Published online Jun 8, 2015. doi: 10.4254/wjh.v7.i10.1355
Peer-review started: October 21, 2014
First decision: November 27, 2014
Revised: December 23, 2014
Accepted: February 10, 2015
Article in press: February 12, 2015
Published online: June 8, 2015
Processing time: 225 Days and 6.6 Hours
The optimal level of immunosuppression in solid organ transplantation, in particular for the liver, is a delicate balance between the benefit of preventing rejection and the adverse side effects of immunosuppression. There is uncertainty about when this level is achieved in any individual recipient. Immunosuppression regimens vary between individual centers and changes with time as new agents and data are available. Presently concerns about the adverse side effects of calcineurin inhibitor, the main class of immunosuppressive agents used in liver transplantation (LT), has led to consideration of the use of antibody induction therapies for patients at higher risk of developing adverse side effects. The longevity of the transplanted organ is potentially improved by better management of rejection episodes and special consideration for tailoring of immunosuppression to the individual with viral hepatitis C, hepatocellular carcinoma or pregnancy. This review provides an overview of the current strategies for post LT immunosuppression and discusses modifications to consider for special patient populations.
Core tip: This manuscript is a review on common aspects and principles of immunosuppression in liver transplantation (LT) including new advents. It covers the sections of induction, maintenance and monitoring of immunosuppression and also discusses on immunosuppression in special populations. In this review, it has been tried to be connected with last updates in the field of immunosuppression in LT.
- Citation: Moini M, Schilsky ML, Tichy EM. Review on immunosuppression in liver transplantation. World J Hepatol 2015; 7(10): 1355-1368
- URL: https://www.wjgnet.com/1948-5182/full/v7/i10/1355.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i10.1355
Liver transplantation (LT) is a life saving procedure for patients with end stage liver disease and its complications, and for liver failure. LT is also curative for some hereditary metabolic disorders like familial hypercholesterolemia and for selected cases of malignancies involving the liver, such as hepatocellular carcinoma (HCC) and hepatoblastoma. Recipients of orthotopic LT have excellent survival rate (83% for 1 year and 75% for 5 years) that has improved markedly over the past three decades[1].
Development of new agents and changes in post transplant immunosuppression regimens are major contributing factors for this improvement. However, while long term post transplant immunosuppression decreases rejection episodes in LT recipients, it also puts the patients at increased risk of infection, malignancies and specific adverse side effects unique to each agent. There are different immunosuppression protocols used by transplant centers worldwide; however, any LT recipient may need an individually tailored immunosuppression regimen to balance the benefits and potential harm of therapy while decreasing the risk of recurrence of their primary disease.
The basis of solid-organ post transplant immunosuppression has commonalities between the different organs, but the liver itself is unique in its immunologic response to provocation. This privilege is called “liver tolerance” which is mostly attributed to the role of the regulatory T-cell[2,3].
LT does not require human leukocyte antigen (HLA) matching between donor and recipient[4], though there is increased interest in understanding HLA and humoral rejection and graft survival. Simultaneous transplantation of liver with another solid organ, for example, a kidney decreases the incidence of rejection episodes for the second organ[5-7] and facilitates minimization of the immunosuppression to a lower level than typically allowed thereby, reducing adverse side effects and cost of therapy. Given these considerations as a general principle, LT recipients are maintained on lower levels of immunosuppression than other solid organ transplant recipients. Moreover, in some selected LT recipients, the allograft may achieve long-term survival even after immunosuppression withdrawal[8].
Living donor LT recipients experience less rejection episodes comparing with deceased donor LT[9,10]. However, the immunological benefit as the only explanation for this observation is in doubt by some researchers[11]. Shorter cold ischemia time and operation in non-emergency conditions are among the factors affecting transplantation outcome from living donors. On the other hand, although, the beneficial effect of HLA similarity between the living donor and recipient has not been proven in LT[11-13], high success rate for weaning from immunosuppression has been reported in pediatric patients receiving liver from their parents[14].
The aim of this review is to provide an overview of strategies for post liver transplant immunosuppression, with special attention to specific populations where modification of standard regimens may be beneficial.
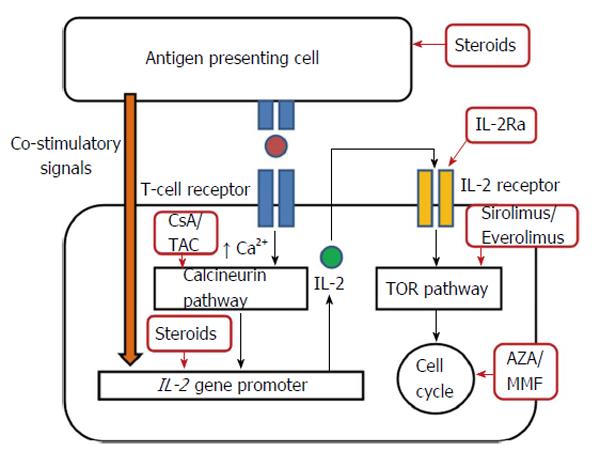
Immunosuppressive agents are required in solid organ transplantation for induction of immunosuppression in the early phase, maintenance of immunosuppression in the late phase or for the treatment of organ rejection. Table 1 summarizes the immunosuppressive agents commonly used in LT and their specific applications. The site of action of these agents is briefly shown in Figure 1.
| Agent | Classification | Indications | Dose |
| Methyl prednisolone (Medrol®), Prednisone or prednisolone[13,16,111] | Corticosteroids | Induction of immunosuppression, treatment of acute cellular rejection, Maintenance of immunosuppression | Variable according to the centers, the etiology of liver disease and history of rejections |
| Tacrolimus (Prograf®, Astagraf®)[53] | CNI | Maintenance of immunosuppression | Starting 0.1-0.15 mg/kg per day divided every 12 h and adjust to the desired trough level |
| Cyclosporine (Neoral®, Sandimmune®, Gengraf®)[52,55] | CNI | Maintenance of immunosuppression | Starting 10-15 mg/kg per day divided every 12 h and adjust to the desired (C2) level |
| Mycophenolate mofetil (Cellcept®, Myfortic®)[60] | Anti-metabolite | Maintenance of immunosuppression, treatment of rejection | Variable doses may be desired in any individual case |
| Azathioprine (Imuran®)[65] | Anti-metabolite | Maintenance of immunosuppression | Variable, maintenance dose may be 1.5-2.5 mg/kg per day, needs to be adjusted for adverse side effects |
| Sirolimus (Rapamune®)[48,68,71] | mTORI | Maintenance of immunosuppression, treatment of rejection, special interests for use in malignancies | Usual dosing is a 6 mg (or 3 mg/m2) oral loading, followed by 2 mg/d (or 1 mg/m2 per day) single dose, higher doses may be administered for individual cases1 |
| Everolimus (Afinitor®)[48,69,72] | mTORI | Maintenance of immunosuppression, treatment of rejection, special interests for use in malignancies | Starting at 1 mg oral every twice a day and adjust to a trough level of 3-8 ng/mL1 |
| 2Muromonab-CD3 (OKT3) | T cell depleting monoclonal antibody | Induction of immunosuppression, treatment of steroid resistant rejection | Withdrawn from the market because of reduced use, no longer available since 2010 |
| Alemtuzumab (campath-1H®)[44-46] | T cell depleting monoclonal antibody | Induction of immunosuppression | Variable between centers, a single dose of 30 mg may be used in operating room |
| ATG (Thymoglobulin®, ATGAM®)[27-30] | T cell depleting polyclonal antibody | Induction of immunosuppression, treatment of steroid resistant rejection | Variable between centers, For induction 1.5 mg/kg per day iv for 3 d and for treatment of rejection 1.5 mg/kg per day iv for 5-7 d of thymoglobulin may be used. For ATGAM a higher dose of 15 mg/kg per day is usually used |
| 2Daclizumab (Zenapax®)[23,115] | IL-2Ra, monoclonal antibody | Induction of immunosuppression, treatment of steroid resistant rejection | For induction the first dose of 1 mg/kg is given within 24 h before Tx and 4 more doses are given after Tx with 2 wk intervals Withdrawn from the market because of reduced use, no longer available |
| Basiliximab (Simulect®)[23,113,114] | IL-2Ra, monoclonal antibody | Induction of immunosuppression, treatment of steroid resistant rejection | For induction a 20 mg iv dose is administered within 2 h prior to reperfusion and another 20 mg on days 4 post Tx |
The definition of induction therapy is intensive peri-operative prophylactic immunosuppression used to prevent acute cellular rejection in the first months following transplantation[15]. Specific agents are discussed individually below.
Corticosteroids have been the mainstay for induction of immunosuppression since the first successful cases of solid organ transplantation[13,16].
Intravenous (iv) injection of corticosteroid are administered in high doses during the transplant operation and in the first few post operation days (usually up to 3 d) in combination with at least one other immunosuppressant agent.
The dose and duration of iv administration of drugs differ according to the local practice among different centers; however a typical dosage is 500 or 1000 mg of methylprednisolone. Corticosteroids are rapidly tapered over the first week to relatively low doses, 10 to 20 mg daily, and are usually maintained in immunosuppression regimen at least for the first 3 to 6 mo post transplant. The major concerns with corticosteroids, especially with high doses, are their adverse side effects. Delirium is a common early problem, and infections and metabolic derangements such as hypertension, hyperlipidemia, diabetes, and obesity may cause significant short and long-term morbidity among liver recipients. In these individuals, steroid reduction or elimination may be indicated. There is also concern that higher doses of steroids increase the risk of disease recurrence in LT patients with chronic viral hepatitis. However, the risk of organ rejection may increase following early corticosteroid dose reduction or withdrawal[17].
Usually, a calcineurin inhibitors (CNI), alone or with an anti-proliferative agent mycophenolic acid (MPA) or azathioprine is started early post transplantation in combination with a corticosteroid to help maintain immunosuppression[18]. More recently, antibody therapies have been combined with corticosteroids or used to facilitate “steroid-free” regimens.
Use of antibodies that are designed specifically to inhibit or deplete recipient T-cells has been reported to decrease acute rejection episodes in the liver allograft[19,20].
Use of antibody induction also provides an opportunity to decrease the dose of other concomitant immunosuppressive agents such as corticosteroids and CNIs[17] thus minimizing the adverse side effects related to these agents. Antibody administration has been used for induction therapy in “steroid-free” protocols where there is elimination of corticosteroid in the induction of immunosuppression in LT patients[21,22].
Compared with corticosteroid induction, less hyperglycemia and diabetes and less cytomegalovirus (CMV) infections are found with antibody induction[17,23].
This “steroid-free” strategy may be especially beneficial for patients with hepatitis C patients and for those with diabetes and hypertension. Antibody induction along with delayed CNI introduction can be used to preserve renal function in LT recipients and reduce renal dysfunction in those with impairment[24].
Overall, no significant increase in adverse side effects was observed in solid transplant recipients receiving antibody induction[23,25]. However, their use adds to the cost of perioperative care.
Antibodies used for induction of immunosuppression in LT are classified into two groups; T-cell depleting and non-depleting [interleukin 2 receptor antagonists (IL-2Ra)][26].
This group includes:
Polyclonal antibodies: Anti-thymocyte globulins (ATG)s are polyclonal animal antibodies against multiple T-cells receptors that are used to achieve circulating lymphocyte depletion. There are two preparations of antithymocyte globulin (ATG) available for clinical use in the United States. Equine ATG (eATG, ATGAM®) is of equine origin and rabbit ATG (rATG, Thymoglobulin®) is generated in rabbits. ATG has been widely used for the treatment of steroid resistance rejections[27,28] as well as induction of immunosuppression in LT[29,30].
rATG is one of most commonly used agents for antibody induction therapy in organ transplantation in the United States. Much of the initial experience with polyclonal antibody induction therapy was learned from kidney transplantation. rATG is superior to the equine originated ATG in prevention of episodes of acute renal rejection[31]. Less severe rejections, fewer serious adverse side effects and even less CMV infection occur, but more profound leucopenia have been observed in renal allograft recipients receiving rATG compared with those who received eATG as induction therapy[32].
The protocol for ATG induction therapy differs between centers. A 10 d course of single infusion of 2.5 mg/kg of rATG was the standard induction in the earlier cyclosporine era, while shorter courses (a 3-d course) was shown to have the same protective effect but less severe life threatening infections[33]. The efficacy of the 3-d induction protocol has been supported even when the first dose is delayed for 48 h post-transplant[34,35]. Intermittent dosing of eATG based on CD3 counting using flow cytometry has been shown to be effective and less costly in induction of immunosuppression for kidney and kidney/pancreas transplantation. In this regimen, the second dose of eATG after transplantation was not given until the CD3 count is above 20 cells/mm3[30].
Administration of rATG may infrequently cause infusion related reactions. Pre-medication with antihistamines and acetaminophen is recommended as rare severe cardiovascular reactions and anaphylaxis have been reported[36]. Serum sickness may develop following rATG administration[37]. More common adverse side effects of AGT are the result of severe cytokine release syndrome induced by the agent. The risk of infectious complications following induction therapy with rATG was comparable to the regimens without antibody induction[38,39]. Although a major concern, a more severe recurrence of hepatitis C virus (HCV) infection has not been observed with rATG induction therapy, and there is even some limited evidence for a decreased risk[40,41]. There are insufficient data regarding the possible increased risk of post transplantation lymphoproliferative disorders (PTLD) following rATG induction in LT patients. However, the overall incidence of PTLD in kidney and heart transplant recipients receiving rATG induction therapy was low and the effect of prophylactic antiviral therapy on the risk reduction has been supported[42].
Monoclonal antibodies: Alemtuzumab (campath-1H) is a humanized rat monoclonal antibody against CD52 receptors on peripheral mononuclear cells. It has a significant depleting effect on peripheral as well as lymph node lymphocytes[43].
As a potent immunosuppressant agent alemtuzumab has its own potential benefits for induction therapy in LT; however, the increased risk for infectious complications may limit its use to special subgroups, in particular for those who need renal sparing regimens[44-46].
The safety of alemtuzumab induction is most in doubt in HCV positive recipients as increased complications and a rapidly progressive recurrence of HCV have been reported[47].
Further studies are required to address the risk benefit issues on use of this agent as induction immunosuppression for LT.
Muromonab-CD3 (OKT3) was a monoclonal antibody directed against CD3 receptors on peripheral T-cells that was successfully used for the treatment of steroid unresponsive acute liver rejection and also for immunosuppression prophylaxis. However, the side-effect profile was considerable and with the availability of newer agents, the manufacturer discontinued its production in 2010.
Non-depleting antibodies: IL-2Ras are humanized monoclonal antibodies that bind to IL-2 receptor on T-cells and thus suppress the proliferative response of T-cells to circulating IL-2. These agents are less immunogenic than other antibodies such as OKT3[48]. For LT, IL-2Ras have a role for patients who need to avoid or to decrease dosages of an accompanied immunosuppressant agent, such as corticosteroids or CNIs. Less frequent diabetes mellitus, less CMV infections and higher glomerular filtration rate were observed among patients receiving IL-2Ra vs those who received corticosteroids as induction therapy. The two IL-2Ra agents, basiliximab and daclizumab did not differ in the mentioned advantages when analyzed by Penninga et al[23] and may be used interchangeably. Although, daclizumab has been off the market since about 2010, the results of few studies using this agent as an IL-2Ra will be reviewed here.
In an analysis of the United Network for Organ Sharing (UNOS) database, Uemura et al[49] compared ATG, daclizumab and steroid alone and ATG plus steroid. They showed a satisfactory short and long-term outcome for daclizumab in LT patients with all etiologies of liver disease including HCV[49]. In a randomized multicenter study, Klintmalm et al[50] concluded that corticosteroid free induction therapy using IL-2Ra (daclizumab) does not increase the risk of fibrosis progression in HCV LT recipients. Basiliximab induction was not associated with increased risk of PTLD, CMV infection or HCV recurrence in another study by Ramirez et al[51], while the rate of acute rejection was decreased and rejection free survival increased.
CNI: CNIs function as immunosuppressants by blocking the signal 2 of T-cell activation by binding to specific receptors and blocking calcineurin, a calcium dependent phosphatase within T-cells. The introduction of the two CNIs, cyclosporine (in 1970s and early 1980s) and tacrolimus (in 1990s) as immunosuppressant agents, greatly improved the outcome of LT. Overall survival of patients undergoing LT on cyclosporine immunosuppressive therapy was 3 times higher than those on azathioprine alone[52].
With the advent of the newer agent, tacrolimus, the outcome of LT improved further. Tacrolimus is superior to cyclosporine in increasing patient and graft survival. Less acute cellular rejection and steroid-resistant rejection episodes are seen with tacrolimus use in the first post-transplant year compared with cyclosporine[53].
Because of their excellent organ protective effects, CNIs, especially tacrolimus, have been included as the main agents in maintenance immunosuppression protocols in most LT centers, worldwide. Tacrolimus is preferred over cyclosporine in most centers because of its greater potency and improved cardiovascular adverse side effect profile. Tacrolimus is usually started with a low (0.1-0.15 mg/kg per day divided in 2 doses every 12 h) oral (or sublingual, if the patient could not tolerate oral medication) dose on the first day post transplant, though some centers delay its start for 2-4 d, and the dose is gradually increased to achieve the desired trough level. An iv formulation of tacrolimus is available, but seizures are a significant risk with use of this form of the medication.
There is controversy, thus center variability, about the optimal trough (C0) level for tacrolimus. Usually levels of 10-15 ng/mL are targeted for the first 4-6 wk, and 5-10 ng/mL thereafter are accepted by many centers; however, it is possible to target lower levels (i.e., 6-10 ng/mL for early post transplantation) without increasing the risk of acute rejections with a benefit toward kidney protection[54].
Cyclosporine is not the CNI of choice for LT recipients; however, in special cases there might be a need to switch from tacrolimus to cyclosporine. When it has to be used the recommended dose is 10-15 mg/kg per day divided in 2 doses, but it may be started at lower doses with gradual increase to achieve the target level. For C0 levels, in the early post transplant period the target level is 250 ng/mL, and 150 ng/mL later on. For cyclosporine, C2 (2 h after dose) monitoring has also been implemented at some centers. The C2 level of cyclosporine may be appropriate in the range of 800-1400 ng/mL for the first 3 mo, 600-1000 ng/mL after 6 mo and 500-700 ng/mL after 1-year post transplantation[55].
The mostly commonly concerning adverse side effect of CNIs is their nephrotoxic effect at high levels. CNIs produce afferent renal arteriolar vasoconstriction that could induce renal dysfunction and also tubular injury. This vasoconstrictor effect is dose dependent and reversible. However, these agents may also have a role in inducing chronic renal injury or at least being a contributing factor along with other factors in non-renal solid organ transplant recipients[56].
Renal insufficiency has a special significance for LT in applying the model for end-stage liver disease as the major driver for organ allocation; more patients with renal dysfunction get priority for LT. These patients with higher risk of post transplant renal dysfunction may need immunosuppression regimens with less risk for nephrotoxicity, such as delayed CNI introduction, use of anti-metabolites for minimization or even elimination of CNI use.
Hypertension, neurotoxicity, metabolic abnormalities including hyperglycemia, electrolyte imbalance and hyperlipidemia are among the common adverse side effects of CNIs. The diabetogenic effect of tacrolimus is greater than cyclosporine[53]. Cyclosporine can cause hirsutism and gingival hyperplasia. Tacrolimus and cyclosporine could be associated with an increased risk of infection, although bone marrow suppression is rarely seen with CNIs in contrast with MPA and azathioprine. The risk of malignancy may be increased after chronic use of CNIs[57,58]. Another important issue while using CNIs is that their metabolism by cytochrome P450 predisposes them to an array of drug interactions that may raise or lower levels. In addition, certain foods (for example grapefruit) that can alter levels of p-glycoprotein can affect the absorption of CNIs and cause significant changes in CNI drug level exposure.
Mycophenolate mofetil: Mycophenolate mofetil (MMF, Cellcept®) and its active compound MPA is a reversible purine synthase inhibitor with anti-proliferative activity against T-cells and B-cells. It blocks the signal 3 of cell activation. There are other presumed mechanisms for immunomodulator effects of this agent. MPA can inhibit monocyte chemo-attraction, destroy activated lymphocytes and induce immune tolerance by affecting regulatory T-cell/helper T-cell balance[59].
As an immunosuppressive agent, MMF was introduced to the field of LT in the 1990s. Its lack of potential nephrotoxicity made it useful for patients with renal dysfunction who need to decrease CNIs doses. MMF is also a useful agent in combination with CNIs in immunosuppressive regimens where corticosteroid withdrawal is desirable. In the United States, the use of MMF for adult LT has been increased to over 80% of transplantations in 2012 usually as an adjunct with CNIs[60]. MMF and an enteric coated formulation mycophenolate sodium (EC-MPS Myfortic®) are the two preparations of MPA available. The bioavailability of MMF is high and monitoring of drug level is not usually recommended, though it may be useful in rare individuals. The average dose for MMF is 1 g every 12 h (720 mg every 12 h for EC-MPS), but it is better tolerated when is started with lower doses and gradually increased. Higher doses may be required but are not usually recommended. There are also reports on the non-linear pharmacokinetics of MPA with decreasing the bioavailability of drug by increasing the dose[59]. The major adverse side effects of MPA are hematologic and gastrointestinal (GI). Bone marrow suppression is usually dose dependent and responds to dose reduction. Nausea, vomiting, abdominal discomfort and diarrhea are common complaints in patients taking MPA derivatives. Dividing the dose to a four times a day schedule may be helpful; however, more serious adverse GI effects as inflammatory bowel disease-like colitis and graft vs host disease-like enteropathy related to MPA have been reported[61,62].
MPA seems to have a protective effect against post transplant de novo malignancies and when included in immunosuppression regimens may increase the time to develop malignancies[63], however, MPA was not shown to be effective in prevention of post liver transplant recurrence of HCC[64].
Azathioprine: Azathioprine is a purine synthase inhibitor and one of the first immunosuppressive agents used in the field of solid organ transplantation. For many years, azathioprine was included in the post organ transplant immunosuppressive maintenance as the only immunomodulatory agent and then later was used as an adjunct with CNIs. However, with the introduction of newer and more potent agents such as tacrolimus, the need for azathioprine was reduced and later it was replaced by MPA when a second agent was needed. Today, azathioprine is less commonly used for LT but may be helpful when there is a need for intensifying immunosuppression and when other agents are not tolerated due to their adverse side effects. In addition, as azathioprine is less costly, in some instances where finances are limited, it is preferred over MPA.
The major adverse side effects of azathioprine are related to bone marrow suppression and its hematologic consequences and hepatotoxicity. A minority of patients are at risk of developing severe bone marrow suppression due to genetically reduced or deficient thiopurine methyl transferase (TPMT) activity, the enzyme responsible for metabolizing 6-mercaptopurine, leading to over-accumulation of 6-thioguanine nucleotides (TGNs). TGNs are the active metabolites of azathioprine. Laboratory genotype or phenotype testing for TPMT may help to recognize these patients. In some other patients TGNs may fail to reach their therapeutic levels despite increasing drug dosage. In these patients who are at increased risk of hepatotoxicity due to accumulation of 6-methy-mercaptopurine (6-MMP), TGN level monitoring during treatment and the ratio of 6-MMP/TGN may be useful in helping to recognize this condition. The complexity, availability and cost of these tests should also be considered[65].
Mammalian target of rapamycin inhibitors: Sirolimus is a macrolide antibiotic which is structurally similar to tacrolimus and binds to FK binding protein but inhibits the mammalian target of rapamycin inhibitors (mTORI), the molecules with kinase activity, instead of inhibiting calcineurin. It acts through blocking signal 3 of cell activation from IL-2 receptors in T-cells and B-cells. Interestingly, despite binding to the same cell receptor, sirolimus and tacrolimus do not compete with each other and act synergistically[48]. Sirolimus was approved for use in renal transplantation by the Food and Drug Administration (FDA) in 1999. It has been considered a non-nephrotoxic immunosuppressant agent that might be replaced by CNIs in liver recipients with renal dysfunction; however, the benefit of this strategy has been questioned by some studies. In a randomized trial, Shenoy et al[66] demonstrated that although the early result of CNI replacing with sirolimus was promising, there was no significant renal function improvement after one year in liver recipients with renal dysfunction on sirolimus. No specific renal improvement benefit by CNI to sirolimus conversion was also demonstrated in another study by Abdelmalek et al[67], while higher biopsy proven rejections and treatment associated adverse side effects were seen in the sirolimus group. Even de novo use of sirolimus with low dose tacrolimus resulted in a high rate of graft loss, death and sepsis when compared with conventional tacrolimus dose, leading to the premature termination of a prospective randomized trial by Asrani et al[68]. Everolimus is another mTORI that when introduced early post LT and combined with low dose tacrolimus, showed promising results with respect to rejection rates and significant beneficial effects on renal function after 2 years[69]. However when used as a single agent without CNI, rejection rates were higher in the everolimus only group.
The other clinical utility of mTORIs in the field of LT is based on their anti-tumor effect. mTOR signaling plays a role in tumor angiogenesis and proliferation that is important in carcinogenesis of HCC[70]. In LT for HCC, sirolimus based immunosuppression was reported to be associated with lower tumor recurrence rate, longer recurrence-free survival and overall survival, and lower recurrence-related mortality compared with CNIs[71]. Everolimus has been used for cancer treatment in HCC. Its protective effect on post liver transplant HCC has not been sufficiently investigated in clinical trials, although reduced recurrence rate has been reported[72].
mTORIs are also possibly beneficial when included in immunosuppression regimen in recipients with malignancies other than HCC, but its use for this purpose needs to be explored by further studies[64].
The common adverse side effects related to mTORIs are edema, hyperlipidemia and oral ulcers. Another less common adverse side effect of mTORIs is proteinuria and glomerular injury that when present may be associated with further impairment of renal function than was present in LT recipients after conversion of CNIs to mTORIs[73]. Acute respiratory distress syndrome has been reported as a rare but lethal complication associated with sirolimus[74,75]. Pleural and pericardial effusion are among other rare complications of sirolimus[76].
mTORIs are also capable of impairing the surgical wound healing and thus may contribute to more wound complications when introduced very early after solid organ transplantation[77].
Preliminary reports of high incidence of complications led to adding a black box warning to sirolimus by the FDA for and increased risk in mortality, graft loss and hepatic artery thrombosis (HAT) following its use in LT recipients. Most cases of HAT in patients who received sirolimus early on occurred within 30 d of LT. The incidence of HAT related to sirolimus use varies in different reports. Molinari et al[78] reported a higher incidence of HAT in sirolimus group with CNI group but no HAT was reported in LT recipients on sirolimus in another study by Harper et al[79]. Similar results were reported by Fischer et al[80], and they did notice no HAT following conversion of CNIs to everolimus.
If use of one of the mTORIs is considered for an individual LT patient, it is preferred that it be started at least 1 mo post-transplant to avoid the risk of HAT and allow enough time for wound healing. Concomitant use of CNIs and mTORIs is suggested in order to decrease the risk of rejection with mTORIs alone.
Recipients with hepatitis C infection: HCV cirrhosis is one of the leading causes of LT in the world and the most common indication for LT in United States and Europe. Post transplantation recurrence of HCV and its complications are major causes of mortality and morbidity in this group of patients. HCV recurs in almost all LT recipients if the viral load is detectable at the time of transplant. In about 10% of LT recipients, recurrence of HCV is associated with rapidly progressive fibrosis leading to early graft loss. HCV recurrence in the remaining patients results in rapidly progressive fibrosis and cirrhosis in approximately 30% of patients within 5 years after LT[81].
Factors influencing the complications resulting from HCV recurrence are multiple and include host, donor, viral and immunosuppression related factors[82,83]. Recipient’s immune response, donor age, pre-transplant viral load and genotype are among implicated important factors.
The role of immunosuppression and tailoring the regimen for HCV recipients are challenging. There is evidence for association of early and severe recurrence of disease following treatment of steroid-responsive acute rejection episodes in liver recipients[83]. Numbers and severity of rejection episodes (both steroid responsive and steroid resistant) are also associated with early recurrence[84].
The beneficial effect of antibody induction in order to avoid steroids in HCV patients has not been documented, although its use has been thought to lower rates of acute cellular rejection. Several studies have shown the safety of ATG and daclizumab as induction agents in HCV patients who undergo LT[49,50].
In the trial by Klintmalm et al[50], severe fibrosis was less frequently observed in HCV recipients receiving steroid free immunosuppression with daclizumab induction compared with a steroid containing regimen at 1 year, but the difference between the groups was not statistically significant. In the same trial, patients without HCV recurrence in the daclizumab group at 1 year had a significantly reduced frequency of severe fibrosis at 2 years after LT[50].
It can be concluded that antibody induction is safe in HCV liver recipients and even may be associated with some benfits in this group of patients, but this needs to be documented in future studies. However, it should be considered that the safety of alemtuzumab in HCV patients is in doubt (as mentioned above).
The role of CNIs on the course of post transplantation HCV recurrence is challenging. There is evidence of in vitro anti replicative effect of cyclosporine on HCV RNA[85,86]. Cyclosporine has also been reported to show less interferrence with the anti-viral effect of interferon-α in HCV infected human hepatocytes when compared with tacrolimus[87]. The results of studies comparing the effect of cyclosporine and tacrolimus on post transplant HCV recurrence, fibrosis progression and sustained virologic response after treatment are conflicting[88].
However, improved long term patient survival was reported with tacrolimus compared to cyclosporine in HCV patients[89] and also, in a UNOS database analysis of 8809 HCV liver recipients by Irish et al[90] where patients on cyclosporine were reported to be at increased risks for patient death, graft loss and biopsy proven acute rejection compared to those on tacrolimus.
Use of mTORI, sirolimus in post LT immunosuppression regimens has raised some interest for its potential benefit in HCV patients. Sirolimus has proven anti-fibrotic effects in animal models[91,92] and has been shown to be associated with a reduced risk of significant fibrosis in post liver transplant HCV[93,94]. However, despite promising results for sirolimus on fibrosis progression in HCV patients post LT, use of sirolimus has been reported to be associated with increased mortality and graft loss both in HCV and non-HCV liver recipients and its use solely for this purpose could not be recommended[95].
Regarding the conflicting results of choice of immunosuppression on outcomes for HCV liver recipients, the advent of new and potent direct acting antiviral agents for the treatment of HCV and their utilization in pre and post-transplant HCV therapy may eliminate the need to focus on the effect of the immunosuppression regimen on HCV recurrence and progression in LT recipients.
Post LT pregnancy: With growing number of LTs as the curative treatment for end-stage liver diseases, more female candidates in child-bearing ages receive organs. There are many issues regarding the management and outcome of pregnancy after solid organ transplantation. Safe contraception, fertility, maternal risks, risk of miscarriage, fetal and neonatal outcome and risk of immunosuppression are among the most important ones.
Post liver transplant pregnancies are classified as high-risk pregnancies due to the increased rate of complications that include hypertension, preeclampsia and pre-term delivery[96].
Fewer complications may be expected when the pregnancy is planned. The time of conception is advised to be at least one year after transplantation, and some suggest waiting 2 years for a better outcome[96].
Overview and management of immunosuppression in liver transplanted female candidates for pregnancy need expertise to balance the risk of rejection and maternal and fetal complications.
Corticosteroids: If the patient is maintained on a low dose of corticosteroids due to the underlying liver disease etiology, like autoimmune disease, or because she has experienced episodes of rejection, there might be a need for an increased dose of steroid during pregnancy[97].
The risks related to use of steroids are gestational diabetes and hypertension that warrant special attention and management.
CNIs: CNIs are the main agents as maintenance immunosuppression in most LT recipients. Potential complications related to CNIs that may adversely affect the maternal outcome are hypertension, diabetes, renal insufficiency and neurotoxicity. Rate of these complications could be minimized by careful drug level monitoring during pregnancy. Reports on comparing the complications resulting from cyclosporine based vs tacrolimus based immunosuppression during pregnancy are conflicting. However, both agents seem to be safe during pregnancy and the complication rates may not be significantly different[98].
Azathioprine: Azathioprine is now less frequently used as an immunosuppressant agent in LT. When patients are on this medication, it seems to be reasonable to continue this agent during pregnancy as there is a low rate of reported risk[97].
MMF: MMF is teratogenic and is reported to be associated with multiple fetal defects and increased risk for miscarriage. It is advised to stop MMF in patients on this medication who wish to get pregnant at least 6 wk before conception.
For female liver recipients on MMF who have a plan for pregnancy, switching of MMF to another immunosuppressant agent rather than abrupt discontinuation may be considered in order to decrease the risk of acute rejection. Although the experience in the field of solid organ transplantation is limited, switching of MMF to azathioprine was reported to be associated with a favorable obstetric outcome in pregnant patients with lupus nephritis[99].
Sirolimus: Data on the safety of sirolimus during pregnancy and its teratogenicity is limited, although no significant fetal malformation has been reported[100].
Prevention and management of post LT recurrence of HCC is of great concern as more patients with HCC are receiving LT. Many factors can affect the risk of tumor recurrence. The role of immunosuppressive therapy in HCC recurrence is an important and challenging issue.
CNIs possess pro-oncogenic effects as documented in experimental models and also in clinical trials both retrospective and prospective[64]. Both tacrolimus and cyclosporine are associated with increased risk of de novo malignancies in solid organ transplantation. The risk of post liver transplant HCC recurrence has been shown to be related to the high blood levels of CNIs, particularly in the early post transplant period rather than the type of CNI[64,101].
The results of studies comparing the effect of the type of CNI (cyclosporine vs tacrolimus) are conflicting and it seems that the role of dosage and blood levels of CNIs is more important than the choice of agent[64,101,102].
Anti-lymphocyte antibodies which are increasingly used for induction of immunosuppression or treatment of steroid resistant rejections are also of concern for increasing the risk of post transplant HCC recurrence. Use of muromonab (although not available anymore) and ATG has been shown to be associated with increased risk of HCC recurrence[103].
Also, Basiliximab has been shown to have a negative impact on tumor recurrence when used as an induction therapy in HCC patients receiving LT[104].
MMF possesses anti-proliferative properties but has not been documented to play a role in prevention of HCC recurrence[64,102].
mTORIs (sirolimus and everolimus) possess anti-tumor properties (refer to above section of Maintenance of Immunosuppression) and are of particular interest for use in LT patients with HCC. The results of preliminary studies on both sirolimus and everolimus and their beneficial effect on post transplantation recurrence of HCC are promising[70-72,105].
Although many centers are already using mTORIs in recipients with HCC, the result of prospective, randomized control trials are required for recommendation on their use for this purpose.
In children both pharmacokinetic and pharmacodynamic of drugs are different from adults. All stages of drug absorption, distribution, metabolism and excretion are affected by a child’s age and will change when they reach adolescence. The major issue for post transplant immunosuppressive therapy in pediatrics is the lack of adequate control trials in this population of patients. Most of the immunosuppressive drugs used for pediatric liver recipients are used based on adult data. However, generally in younger children the clearance of most drugs is increased and most children need higher weight based doses of immunosuppressive medication.
CNIs, tacrolimus and cyclosporine are approved for use in pediatric liver recipients[106]. Tacrolimus based regimen is superior to cyclosporine based with significant improvement in patient and graft survival, less rejections, less hypertension and decreased dose of required corticosteroids.
Other immunosuppressant agents as MMF or mTORIs may be used off-label in pediatric LT for specific indications. Confronting the adverse side effects of CNIs, need for an auxiliary agent for management and further prevention of acute rejections or withdrawal from corticosteroids are among these indications.
T-cell depleting antibodies as ATG and IL-2Ra are increasingly used in children by some LT centers for indications similar to adults, though the experience with their use in this population is limited[106,107].
There are major issues specific for pediatric patients receiving post transplant immunosuppression that need special consideration.
Linear growth may be affected by immunosuppression therapy especially with corticosteroids. Thus, there is a desire for steroid-free immunosuppression or rapid withdrawal in pediatrics if possible[108].
Children are at increased risk of developing PTLD in comparison with adult due to higher risk of primary exposure to Epstein-Barr virus (EBV). Prolonged high-dose immunosuppressive therapy is also a major risk factor[106]. Special attention is needed for prevention of this complication (EBV screening) and early diagnosis in pediatric patients.
Treatment of rejection: With the advent of high efficacy immunosuppression regimen, the rate of acute cellular rejection (ACR) has been decreasing but still complicates up to about 25% of liver transplants, with less frequent rejections rates following living liver donation[9,10].
The diagnosis of ACR should be considered when there is evidence of abnormal liver tests and confirmed by liver biopsy. The other possible causes of abnormal liver test in a liver transplant patient should always be considered in the differential diagnoses and excluded. Among these, the most important are HAT, primary graft non-function, biliary leakage and sepsis in the early post transplant phase. Primary disease recurrence, particularly for HCV, viral infections such as CMV and EBV, recurrence of HBV, biliary strictures and drug toxicity occur later on post transplantation. An important challenge exists in differentiating HCV recurrence from acute cellular rejection, though acutely higher viral loads and presence of apoptotic bodies on biopsy favor HCV over rejection.
The histologic grading of acute cellular rejection is not always correlated with clinical outcomes[109]. It also may not precisely predict the response to medical management[110].
High dose iv corticosteroid is the first line of treatment for moderate to severe ACR in most centers. About 60%-80% of episodes of ACR are responsive to the first course of high dose corticosteroids, and the remainder may need more than one course or might be resistant to steroids. The method of corticosteroid pulse therapy (dosage and duration) varies between centers. Usually a 3 d course of 500-1000 mg of methylprednisolone per day is given to the patient. Volpin et al[111] have suggested a single dose of 1000 mg methylprednisolone followed by a 6 d tapering from 200 to 20 mg/d is more effective and associated with less infectious complications.
About 5%-15% of episodes of liver allograft ACRs are unresponsive to more than one course of dose steroids and thus called steroid resistant rejections (SRR). SRR episodes although rare are associated with poor outcomes[112].
Muromonab was among the first agents used in liver SRR with acceptable success rate; however, with withdrawal of muromonab from the market, this agent is no longer available. Following the promising results from renal transplantation, the polyclonal antibody, ATG was used for the treatment of SRR in most LT centers. The results have been promising with most cases of SRR responding to ATG[27,28]. For treatment of rejection, ATG is usually started with a dose of 1.5 mg/kg per day intravenously. The duration of treatment is variable between centers and also for any individual case ranging from 5 to 14 d. The absolute lymphocyte count may be monitored to achieve a goal of 200/mm3 or less.
The important issue in the treatment of SRR is monitoring for the treatment associated adverse side effects. Patients who have received treatment for SRR are at increased risk of infectious complications and malignancy.
IL-2Ras have been used for the treatment of SRR in LT. There are reports of successful treatment of SRR using basiliximab both in pediatric and adult LT groups[113,114]. Daclizumab has been also used as the rescue therapy for SRR with good results[115]. The promising results of using IL-2Ra for the treatment of SRR in LT and the limited related complication rates has been resulted in increasing use of these agents and their preference over anti-lymphocyte antibodies for this purpose[107].
Increasing the individual base level of immunosuppression is usually required in cases of ACR. This usually includes one or more of the following strategies: (1) Adjusting the CNIs to higher trough levels, if there is no contraindication; (2) Changing CNI to tacrolimus if the patient is already on cyclosporine; (3) Adding MMF (or azathioprine) or increasing their dose; and (4) Adding mTORI.
Withdrawal of immunosuppression: Withdrawal of immunosuppression is of particular interest with the aim of minimizing the adverse side effects and improving the quality of life in LT patients and the unique immune tolerant character of the liver makes this potentially possible. According to the published data, weaning of immunosuppression may be possible in up to 40% of liver recipients[116,117].
The success rate of weaning immune suppression may be even higher in pediatric patients receiving parental organs[14]. However, it is important to select the right candidate for weaning and perform the weaning at the best time.
The most important factor that correlates with successful immunosuppression weaning is the time from transplantation[8,118]. Other factors that have been implicated to have a role in a successful weaning in other studies are male gender, older age at the time of transplantation, and some have suggested the significance of biochemical indices as lower HLA mismatch between donor and recipient[117] and indexes of lymphocytes stimulation[8]. It should be considered that candidates for immunosuppression weaning have been carefully selected in most studies among patients with non-immune causes of liver disease, those with stable post transplantation liver function and less rejection episodes.
In general, it could be stated that complete immunosuppression withdrawal is possible in highly selected liver recipients. Further studies are required to identify the important clinical and paraclinical factors for a confident and successful weaning.
Transplant immunology is an ever changing science. Further elucidation of interacting cellular mechanisms involved in rejection and graft tolerance would be welcomed by transplant physicians and patients alike. The aim of treatment is to maximize the effectiveness and minimize the adverse side effects of immunosuppressive therapy with the hope for a higher quality and increased longevity of the graft and patient’s life. Any individual recipient needs a customized immunosuppression regimen according to: (1) age that will change over time; (2) the co-morbid conditions as renal failure and diabetes with the preference of CNI minimizing or avoiding; (3) transplantation indications as autoimmune hepatitis that need a longer duration of steroid therapy, HCV infection requiring early steroid withdrawal and concomitant HCC with possible benefit from mTORIs; (4) the behavior of the graft over time; intensifying the immunosuppression with repeated rejection episodes and lowering in the absence of rejection; (5) occurrence of immunosuppression related complications with the need for lowering the dose or changing the specific agents to another one; and (6) physiologic conditions after transplantation as pregnancy, weight loss or gain requiring changes in dosage or agent choice.
Future studies would enable the art of individually designed immunosuppression for individual transplant recipients.
P- Reviewer: Ma L, Schuurman HJ S- Editor: Song XX L- Editor: A E- Editor: Liu SQ
| 1. | Adam R, Hoti E. Liver transplantation: the current situation. Semin Liver Dis. 2009;29:3-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 2. | Crispe IN. Immune tolerance in liver disease. Hepatology. 2014;60:2109-2117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 210] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 3. | Crispe IN, Giannandrea M, Klein I, John B, Sampson B, Wuensch S. Cellular and molecular mechanisms of liver tolerance. Immunol Rev. 2006;213:101-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 181] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 4. | Opelz G, Wujciak T, Döhler B, Scherer S, Mytilineos J. HLA compatibility and organ transplant survival. Collaborative Transplant Study. Rev Immunogenet. 1999;1:334-342. [PubMed] |
| 5. | Opelz G, Margreiter R, Döhler B. Prolongation of long-term kidney graft survival by a simultaneous liver transplant: the liver does it, and the heart does it too. Transplantation. 2002;74:1390-1394; discussion 1370-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 6. | Raichlin E, Daly RC, Rosen CB, McGregor CG, Charlton MR, Frantz RP, Clavell AL, Rodeheffer RJ, Pereira NL, Kremers WK. Combined heart and liver transplantation: a single-center experience. Transplantation. 2009;88:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 102] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Rana A, Robles S, Russo MJ, Halazun KJ, Woodland DC, Witkowski P, Ratner LE, Hardy MA. The combined organ effect: protection against rejection? Ann Surg. 2008;248:871-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 8. | de la Garza RG, Sarobe P, Merino J, Lasarte JJ, D’Avola D, Belsue V, Delgado JA, Silva L, Iñarrairaegui M, Sangro B. Trial of complete weaning from immunosuppression for liver transplant recipients: factors predictive of tolerance. Liver Transpl. 2013;19:937-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 9. | Fisher RA, Cotterell AH, Maluf DG, Stravitz RT, Ashworth A, Nakatsuka M, Sterling RK, Luketic VA, Behnke MK, Posner MP. Adult living donor versus deceased donor liver transplantation: a 10-year prospective single center experience. Ann Hepatol. 2009;8:298-307. [PubMed] |
| 10. | Maluf DG, Stravitz RT, Cotterell AH, Posner MP, Nakatsuka M, Sterling RK, Luketic VA, Shiffman ML, Ham JM, Marcos A. Adult living donor versus deceased donor liver transplantation: a 6-year single center experience. Am J Transplant. 2005;5:149-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 125] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 11. | Shaked A, Ghobrial RM, Merion RM, Shearon TH, Emond JC, Fair JH, Fisher RA, Kulik LM, Pruett TL, Terrault NA. Incidence and severity of acute cellular rejection in recipients undergoing adult living donor or deceased donor liver transplantation. Am J Transplant. 2009;9:301-308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 128] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 12. | Sieders E, Hepkema BG, Peeters PM, TenVergert EM, de Jong KP, Porte RJ, Bijleveld CM, van den Berg AP, Lems SP, Gouw AS. The effect of HLA mismatches, shared cross-reactive antigen groups, and shared HLA-DR antigens on the outcome after pediatric liver transplantation. Liver Transpl. 2005;11:1541-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Starzl TE, Marchioro TL, Vonkaulla KN, Hermann G, Brittain RS, Waddell WR. Homotransplantation of the liver in humans. Surg Gynecol Obstet. 1963;117:659-676. [PubMed] |
| 14. | Feng S, Ekong UD, Lobritto SJ, Demetris AJ, Roberts JP, Rosenthal P, Alonso EM, Philogene MC, Ikle D, Poole KM. Complete immunosuppression withdrawal and subsequent allograft function among pediatric recipients of parental living donor liver transplants. JAMA. 2012;307:283-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 263] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 15. | Kirk AD. Induction immunosuppression. Transplantation. 2006;82:593-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 101] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Starzl TE, Marchioro TL, Waddell WR. The reversal of rejection in human renal homografts with subsequent development of homograft tolerance. Surg Gynecol Obstet. 1963;117:385-395. [PubMed] |
| 17. | Liu CL, Fan ST, Lo CM, Chan SC, Ng IO, Lai CL, Wong J. Interleukin-2 receptor antibody (basiliximab) for immunosuppressive induction therapy after liver transplantation: a protocol with early elimination of steroids and reduction of tacrolimus dosage. Liver Transpl. 2004;10:728-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Bush WW. Overview of transplantation immunology and the pharmacotherapy of adult solid organ transplant recipients: focus on immunosuppression. AACN Clin Issues. 1999;10:253-269; quiz 304-306. [PubMed] |
| 19. | Eckhoff DE, McGuire B, Sellers M, Contreras J, Frenette L, Young C, Hudson S, Bynon JS. The safety and efficacy of a two-dose daclizumab (zenapax) induction therapy in liver transplant recipients. Transplantation. 2000;69:1867-1872. [PubMed] |
| 20. | Vincenti F, Kirkman R, Light S, Bumgardner G, Pescovitz M, Halloran P, Neylan J, Wilkinson A, Ekberg H, Gaston R. Interleukin-2-receptor blockade with daclizumab to prevent acute rejection in renal transplantation. Daclizumab Triple Therapy Study Group. N Engl J Med. 1998;338:161-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 721] [Cited by in RCA: 644] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 21. | Kato T, Gaynor JJ, Yoshida H, Montalvano M, Takahashi H, Pyrsopoulos N, Nishida S, Moon J, Selvaggi G, Levi D. Randomized trial of steroid-free induction versus corticosteroid maintenance among orthotopic liver transplant recipients with hepatitis C virus: impact on hepatic fibrosis progression at one year. Transplantation. 2007;84:829-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Lupo L, Panzera P, Tandoi F, Carbotta G, Giannelli G, Santantonio T, Rendina M, Gentile A, Memeo V. Basiliximab versus steroids in double therapy immunosuppression in liver transplantation: a prospective randomized clinical trial. Transplantation. 2008;86:925-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Penninga L, Wettergren A, Wilson CH, Chan AW, Steinbrüchel DA, Gluud C. Antibody induction versus corticosteroid induction for liver transplant recipients. Cochrane Database Syst Rev. 2014;5:CD010252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Beckebaum S, Cicinnati VR, Radtke A, Kabar I. Calcineurin inhibitors in liver transplantation - still champions or threatened by serious competitors? Liver Int. 2013;33:656-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Kaden J, Völp A, Wesslau C. High graft protection and low incidences of infections, malignancies and other adverse effects with intra-operative high dose ATG-induction: a single centre cohort study of 760 cases. Ann Transplant. 2013;18:9-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Gotthardt DN, Bruns H, Weiss KH, Schemmer P. Current strategies for immunosuppression following liver transplantation. Langenbecks Arch Surg. 2014;399:981-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Bijleveld CG, Klompmaker IJ, van den Berg AP, Gouw AS, Hepkema BG, Haagsma EB, Verwer R, Slooff MJ. Incidence, risk factors, and outcome of antithymocyte globulin treatment of steroid-resistant rejection after liver transplantation. Transpl Int. 1996;9:570-575. [PubMed] |
| 28. | Schmitt TM, Phillips M, Sawyer RG, Northup P, Hagspiel KD, Pruett TL, Bonatti HJ. Anti-thymocyte globulin for the treatment of acute cellular rejection following liver transplantation. Dig Dis Sci. 2010;55:3224-3234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Langrehr JM, Nüssler NC, Neumann U, Guckelberger O, Lohmann R, Radtke A, Jonas S, Klupp J, Steinmüller T, Lobeck H. A prospective randomized trial comparing interleukin-2 receptor antibody versus antithymocyte globulin as part of a quadruple immunosuppressive induction therapy following orthotopic liver transplantation. Transplantation. 1997;63:1772-1781. [PubMed] |
| 30. | Benítez CE, Puig-Pey I, López M, Martínez-Llordella M, Lozano JJ, Bohne F, Londoño MC, García-Valdecasas JC, Bruguera M, Navasa M. ATG-Fresenius treatment and low-dose tacrolimus: results of a randomized controlled trial in liver transplantation. Am J Transplant. 2010;10:2296-2304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 31. | Deeks ED, Keating GM. Rabbit antithymocyte globulin (thymoglobulin): a review of its use in the prevention and treatment of acute renal allograft rejection. Drugs. 2009;69:1483-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 32. | Brennan DC, Flavin K, Lowell JA, Howard TK, Shenoy S, Burgess S, Dolan S, Kano JM, Mahon M, Schnitzler MA. A randomized, double-blinded comparison of Thymoglobulin versus Atgam for induction immunosuppressive therapy in adult renal transplant recipients. Transplantation. 1999;67:1011-1018. [PubMed] |
| 33. | Soliman T, Hetz H, Burghuber C, Györi G, Silberhumer G, Steininger R, Mühlbacher F, Berlakovich GA. Short-term versus long-term induction therapy with antithymocyte globulin in orthotopic liver transplantation. Transpl Int. 2007;20:447-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Mangus RS, Fridell JA, Vianna RM, Kwo PY, Chen J, Tector AJ. Immunosuppression induction with rabbit anti-thymocyte globulin with or without rituximab in 1000 liver transplant patients with long-term follow-up. Liver Transpl. 2012;18:786-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 35. | Peddi VR, Bryant M, Roy-Chaudhury P, Woodle ES, First MR. Safety, efficacy, and cost analysis of thymoglobulin induction therapy with intermittent dosing based on CD3+ lymphocyte counts in kidney and kidney-pancreas transplant recipients. Transplantation. 2002;73:1514-1518. [PubMed] |
| 36. | Busani S, Rinaldi L, Begliomini B, Pasetto A, Girardis M. Thymoglobulin-induced severe cardiovascular reaction and acute renal failure in a patient scheduled for orthotopic liver transplantation. Minerva Anestesiol. 2006;72:243-248. [PubMed] |
| 37. | Lundquist AL, Chari RS, Wood JH, Miller GG, Schaefer HM, Raiford DS, Wright KJ, Gorden DL. Serum sickness following rabbit antithymocyte-globulin induction in a liver transplant recipient: case report and literature review. Liver Transpl. 2007;13:647-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 38. | Soliman T, Hetz H, Burghuber C, Györi G, Silberhumer G, Steininger R, Mühlbacher F, Berlakovich GA. Short-term induction therapy with anti-thymocyte globulin and delayed use of calcineurin inhibitors in orthotopic liver transplantation. Liver Transpl. 2007;13:1039-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 39. | Eason JD, Blazek J, Mason A, Nair S, Loss GE. Steroid-free immunosuppression through thymoglobulin induction in liver transplantation. Transplant Proc. 2001;33:1470-1471. [PubMed] |
| 40. | Garcia-Saenz-de-Sicilia M, Olivera-Martinez MA, Grant WJ, Mercer DF, Baojjang C, Langnas A, McCashland T. Impact of anti-thymocyte globulin during immunosuppression induction in patients with hepatitis C after liver transplantation. Dig Dis Sci. 2014;59:2804-2812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 41. | De Ruvo N, Cucchetti A, Lauro A, Masetti M, Cautero N, Di Benedetto F, Dazzi A, Del Gaudio M, Ravaioli M, Zanello M. Preliminary results of immunosuppression with thymoglobuline pretreatment and hepatitis C virus recurrence in liver transplantation. Transplant Proc. 2005;37:2607-2608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 42. | Marks WH, Ilsley JN, Dharnidharka VR. Posttransplantation lymphoproliferative disorder in kidney and heart transplant recipients receiving thymoglobulin: a systematic review. Transplant Proc. 2011;43:1395-1404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 43. | Magliocca JF, Knechtle SJ. The evolving role of alemtuzumab (Campath-1H) for immunosuppressive therapy in organ transplantation. Transpl Int. 2006;19:705-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 98] [Article Influence: 5.2] [Reference Citation Analysis (1)] |
| 44. | Dhesi S, Boland B, Colquhoun S. Alemtuzumab and liver transplantation: a review. Curr Opin Organ Transplant. 2009;14:245-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 45. | Tzakis AG, Tryphonopoulos P, Kato T, Nishida S, Levi DM, Madariaga JR, Gaynor JJ, De Faria W, Regev A, Esquenazi V. Preliminary experience with alemtuzumab (Campath-1H) and low-dose tacrolimus immunosuppression in adult liver transplantation. Transplantation. 2004;77:1209-1214. [PubMed] |
| 46. | Levitsky J, Thudi K, Ison MG, Wang E, Abecassis M. Alemtuzumab induction in non-hepatitis C positive liver transplant recipients. Liver Transpl. 2011;17:32-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 47. | Marcos A, Eghtesad B, Fung JJ, Fontes P, Patel K, Devera M, Marsh W, Gayowski T, Demetris AJ, Gray EA. Use of alemtuzumab and tacrolimus monotherapy for cadaveric liver transplantation: with particular reference to hepatitis C virus. Transplantation. 2004;78:966-971. [PubMed] |
| 48. | Mukherjee S, Mukherjee U. A comprehensive review of immunosuppression used for liver transplantation. J Transplant. 2009;2009:701464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 49. | Uemura T, Schaefer E, Hollenbeak CS, Khan A, Kadry Z. Outcome of induction immunosuppression for liver transplantation comparing anti-thymocyte globulin, daclizumab, and corticosteroid. Transpl Int. 2011;24:640-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 50. | Klintmalm GB, Davis GL, Teperman L, Netto GJ, Washburn K, Rudich SM, Pomfret EA, Vargas HE, Brown R, Eckhoff D. A randomized, multicenter study comparing steroid-free immunosuppression and standard immunosuppression for liver transplant recipients with chronic hepatitis C. Liver Transpl. 2011;17:1394-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 51. | Ramirez CB, Doria C, di Francesco F, Iaria M, Kang Y, Marino IR. Basiliximab induction in adult liver transplant recipients with 93% rejection-free patient and graft survival at 24 months. Transplant Proc. 2006;38:3633-3635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 52. | Iwatsuki S, Starzl TE, Todo S, Gordon RD, Esquivel CO, Tzakis AG, Makowka L, Marsh JW, Koneru B, Stieber A. Experience in 1,000 liver transplants under cyclosporine-steroid therapy: a survival report. Transplant Proc. 1988;20:498-504. [PubMed] |
| 53. | Haddad EM, McAlister VC, Renouf E, Malthaner R, Kjaer MS, Gluud LL. Cyclosporin versus tacrolimus for liver transplanted patients. Cochrane Database Syst Rev. 2006;CD005161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 96] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 54. | Rodríguez-Perálvarez M, Germani G, Darius T, Lerut J, Tsochatzis E, Burroughs AK. Tacrolimus trough levels, rejection and renal impairment in liver transplantation: a systematic review and meta-analysis. Am J Transplant. 2012;12:2797-2814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 136] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 55. | Villamil F, Pollard S. C2 monitoring of cyclosporine in de novo liver transplant recipients: the clinician’s perspective. Liver Transpl. 2004;10:577-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 56. | Issa N, Kukla A, Ibrahim HN. Calcineurin inhibitor nephrotoxicity: a review and perspective of the evidence. Am J Nephrol. 2013;37:602-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 173] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 57. | Doesch AO, Müller S, Konstandin M, Celik S, Kristen A, Frankenstein L, Ehlermann P, Sack FU, Katus HA, Dengler TJ. Malignancies after heart transplantation: incidence, risk factors, and effects of calcineurin inhibitor withdrawal. Transplant Proc. 2010;42:3694-3699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 58. | Berardinelli L, Messa PG, Pozzoli E, Beretta C, Montagnino G. Malignancies in 2,753 kidney recipients transplanted during a 39-year experience. Transplant Proc. 2009;41:1231-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 59. | Staatz CE, Tett SE. Pharmacology and toxicology of mycophenolate in organ transplant recipients: an update. Arch Toxicol. 2014;88:1351-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 60. | Kim WR, Smith JM, Skeans MA, Schladt DP, Schnitzler MA, Edwards EB, Harper AM, Wainright JL, Snyder JJ, Israni AK. OPTN/SRTR 2012 Annual Data Report: liver. Am J Transplant. 2014;14 Suppl 1:69-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 170] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 61. | Parfitt JR, Jayakumar S, Driman DK. Mycophenolate mofetil-related gastrointestinal mucosal injury: variable injury patterns, including graft-versus-host disease-like changes. Am J Surg Pathol. 2008;32:1367-1372. [PubMed] |
| 62. | Al-Absi AI, Cooke CR, Wall BM, Sylvestre P, Ismail MK, Mya M. Patterns of injury in mycophenolate mofetil-related colitis. Transplant Proc. 2010;42:3591-3593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 63. | Robson R, Cecka JM, Opelz G, Budde M, Sacks S. Prospective registry-based observational cohort study of the long-term risk of malignancies in renal transplant patients treated with mycophenolate mofetil. Am J Transplant. 2005;5:2954-2960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 162] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 64. | Rodríguez-Perálvarez M, De la Mata M, Burroughs AK. Liver transplantation: immunosuppression and oncology. Curr Opin Organ Transplant. 2014;19:253-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 65. | Ford LT, Berg JD. Thiopurine S-methyltransferase (TPMT) assessment prior to starting thiopurine drug treatment; a pharmacogenomic test whose time has come. J Clin Pathol. 2010;63:288-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 66. | Shenoy S, Hardinger KL, Crippin J, Desai N, Korenblat K, Lisker-Melman M, Lowell JA, Chapman W. Sirolimus conversion in liver transplant recipients with renal dysfunction: a prospective, randomized, single-center trial. Transplantation. 2007;83:1389-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 67. | Abdelmalek MF, Humar A, Stickel F, Andreone P, Pascher A, Barroso E, Neff GW, Ranjan D, Toselli LT, Gane EJ. Sirolimus conversion regimen versus continued calcineurin inhibitors in liver allograft recipients: a randomized trial. Am J Transplant. 2012;12:694-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 68. | Asrani SK, Wiesner RH, Trotter JF, Klintmalm G, Katz E, Maller E, Roberts J, Kneteman N, Teperman L, Fung JJ. De novo sirolimus and reduced-dose tacrolimus versus standard-dose tacrolimus after liver transplantation: the 2000-2003 phase II prospective randomized trial. Am J Transplant. 2014;14:356-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 69. | Saliba F, De Simone P, Nevens F, De Carlis L, Metselaar HJ, Beckebaum S, Jonas S, Sudan D, Fischer L, Duvoux C. Renal function at two years in liver transplant patients receiving everolimus: results of a randomized, multicenter study. Am J Transplant. 2013;13:1734-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 135] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 70. | Finn RS. Current and Future Treatment Strategies for Patients with Advanced Hepatocellular Carcinoma: Role of mTOR Inhibition. Liver Cancer. 2012;1:247-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 71. | Menon KV, Hakeem AR, Heaton ND. Meta-analysis: recurrence and survival following the use of sirolimus in liver transplantation for hepatocellular carcinoma. Aliment Pharmacol Ther. 2013;37:411-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 106] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 72. | Jeng LB, Thorat A, Hsieh YW, Yang HR, Yeh CC, Chen TH, Hsu SC, Hsu CH. Experience of using everolimus in the early stage of living donor liver transplantation. Transplant Proc. 2014;46:744-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 73. | Wadei HM, Zaky ZS, Keaveny AP, Rosser B, Jones M, Mai ML, Bulatao I, Gonwa TA. Proteinuria following sirolimus conversion is associated with deterioration of kidney function in liver transplant recipients. Transplantation. 2012;93:1006-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 74. | Das BB, Shoemaker L, Subramanian S, Johnsrude C, Recto M, Austin EH. Acute sirolimus pulmonary toxicity in an infant heart transplant recipient: case report and literature review. J Heart Lung Transplant. 2007;26:296-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 75. | Amigues L, Klouche K, Massanet P, Gaillard N, Garrigue V, Beraud JJ, Mourad G. Sirolimus-associated acute respiratory distress syndrome in a renal transplant recipient. Transplant Proc. 2005;37:2830-2831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 76. | Montalbano M, Neff GW, Yamashiki N, Meyer D, Bettiol M, Slapak-Green G, Ruiz P, Manten E, Safdar K, O’Brien C. A retrospective review of liver transplant patients treated with sirolimus from a single center: an analysis of sirolimus-related complications. Transplantation. 2004;78:264-268. [PubMed] |
| 77. | Pengel LH, Liu LQ, Morris PJ. Do wound complications or lymphoceles occur more often in solid organ transplant recipients on mTOR inhibitors? A systematic review of randomized controlled trials. Transpl Int. 2011;24:1216-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 78. | Molinari M, Berman K, Meeberg G, Shapiro JA, Bigam D, Trotter JF, Kneteman N. Multicentric outcome analysis of sirolimus-based immunosuppression in 252 liver transplant recipients. Transpl Int. 2010;23:155-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 79. | Harper SJ, Gelson W, Harper IG, Alexander GJ, Gibbs P. Switching to sirolimus-based immune suppression after liver transplantation is safe and effective: a single-center experience. Transplantation. 2011;91:128-132. [PubMed] |
| 80. | Fischer L, Klempnauer J, Beckebaum S, Metselaar HJ, Neuhaus P, Schemmer P, Settmacher U, Heyne N, Clavien PA, Muehlbacher F. A randomized, controlled study to assess the conversion from calcineurin-inhibitors to everolimus after liver transplantation--PROTECT. Am J Transplant. 2012;12:1855-1865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 156] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 81. | Rodriguez-Luna H, Douglas DD. Natural history of hepatitis C following liver transplantation. Curr Opin Infect Dis. 2004;17:363-371. [PubMed] |
| 82. | Joshi D, Pinzani M, Carey I, Agarwal K. Recurrent HCV after liver transplantation-mechanisms, assessment and therapy. Nat Rev Gastroenterol Hepatol. 2014;11:710-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 83. | Gane EJ. The natural history of recurrent hepatitis C and what influences this. Liver Transpl. 2008;14 Suppl 2:S36-S44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 192] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 84. | Sheiner PA, Schwartz ME, Mor E, Schluger LK, Theise N, Kishikawa K, Kolesnikov V, Bodenheimer H, Emre S, Miller CM. Severe or multiple rejection episodes are associated with early recurrence of hepatitis C after orthotopic liver transplantation. Hepatology. 1995;21:30-34. [PubMed] |
| 85. | Nakagawa M, Sakamoto N, Enomoto N, Tanabe Y, Kanazawa N, Koyama T, Kurosaki M, Maekawa S, Yamashiro T, Chen CH. Specific inhibition of hepatitis C virus replication by cyclosporin A. Biochem Biophys Res Commun. 2004;313:42-47. [PubMed] |
| 86. | Watashi K, Hijikata M, Hosaka M, Yamaji M, Shimotohno K. Cyclosporin A suppresses replication of hepatitis C virus genome in cultured hepatocytes. Hepatology. 2003;38:1282-1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 409] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 87. | Hirano K, Ichikawa T, Nakao K, Matsumoto A, Miyaaki H, Shibata H, Eguchi S, Takatsuki M, Ikeda M, Yamasaki H. Differential effects of calcineurin inhibitors, tacrolimus and cyclosporin a, on interferon-induced antiviral protein in human hepatocyte cells. Liver Transpl. 2008;14:292-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 88. | Duvoux C, Firpi R, Grazi GL, Levy G, Renner E, Villamil F. Recurrent hepatitis C virus infection post liver transplantation: impact of choice of calcineurin inhibitor. Transpl Int. 2013;26:358-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 89. | Wiesner RH. A long-term comparison of tacrolimus (FK506) versus cyclosporine in liver transplantation: a report of the United States FK506 Study Group. Transplantation. 1998;66:493-499. [PubMed] |
| 90. | Irish WD, Arcona S, Bowers D, Trotter JF. Cyclosporine versus tacrolimus treated liver transplant recipients with chronic hepatitis C: outcomes analysis of the UNOS/OPTN database. Am J Transplant. 2011;11:1676-1685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 91. | Biecker E, De Gottardi A, Neef M, Unternährer M, Schneider V, Ledermann M, Sägesser H, Shaw S, Reichen J. Long-term treatment of bile duct-ligated rats with rapamycin (sirolimus) significantly attenuates liver fibrosis: analysis of the underlying mechanisms. J Pharmacol Exp Ther. 2005;313:952-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 92. | Neef M, Ledermann M, Saegesser H, Schneider V, Reichen J. Low-dose oral rapamycin treatment reduces fibrogenesis, improves liver function, and prolongs survival in rats with established liver cirrhosis. J Hepatol. 2006;45:786-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 115] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 93. | Kelly MA, Kaplan M, Nydam T, Wachs M, Bak T, Kam I, Zimmerman MA. Sirolimus reduces the risk of significant hepatic fibrosis after liver transplantation for hepatitis C virus: a single-center experience. Transplant Proc. 2013;45:3325-3328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 94. | McKenna GJ, Trotter JF, Klintmalm E, Onaca N, Ruiz R, Jennings LW, Neri M, O’Leary JG, Davis GL, Levy MF. Limiting hepatitis C virus progression in liver transplant recipients using sirolimus-based immunosuppression. Am J Transplant. 2011;11:2379-2387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 95. | Watt KD, Dierkhising R, Heimbach JK, Charlton MR. Impact of sirolimus and tacrolimus on mortality and graft loss in liver transplant recipients with or without hepatitis C virus: an analysis of the Scientific Registry of Transplant Recipients Database. Liver Transpl. 2012;18:1029-1036. [PubMed] |
| 96. | Deshpande NA, James NT, Kucirka LM, Boyarsky BJ, Garonzik-Wang JM, Cameron AM, Singer AL, Dagher NN, Segev DL. Pregnancy outcomes of liver transplant recipients: a systematic review and meta-analysis. Liver Transpl. 2012;18:621-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 97. | Parhar KS, Gibson PS, Coffin CS. Pregnancy following liver transplantation: review of outcomes and recommendations for management. Can J Gastroenterol. 2012;26:621-626. [PubMed] |
| 98. | Christopher V, Al-Chalabi T, Richardson PD, Muiesan P, Rela M, Heaton ND, O’Grady JG, Heneghan MA. Pregnancy outcome after liver transplantation: a single-center experience of 71 pregnancies in 45 recipients. Liver Transpl. 2006;12:1138-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 110] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 99. | Fischer-Betz R, Specker C, Brinks R, Aringer M, Schneider M. Low risk of renal flares and negative outcomes in women with lupus nephritis conceiving after switching from mycophenolate mofetil to azathioprine. Rheumatology (Oxford). 2013;52:1070-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 100. | Sifontis NM, Coscia LA, Constantinescu S, Lavelanet AF, Moritz MJ, Armenti VT. Pregnancy outcomes in solid organ transplant recipients with exposure to mycophenolate mofetil or sirolimus. Transplantation. 2006;82:1698-1702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 276] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 101. | Rodríguez-Perálvarez M, Tsochatzis E, Naveas MC, Pieri G, García-Caparrós C, O’Beirne J, Poyato-González A, Ferrín-Sánchez G, Montero-Álvarez JL, Patch D. Reduced exposure to calcineurin inhibitors early after liver transplantation prevents recurrence of hepatocellular carcinoma. J Hepatol. 2013;59:1193-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 188] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 102. | Chen K, Man K, Metselaar HJ, Janssen HL, Peppelenbosch MP, Pan Q. Rationale of personalized immunosuppressive medication for hepatocellular carcinoma patients after liver transplantation. Liver Transpl. 2014;20:261-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 103. | Decaens T, Roudot-Thoraval F, Bresson-Hadni S, Meyer C, Gugenheim J, Durand F, Bernard PH, Boillot O, Compagnon P, Calmus Y. Role of immunosuppression and tumor differentiation in predicting recurrence after liver transplantation for hepatocellular carcinoma: a multicenter study of 412 patients. World J Gastroenterol. 2006;12:7319-7325. [PubMed] |
| 104. | Lee JY, Kim YH, Yi NJ, Kim HS, Lee HS, Lee BK, Kim H, Choi YR, Hong G, Lee KW. Impact of immunosuppressant therapy on early recurrence of hepatocellular carcinoma after liver transplantation. Clin Mol Hepatol. 2014;20:192-203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 105. | Chinnakotla S, Davis GL, Vasani S, Kim P, Tomiyama K, Sanchez E, Onaca N, Goldstein R, Levy M, Klintmalm GB. Impact of sirolimus on the recurrence of hepatocellular carcinoma after liver transplantation. Liver Transpl. 2009;15:1834-1842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 106. | Dhawan A. Immunosuppression in pediatric liver transplantation: are little people different? Liver Transpl. 2011;17 Suppl 3:S13-S19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 107. | Thangarajah D, O’Meara M, Dhawan A. Management of acute rejection in paediatric liver transplantation. Paediatr Drugs. 2013;15:459-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 108. | Al-Sinani S, Dhawan A. Corticosteroids usage in pediatric liver transplantation: To be or not to be! Pediatr Transplant. 2009;13:160-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 109. | Ludwig J. Classification and terminology of hepatic allograft rejection: whither bound? Mayo Clin Proc. 1989;64:676-679. [PubMed] |
| 110. | Höroldt BS, Burattin M, Gunson BK, Bramhall SR, Nightingale P, Hübscher SG, Neuberger JM. Does the Banff rejection activity index predict outcome in patients with early acute cellular rejection following liver transplantation? Liver Transpl. 2006;12:1144-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 111. | Volpin R, Angeli P, Galioto A, Fasolato S, Neri D, Barbazza F, Merenda R, Del Piccolo F, Strazzabosco M, Casagrande F. Comparison between two high-dose methylprednisolone schedules in the treatment of acute hepatic cellular rejection in liver transplant recipients: a controlled clinical trial. Liver Transpl. 2002;8:527-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 112. | Wu L, Tam N, Deng R, Wu C, Chen P, Wang D, He X. Steroid-resistant acute rejection after cadaveric liver transplantation: experience from one single center. Clin Res Hepatol Gastroenterol. 2014;38:592-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 113. | Aw MM, Taylor RM, Verma A, Parke A, Baker AJ, Hadzic D, Muiesan P, Rela M, Heaton ND, Mieli-Vergani G. Basiliximab (Simulect) for the treatment of steroid-resistant rejection in pediatric liver transpland recipients: a preliminary experience. Transplantation. 2003;75:796-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 114. | Fernandes ML, Lee YM, Sutedja D, Wai CT, Isacc J, Prabhakaran K, Lim SG, Lee KH. Treatment of steroid-resistant acute liver transplant rejection with basiliximab. Transplant Proc. 2005;37:2179-2180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 115. | Orr DW, Portmann BC, Knisely AS, Stoll S, Rela M, Muiesan P, Bowles MJ, Heaton ND, O’Grady JG, Heneghan MA. Anti-interleukin 2 receptor antibodies and mycophenolate mofetil for treatment of steroid-resistant rejection in adult liver transplantation. Transplant Proc. 2005;37:4373-4379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 116. | Takatsuki M, Uemoto S, Inomata Y, Egawa H, Kiuchi T, Fujita S, Hayashi M, Kanematsu T, Tanaka K. Weaning of immunosuppression in living donor liver transplant recipients. Transplantation. 2001;72:449-454. [PubMed] |
| 117. | Devlin J, Doherty D, Thomson L, Wong T, Donaldson P, Portmann B, Williams R. Defining the outcome of immunosuppression withdrawal after liver transplantation. Hepatology. 1998;27:926-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 173] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 118. | Benítez C, Londoño MC, Miquel R, Manzia TM, Abraldes JG, Lozano JJ, Martínez-Llordella M, López M, Angelico R, Bohne F. Prospective multicenter clinical trial of immunosuppressive drug withdrawal in stable adult liver transplant recipients. Hepatology. 2013;58:1824-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 250] [Article Influence: 20.8] [Reference Citation Analysis (0)] |