should be aware that acute AITP can occur both during and after treatment; in the case of suspected AITP, presence of anti-platelet antibodies should be checked not only during treatment but also after discontinuation.
- Citation: Arena R, Cecinato P, Lisotti A, Buonfiglioli F, Calvanese C, Grande G, Montagnani M, Azzaroli F, Mazzella G. Severe immune thrombocytopenia after peg-interferon-alpha2a, ribavirin and telaprevir treatment completion: A case report and systematic review of literature. World J Hepatol 2015; 7(12): 1718-1722
- URL: https://www.wjgnet.com/1948-5182/full/v7/i12/1718.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i12.1718
Presence of autoimmune thrombocytopenia (AITP) could be directly related to hepatitis C virus (HCV) infection, even in early stages of the disease. To date, AITP was not considered an absolute contraindication for interferon-based antiviral treatment and usually resolves after virus clearance[1,2]; however, with the registration of interferon-free regimen, patients with severe thrombocytopenia should be considered for all-oral new direct antiviral agents therapy.
On the other side, mild to moderate thrombocytopenia could be observed in patients receiving antiviral treatment with interferon alpha, due to bone marrow suppression[3]. However, severe life-threatening immune thrombocytopenia has rarely been associated with interferon treatment[4-26]. Finally, thrombocytopenia is a common finding in liver cirrhosis, usually related to congestive splenomegaly and to inadequate liver thrombopoietin synthesis[27].
We present a case of a chronic HCV infected patient in which severe AITP occurred 3 wk after pegylated-interferon (Peg-IFN)α-2a, ribavirin (RBV) and telaprevir treatment completion.
We describe the case of a 68-year-old female patient affected by genotype 1b chronic HCV infection (on histology, fibrosis staging F3 according to Metavir); the patient reported a partial response (fall of 4 log10 HCV-RNA) to a previous course of therapy with Peg-IFNα-2a plus RBV and, in February 2012, started treatment with Peg-INFα-2a (180 mcg/wk) plus RBV (1200 mg/d) and telaprevir (2250 mg/d during the first 12 wk).
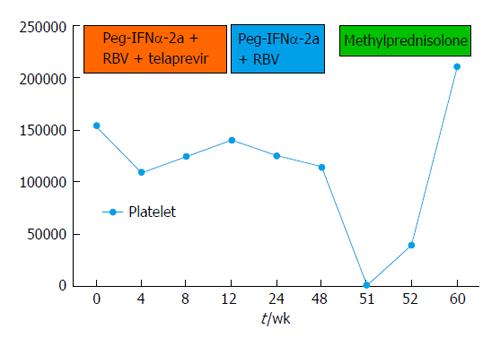
Before starting treatment the patient had normal laboratory tests, in particular: haemoglobin 14 g/dL, white blood cell count 6.840/mmc, platelet count 155.000/microl, aspartate aminotransferase (AST) 32 IU/L, alanine aminotransferase (ALT) 52 IU/L, γ-glutamyl transpeptidase 48 IU/L, total bilirubin 0.89 mg/dL, albumin 4.1 g/dL, gamma-globulin 1550 mg/dL, HCV-RNA 256665 IU/mL, thyroid stimulating hormone 2.08 microU/mL; anti-mitochondrial antibodies, anti-smooth muscle antibodies, anti-thyroid autoantibodies were negative while anti-nuclear antibodies were found positive at a title of 1:80 with speckled pattern. Anti-platelet antibodies were not assessed. HCV-RNA level decreased rapidly and became undetectable after 2 wk of treatment (-5.4 log UI/mL HCV-RNA in 2 wk). After completing the 12-wk course of triple therapy, the patient continued Peg-INFα-2a and RBV for other 36 wk.
During treatment, the patient developed severe anemia without clinical signs of blood loss that required two blood transfusions and then therapy with erythropoietin 40000 U/wk for 14 wk.
At the end of the treatment, laboratory values showed: HCV RNA not detectable, normal ALT and AST, hemoglobin 11 g/dL, platelets 115.000/mmc, white blood 2.560/mmc, gamma-globulins 1280 mg/dL.
Three weeks after treatment withdrawal, an episode of gingival bleeding occurred; laboratory finding showed severe thrombocytopenia (1.000/microl) with normal white and red blood cells count; liver and kidney function and coagulation tests were normal. Anti-platelets auto-antibodies, both immunoglobulin M (IgM) and IgG, were found positive, while direct Coomb’s test and irregular antibodies against erythrocytes were negative. Anti-nuclear antibodies were still positive with a speckled pattern. Antibodies anti-Helicobacter pylori were negative.
Clinical and laboratory findings were consistent with the diagnosis of AITP; therefore, intravenous methyl-prednisolone 60 mg/d was started for one week, followed by 30 mg/d of oral prednisone, gradually tapered for three months. No further bleeding and a gradual increase in platelet count were observed; after one week of treatment, platelet count was 40.000/microl. After eight weeks, platelet count was within normal range (211.000/microl), anti-platelet as well as antinuclear antibodies became negative and HCV-RNA was persistently undetectable (the patient achieved a sustained virological response).
This is the second case of AITP occurred after Peg-IFN and RBV treatment completion: generally, this side effect is observed in course of interferon treatment. Thrombocytopenia is usually defined as a platelet count less than 100000/microl. Severe thrombocytopenia (< 50000/microl) is associated with increased bleeding risk during invasive procedures, while spontaneous and even severe bleeding could be observed in patients with platelet count less than 1000/microl.
Thrombocytopenia is a frequently observed in patients with haematological disorders, with HCV infection, with hypersplenism associated to liver cirrhosis or related to drug assumption.
Drug-induced thrombocytopenia develop through two main mechanisms: (1) bone marrow toxicity (i.e., cytotoxic drugs) resulting in reduced production of all blood cells (red cells, white cells and platelets); and (2) increased destruction of normal platelets (both immune-mediated or not)[28].
The incidence of drug induced AITP in the general population is approximately 10 cases per million inhabitants per year, and its pathogenesis is not completely understood yet; however, IgG-type antibodies against platelet glycoprotein (GP) IIb/IIIa, GP Ia/IIa, and/or GP Ib/IX seem to play an important role[29].
In literature, we found several cases of PegIFN-induced autoimmune cytopenias; among those, severe life-threatening AITP, although rare, is a well-documented and recognized adverse event.
To our knowledge, 24 cases of AITP are related to interferon-based treatment for HCV; among those, only one occurred after treatment discontinuation[16] (Table 1).
| Ref. | Sex | Age | HCV genotype | Antiviral therapy | Occurrence of AITP | Clinical outcome |
| de Manuel Moreno et al[5] | F | 46 | 1b | Peg-IFNα-2b + RBV | 12 wk | Corticosteroids responsive |
| Kim et al[6] | F | 72 | NR | Peg-IFNα-2a | 120 wk | Corticosteroids responsive |
| Li et al[26] | F | 54 | 1b | Peg-IFNα-2a + RBV | 12 wk | CR |
| Elefsiniotis et al[16] | M | 27 | NR | Peg-IFNα-2b + RBV | 24 wk after therapy discontinuation | CR |
| Huang et al[7] | F | 48 | 2 | Peg-IFNα-2a + RBV | 1 wk | Corticosteroids responsive |
| Naz et al[8] | F | 60 | NR | Peg-IFNα-2b + RBV | 7 wk | CR (treated with ursodeoxycholic acid) |
| Enomoto et al[9] | F | 69 | 1b | Peg-IFNα-2b + RBV | 12 wk | Corticosteroids responsive |
| Carnero-Fernández et al[10] | M | 20 | NR | Peg-IFNα-2b + RBV | 20 wk | CR (treated with iv immunoglobulin ) |
| Alves Couto et al[11] | M | 44 | NR | Peg-IFNα-2b + RBV | 16 wk | CR |
| Weitz et al[12] | F | 43 | 1b | Peg-INF + RBV | 48 wk | CR (treated with Rituximab) |
| Lambotte et al[24] | F | 73 | 1b | Peg-IFNα-2a + RBV | 8 wk | CR |
| Nakajima et al[23] | M | 47 | 1b | IFNα-2a + RBV | 8 wk | Incomplete response |
| Medeiros et al[13] | M | 40 | NR | IFN-α + RBV (for 24 wk) and Peg-IFNα-2a + RBV | 36 wk | CR |
| Dimitroulopoulos et al[22] | F | 20 | 3 | IFNα | 28 wk | CR |
| Sevastianos et al[14] | F | 38 | 4 | Peg-IFNα-2b | 4 wk | CR |
| Fujii et al[21] | F | 24 | NR | IFNα | 4 wk | CR |
| Sagir et al[15] | M | 45 | NR | Peg-IFNα-2b + RBV | 10 wk | CR |
| Pockros et al[20] | M | 61 | 1b | IFNα | 16 wk | CR |
| Jiménez-Sáenz et al[19] | M | 46 | NR | IFNα-2b | 144 wk | CR |
| Tappero et al[18] | F | NR | NR | IFNα-2a | 8 wk | CR |
| Dourakis et al[25] | M | 39 | NR | IFNα | 32 wk | CR |
| Dourakis et al[25] | F | 64 | NR | IFNα | 24 wk | CR |
| Shrestha et al[17] | M | 41 | NR | IFNα | NR | CR |
| Demirturk et al[27] | NR | NR | NR | Peg-IFN + RBV | NR | CR |
In the reported case, the patient experienced an episode of severe thrombocytopenia three weeks after antiviral therapy completion, despite negative HCV-RNA (the patient achieved a sustained virological response): the concomitant presence of spontaneous bleeding, anti-platelets antibodies positivity, the exclusion of all other possible causes (i.e., viral and Helicobacter pylori infection, haematological or autoimmune disorders and other concomitant therapies) led to the diagnosis of Peg-IFN-induced AITP.
The relationship between AITP and discontinuation of the drug, in our case, could be explained by the long half-life of Peg-IFNα-2a (T1/2 = approximately 160 h); since five plasma half-lives are necessary to eliminate about 99% of administered drug, 33 d are necessary for a 99% clearance of Peg-IFNα-2a[30].
Peg-IFN-induced autoimmune diseases may appear even after treatment withdrawal; however, we encourage the investigation all other causes of autoimmunity, especially when a tight time-correlation with the event is absent.
Based on the latest guidelines on the management of AITP[31], we prescribed a first-line long course of corticosteroids (e.g., prednisone 1 mg/kg orally for 21 d then tapered off); in this case, the patient presented a rapid improvement in platelet count after first-line treatment (Figure 1); therefore the administration of IV immunoglobulin was not necessary. In fact, immunoglobulin infusion should be prescribed, together with corticosteroids, when a rapid increase in platelet count is clinically required[30]. In non-responsive cases, rituximab may be an option. Platelets infusion should be considered only in cases of severe thrombocytopenia associated with active bleeding. In cases of AITP survival time of infused platelets is reduced to a few hours. However, this practice can help to control acute bleeding[32].
Literature review shows a higher incidence of AITP in patients treated with Peg-IFNα-2b compared to those treated with α-2a; however, published data are insufficient to assume a greater immunogenicity of Peg-IFNα-2b over Peg-IFNα-2a[30].
In conclusion, physicians prescribing pegylated interferon should be aware that acute AITP can occur both during and after treatment. Consequently, it seems logical to us that anti-platelet antibodies dosage should be determined in all patients presenting with thrombocytopenia both during treatment and after discontinuation. A deep knowledge and prompt recognition of interferon-related adverse events is even more important with the availability of interferon-sparing treatment regimens.
Gingival spontaneous bleeding was the first finding in this patient; severe thrombocytopenia coupled with positive anti-platelet antibodies led to the diagnosis.
All other causes of autoimmune thrombocytopenia (AITP) had been evaluated; moreover, the not-tight time-correlation required an accurate evaluation of possible hematological or autoimmune disorders, assumption of concomitant drugs.
Severe thrombocytopenia (1000/mmc) and positive anti-platelet antibodies suggested the diagnosis of AITP.
Treatment with intravenous corticosteroids led to prompt total platelet count increase within one week; after 8 wk of treatment, normal platelet count and negative anti-platelet antibodies were observed.
All related cases of interferon-related AITP were reviewed and summarized in Table 1.
IFN: Interferon; Peg-IFN: Pegylated-IFN; AITP: Autoimmune thrombocytopenia; RBV: Ribavirin.
AITP can occur even after interferon withdrawal; physicians must be aware of this unusual clinical manifestation and consider interferon-related AITP also in this setting.
Authors report a rare case of AITP in a very particular population.
P- Reviewer: Imashuku S, Lesmana CRA, Redondo PC, Zhu X S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Durand JM. Extrahepatic manifestations of hepatitis C virus infection. Am J Gastroenterol. 1997;92:1402. [PubMed] |
| 2. | Hernández F, Blanquer A, Linares M, López A, Tarín F, Cerveró A. Autoimmune thrombocytopenia associated with hepatitis C virus infection. Acta Haematol. 1998;99:217-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Raanani P, Ben-Bassat I. Immune-mediated complications during interferon therapy in hematological patients. Acta Haematol. 2002;107:133-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 89] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Peck-Radosavljevic M, Zacherl J, Meng YG, Pidlich J, Lipinski E, Längle F, Steininger R, Mühlbacher F, Gangl A. Is inadequate thrombopoietin production a major cause of thrombocytopenia in cirrhosis of the liver? J Hepatol. 1997;27:127-131. [PubMed] |
| 5. | de Manuel Moreno J, Costero Pastor B, Sandoval Martínez A, Villafruela Cives M, Bejarano Redondo L, del Pozo Prieto D, Becerro Pedreño I, Borrego Rodríguez G, Poves Martínez E. [Idiopathic thrombocytopenic purpura induced by PEG interferon]. Rev Esp Enferm Dig. 2010;102:400-401. [PubMed] |
| 6. | Kim SR, Imoto S, Kudo M, Nakajima T, Ando K, Mita K, Fukuda K, Hong HS, Lee YH, Nakashima K. Autoimmune thrombocytopenic purpura during pegylated interferon α treatment for chronic hepatitis C. Intern Med. 2010;49:1119-1122. [PubMed] |
| 7. | Huang HH, Lin CF, Shih YL, Chang WK, Hsieh TY. Rapid development of severe thrombocytopenia in a female with chronic hepatitis C after single-dose pegylated interferon therapy. Dig Liver Dis. 2010;42:233-234. [PubMed] |
| 8. | Naz H, Aslan V. Treatment of autoimmune thrombocytopenia in a case of chronic hepatitis C with ursodeoxycholic acid. J Infect Dev Ctries. 2009;3:644-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Enomoto M, Yamane T, Hino M, Ohnishi M, Tamori A, Kawada N. Platelet-associated IgG for the diagnosis of immune thrombocytopaenic purpura during peginterferon alpha and ribavirin treatment for chronic hepatitis C. Liver Int. 2008;28:1314-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Carnero-Fernández M, Pineda JR, Lite-Alvarez JM. [Severe thrombocytopenia with probable autoimmune mechanism associated with interferon therapy in a patient with chronic hepatitis C]. Gastroenterol Hepatol. 2006;29:297-298. [PubMed] |
| 11. | Alves Couto C, Costa Faria L, Dias Ribeiro D, de Paula Farah K, de Melo Couto OF, de Abreu Ferrari TC. Life-threatening thrombocytopenia and nephrotic syndrome due to focal segmental glomerulosclerosis associated with pegylated interferon alpha-2b and ribavirin treatment for hepatitis C. Liver Int. 2006;26:1294-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Weitz IC. Treatment of immune thrombocytopenia associated with interferon therapy of hepatitis C with the anti-CD20 monoclonal antibody, rituximab. Am J Hematol. 2005;78:138-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Medeiros BC, Seligman PA, Everson GT, Forman LM. Possible autoimmune thrombocytopenia associated with pegylated interferon-alpha2a plus ribavarin treatment for hepatitis C. J Clin Gastroenterol. 2004;38:84-86. [PubMed] |
| 14. | Sevastianos VA, Deutsch M, Dourakis SP, Manesis EK. Pegylated interferon-2b-associated autoimmune thrombocytopenia in a patient with chronic hepatitis C. Am J Gastroenterol. 2003;98:706-707. [PubMed] |
| 15. | Sagir A, Wettstein M, Heintges T, Häussinger D. Autoimmune thrombocytopenia induced by PEG-IFN-alpha2b plus ribavirin in hepatitis C. Dig Dis Sci. 2002;47:562-563. [PubMed] |
| 16. | Elefsiniotis IS, Pantazis KD, Fotos NV, Moulakakis A, Mavrogiannis C. Late onset autoimmune thrombocytopenia associated with pegylated interferon-alpha-2b plus ribavirin treatment for chronic hepatitis C. J Gastroenterol Hepatol. 2006;21:622-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Shrestha R, McKinley C, Bilir BM, Everson GT. Possible idiopathic thrombocytopenic purpura associated with natural alpha interferon therapy for chronic hepatitis C infection. Am J Gastroenterol. 1995;90:1146-1147. [PubMed] |
| 18. | Tappero G, Negro F, Farina M, Gallo M, Angeli A, Hadengue A. Safe switch to beta-interferon treatment of chronic hepatitis C after alpha-interferon-induced autoimmune thrombocytopenia. J Hepatol. 1996;25:270. [PubMed] |
| 19. | Jiménez-Sáenz M, Rojas M, Piñar A, Salas E, Rebollo J, Carmona I, Herrerías-Esteban JM, Herrerías-Gutiérrez JM. Sustained response to combination therapy in a patient with chronic hepatitis C and thrombocytopenia secondary to alpha-interferon. J Gastroenterol Hepatol. 2000;15:567-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Pockros PJ, Duchini A, McMillan R, Nyberg LM, McHutchison J, Viernes E. Immune thrombocytopenic purpura in patients with chronic hepatitis C virus infection. Am J Gastroenterol. 2002;97:2040-2045. [PubMed] |
| 21. | Fujii H, Kitada T, Yamada T, Sakaguchi H, Seki S, Hino M. Life-threatening severe immune thrombocytopenia during alpha-interferon therapy for chronic hepatitis C. Hepatogastroenterology. 2003;50:841-842. [PubMed] |
| 22. | Dimitroulopoulos D, Dourakis SP, Xinopoulos D, Tsamakidis K, Paraskevas E. Immune thrombocytopenic purpura in a patient treated with interferon alfacon-1. J Viral Hepat. 2004;11:477-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Nakajima H, Takagi H, Yamazaki Y, Toyoda M, Takezawa J, Nagamine T, Mori M. Immune thrombocytopenic purpura in patients with hepatitis C virus infection. Hepatogastroenterology. 2005;52:1197-1200. [PubMed] |
| 24. | Lambotte O, Gelu-Simeon M, Maigne G, Kotb R, Buffet C, Delfraissy JF, Goujard C. Pegylated interferon alpha-2a-associated life-threatening Evans’ syndrome in a patient with chronic hepatitis C. J Infect. 2005;51:e113-e115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Dourakis SP, Deutsch M, Hadziyannis SJ. Immune thrombocytopenia and alpha-interferon therapy. J Hepatol. 1996;25:972-975. [PubMed] |
| 26. | Li L, Han DK, Lu J. Interferon-alpha induced severe thrombocytopenia: a case report and review of the literature. World J Gastroenterol. 2010;16:1414-1417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 27. | Demirturk N, Cevik F, Demirdal T, Aykin N, Aslan V. Autoimmune thrombocytopenia induced by PEG-IFN-alpha plus ribavirin in hepatitis C. Platelets. 2006;17:340-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | van den Bemt PM, Meyboom RH, Egberts AC. Drug-induced immune thrombocytopenia. Drug Saf. 2004;27:1243-1252. [PubMed] |
| 29. | McCarthy LJ, Dzik W. Immune thrombocytopenic purpura. N Engl J Med. 2002;347:449-450; author reply 449-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Foster GR. Pegylated interferons for the treatment of chronic hepatitis C: pharmacological and clinical differences between peginterferon-alpha-2a and peginterferon-alpha-2b. Drugs. 2010;70:147-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 31. | Neunert C, Lim W, Crowther M, Cohen A, Solberg L Jr, Crowther MA. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117:4190-4207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1255] [Cited by in RCA: 1296] [Article Influence: 92.6] [Reference Citation Analysis (0)] |
| 32. | Wazny LD, Ariano RE. Evaluation and management of drug-induced thrombocytopenia in the acutely ill patient. Pharmacotherapy. 2000;20:292-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |