Published online Jun 28, 2015. doi: 10.4254/wjh.v7.i12.1652
Peer-review started: March 16, 2015
First decision: April 27, 2015
Revised: May 5, 2015
Accepted: June 4, 2015
Article in press: June 8, 2015
Published online: June 28, 2015
Processing time: 107 Days and 19.2 Hours
Metformin, a biguanide derivative, is the most commonly prescribed medication in the treatment of type 2 diabetes mellitus. More recently, the use of metformin has shown potential as a preventive and therapeutic agent for a broad spectrum of conditions, including liver disease and hepatic malignancies. In this systematic review, we critically analyze the literature behind the potential use of metformin across the spectrum of liver disease and malignancies. The PubMed and Ovid MEDLINE databases were searched from 2000 to March 2015, using a combination of relevant text words and MeSH terms: metformin and mammalian target of rapamycin, hepatitis B virus (HBV), hepatitis B virus (HCV), non-alcoholic fatty liver disease (NAFLD), hepatocellular carcinoma (HCC) or cholangiocarcinoma. The search results were evaluated for pertinence to the issue of metformin in liver disease as well as for quality of study design. Metformin has a number of biochemical effects that would suggest a benefit in treating chronic liver diseases, particularly in the context of insulin resistance and inflammation. However, the literature thus far does not support any independent therapeutic role in NAFLD or HCV. Nonetheless, there is Level III evidence for a chemopreventive role in patients with diabetes and chronic liver disease, with decreased incidence of HCC and cholangiocarcinoma. The use of metformin seems to be safe in patients with cirrhosis, and provides a survival benefit. Once hepatic malignancies are already established, metformin does not offer any therapeutic potential. In conclusion, there is insufficient evidence to recommend use of metformin in the adjunctive treatment of chronic liver diseases, including NAFLD and HCV. However, there is good evidence for a chemopreventive role against HCC among patients with diabetes and chronic liver disease, and metformin should be continued in patients even with cirrhosis to provide this benefit.
Core tip: There has recently been a growing literature on the use of metformin as a potential preventive and therapeutic agent for various chronic liver diseases and hepatic malignancies. We therefore decided to review the efficacy of metformin across the spectrum of liver disease and malignancies. Based on our systematic review, there is insufficient evidence to recommend use of metformin in the adjunctive treatment of non-alcoholic fatty liver disease and hepatitis C. However, there is good evidence for a chemopreventive role against hepatocellular carcinoma among patients with diabetes and chronic liver disease, and metformin should be continued in patients even with cirrhosis to provide this benefit.
- Citation: Bhat A, Sebastiani G, Bhat M. Systematic review: Preventive and therapeutic applications of metformin in liver disease. World J Hepatol 2015; 7(12): 1652-1659
- URL: https://www.wjgnet.com/1948-5182/full/v7/i12/1652.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i12.1652
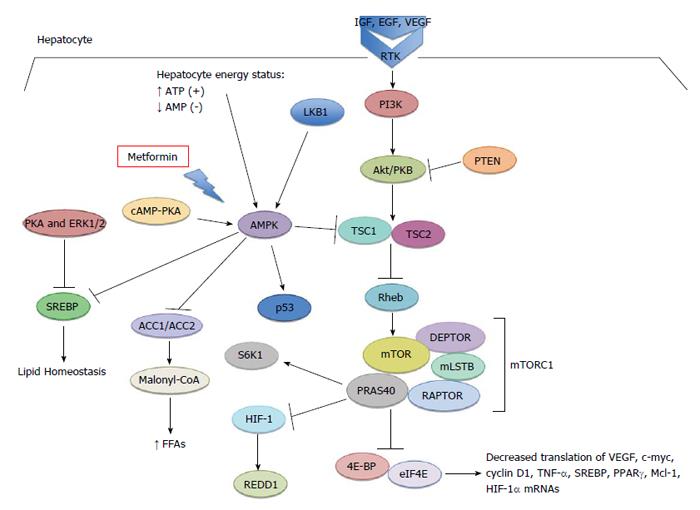
Metformin as an oral hypoglycemic medication has been typically prescribed for type 2 diabetes and insulin resistance in polycystic ovarian disease. Its hypoglycemic action occurs by virtue of its ability to inhibit both gluconeogenesis and glycogenolysis in hepatocytes[1] (Figure 1). It also indirectly downregulates circulating insulin and insulin growth factor-1, by virtue of decreasing serum glucose[2-4]. Additionally, metformin binds reversibly to complex I of the mitochondrial electron transport chain in hepatocytes, giving rise to cellular stress[1,5]. This inhibition leads to an increase in adenosine monophosphate (AMP) generation, with a concomitant decrease in adenosine triphosphate (ATP) production. Increasing levels of AMP bind to AMP kinase (AMPK), a key regulator of cellular metabolism in both normal and cancer cells, resulting in a conformational change and activation[1,6,7]. Cellular stress, such as nutrient deprivation and hypoxic conditions, also increase AMP levels[7]. Once activated by AMP, the conformational change facilitates liver kinase B1 (LKB1) phosphorylation of AMPK, which in turn phosphorylates Tsc1/2. This leads to negative regulation of the mammalian target of rapamycin (mTOR) pathway[8]. Metformin improves insulin resistance through activation of AMPK, which subsequently blocks hepatic glucose release and promotes glucose uptake in skeletal muscle[9]. It also likely uses various mechanisms to restore insulin sensitivity by limiting lipid storage through the inhibition of free fatty acid formation; AMPK suppression of acetyl-CoA carboxylase 1 (ACC1), ACC2, and HMG-CoA reductase decreases fatty acid synthase expression and activates malonyl-CoA carboxylase[3,10,11]. Moreover, AMPK suppresses fatty acid synthesis through the inhibition of transcription factor SREBP-1c. This transcription factor is induced by glucose and insulin excess, and is therefore inappropriately elevated in patients with non-alcoholic fatty liver disease (NAFLD)[12,13]. Interestingly, metformin has also been shown to modulate adipokine synthesis (cytokines that have action on adipose tissue). Adipokines such as tumor necrosis factor-α and interleukin-6 can directly stimulate AMPK, thereby preventing hepatic fat accumulation through an increase in β-oxidation of free fatty acids (FFAs) and/or by decreasing de novo synthesis[14]. Zhang et al[15] have shown that metformin exerts antagonistic effects on catecholamine-induced lipolysis via decrease in cAMP production.
Recent years have seen investigation of its use in a variety of conditions, such as NAFLD, in addition to potential use as a chemopreventive and chemotherapeutic agent. The exploration into the use of metformin in chemoprevention began in 2005, with Evans et al[16] demonstrating that metformin significantly reduced the risk of cancer development in diabetic patients. The findings of this large cohort study demonstrated a 23% reduction in overall cancer incidence among diabetic patients treated with metformin as compared to those treated with sulfonylurea derivatives[4]. The discovery gave rise to a number of studies focusing on the ability of metformin to lower the risk of cancer in long-term users of the medication. A prospective cohort study by Libby et al[17] demonstrated that 7.3% of patients taking metformin had a diagnosis of cancer, as compared to 11.6% of a control population. After adjusting for confounding variables, patients on metformin still had a significantly decreased risk of cancer, with a hazard ratio of 0.63 (95%CI: 0.53-0.75). A second prospective cohort study by Bowker et al[18] similarly showed that the use of metformin reduced cancer-related mortality as compared to sulfonylureas or insulin in diabetic patients, with an adjusted hazard ratio of 0.80 (95%CI: 0.65-0.98). These studies fuelled further investigation into chemopreventive use in hepatic malignancies, as well as applications to target insulin resistance in chronic liver disease. The biochemical basis of these effects, along with the literature behind potential applications in hepatology, are systematically detailed in this review.
We systematically searched PubMed and Ovid MEDLINE databases from 2000 to March 2015, using a combination of metformin with the following relevant text words and MeSH terms: hepatitis B virus (HBV), HCV, NAFLD, hepatocellular carcinoma (HCC) or cholangiocarcinoma. The search results were evaluated for pertinence to the issue of metformin in liver disease as well as for quality of study design, and we used material written in English. The reference lists from all identified studies were searched for further relevant papers. Review articles were used as a reference, but not as primary sources of information.
Although the pathogenesis of NAFLD is not clearly understood, it is known that insulin resistance assumes a pivotal role. Importantly, NAFLD is associated with an increased risk of type 2 diabetes mellitus and cardiovascular disease[19]. In insulin-resistant patients, the increased influx of FFAs into the liver due to peripheral lipolysis leads to hepatic steatosis. Insulin resistance is said to increase hepatic lipogenesis through activation of the lipogenic transcription factor sterol-regulator element binding protein-1 (SREBP1). The state of hyperinsulinemia upregulates glycogenolysis, leading to increased fatty acid synthesis in the hepatocytes[20]. The “two-hit” hypothesis of non-alcoholic steatohepatitis (NASH) pathophysiology proposes a synergistic effect of “first-hit” obesity and diet, followed by the “second-hit” of inflammation and cellular injury. The “second-hit” is thought to contribute to the insulin resistance by releasing cytokines and free fatty acids, and increasing intracellular oxidative stress[20,21].
Current management of patients with NAFLD principally involves weight loss through diet and exercise[13]. Vitamin E is recommended as first line pharmacotherapy in non-diabetic adults with biopsy-proven NASH but not in diabetic patients due to lack of ad hoc data[22]. Hence, there is no pharmacotherapy as yet for diabetic NASH patients. There have been several pharmacotherapeutic attempts to target the insulin resistance thought to be the underlying pathophysiologic mechanism of NAFLD[23]. In a 6-mo, prospective, randomized study (level II) that compared low-dose metformin (1 g/d) with diet to diet alone, both groups achieved a significant reduction in the proportion of patients with ultrasonographic evidence of hepatic steatosis[24]. Although metabolic parameters were significantly better in the metformin group, this study illustrated that diet in itself could ameliorate hepatic steatosis. Improvement in liver histology of NAFLD patients on metformin treatment has been documented (level IV - open-label, single arm)[25-27]. In a pilot study, 26 NASH patients were treated with 48 wk of metformin 2000 mg a day[26]. This resulted in a histological response and improvement in alanine aminotransferase levels in only 30% of the patients, and correlated with weight loss rather than with improvement in insulin sensitivity. Histological improvement was limited to hepatocellular injury and parenchymal inflammation. Along with histological improvement, there was a significant decrease in the aminotransferase levels observed[26]. Another uncontrolled trial of metformin (850-1000 mg/d) with N-acetylcysteine (1.2 g/d) for 12 mo demonstrated significant improvement in steatosis and fibrosis, although lobular inflammation and hepatocellular ballooning remained unchanged. Aminotransferase levels also were not significantly different following the treatment course[27]. In three randomized trials (level II), metformin treatment had very little effect on liver histology, but it did ameliorate the liver aminotransferases and insulin resistance[28-30]. A 12-mo, randomized, placebo-controlled trial of diet, exercise and metformin vs diet and exercise only in 19 non-diabetic patients with insulin resistance and NASH failed to show any improvement in histology and liver enzyme levels[28]. It was rather weight loss in itself through diet and exercise that correlated with improved liver histology, aminotransferases and insulin resistance. Another randomized trial (not placebo-controlled) of metformin (850 mg twice daily) and dietary treatment vs dietary treatment alone in 36 patients showed histological improvement in both groups[29]. The metformin group did nonetheless have significantly decreased insulin resistance and aminotransferases. An additional randomized, placebo-controlled trial of metformin in 48 patients with biopsy-proven NAFLD failed to show any histological improvement as compared to placebo[30].
In the pediatric context as well, metformin has failed to show any histological benefit. In a small observational pilot study of 57 overweight or obese children between ages of 9 to 18 years with biopsy-proven NAFLD or NASH for 24 mo, metformin was no more efficacious than lifestyle modifications in improving serum transaminases, hepatic steatosis or liver histology in patients (level IV - open label trial)[31]. A meta-analysis by Mazza et al[13] concluded that the addition of metformin may still be an attractive option to patients who have prediabetes or diabetes, due to clear evidence of improvement in insulin resistance associated with NAFLD. However, metformin has not demonstrated concrete improvement in liver histology in randomized, controlled studies, and therefore cannot be recommended for treatment of NASH[22].
There have been conflicting reports as to whether chronic HBV infection is correlated with insulin resistance[32,33]. Insulin resistance in patients with HBV is more likely concordant with their individual metabolic profiles. There are therefore no clinical studies of metformin on patients with HBV infection. Nonetheless, an in vitro study demonstrated that metformin transcriptionally downregulated hepatitis B surface antigen expression and HBV replication when used on a human hepatoma cell line. Additionally, it was found to act synergistically with antiviral effects of Lamivudine and interferon (IFN) alpha-2b[34]. Based on in vitro studies, metformin may have potential benefit for patients with HBV, however clinical trials are needed to further explore this therapy.
It is well established that HCV infection can induce a state of insulin resistance, ultimately leading to hepatic steatosis. It is hypothesized that HCV utilizes host cell glucose or lipid metabolism in order to complete its own life cycle, giving rise to the high prevalence of diabetes mellitus in patients with chronic HCV[35]. Evidence indicates that there is an association between patient metabolic profiles and the severity of hepatic fibrosis in HCV patients[36]. One of the key metabolic factors is insulin resistance, known to aggravate hepatic steatosis, which promotes liver fibrosis progression and increases the risk of HCC. It is also associated with high HCV viral load and poor virologic response to interferon treatment[37]. As oral hypoglycemic agents are the treatment of choice for insulin resistance, Romero-Gómez et al[37] hypothesized that metformin would aid in improved responses to peg-IFN (PEG-IFN) alfa-2a plus ribavirin (RBV) treatment in patients with naïve genotype 1 CHC patients. Although addition of metformin to peginterferon and RBV improved insulin sensitivity in this randomized, placebo-controlled trial of 123 patients, the results failed to show a significant difference in sustained virological response (SVR) between treatment and control groups[37]. Though the aforementioned results were not as promising as anticipated, additional trials were conducted to study whether metformin could help improve HCV treatment outcomes by correcting insulin resistance. A randomized, double-blind controlled trial of metformin vs placebo in addition to PEG-IFN and RBV treatment showed that SVR was no different between the 2 groups (75% vs 79%)[38]. In a small, randomized controlled trial by Hsu et al[35], various oral hypoglycemic agents, including metformin, were combined with the standard IFN-based therapy in patients with HCV genotype 1 and insulin resistance. Although the study was too small to derive definite conclusions, the data suggested that the addition of an oral hypoglycemic agent to PEG-IFN alfa-2a plus RBV achieved a better SVR (level II - randomized, not placebo-controlled). Although new oral interferon-free regimens are rapidly changing the therapeutic landscape of HCV treatment, these findings suggest metformin may play a role in improving HCV treatment response specifically in the subgroup of patients with insulin resistance. Furthermore, metformin has an impact on the prognosis of HCV-induced liver cirrhosis, as shown by a reduction in the incidence of HCC and liver-related death or transplantation (level II)[39].
The PI3K/Akt/mTOR pathway is often activated in malignancies, and phosphorylates downstream signaling effector molecules involved in cell cycle progression, protein synthesis, cell growth, and angiogenesis[4]. Metformin is known to inhibit the mTOR pathway at least partly through an LKB1-AMPK-dependent mechanism, as illustrated by the lack of its effects in LKB1-deficient mice[7]. The effects of metformin on cell survival and metabolism can be explained both by this LKB1-AMPK-dependent mechanism as well as its insulin-lowering effects. Insulin has mitogenic and pro-survival effects on cells, and tumor cells often express insulin receptors at higher levels, making them highly sensitive to insulin stimulation[40]. Ultimately, metformin results in inhibition of the mTOR pathway and downstream effectors. These downstream effectors include eIF4E, which is normally bound to the 4E-BPs (eIF4E-binding proteins). When phosphorylated by mTORC1, the 4E-BPs detach from eIF4E, which is then free to complex with other proteins to initiate translation. The translation of mRNAs coding for proteins involved in processes pertinent to tumorigenesis, such as the cell cycle, angiogenesis, and apoptosis, is particularly favored[41]. Other effectors of mTORC1 are cyclin D1 (cell cycle regulation), p70S6K => phospho-S6 (ribosome biogenesis), and SREBP (lipid synthesis), which all contribute to fuelling tumorigenesis.
Specific effects of metformin on the hallmarks of cancer as defined by Weinberg and Hanahan have been elucidated[42]. In tumor development, the growth of the cell mass quickly exceeds its supply of nutrients and oxygen. Rapidly growing tumors encounter hypoxic conditions that hinder their ability to grow. However, cancer cells are able to circumvent these metabolic limitations. Despite conditions of cellular stress, there may be insufficient activity of AMPK, enabling the mTOR pathway-activated and uncontrolled cell growth. This makes metformin an attractive chemopreventive agent as it is an AMPK activator[8]. Angiogenesis is essential in the growth and invasive properties of tumor cells. Studies have shown that metformin negatively regulates hypoxia-inducible factor-1α, tumor necrosis factor-α, plasminogen activator inhibitor-1, and von Willebrand Factor, which decreases the levels of vascular endothelial growth factor (VEGF) and ultimately angiogenesis[8].
Metformin plays a role in induction of apoptosis through either p53-dependent or independent mechanisms. The tumor suppressor p53 is involved in DNA damage repair and cell cycle regulation. Ultimately, the activation of p53 induces apoptosis of cells under low nutrient conditions[2,8]. Tumor suppressor p53 regulates synthesis of cytochrome c oxidase 2 (SCO2) activity, allowing the cell to efficiently couple mitochondrial oxidative phosphorylation. Hence p53-defective cells, as is seen in over 50% of tumors, have a decrease in SCO2 activity and a deregulation of cell metabolism in a hypoxic environment. The inability of these cells to conserve energy when exposed to metformin-induced energetic crisis ultimately leads to apoptosis[1,5]. Metformin blocks the cell cycle partly through decreased levels of cyclin D1 expression, with a dose-dependent inhibition of cell proliferation[8]. Hence, metformin inhibits all of these processes that are key to tumorigenesis.
HCC is one of the leading causes of cancer-related deaths[43] and its incidence is on the rise in North America particularly due to the increasing burden of HCV cirrhosis[44]. Most HCC tumors are diagnosed at an advanced stage, when curative therapy is no longer an option. The only chemotherapy with survival benefit, though minimal, is the Ras-Raf kinase inhibitor sorafenib. The mTOR pathway is upregulated in up to 50% of HCCs. The mTOR pathway has also been found to play an essential role specifically in hepatocarcinogenesis arising in the context of NASH[45].
In vitro data have shown metformin to be a potent inhibitor of HepG2 and Huh7 liver cancer cell proliferation[7,46]. An apoptotic effect has also been noted through increased expression of cleaved caspase-3 and a significantly increased percentage of early apoptotic cells[44]. Cell cycle arrest in G0/G1 phase in several HCC cell lines has also been found in vitro, correlating with a strong decrease in expression of G1 cyclins, specifically cyclin D1, cyclin E and cyclin-dependent kinase 4[47]. In vivo studies have shown that metformin also exerts effects on apoptosis, cell cycle, and proliferation, likely through the mTOR pathway. In an in vivo study on HepG2 cells xenografted into nude mice, Xiong et al[44] demonstrated that metformin treatment at 200-mg/kg per day dose led to a 40.8% reduction in tumor volume. In this study as well, metformin was shown to suppress the protein synthesis machinery downstream of mTOR, inhibit cell proliferation and induce apoptosis. In a diethylnitrosamine-induced HCC mouse model, metformin was shown to down-regulate lipogenesis through decreased expression of lipogenic enzymes[48]. In addition, the restoration of these lipogenic enzymes through ectopic expression rescued the metformin-mediated growth inhibition. These findings provide an interesting application of metformin in patients with HCC in the context of disorders where there is upregulation of lipid synthesis such as NAFLD[48].
Population data have suggested a role for metformin as a chemopreventive agent against HCC among patients with diabetes[49-56] and chronic liver disease[57,58]. In a meta-analysis of 8 observational studies, including 22650 cases of HCC in 334307 patients with type 2 diabetes, it was found that metformin given to diabetic patients resulted in a 50% risk reduction in HCC incidence (OR = 0.50, 95%CI: 0.34-0.73)[53,54]. A similar meta-analysis of 7 studies (3 cohort, 4 case-control, with 562 HCC cases out of 16549 patients) reported an even further reduced risk of HCC in diabetic patients on metformin vs those using other hypoglycemic agents (OR = 0.24, 95%CI: 0.13-0.46)[55]. Although these data are striking, it should be kept in mind that the studies used in these meta-analysis were observational, and higher quality randomized trials would be optimal to consolidate whether metformin has a chemopreventive benefit.
In already-diagnosed HCC, the clinical literature is sparse. A retrospective clinical study determined that in patients with already-established HCC[59], though duration of exposure to metformin prior to diagnosis was not available. Combination therapy of metformin with radiofrequency ablation (RFA) has also been attempted prospectively. In a prospective case cohort study, diabetic patients with early stage HCC were treated with RFA concurrently with metformin[60]. The use of metformin as a chemotherapeutic adjunct in these patients was observed to have a lower mortality rate as compared to the untreated diabetic patients with early stage HCC. Furthermore, patients with early stage HCC receiving sulfonylureas and insulin exposures did not achieve the same effects as metformin (level III - prospective case cohort study)[60].
Physicians are often hesitant to use metformin in cirrhotic patients, given previous reports of lactic acidosis. A recent study has disproven this concern, revealing that cirrhotic patients may safely take metformin[61]. Additionally, use of metformin significantly extended survival in cirrhotic patients, with continuation decreasing risk of death by 57%. Hence, using metformin as a chemopreventive agent against HCC is a reasonable option in patients with chronic liver disease. However, it is unclear at this time whether metformin is beneficial as an adjunct in the treatment of HCC.
Cholangiocarcinoma is associated with a dismal prognosis, serving as an impetus to study its molecular basis and thereby develop therapeutic avenues. McKay et al[62] performed an array of comparative genomic hybridization to discover any potential therapeutic targets. The DNA extracted from 32 cholangiocarcinoma tumors was discovered to have copy number gains in several genes along the mTOR pathway[62]. Frequent gain of function mutations in genes related to the mTOR pathway, including mTOR, VEGF receptor, platelet-derived growth factor, and epidermal growth factor receptor, were identified making this a novel target for treatment options[41,62]. In a case-control study, it was established that type 2 diabetes mellitus independently predicts risk of intrahepatic cholangiocarcinoma (ICC), along with primary sclerosing cholangitis, cirrhosis, and smoking[63]. Diabetic patients with metformin had a significantly decreased odds ratio for ICC as compared to diabetics not on metformin (OR = 0.4; 95%CI: 0.2-0.9; P = 0.04). An in vitro study demonstrated a dose and time-dependent antiproliferative action of metformin through apoptosis induction and cell cycle arrest via the AMPK-mTORC1 pathway in ICC cell lines[64]. Additionally, metformin was found to enhance the sensitivity of ICC cells to sorafenib, 5-fluorouracil and As2O3. Hence, one might anticipate at least a chemopreventive role for metformin in ICC, although it would be interesting to determine whether such a role exists in prevention of perihilar and extrahepatic cholangiocarcinoma.
In conclusion, metformin is a widely used hypoglycemic agent with benefits beyond the management of diabetes. Given its effects on insulin resistance, there are data suggestive of benefit in HCV and NAFLD. However, at this time, there is insufficient evidence to recommend use of metformin in the adjunctive treatment of these chronic liver diseases. Nonetheless, by virtue of its inhibitory effect on the mTOR pathway, there is good evidence for a chemopreventive role against HCC among patients with diabetes and chronic liver disease, and metformin should be continued in patients even with cirrhosis to provide this benefit.
P- Reviewer: Chuang WL, Czaja MJ, Han T, Koch TR, Narciso-Schiavon JL S- Editor: Tian YL L- Editor: A E- Editor: Liu SQ
| 1. | Buzzai M, Jones RG, Amaravadi RK, Lum JJ, DeBerardinis RJ, Zhao F, Viollet B, Thompson CB. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 2007;67:6745-6752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 673] [Cited by in RCA: 723] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 2. | Li D. Metformin as an antitumor agent in cancer prevention and treatment. J Diabetes. 2011;3:320-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Kohjima M, Higuchi N, Kato M, Kotoh K, Yoshimoto T, Fujino T, Yada M, Yada R, Harada N, Enjoji M. SREBP-1c, regulated by the insulin and AMPK signaling pathways, plays a role in nonalcoholic fatty liver disease. Int J Mol Med. 2008;21:507-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Pawałowska M, Markowska A. The influence of metformin in the etiology of selected cancers. Contemp Oncol (Pozn). 2012;16:223-229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Pollak M. Potential applications for biguanides in oncology. J Clin Invest. 2013;123:3693-3700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 156] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 6. | Rosilio C, Ben-Sahra I, Bost F, Peyron JF. Metformin: a metabolic disruptor and anti-diabetic drug to target human leukemia. Cancer Lett. 2014;346:188-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 7. | Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9:563-575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1555] [Cited by in RCA: 1524] [Article Influence: 95.3] [Reference Citation Analysis (0)] |
| 8. | Jalving M, Gietema JA, Lefrandt JD, de Jong S, Reyners AK, Gans RO, de Vries EG. Metformin: taking away the candy for cancer? Eur J Cancer. 2010;46:2369-2380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 300] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 9. | Aljada A, Mousa SA. Metformin and neoplasia: implications and indications. Pharmacol Ther. 2012;133:108-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 10. | Fullerton MD, Galic S, Marcinko K, Sikkema S, Pulinilkunnil T, Chen ZP, O’Neill HM, Ford RJ, Palanivel R, O’Brien M. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat Med. 2013;19:1649-1654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 629] [Cited by in RCA: 669] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 11. | Shaw RJ. Metformin trims fats to restore insulin sensitivity. Nat Med. 2013;19:1570-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Li Y, Xu S, Mihaylova MM, Zheng B, Hou X, Jiang B, Park O, Luo Z, Lefai E, Shyy JY. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011;13:376-388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1426] [Cited by in RCA: 1365] [Article Influence: 97.5] [Reference Citation Analysis (0)] |
| 13. | Mazza A, Fruci B, Garinis GA, Giuliano S, Malaguarnera R, Belfiore A. The role of metformin in the management of NAFLD. Exp Diabetes Res. 2012;2012:716404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 14. | Huypens P, Quartier E, Pipeleers D, Van de Casteele M. Metformin reduces adiponectin protein expression and release in 3T3-L1 adipocytes involving activation of AMP activated protein kinase. Eur J Pharmacol. 2005;518:90-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Zhang S, Liu X, Brickman WJ, Christoffel KK, Zimmerman D, Tsai HJ, Wang G, Wang B, Li Z, Tang G. Association of plasma leptin concentrations with adiposity measurements in rural Chinese adolescents. J Clin Endocrinol Metab. 2009;94:3497-3504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1692] [Cited by in RCA: 1822] [Article Influence: 91.1] [Reference Citation Analysis (0)] |
| 17. | Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care. 2009;32:1620-1625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 839] [Cited by in RCA: 809] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 18. | Bowker SL, Yasui Y, Veugelers P, Johnson JA. Glucose-lowering agents and cancer mortality rates in type 2 diabetes: assessing effects of time-varying exposure. Diabetologia. 2010;53:1631-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 128] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 19. | Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, Natale S, Vanni E, Villanova N, Melchionda N. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1907] [Cited by in RCA: 1917] [Article Influence: 87.1] [Reference Citation Analysis (0)] |
| 20. | James OF, Day CP. Non-alcoholic steatohepatitis (NASH): a disease of emerging identity and importance. J Hepatol. 1998;29:495-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 266] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 21. | Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2953] [Cited by in RCA: 3129] [Article Influence: 115.9] [Reference Citation Analysis (36)] |
| 22. | Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1226] [Cited by in RCA: 1357] [Article Influence: 104.4] [Reference Citation Analysis (4)] |
| 23. | Doycheva I, Loomba R. Effect of metformin on ballooning degeneration in nonalcoholic steatohepatitis (NASH): when to use metformin in nonalcoholic fatty liver disease (NAFLD). Adv Ther. 2014;31:30-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 24. | Garinis GA, Fruci B, Mazza A, De Siena M, Abenavoli S, Gulletta E, Ventura V, Greco M, Abenavoli L, Belfiore A. Metformin versus dietary treatment in nonalcoholic hepatic steatosis: a randomized study. Int J Obes (Lond). 2010;34:1255-1264. [PubMed] [DOI] [Full Text] |
| 25. | Nair S, Diehl AM, Wiseman M, Farr GH, Perrillo RP. Metformin in the treatment of non-alcoholic steatohepatitis: a pilot open label trial. Aliment Pharmacol Ther. 2004;20:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 249] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 26. | Loomba R, Lutchman G, Kleiner DE, Ricks M, Feld JJ, Borg BB, Modi A, Nagabhyru P, Sumner AE, Liang TJ. Clinical trial: pilot study of metformin for the treatment of non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2009;29:172-182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 232] [Cited by in RCA: 222] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 27. | de Oliveira CP, Stefano JT, de Siqueira ER, Silva LS, de Campos Mazo DF, Lima VM, Furuya CK, Mello ES, Souza FG, Rabello F. Combination of N-acetylcysteine and metformin improves histological steatosis and fibrosis in patients with non-alcoholic steatohepatitis. Hepatol Res. 2008;38:159-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Shields WW, Thompson KE, Grice GA, Harrison SA, Coyle WJ. The Effect of Metformin and Standard Therapy versus Standard Therapy alone in Nondiabetic Patients with Insulin Resistance and Nonalcoholic Steatohepatitis (NASH): A Pilot Trial. Therap Adv Gastroenterol. 2009;2:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 29. | Uygun A, Kadayifci A, Isik AT, Ozgurtas T, Deveci S, Tuzun A, Yesilova Z, Gulsen M, Dagalp K. Metformin in the treatment of patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2004;19:537-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 276] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 30. | Haukeland JW, Konopski Z, Eggesbø HB, von Volkmann HL, Raschpichler G, Bjøro K, Haaland T, Løberg EM, Birkeland K. Metformin in patients with non-alcoholic fatty liver disease: a randomized, controlled trial. Scand J Gastroenterol. 2009;44:853-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 248] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 31. | Nobili V, Manco M, Ciampalini P, Alisi A, Devito R, Bugianesi E, Marcellini M, Marchesini G. Metformin use in children with nonalcoholic fatty liver disease: an open-label, 24-month, observational pilot study. Clin Ther. 2008;30:1168-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 32. | Kumar M, Choudhury A, Manglik N, Hissar S, Rastogi A, Sakhuja P, Sarin SK. Insulin resistance in chronic hepatitis B virus infection. Am J Gastroenterol. 2009;104:76-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Lee JG, Lee S, Kim YJ, Cho BM, Park JS, Kim HH, Cheong J, Jeong DW, Lee YH, Cho YH. Association of chronic viral hepatitis B with insulin resistance. World J Gastroenterol. 2012;18:6120-6126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 34. | Xun YH, Zhang YJ, Pan QC, Mao RC, Qin YL, Liu HY, Zhang YM, Yu YS, Tang ZH, Lu MJ. Metformin inhibits hepatitis B virus protein production and replication in human hepatoma cells. J Viral Hepat. 2014;21:597-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 35. | Hsu CS, Hsu SJ, Lin HH, Tseng TC, Wang CC, Chen DS, Kao JH. A pilot study of add-on oral hypoglycemic agents in treatment-naïve genotype-1 chronic hepatitis C patients receiving peginterferon alfa-2b plus ribavirin. J Formos Med Assoc. 2014;113:716-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 36. | Hwang SJ, Lee SD. Hepatic steatosis and hepatitis C: Still unhappy bedfellows? J Gastroenterol Hepatol. 2011;26 Suppl 1:96-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 37. | Romero-Gómez M, Diago M, Andrade RJ, Calleja JL, Salmerón J, Fernández-Rodríguez CM, Solà R, García-Samaniego J, Herrerías JM, De la Mata M. Treatment of insulin resistance with metformin in naïve genotype 1 chronic hepatitis C patients receiving peginterferon alfa-2a plus ribavirin. Hepatology. 2009;50:1702-1708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 120] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 38. | Sharifi AH, Mohammadi M, Fakharzadeh E, Zamini H, Zaer-Rezaee H, Jabbari H, Merat S. Efficacy of adding metformin to pegylated interferon and ribavirin in treatment naïve patients with chronic hepatitis C: a randomized double-blind controlled trial. Middle East J Dig Dis. 2014;6:13-17. [PubMed] |
| 39. | Nkontchou G, Cosson E, Aout M, Mahmoudi A, Bourcier V, Charif I, Ganne-Carrie N, Grando-Lemaire V, Vicaut E, Trinchet JC. Impact of metformin on the prognosis of cirrhosis induced by viral hepatitis C in diabetic patients. J Clin Endocrinol Metab. 2011;96:2601-2608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 40. | Dowling RJ, Goodwin PJ, Stambolic V. Understanding the benefit of metformin use in cancer treatment. BMC Medicine. 2011;9:33. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 257] [Cited by in RCA: 279] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 41. | Bhat M, Sonenberg N, Gores GJ. The mTOR pathway in hepatic malignancies. Hepatology. 2013;58:810-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 42. | Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19834] [Cited by in RCA: 19513] [Article Influence: 780.5] [Reference Citation Analysis (0)] |
| 43. | Cheng J, Huang T, Li Y, Guo Y, Zhu Y, Wang Q, Tan X, Chen W, Zhang Y, Cheng W. AMP-activated protein kinase suppresses the in vitro and in vivo proliferation of hepatocellular carcinoma. PLoS One. 2014;9:e93256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 44. | Xiong Y, Lu QJ, Zhao J, Wu GY. Metformin inhibits growth of hepatocellular carcinoma cells by inducing apoptosis via mitochondrion-mediated pathway. Asian Pac J Cancer Prev. 2012;13:3275-3279. [PubMed] |
| 45. | Villanueva A, Chiang DY, Newell P, Peix J, Thung S, Alsinet C, Tovar V, Roayaie S, Minguez B, Sole M. Pivotal role of mTOR signaling in hepatocellular carcinoma. Gastroenterology. 2008;135:1972-1983, 1983.e1-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 564] [Cited by in RCA: 591] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 46. | Qu Z, Zhang Y, Liao M, Chen Y, Zhao J, Pan Y. In vitro and in vivo antitumoral action of metformin on hepatocellular carcinoma. Hepatol Res. 2012;42:922-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 47. | Miyoshi H, Kato K, Iwama H, Maeda E, Sakamoto T, Fujita K, Toyota Y, Tani J, Nomura T, Mimura S. Effect of the anti-diabetic drug metformin in hepatocellular carcinoma in vitro and in vivo. Int J Oncol. 2014;45:322-332. [PubMed] |
| 48. | Bhalla K, Hwang BJ, Dewi RE, Twaddel W, Goloubeva OG, Wong KK, Saxena NK, Biswal S, Girnun GD. Metformin prevents liver tumorigenesis by inhibiting pathways driving hepatic lipogenesis. Cancer Prev Res (Phila). 2012;5:544-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 49. | Chen HP, Shieh JJ, Chang CC, Chen TT, Lin JT, Wu MS, Lin JH, Wu CY. Metformin decreases hepatocellular carcinoma risk in a dose-dependent manner: population-based and in vitro studies. Gut. 2013;62:606-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 307] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 50. | Donadon V, Balbi M, Ghersetti M, Grazioli S, Perciaccante A, Della Valentina G, Gardenal R, Dal Mas M, Casarin P, Zanette G. Antidiabetic therapy and increased risk of hepatocellular carcinoma in chronic liver disease. World J Gastroenterol. 2009;15:2506-2511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 109] [Cited by in RCA: 118] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 51. | Lee MS, Hsu CC, Wahlqvist ML, Tsai HN, Chang YH, Huang YC. Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: a representative population prospective cohort study of 800,000 individuals. BMC Cancer. 2011;11:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 314] [Cited by in RCA: 366] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 52. | Hassan MM, Curley SA, Li D, Kaseb A, Davila M, Abdalla EK, Javle M, Moghazy DM, Lozano RD, Abbruzzese JL. Association of diabetes duration and diabetes treatment with the risk of hepatocellular carcinoma. Cancer. 2010;116:1938-1946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 241] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 53. | Singh S, Singh PP, Singh AG, Murad MH, Sanchez W. Anti-diabetic medications and the risk of hepatocellular cancer: a systematic review and meta-analysis. Am J Gastroenterol. 2013;108:881-891; quiz 892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 235] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 54. | Zhang H, Gao C, Fang L, Zhao HC, Yao SK. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients: a meta-analysis. Scand J Gastroenterol. 2013;48:78-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 55. | Lai SW, Chen PC, Liao KF, Muo CH, Lin CC, Sung FC. Risk of hepatocellular carcinoma in diabetic patients and risk reduction associated with anti-diabetic therapy: a population-based cohort study. Am J Gastroenterol. 2012;107:46-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 252] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 56. | Donadon V, Balbi M, Valent F, Avogaro A. Glycated hemoglobin and antidiabetic strategies as risk factors for hepatocellular carcinoma. World J Gastroenterol. 2010;16:3025-3032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 57. | Donadon V, Balbi M, Mas MD, Casarin P, Zanette G. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients with chronic liver disease. Liver Int. 2010;30:750-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 223] [Article Influence: 14.9] [Reference Citation Analysis (1)] |
| 58. | Chen CI, Kuan CF, Fang YA, Liu SH, Liu JC, Wu LL, Chang CJ, Yang HC, Hwang J, Miser JS. Cancer risk in HBV patients with statin and metformin use: a population-based cohort study. Medicine (Baltimore). 2015;94:e462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 59. | Bhat M, Chaiteerakij R, Harmsen WS, Schleck CD, Yang JD, Giama NH, Therneau TM, Gores GJ, Roberts LR. Metformin does not improve survival in patients with hepatocellular carcinoma. World J Gastroenterol. 2014;20:15750-15755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 60. | Chen TM, Lin CC, Huang PT, Wen CF. Metformin associated with lower mortality in diabetic patients with early stage hepatocellular carcinoma after radiofrequency ablation. J Gastroenterol Hepatol. 2011;26:858-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 61. | Zhang X, Harmsen WS, Mettler TA, Kim WR, Roberts RO, Therneau TM, Roberts LR, Chaiteerakij R. Continuation of metformin use after a diagnosis of cirrhosis significantly improves survival of patients with diabetes. Hepatology. 2014;60:2008-2016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 142] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 62. | McKay SC, Unger K, Pericleous S, Stamp G, Thomas G, Hutchins RR, Spalding DR. Array comparative genomic hybridization identifies novel potential therapeutic targets in cholangiocarcinoma. HPB (Oxford). 2011;13:309-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 63. | Chaiteerakij R, Yang JD, Harmsen WS, Slettedahl SW, Mettler TA, Fredericksen ZS, Kim WR, Gores GJ, Roberts RO, Olson JE. Risk factors for intrahepatic cholangiocarcinoma: association between metformin use and reduced cancer risk. Hepatology. 2013;57:648-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 117] [Article Influence: 9.8] [Reference Citation Analysis (1)] |
| 64. | Ling S, Feng T, Ke Q, Fan N, Li L, Li Z, Dong C, Wang C, Xu F, Li Y. Metformin inhibits proliferation and enhances chemosensitivity of intrahepatic cholangiocarcinoma cell lines. Oncol Rep. 2014;31:2611-2618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |