Published online Jun 18, 2015. doi: 10.4254/wjh.v7.i11.1509
Peer-review started: October 3, 2014
First decision: November 14, 2014
Revised: March 2, 2015
Accepted: March 18, 2015
Article in press: March 20, 2015
Published online: June 18, 2015
Processing time: 258 Days and 8.5 Hours
In 1953, the pioneer of human orthotopic liver transplantation (LT), Thomas E Starzl, was the first to attempt an orthotopic liver transplant into a 3 years old patient suffering from biliary atresia. Thus, the first LT in humans was attempted in a disease, which, up until today, remains the main indication for pediatric LT (pLT). During the last sixty years, refinements in diagnostics and surgical technique, the introduction of new immunosuppressive medications and improvements in perioperative pediatric care have established LT as routine procedure for childhood acute and chronic liver failure as well as inherited liver diseases. In contrast to adult recipients, pLT differs greatly in indications for LT, allocation practice, surgical technique, immunosuppression and post-operative life-long aftercare. Many aspects are focus of ongoing preclinical and clinical research. The present review gives an overview of current developments and the clinical outcome of pLT, with a focus on alternatives to full-size deceased-donor organ transplantation.
Core tip: As of today, pediatric liver transplantation (pLT) has become a safe and routine procedure for the treatment of childhood acute and chronic liver failure as well as inherited liver diseases. In contrast to adult recipients, pLT differs greatly in indications for LT, allocation practice, surgical technique, immunosuppression and post-operative life-long aftercare. Long-term survival after pLT implies life-long aftercare in an interdisciplinary team. The present review gives an insight into current indications for pLT, outcome after living-donor and deceased-donor organ transplantation and of ongoing clinical and preclinical developments to improve long-term outcome.
- Citation: Hackl C, Schlitt HJ, Melter M, Knoppke B, Loss M. Current developments in pediatric liver transplantation. World J Hepatol 2015; 7(11): 1509-1520
- URL: https://www.wjgnet.com/1948-5182/full/v7/i11/1509.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i11.1509
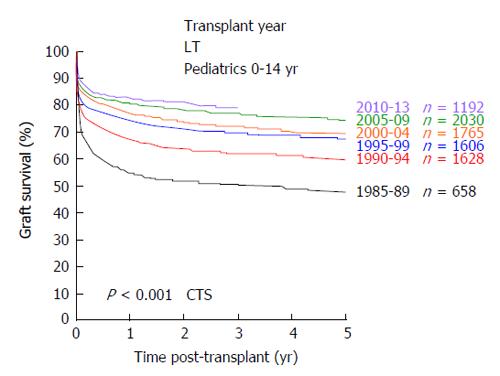
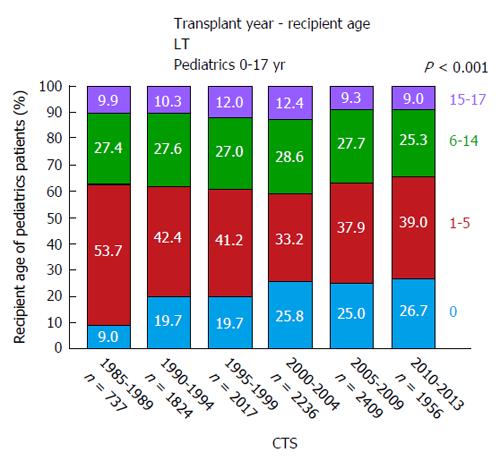
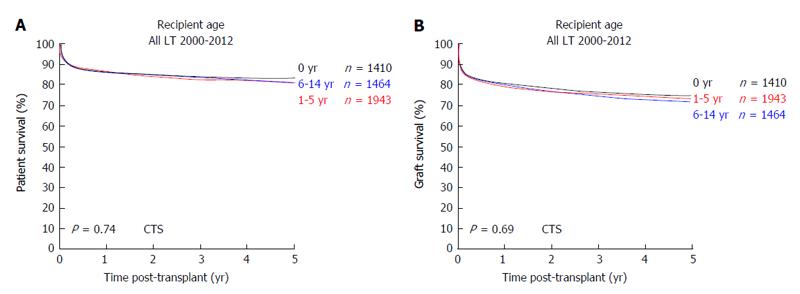
In 1953, the pioneer of human orthotopic liver transplantation (LT), Thomas E Starzl, was the first to attempt an orthotopic liver transplant into a 3 years old patient suffering from biliary atresia[1]. LT is the only curative treatment option for patients with irrevocable acute or chronic liver failure and, in the last six decades, has developed from an experimental approach with very high mortality to an almost routine procedure with good short and long-term survival rates. In the early years, long-term survival rates after pediatric LT (pLT) were 11%-39%[2-4] and, since then, have improved to up to 90% with long-term graft survival rates of > 80% (Figure 1)[5,6]. Due to continuing improvements of surgical and interventional techniques as well as perioperative neonatal and pediatric intensive care medicine, the average age of pediatric transplant recipients has steadily declined, with a continuous increase of patients transplanted within the first year of life. As of today, approximately 27% of pLT are performed in recipients younger than 12 mo (Figure 2). Patients in this young age, which in former years could not be transplanted (and mostly died before reaching the size and age of transplantability), today show a long-term survival of almost 90%, which is comparable to older children (Figure 3). At the same time, long-term survival after pLT implies life-long aftercare in an interdisciplinary team to ensure a life with as little as possible secondary morbidity. The present review gives an insight into current indications for pLT, outcome after living-donor and deceased-donor organ transplantation and of ongoing clinical and preclinical developments to improve long-term outcome after pLT.
Indications for LT in pediatric patients are manifold and can be classified into cholestatic disorders, metabolic liver diseases causing liver cirrhosis, metabolic liver diseases without liver cirrhosis, acute liver failure, acute and chronic hepatitis, and liver tumors (Table 1). With approximately 40%, the main indication for pLT is biliary atresia. Thus, the indications for pLT are significantly different to indications in adult LT recipients.
| Cholestatic disorders | Extrahepatic biliary atresia |
| Intrahepatic biliary hypoplasia (Alagille disease, other) | |
| Progressive familial intrahepatic cholestasis | |
| Sclerosing cholangitis (primary, neonatal, secondary) | |
| Nutritive-toxic cirrhosis | |
| Caroli disease | |
| Cholangiodysplasia | |
| Congenital liver fibrosis | |
| Langerhans cell histiocytosis | |
| Acute liver failure | |
| Metabolic, with cirrhosis | Alpha 1-antitrypsin deficiency |
| Wilson's disease | |
| Tyrosinemia | |
| Galactosemia | |
| Neonatal hemochromatosis | |
| Cystic fibrosis | |
| Glycogenosis type IV | |
| Metabolic bile acid dysfunction | |
| Niemann-Pick's disease | |
| Gaucher's disease | |
| Metabolic, without cirrhosis | Hyperoxaluria |
| Crigler-Najjar syndrome | |
| Urea cycle disorders | |
| Familial hypercholesteremia type IIA | |
| Glycogenosis type IA | |
| Hemophilia type A, type B | |
| Protein C deficiency | |
| Wolman's disease | |
| Organic acidemia | |
| Hepatitis | Hepatitis B |
| Hepatitis C | |
| Hepatitis non-ABC | |
| Autoimmune hepatitis | |
| Neonatal hepatitis | |
| Liver tumors | Hepatoblastoma |
| Hepatocellular carcinoma | |
| Fibrolamellar carcinoma | |
| Hemangioendothelioma | |
| Various | Budd-Chiari syndrome |
| Cryptogenic liver cirrhosis | |
| Infantile copper overload |
In former years, pLT was only performed in curative intent. Today, pLT is also performed, if life expectancy and/or quality of life can be significantly improved. In patients diagnosed with metabolic liver diseases not resulting in liver cirrhosis, the indication for LT has to be carefully evaluated. LT should be performed if the disease can either be cured or extrahepatic manifestations can be significantly improved. A contraindication in this setting would be advanced stage of irreversible extrahepatic manifestations.
In patients with chronic liver disease, listing for LT should be performed in case of (1) reduced liver synthesis function (e.g., decreased cholinesterase, decreased factor V); (2) portal hypertension with or without gastrointestinal bleeding, severe hypersplenism, and/or refractory ascites; (3) failure to thrive despite adequate nutritional therapy; (4) recurrent cholangitis; (5) development of hepatorenal/hepatopulmonary syndrome; (6) recurrent or persistent hepatic encephalopathy; (7) significantly reduced quality of life; and/or (8) early in metabolic liver diseases resulting in life-threatening conditions[7]. Pre- and perioperative morbidity and nutritional status significantly correlate with long-term survival, morbidity as well as physical and cognitive function after pLT[8-13]. Therefore, accurately timed listing and meticulous pediatric management before and after pLT is crucial for long-term outcome.
In pediatric patients with acute liver failure, listing criteria, as in adults, focus more on acute metabolic and synthetic liver function, including the following criteria: hepatic encephalopathy (> grade 2), factor V < 20% without adequate increase after sufficient substitution, hyperbilirubinemia (> 17.5 mg/dL), phosphate level above upper reference and/or significant renal failure[7].
Due to shortage of deceased-donor organs, different allocation solutions are intensively discussed and permanently adapted. In adult LT, a model for the sickest first policy, the Model of End Stage Liver Disease (MELD), was implemented in the allocation procedure within the United Network for Organ Sharing (UNOS) in 2002 and within the Eurotransplant (ET) network in 2007[14,15]. The MELD allocation system is not applicable to all patient groups, especially not to those who have progressive liver disease but no significant impairment of liver or renal function (e.g., patients with liver tumor, some metabolic and/or inherited diseases as well as patients with primary sclerosing cholangitis). For these patients a special allocation system by an exceptional MELD (eMELD) calculation has been implemented[16].
Center based allocation is in use especially in countries with few transplant centers, e.g., in Australia, United Kingdom, and Austria. Moreover, it is used in parallel to the MELD system for extended criteria donor organs. The advantage of the center-based allocation is that the physicians can match the organ to the patient and therefore enable transplantation in patients not well represented by the MELD allocation system. Yet, this system is prone to a more subjective decision making when allocating an organ and must be assessed critically.
Due to special characteristics in infants and children, especially concerning the inability to develop high serum creatinine values as a marker of severe liver and overall disease, the MELD allocation system can not be applied for this patient group[17,18]. Therefore a special liver allocation system for patients younger than 12 years of age was developed within the UNOS network, not including creatinine as a major component. The so called Pediatric Model for End Stage Liver Disease (PELD) is calculated from serum albumin, bilirubin, INR, age at listing and failure to thrive (based on height, weight and gender) and was implemented for pediatric liver allocation within the UNOS network in 2002[18-20]. Based on multivariate analyses of the Studies of Pediatric LT (SPLIT) database, the PELD score predicts the probability of death or hospitalization to the intensive care unit within 3 mo of listing for LT[19].
When the MELD system was introduced in the ET network in 2007, allocation via PELD was not implemented for pediatric liver transplant patients. Alternatively, the so-called matchMELD was introduced, a system comparable to the eMELD granted to defined subgroups of adult recipients not adequately represented by the MELD system. The initial matchMELD at the time of listing is set at a calculated 3-mo-mortality of 35% for children younger than 12 years of age and 15% for children aged 12 to 16 years. Every three months (90 d), the matchMELD increases according to a calculated increase in 3-mo-mortality of 15% (children < 12 years) or 10% (children aged 12 to 16 years). Furthermore, organs derived from small adults or pediatric donors (< 46 kg body weight) are allocated with priority to pediatric recipients[21,22]. High urgency allocation is generally only possible in case of acute liver failure or for re-transplantation due to graft impairment within 14 d of transplantation.
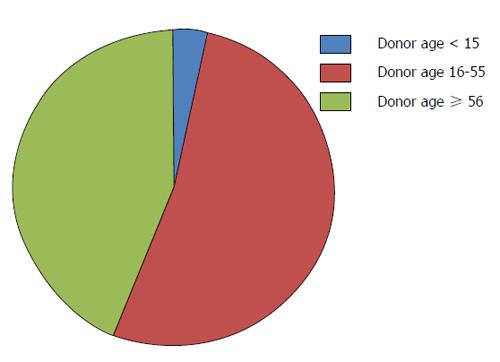
However, due to a significant mismatch in available pediatric donor organs compared to organs needed for pLT (Figure 4), alternative techniques to increase the donor pool must be applied. Here, living-donor-LT (LDLT) is of particular interest in pLT. In many East-Asian countries deceased-donor liver transplant (DDLT) is rarely performed due to religious and other reasons, which has led to a broad establishment of LDLT in these countries[23-25] and might serve as an example for Western countries to expand the donor pool especially in pLT.
Before the technique of liver splitting was established, pediatric patients were dependent on donors with similar age or size. In the early 1980’s, Christoph Broelsch and Henri Bismuth were the first applying the technique of reduced-size LT in children[26,27]. In 1988, Rudolf Pichlmayr performed the first split LT offering one cadaveric liver to two recipients[28]. However, pediatric deceased donors as well as organs suitable for split-LT remain rare. Figure 4 demonstrates the age of deceased liver donors within the ET network in 2013. Numbers of pLT performed significantly exceed the number of available pediatric organ donors[21].
The technique of full size LT in children is equivalent to adult LT (piggy back or conventional technique). Partial liver grafts can be obtained either by splitting a cadaveric donor organ or by living-donor liver donation. For liver splitting, the anatomical determination of the eight liver segments first described by Couinaud[29,30] in 1957 is essential. Two standard splitting procedures exist: the anatomical splitting (dividing the liver at Cantlie’s line) and splitting along the falciform ligament[31]. Splitting of the left lateral segment is technically easier to perform than the true right/left lobe split procedure. Furthermore, the left lateral segment is the smallest part of the liver compared to the extended right, the anatomical left or the right liver lobe and is preferentially used in pLT. In small infants, even the left lateral segment of the liver often is too large and techniques to cut down left lateral lobes may be used to prevent graft-size mismatching and the so-called “large-for-size” syndrome[32]. Due to size mismatch (large graft in small recipient), primary closure of the abdominal wall after pLT is often not possible and should not be enforced in order to prevent compromising graft perfusion by external pressure. In these cases, abdominal wall closure is performed in stages during the first week post-transplant after continuous recovery of the graft from reperfusion injury and edema or accomplished by using mesh grafts[33].
A special surgical technique is auxilliary LT [auxilliary partial orthotopic LT (APOLT)] with implantation of a partial graft without fully removing the native liver. Gubernatis et al[34] reported the first successful case in a patient with acute liver failure. She recovered, her native liver regenerated and immunosuppressive treatment could be withdrawn[34]. APOLT can be successfully performed in children with acute fulminant liver failure or in children with metabolic liver diseases without primary hepatocellular dysfunction or cirrhosis[7,35]. The rationale to perform APOLT in patients with metabolic diseases is to provide sufficient liver mass containing the missing enzyme to correct metabolic function. In case of graft failure, the patient’s native liver is still present to secure general liver function. Furthermore these patients preserve the option for later genetic therapy if this can be provided to correct metabolic function in the future[35]. If APOLT is performed in acute fulminant liver failure, e.g., due to severe hepatic necrosis (viral/toxic), the immunosuppressive therapy can be ceased in case the native liver recovers, resulting in an atrophy of the transplanted liver[36]. Yet it must be mentioned that APOLT is technically highly demanding and associated with a higher rate of complications.
Complementary to splitting organs obtained from donors after brain death, organ donation after circulatory death (DCD) has been shown to increase the organ donor pool. DCD can be performed either as “controlled donation”, i.e., planned withdrawal of medical support (ventilation, inotropic support) in the context of catastrophic illness[37], or as “uncontrolled donation” in patients with uncontrolled, out-of-hospital circulatory arrest. Although multiple ethical concerns are connected with donation after circulatory arrest[38,39], the World Health Organization encourages implementation of DCD worldwide[40]. DCD is currently performed in the United States, in 10 of 27 European nations, in Canada, Australia, Japan, China, the Far East and selected South American nations[41].
DCD LT after meticulous donor selection has reached outcomes only mildly inferior to LT after brain death[42], with increased rates of ischemic cholangiopathy and mildly reduced graft survival due to prolonged warm ischemia time[43-45]. Absolute numbers of LT performed after DCD are limited. Within UNOS, pLT after DCD has been performed in 45 cases, compared to 8120 pLT after brain death liver donations from 1996-2012. However, numbers are increasing with 12 transplanted livers (adult and pediatric recipients) from 70 recovered DCD donors in 1996 compared to 2789 transplanted livers from 8297 recovered DCD donors in 2012 within UNOS[41].
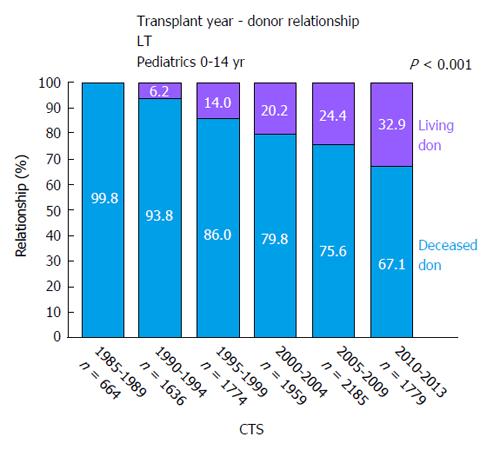
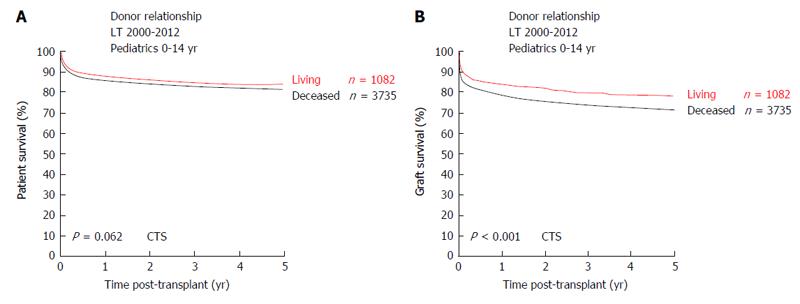
After successful implementation of split-liver LT in pLT, this technique lead to the first LDLT. In 1989, the first series of LDLT in pediatric recipients were performed in Chicago[46]. As of today, LDLT is an established procedure and the main form of LT due to scarcity of deceased donor organs in most East-Asian countries[23]. In western countries and especially in the UNOS area, use of living-donor organs for LT is less frequent and within UNOS constantly < 5% of LT over the last years[47]. Within the ET network, rates of LDLT in pLT are steadily increasing. Analyses of the collaborative transplant study (CTS) database show LDLT rates in pLT of 33% (Figure 5). Retrospective analyses have shown favorable or equal results as compared to pLT after DDLT[48-56]. CTS database analyses show a similar long-term patient survival of pLT after LDLT vs DDLT (5-year patient survival 83.7% after LDLT and 81% after DDLT, P = 0.062) (Figure 6A). However, long-term graft survival is significantly better after LDLT vs DDLT (5-year graft survival 78.2% in LDLT vs 71.4% in DDLT, P < 0.001) (Figure 6B). The advantages of LDLT are the use of an optimal healthy donor, minimal ischemic time, elective surgery and timing of transplantation according to the recipients’ need, which is particularly relevant for pediatric patients, as during a waiting time for pLT, the underlying disease can cause significant somatic and psycho-social long-term morbidity in the developing pediatric organism.
It has been shown that long-term-outcome after pLT significantly correlates with the severity of morbidity at pLT[11]. LDLT offers the possibility and advantage of optimal timing of the transplant procedure before severe morbidity develops. Therefore the main advantage of LDLT is the immediate organ availability for the patient in need. Recipients of living donor livers have a shorter waiting time than recipients of organs from deceased donors. Thus, waiting time mortality can be reduced. However, living donation is not without risk for the healthy donor and LDLT is surgically more demanding than whole organ transplantation. For the donor, major complications (exceeding Clavien grade II) were described in up to 44% after right-lobe LDLT and mortality risk was up to 0.8%[57-59]. Right lobe donors undergo operating procedures of longer duration, have significant longer hospital stay and require more blood transfusions[60,61]. However, for pLT, in most cases left-lobe liver donation is performed and the complication rates after full left lobe or left lateral lobectomy are significantly lower[62-64]. Overall biliary complications are one of the major concerns in LDLT donors. In order to decrease morbidity and mortality after liver donation, a thorough evaluation of the potential donor is essential to detect and exclude potential increased medical risk factors for the otherwise healthy donor. Furthermore, complications decrease as surgeon and center experience grows.
Analyses of 2192 pLT within UNOS between 1995 and 2006 (1832 DDLT, thereof 1183 whole organs, 261 split organs, 388 reduced size organs; 360 LDLT) showed, that only 33.9% of patients younger than 1 year of age received a full organ, with increasing numbers in older recipients (49.1% in patients 1-5 years of age; 65.3% in patients 5-12 years of age, 79.4% in patients older than 12 years[65]). Operating time, ischemia time and anhepatic time were significantly longer in reduced size or split organs, but with no clinically relevant significance.
Acute graft rejections are observed in 30%-50% during the first year after pLT, but become rare in the long-term outcome. In contrast to adult LT and to transplantation of other organs, acute rejections in pLT do not correlate with long-term outcome or long-term chronic rejection[7,11].
Analyses of the SPLIT database have shown a long-term patient survival after pLT of almost 90%[65], which is in line with CTS database analyses (Figure 3). Mortality in patients > 1 year after pLT is below 5%[66] and mainly caused by posttransplant lymphoproliferative disease (PTLD), recurrent malignancy, sepsis and multi-organ failure. Loss of graft function is observed in 20%-30% after pLT, with < 5% graft loss > 1 year after pLT[66]. In multivariate analyses, predictors of graft loss have been shown to be DDLT split graft, reduced size DDLT graft, fulminant liver failure as indication for pLT, donor age < 5 mo and prolonged warm ischemia time[65].
Main reasons for patient mortality are early postoperative complications, primary non-function and infections. Reasons for repeated surgical interventions after pLT are complications caused by anatomical-technical aspects. Overall rates of complications are observed in 45.1% after full organ pLT, vs 51.9% in LDLT pLT, vs 66.7% in DDLT split organ pLT. Repeated surgery within the first 3 mo after pLT is performed in 29.5% after full organ pLT vs 41.9% after LDLT pLT and 47.1% after DDLT split pLT. Biliary complications have been observed in 7.5% after full-organ DDLT pLT, which was significantly lower than after DDLT split organ pLT (18.8%) or LDLT pLT (17.5%)[65]. In overall vascular complications and arterial thrombosis, no significant difference was seen between full organ DDLT, split organ DDLT, and LDLT. Portal vein thrombosis has been shown to be significantly lower in full-organ pLT (3.6%) vs split DDLT (14.6%) or LDLT (11.1%). Although overall complications, biliary complications and portal vein thrombosis happen significantly more often after LDLT vs DDLT, there is no significant difference in 30-d post-LT mortality after full-organ pLT (3%) vs LDLT (3.6%), but significantly less in both techniques compared to DDLT split organ pLT (6.9%)[65].
Graft fibrosis has been described in 60% of patients at 10 years after pLT and has been shown to correlate significantly with (1) partial organ graft; (2) young age of recipient; (3) increased donor/recipient age mismatch; and (4) prolonged cold ischemia time[67]. Additionally, graft fibrosis seems to be associated with high de novo donor specific antibodies[67,68]. Comparing graft fibrosis after LDLT vs DDLT, no significant difference has been described.
Acute rejections are responsible for 10% of late organ losses[66]. Another 10% of late organ losses are caused by arterial thrombosis and biliary complications[7].
Chronic rejection, even if rare in absolute numbers, develops in 5%-10% of patients and is responsible for 30% of late graft failures[69]. Positive predictors for late graft failure are (1) pLT for malignant disease; (2) pLT for acute liver failure; (3) repeated surgery within the first 30 d after pLT (other than scheduled 2nd look surgery); (4) > 5 hospital admissions during the first year after pLT; and (5) steroid-resistant acute rejections[66].
In addition to direct transplant-related complications, long-term morbidity and quality of life is a main focus in ongoing research in pLT. Major long-term complications after pLT are reduction of kidney function (17%-32% of patients after pLT[70,71], arterial hypertension (15%-30% of patients after LT)[72,73] and development of secondary neoplasias, particularly PTLD (5%-10% of patients after LT)[74,75].
Kidney function can be reduced as a consequence of long-term immunosuppression, but may also be caused by the underlying disease (e.g., Alagille’s disease). Furthermore, long-term influence on kidney function of many chronic liver diseases before LT is unknown. Therefore development of kidney protective new immunosuppressive regiments (see below) and close post-pLT aftercare including translation of care into adulthood are crucial for long-term morbidity and quality of life.
PTLD is seen in up to 15% of patients after pLT and mortality rates of 30%, in single reports of up to 50% have been described[76,77]. Main risk-factors for the development of PTLD are Epstein-Barr virus-naïve recipients, high total immuosuppressive load and the intensity of active viral load[78]. In addition to optimal antiviral therapy, the choice of the immunosuppressive regimen can significantly influence the risk of PTLD and is an ongoing focus of preclinical and clinical research.
Of special importance in pediatric organ transplantation is the problem of achieving a successful transition into adult care. Medication nonadherence as one of the main problems has been described in 17%-53% adolescents after LT[79]. Nonadherence to medical regimens post transplantation increases rates of complications, graft rejection, health care utilization and mortality. Therefore targeting problems of nonadherence should be the main focus in strategies to improve the transition process[80].
Equal to patients after LT from a deceased donor, patients after living liver donation require immunosuppression to avoid immediate as well as long-term rejection of the transplanted organ. Therefore all patients, adults and children, are treated according to standardized immunosuppression protocols consisting of protocols for the early post-transplant period and protocols for long-term maintanance therapy.
As in adult LT, the introduction of calcineurin inhibitors (CNI) in the early 1980s gave way to long-term survival also for pediatric transplant recipients and until today remain the backbone of immunosuppression protocols[81,82]. The early post-transplant phase is the time of highest risk for immunologic reactions between graft and host and therefore the highest immunosuppression is required during this period. Most protocols comprise of induction therapy, dominated by interleukin-2 receptor antibodies especially in the pediatric transplant population (Basiliximab® and Daclizumab®), combined with corticosteroids and calcineurin inhibitors (cyclosporine A and tacrolimus) as maintenance therapy[83-88].
In contrast to adults, the use of other mono- or polyclonal antibodies [e.g., monoclonal anti-CD3 antibody preparations (OKT3) and rabbit or equine anti-thymocyte globulin] for induction therapy has not been adopted by the pediatric transplantation community because of concern of undesired short - and uncertain long-term effects of such potent drugs on the developing organism and immune system[89].
Over the past years many studies could show that an overall minimization of immunosuppression is possible, especially in pediatric liver transplant patients, which may be of significant advantage for long-term quality of life. Especially in pediatric recipients, it is of great concern to compose the immunosuppressant drugs according to the individual need to minimize long-term undesired side effects[90-93]. The main goal of drug minimization is reduction of negative side effects, especially on the growing organism, and avoiding long-term morbidity while preserving graft function. The most significant side-effects of different immunosuppressants are nephrotoxicity, diabetes, development of hypertension, hyperlipidemia, impairment of growth, neurologic alterations, hypertrichosis and bone marrow suppression. Yet, up to date we are missing appropriate tools to determine the optimal level of immunosuppression due to great differences between individuals as well as within the same individual over time.
Regarding these aspects and based on increasing data to safety aspects in the use of different immunosuppressant drugs in the adult population, multiple combination treatments, such as mycophenolate-mofetil and mammalian target of rapamycin inhibitors (Sirolimus and Everolimus), with and without CNIs have been introduced for maintenance therapy also in pediatric solid organ transplant patients and are topic of ongoing studies[94-101]. By this strategy the single immunosuppressive drugs may be decreased to levels that do not cause significant clinical side-effects but are sufficient to avoid rejection.
Up to date, life-long immunosuppression is suggested after solid organ transplantation, but more and more data is evolving that especially patients who are transplanted early in life or receive a parental living liver donation may develop a certain extent of immune tolerance towards the transplanted graft. Single center experiences in which patients were withdrawn from immunosuppression because of medical reasons (e.g., PTLD or renal insufficiency) or had self-withdrawn their medication due to non-compliance suggest that approximately 20% of liver transplant patients become operationally tolerant towards the graft[102-107]. Yet, up to date there are no reliable markers to determine, which patient has developed tolerance and which patient should remain on immunosuppressive drugs. Clinical experience shows that graft rejection may occur even years after weaning of immunosuppression and a focus of ongoing research is the definition of robust markers for distinguishing tolerant from non-tolerant liver transplant patients[107,108].
Another, more aggressive approach to induce immune tolerance in solid organ transplantation is to combine solid organ transplantation with hematopoietic stem cell transplantation from the same donor[109-111].
In our opinion future immunosuppressive strategies in pLT have to imply 3 main goals: (1) minimization as well as individualization of immunosuppression to reduce long-term negative side effects; (2) preservation of long-term allograft function; and (3) development of strategies to monitor and induce tolerance as well as differentiate between operationally tolerant and non-tolerant patients.
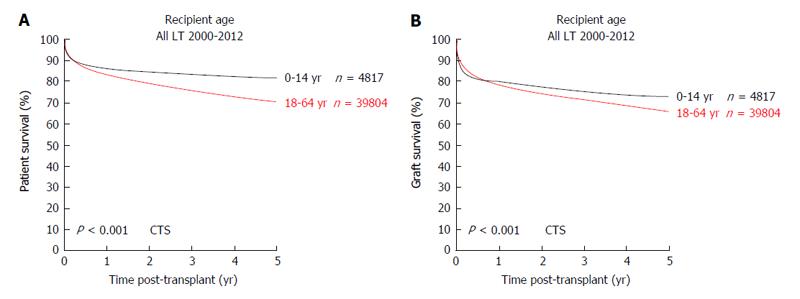
pLT is a routine and safe procedure to treat acute or chronic liver failure or selected metabolic liver diseases in children. Short and long-term survival are significantly better in pLT compared to LT in adult recipients (Figure 7) and patient survival curves plateau at 4 years after pLT. A main problem of pLT, especially within the ET network, is the scarcity of pediatric donor organs or organs suitable for splitting after DDLT. Here, LDLT is a valid solution and should further be promoted. In conspect with the comparable long-term patient survival after LDLT and increased graft survival after LDLT vs DDLT pLT, results discussed in this review on outcome after pLT lead us to the following conclusions: (1) In pediatric LT, LDLT is a safe procedure with long-term outcomes equal to or even better than DDLT; (2) In small infants, where full-organ LT is not an option due to donor/recipient size mismatch, LDLT enables LT in patients which in former times could not be transplanted due to the scarcity of deceased donor livers suitable for splitting; (3) LDLT enables pLT at a recipient-controlled time, when perioperative morbidity can be minimized and long-term negative effects of the underlying disease may be prevented; (4) Immunosuppression after LDLT can often be significantly reduced in pediatric recipients and further research in immunosuppressive therapies may in future minimize immunosuppression-related morbidity and PTLD and, in some cases, may induce immune tolerance; (5) Microsurgical techniques and interdisciplinary management of pLT recipient need to be further improved to reduce acute complications due to biliary or portal vein complications and to further increase long-term patient and graft survival; (6) In line with the latter argument, pLT should be exclusively performed in highly specialized centers, where several disciplines (pediatric transplant surgery, pediatric and adolescent medicine, pediatric intensive care medicine, interventional radiology and anesthesiology trained in pediatric treatment) closely interact and are on call 24/7/365; and (7) Meticulous donor selection and donor safety must continue to have highest priority in LDLT.
The authors sincerely thank Dr. Bernd Döhler and Professor, Dr. Gerhard Opelz for compiling and providing CTS data.
P- Reviewer: Yagi H, Wong GLH S- Editor: Song XX L- Editor: A E- Editor: Liu SQ
| 1. | Starzl TE, Marchioro TL, Vonkaulla KN, Hermann G, Brittain RS, Waddell WR. Homotransplantation of the liver in humans. Surg Gynecol Obstet. 1963;117:659-676. [PubMed] |
| 2. | Starzl TE, Groth CG, Brettschneider L, Penn I, Fulginiti VA, Moon JB, Blanchard H, Martin AJ, Porter KA. Orthotopic homotransplantation of the human liver. Ann Surg. 1968;168:392-415. [PubMed] |
| 3. | Starzl TE, Koep LJ, Schröter GP, Halgrimson CG, Porter KA, Weil R. Liver replacement for pediatric patients. Pediatrics. 1979;63:825-829. [PubMed] |
| 4. | Pichlmayr R, Brölsch C, Wonigeit K, Neuhaus P, Siegismund S, Schmidt FW, Burdelski M. Experiences with liver transplantation in Hannover. Hepatology. 1984;4:56S-60S. [PubMed] |
| 5. | Yazigi NA. Long term outcomes after pediatric liver transplantation. Pediatr Gastroenterol Hepatol Nutr. 2013;16:207-218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 6. | Abramson O, Rosenthal P. Current status of pediatric liver transplantation. Clin Liver Dis. 2000;4:533-552. [PubMed] |
| 7. | Melter M, Grothues D, Knoppke B. Pädiatrische Lebertransplantation. Monatsschr Kinderheilkd. 2012;160:343-357. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Fine RN. Growth following solid-organ transplantation. Pediatr Transplant. 2002;6:47-52. [PubMed] |
| 9. | Moukarzel AA, Najm I, Vargas J, McDiarmid SV, Busuttil RW, Ament ME. Effect of nutritional status on outcome of orthotopic liver transplantation in pediatric patients. Transplant Proc. 1990;22:1560-1563. [PubMed] |
| 10. | Moukarzel AA, Najm I, Vargas J, McDiarmid SV, Busuttil RW, Ament ME. Prediction of long-term linear growth following liver transplantation. Transplant Proc. 1990;22:1558-1559. [PubMed] |
| 11. | Rodeck B, Melter M, Kardorff R, Hoyer PF, Ringe B, Burdelski M, Oldhafer KJ, Pichlmayr R, Brodehl J. Liver transplantation in children with chronic end stage liver disease: factors influencing survival after transplantation. Transplantation. 1996;62:1071-1076. [PubMed] |
| 12. | Stewart SM, Uauy R, Waller DA, Kennard BD, Benser M, Andrews WS. Mental and motor development, social competence, and growth one year after successful pediatric liver transplantation. J Pediatr. 1989;114:574-581. [PubMed] |
| 13. | Wayman KI, Cox KL, Esquivel CO. Neurodevelopmental outcome of young children with extrahepatic biliary atresia 1 year after liver transplantation. J Pediatr. 1997;131:894-898. [PubMed] |
| 14. | Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 2064] [Article Influence: 82.6] [Reference Citation Analysis (0)] |
| 15. | Said A, Williams J, Holden J, Remington P, Gangnon R, Musat A, Lucey MR. Model for end stage liver disease score predicts mortality across a broad spectrum of liver disease. J Hepatol. 2004;40:897-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 281] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 16. | Rodeck B, Melter M, Kardorff R, Hoyer PF, Ringe B, Burdelski M, Oldhafer KJ, Pichlmayr R, Brodehl J. Liver transplantation in children with chronic end stage liver disease: factors influencing survival after transplantation. Transplantation. 1996;62:1071-1076. [PubMed] |
| 17. | Freeman RB, Wiesner RH, Harper A, McDiarmid SV, Lake J, Edwards E, Merion R, Wolfe R, Turcotte J, Teperman L; UNOS/OPTN Liver Disease Severity Score. The new liver allocation system: moving toward evidence-based transplantation policy. Liver Transpl. 2002;8:851-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 545] [Cited by in RCA: 534] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 18. | Wiesner RH, McDiarmid SV, Kamath PS, Edwards EB, Malinchoc M, Kremers WK, Krom RA, Kim WR. MELD and PELD: application of survival models to liver allocation. Liver Transpl. 2001;7:567-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 673] [Cited by in RCA: 605] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 19. | McDiarmid SV, Anand R, Lindblad AS; Principal Investigators and Institutions of the Studies of Pediatric Liver Transplantation (SPLIT) Research Group. Development of a pediatric end-stage liver disease score to predict poor outcome in children awaiting liver transplantation. Transplantation. 2002;74:173-181. [PubMed] |
| 20. | McDiarmid SV. New liver allocation policies and their potential effect on pediatric patients awaiting liver transplantation. Pediatr Transplant. 2002;6:180-186. [PubMed] |
| 21. | Eurotransplant International Foundation. EAR 2013. Available from: http://www.eurotransplant.org/cms/index.php?page=annual_reports. |
| 22. | McDiarmid SV, Merion RM, Dykstra DM, Harper AM. Selection of pediatric candidates under the PELD system. Liver Transplantation. 2004;10:S23-S30. [RCA] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 74] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Lee SG, Moon DB. Living donor liver transplantation for hepatocellular carcinoma. Recent Results Cancer Res. 2013;190:165-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Moon DB, Lee SG, Hwang S, Kim KH, Ahn CS, Ha TY, Song GW, Jung DH, Park GC, Namkoong JM. More than 300 consecutive living donor liver transplants a year at a single center. Transplant Proc. 2013;45:1942-1947. [PubMed] |
| 25. | Song GW, Lee SG, Hwang S, Ahn CS, Moon DB, Kim KH, Ha TY, Jung DH, Park GC, Namgung JM. Successful experiences of ABO-incompatible adult living donor liver transplantation in a single institute: no immunological failure in 10 consecutive cases. Transplant Proc. 2013;45:272-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 26. | Broelsch CE, Emond JC, Thistlethwaite JR, Whitington PF, Zucker AR, Baker AL, Aran PF, Rouch DA, Lichtor JL. Liver transplantation, including the concept of reduced-size liver transplants in children. Ann Surg. 1988;208:410-420. [PubMed] |
| 27. | Broelsch CE, Emond JC, Thistlethwaite JR, Rouch DA, Whitington PF, Lichtor JL. Liver transplantation with reduced-size donor organs. Transplantation. 1988;45:519-524. [PubMed] |
| 28. | Pichlmayr R, Ringe B, Gubernatis G, Hauss J, Bunzendahl H. [Transplantation of a donor liver to 2 recipients (splitting transplantation)--a new method in the further development of segmental liver transplantation]. Langenbecks Arch Chir. 1988;373:127-130. [PubMed] |
| 29. | Couinaud C. [Liver lobes and segments: notes on the anatomical architecture and surgery of the liver]. Presse Med. 1954;62:709-712. [PubMed] |
| 30. | Couinaud C. Liver anatomy: portal (and suprahepatic) or biliary segmentation. Dig Surg. 1999;16:459-467. [PubMed] |
| 32. | Kanazawa H, Sakamoto S, Fukuda A, Uchida H, Hamano I, Shigeta T, Kobayashi M, Karaki C, Tanaka H, Kasahara M. Living-donor liver transplantation with hyperreduced left lateral segment grafts: a single-center experience. Transplantation. 2013;95:750-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | Caso Maestro O, Abradelo de Usera M, Justo Alonso I, Calvo Pulido J, Manrique Municio A, Cambra Molero F, García Sesma A, Loinaz Segurola C, Moreno González E, Jiménez Romero C. Porcine acellular dermal matrix for delayed abdominal wall closure after pediatric liver transplantation. Pediatr Transplant. 2014;18:594-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Gubernatis G, Pichlmayr R, Kemnitz J, Gratz K. Auxiliary partial orthotopic liver transplantation (APOLT) for fulminant hepatic failure: first successful case report. World J Surg. 1991;15:660-665; discussion 665-666. [PubMed] |
| 35. | Faraj W, Dar F, Bartlett A, Melendez HV, Marangoni G, Mukherji D, Vergani GM, Dhawan A, Heaton N, Rela M. Auxiliary liver transplantation for acute liver failure in children. Ann Surg. 2010;251:351-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 36. | Shanmugam NP, Dhawan A. Selection criteria for liver transplantation in paediatric acute liver failure: the saga continues. Pediatr Transplant. 2011;15:5-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 37. | Reich DJ, Mulligan DC, Abt PL, Pruett TL, Abecassis MM, D’Alessandro A, Pomfret EA, Freeman RB, Markmann JF, Hanto DW. ASTS recommended practice guidelines for controlled donation after cardiac death organ procurement and transplantation. Am J Transplant. 2009;9:2004-2011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 267] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 38. | Snoeijs MG, Wind T, van Heurn E. Protocols for uncontrolled donation after circulatory death. Lancet. 2012;380:974-975; author reply 975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 39. | Bernat JL, Capron AM, Bleck TP, Blosser S, Bratton SL, Childress JF, DeVita MA, Fulda GJ, Gries CJ, Mathur M. The circulatory-respiratory determination of death in organ donation. Crit Care Med. 2010;38:963-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 164] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 40. | WHO; Transplantation Society (TTS); Organizatión Nacional de Transplantes (ONT). Third WHO Global Consultation on Organ Donation and Transplantation: striving to achieve self-sufficiency, March 23–25, 2010, Madrid, Spain. Transplantation. 2011;91 Suppl 11:S27-S28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 41. | Morrissey PE, Monaco AP. Donation after circulatory death: current practices, ongoing challenges, and potential improvements. Transplantation. 2014;97:258-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 185] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 42. | Fondevila C, Hessheimer AJ, Flores E, Ruiz A, Mestres N, Calatayud D, Paredes D, Rodríguez C, Fuster J, Navasa M. Applicability and results of Maastricht type 2 donation after cardiac death liver transplantation. Am J Transplant. 2012;12:162-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 147] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 43. | Waki K. UNOS Liver Registry: ten year survivals. Clin Transpl. 2006;29-39. [PubMed] |
| 44. | Dubbeld J, Hoekstra H, Farid W, Ringers J, Porte RJ, Metselaar HJ, Baranski AG, Kazemier G, van den Berg AP, van Hoek B. Similar liver transplantation survival with selected cardiac death donors and brain death donors. Br J Surg. 2010;97:744-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 164] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 45. | Jay CL, Lyuksemburg V, Ladner DP, Wang E, Caicedo JC, Holl JL, Abecassis MM, Skaro AI. Ischemic cholangiopathy after controlled donation after cardiac death liver transplantation: a meta-analysis. Ann Surg. 2011;253:259-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 256] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 46. | Broelsch CE, Emond JC, Whitington PF, Thistlethwaite JR, Baker AL, Lichtor JL. Application of reduced-size liver transplants as split grafts, auxiliary orthotopic grafts, and living related segmental transplants. Ann Surg. 1990;212:368-375; discussion 375-377. [PubMed] |
| 47. | Organ Procurement and Transplantation Network. [Accessed 2014 Dec 1]. Available from: http://optn.transplant.hrsa.gov/converge/latestdata/rptData.asp. |
| 48. | Bhangui P, Vibert E, Majno P, Salloum C, Andreani P, Zocrato J, Ichai P, Saliba F, Adam R, Castaing D. Intention-to-treat analysis of liver transplantation for hepatocellular carcinoma: living versus deceased donor transplantation. Hepatology. 2011;53:1570-1579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 49. | Gondolesi GE, Roayaie S, Muñoz L, Kim-Schluger L, Schiano T, Fishbein TM, Emre S, Miller CM, Schwartz ME. Adult living donor liver transplantation for patients with hepatocellular carcinoma: extending UNOS priority criteria. Ann Surg. 2004;239:142-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 162] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 50. | Grant RC, Sandhu L, Dixon PR, Greig PD, Grant DR, McGilvray ID. Living vs. deceased donor liver transplantation for hepatocellular carcinoma: a systematic review and meta-analysis. Clin Transplant. 2013;27:140-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 51. | Kaihara S, Kiuchi T, Ueda M, Oike F, Fujimoto Y, Ogawa K, Kozaki K, Tanaka K. Living-donor liver transplantation for hepatocellular carcinoma. Transplantation. 2003;75:S37-S40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 121] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 52. | Sandhu L, Sandroussi C, Guba M, Selzner M, Ghanekar A, Cattral MS, McGilvray ID, Levy G, Greig PD, Renner EL. Living donor liver transplantation versus deceased donor liver transplantation for hepatocellular carcinoma: comparable survival and recurrence. Liver Transpl. 2012;18:315-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 53. | Todo S, Furukawa H; Japanese Study Group on Organ Transplantation. Living donor liver transplantation for adult patients with hepatocellular carcinoma: experience in Japan. Ann Surg. 2004;240:451-459; discussion 459-461. [PubMed] |
| 54. | Saidi RF, Jabbour N, Li Y, Shah SA, Bozorgzadeh A. Is left lobe adult-to-adult living donor liver transplantation ready for widespread use? The US experience (1998-2010). HPB (Oxford). 2012;14:455-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 55. | Saidi RF, Markmann JF, Jabbour N, Li Y, Shah SA, Cosimi AB, Bozorgzadeh A. The faltering solid organ donor pool in the United States (2001-2010). World J Surg. 2012;36:2909-2913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 56. | Muzaale AD, Dagher NN, Montgomery RA, Taranto SE, McBride MA, Segev DL. Estimates of early death, acute liver failure, and long-term mortality among live liver donors. Gastroenterology. 2012;142:273-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 57. | Roll GR, Roberts JP. Left versus right lobe liver donation. Am J Transplant. 2014;14:251-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 58. | Roll GR, Parekh JR, Parker WF, Siegler M, Pomfret EA, Ascher NL, Roberts JP. Left hepatectomy versus right hepatectomy for living donor liver transplantation: shifting the risk from the donor to the recipient. Liver Transpl. 2013;19:472-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 59. | Ghobrial RM, Freise CE, Trotter JF, Tong L, Ojo AO, Fair JH, Fisher RA, Emond JC, Koffron AJ, Pruett TL. Donor morbidity after living donation for liver transplantation. Gastroenterology. 2008;135:468-476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 347] [Cited by in RCA: 325] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 60. | Kousoulas L, Becker T, Richter N, Emmanouilidis N, Schrem H, Barg-Hock H, Klempnauer J, Lehner F. Living donor liver transplantation: effect of the type of liver graft donation on donor mortality and morbidity. Transpl Int. 2011;24:251-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 61. | Kousoulas L, Emmanouilidis N, Klempnauer J, Lehner F. Living-donor liver transplantation: impact on donor’s health-related quality of life. Transplant Proc. 2011;43:3584-3587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 62. | Lo CM. Complications and long-term outcome of living liver donors: a survey of 1,508 cases in five Asian centers. Transplantation. 2003;75:S12-S15. [PubMed] |
| 63. | Morioka D, Egawa H, Kasahara M, Ito T, Haga H, Takada Y, Shimada H, Tanaka K. Outcomes of adult-to-adult living donor liver transplantation: a single institution’s experience with 335 consecutive cases. Ann Surg. 2007;245:315-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 156] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 64. | Umeshita K, Fujiwara K, Kiyosawa K, Makuuchi M, Satomi S, Sugimachi K, Tanaka K, Monden M; Japanese Liver Transplantation Society. Operative morbidity of living liver donors in Japan. Lancet. 2003;362:687-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 209] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 65. | Diamond IR, Fecteau A, Millis JM, Losanoff JE, Ng V, Anand R, Song C; SPLIT Research Group. Impact of graft type on outcome in pediatric liver transplantation: a report From Studies of Pediatric Liver Transplantation (SPLIT). Ann Surg. 2007;246:301-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 177] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 66. | Soltys KA, Mazariegos GV, Squires RH, Sindhi RK, Anand R. Late graft loss or death in pediatric liver transplantation: an analysis of the SPLIT database. Am J Transplant. 2007;7:2165-2171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 114] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 67. | Scheenstra R, Peeters PM, Verkade HJ, Gouw AS. Graft fibrosis after pediatric liver transplantation: ten years of follow-up. Hepatology. 2009;49:880-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 137] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 68. | Ekong UD, Melin-Aldana H, Seshadri R, Lokar J, Harris D, Whitington PF, Alonso EM. Graft histology characteristics in long-term survivors of pediatric liver transplantation. Liver Transpl. 2008;14:1582-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 69. | Spada M, Riva S, Maggiore G, Cintorino D, Gridelli B. Pediatric liver transplantation. World J Gastroenterol. 2009;15:648-674. [PubMed] |
| 70. | Campbell KM, Bucuvalas JC. Renal function in the long term after pediatric liver transplantation: is there a need for protocol kidney biopsies? Curr Opin Organ Transplant. 2010;15:608-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 71. | Harambat J, Ranchin B, Dubourg L, Liutkus A, Hadj-Haïssa A, Rivet C, Boillot O, Lachaux A, Cochat P. Renal function in pediatric liver transplantation: a long-term follow-up study. Transplantation. 2008;86:1028-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 72. | Bayrakci US, Baskin E, Ozcay F, Gulleroglu K, Ozbay F, Sevmis S, Karakayali H, Haberal M. Abnormal circadian blood pressure regulation in liver transplanted children. Pediatr Transplant. 2012;16:160-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 73. | McLin VA, Anand R, Daniels SR, Yin W, Alonso EM; SPLIT Research Group. Blood pressure elevation in long-term survivors of pediatric liver transplantation. Am J Transplant. 2012;12:183-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 74. | Gross TG, Hinrichs SH, Winner J, Greiner TC, Kaufman SS, Sammut PH, Langnas AN. Treatment of post-transplant lymphoproliferative disease (PTLD) following solid organ transplantation with low-dose chemotherapy. Ann Oncol. 1998;9:339-340. [PubMed] |
| 75. | Gross TG, Orjuela MA, Perkins SL, Park JR, Lynch JC, Cairo MS, Smith LM, Hayashi RJ. Low-dose chemotherapy and rituximab for posttransplant lymphoproliferative disease (PTLD): a Children’s Oncology Group Report. Am J Transplant. 2012;12:3069-3075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 122] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 76. | Kamdar KY, Rooney CM, Heslop HE. Posttransplant lymphoproliferative disease following liver transplantation. Curr Opin Organ Transplant. 2011;16:274-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 77. | Taylor AL, Marcus R, Bradley JA. Post-transplant lymphoproliferative disorders (PTLD) after solid organ transplantation. Crit Rev Oncol Hematol. 2005;56:155-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 286] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 78. | Guthery SL, Heubi JE, Bucuvalas JC, Gross TG, Ryckman FC, Alonso MH, Balistreri WF, Hornung RW. Determination of risk factors for Epstein-Barr virus-associated posttransplant lymphoproliferative disorder in pediatric liver transplant recipients using objective case ascertainment. Transplantation. 2003;75:987-993. [PubMed] |
| 79. | Dobbels F, Vanhaecke J, Desmyttere A, Dupont L, Nevens F, De Geest S. Prevalence and correlates of self-reported pretransplant nonadherence with medication in heart, liver, and lung transplant candidates. Transplantation. 2005;79:1588-1595. [PubMed] |
| 80. | Fredericks EM. Nonadherence and the transition to adulthood. Liver Transpl. 2009;15 Suppl 2:S63-S69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 81. | Starzl TE, Iwatsuki S, Klintmalm G, Schröter GP, Weil R, Koep LJ, Porter KA. Liver transplantation, 1980, with particular reference to cyclosporin-A. Transplant Proc. 1981;13:281-285. [PubMed] |
| 82. | Starzl TE, Todo S, Tzakis AG, Gordon RD, Makowka L, Stieber A, Podesta L, Yanaga K, Concepcion W, Iwatsuki S. Liver transplantation: an unfinished product. Transplant Proc. 1989;21:2197-2200. [PubMed] |
| 83. | Ganschow R, Grabhorn E, Burdelski M. Basiliximab in paediatric liver-transplant recipients. Lancet. 2001;357:388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 84. | Ganschow R, Grabhorn E, Schulz A, Von Hugo A, Rogiers X, Burdelski M. Long-term results of basiliximab induction immunosuppression in pediatric liver transplant recipients. Pediatr Transplant. 2005;9:741-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 85. | Grabhorn E, Schulz A, Helmke K, Hinrichs B, Rogiers X, Broering DC, Burdelski M, Ganschow R. Short- and long-term results of liver transplantation in infants aged less than 6 months. Transplantation. 2004;78:235-241. [PubMed] |
| 86. | Heffron TG, Pillen T, Smallwood GA, Welch D, Oakley B, Romero R. Pediatric liver transplantation with daclizumab induction. Transplantation. 2003;75:2040-2043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 87. | Schuller S, Wiederkehr JC, Coelho-Lemos IM, Avilla SG, Schultz C. Daclizumab induction therapy associated with tacrolimus-MMF has better outcome compared with tacrolimus-MMF alone in pediatric living donor liver transplantation. Transplant Proc. 2005;37:1151-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 88. | Strassburg CP, Manns MP. [Partial liver transplantation and living donation from the viewpoint of internal medicine]. Der Internist. 2002;43:1551-1558. |
| 89. | Di Filippo S. Anti-IL-2 receptor antibody vs. polyclonal anti-lymphocyte antibody as induction therapy in pediatric transplantation. Pediatr Transplant. 2005;9:373-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 90. | Dell-Olio D, Kelly DA. Calcineurin inhibitor minimization in pediatric liver allograft recipients. Pediatr Transplant. 2009;13:670-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 91. | Tönshoff B, Höcker B. Treatment strategies in pediatric solid organ transplant recipients with calcineurin inhibitor-induced nephrotoxicity. Pediatr Transplant. 2006;10:721-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 92. | Tredger JM, Brown NW, Dhawan A. Immunosuppression in pediatric solid organ transplantation: opportunities, risks, and management. Pediatr Transplant. 2006;10:879-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 93. | Turmelle YP, Nadler ML, Anderson CD, Doyle MB, Lowell JA, Shepherd RW. Towards minimizing immunosuppression in pediatric liver transplant recipients. Pediatr Transplant. 2009;13:553-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 94. | Evans HM, McKiernan PJ, Kelly DA. Mycophenolate mofetil for renal dysfunction after pediatric liver transplantation. Transplantation. 2005;79:1575-1580. [PubMed] |
| 95. | Ferraris JR, Duca P, Prigoshin N, Tambutti ML, Boldrini G, Cardoni RL, D’Agostino D. Mycophenolate mofetil and reduced doses of cyclosporine in pediatric liver transplantation with chronic renal dysfunction: changes in the immune responses. Pediatr Transplant. 2004;8:454-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 96. | Filler G, Gellermann J, Zimmering M, Mai I. Effect of adding Mycophenolate mofetil in paediatric renal transplant recipients with chronical cyclosporine nephrotoxicity. Transpl Int. 2000;13:201-206. [PubMed] |
| 97. | Hoyer PF, Ettenger R, Kovarik JM, Webb NJ, Lemire J, Mentser M, Mahan J, Loirat C, Niaudet P, VanDamme-Lombaerts R. Everolimus in pediatric de nova renal transplant patients. Transplantation. 2003;75:2082-2085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 98. | Jiménez-Rivera C, Avitzur Y, Fecteau AH, Jones N, Grant D, Ng VL. Sirolimus for pediatric liver transplant recipients with post-transplant lymphoproliferative disease and hepatoblastoma. Pediatr Transplant. 2004;8:243-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 99. | Scheenstra R, Torringa ML, Waalkens HJ, Middelveld EH, Peeters PM, Slooff MJ, Gouw AS, Verkade HJ, Bijleveld CM. Cyclosporine A withdrawal during follow-up after pediatric liver transplantation. Liver Transpl. 2006;12:240-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 100. | Sindhi R, Ganjoo J, McGhee W, Mazariegos G, Reyes J. Preliminary immunosuppression withdrawal strategies with sirolimus in children with liver transplants. Transplant Proc. 2002;34:1972-1973. [PubMed] |
| 101. | Vester U, Kranz B, Wehr S, Boger R, Hoyer PF; RAD B 351 Study Group. Everolimus (Certican) in combination with neoral in pediatric renal transplant recipients: interim analysis after 3 months. Transplant Proc. 2002;34:2209-2210. [PubMed] |
| 102. | Feng S, Ekong UD, Lobritto SJ, Demetris AJ, Roberts JP, Rosenthal P, Alonso EM, Philogene MC, Ikle D, Poole KM. Complete immunosuppression withdrawal and subsequent allograft function among pediatric recipients of parental living donor liver transplants. JAMA. 2012;307:283-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 262] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 103. | Koshiba T, Li Y, Takemura M, Wu Y, Sakaguchi S, Minato N, Wood KJ, Haga H, Ueda M, Uemoto S. Clinical, immunological, and pathological aspects of operational tolerance after pediatric living-donor liver transplantation. Transpl Immunol. 2007;17:94-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 144] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 104. | Mazariegos GV, Reyes J, Marino IR, Demetris AJ, Flynn B, Irish W, McMichael J, Fung JJ, Starzl TE. Weaning of immunosuppression in liver transplant recipients. Transplantation. 1997;63:243-249. [PubMed] |
| 105. | Mazariegos GV, Sindhi R, Thomson AW, Marcos A. Clinical tolerance following liver transplantation: long term results and future prospects. Transpl Immunol. 2007;17:114-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 106. | Starzl TE, Demetris AJ, Trucco M, Ricordi C, Murase N, Thomson AW. The Role of Cell Migration and Chimerism in Organ Transplant Acceptance and Tolerance Induction. Transplant Sci. 1993;3:47-50. [PubMed] |
| 107. | Takatsuki M, Uemoto S, Inomata Y, Egawa H, Kiuchi T, Fujita S, Hayashi M, Kanematsu T, Tanaka K. Weaning of immunosuppression in living donor liver transplant recipients. Transplantation. 2001;72:449-454. [PubMed] |
| 108. | Martínez-Llordella M, Lozano JJ, Puig-Pey I, Orlando G, Tisone G, Lerut J, Benítez C, Pons JA, Parrilla P, Ramírez P. Using transcriptional profiling to develop a diagnostic test of operational tolerance in liver transplant recipients. J Clin Invest. 2008;118:2845-2857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 109. | Donckier V, Troisi R, Le Moine A, Toungouz M, Ricciardi S, Colle I, Van Vlierberghe H, Craciun L, Libin M, Praet M. Early immunosuppression withdrawal after living donor liver transplantation and donor stem cell infusion. Liver Transpl. 2006;12:1523-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 110. | Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, Shaffer J, Preffer FI, Ding R, Sharma V. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358:353-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 890] [Cited by in RCA: 831] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 111. | Scandling JD, Busque S, Dejbakhsh-Jones S, Benike C, Millan MT, Shizuru JA, Hoppe RT, Lowsky R, Engleman EG, Strober S. Tolerance and chimerism after renal and hematopoietic-cell transplantation. N Engl J Med. 2008;358:362-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 395] [Article Influence: 23.2] [Reference Citation Analysis (0)] |