Published online Jan 27, 2015. doi: 10.4254/wjh.v7.i1.121
Peer-review started: July 17, 2014
First decision: July 29, 2014
Revised: August 15, 2014
Accepted: November 7, 2014
Article in press: November 10, 2014
Published online: January 27, 2015
Processing time: 177 Days and 21.2 Hours
The consequences of hepatitis B virus (HBV) and human immunodeficiency virus (HIV) co-infection on progression of severe liver diseases is a serious public health issue, worldwide. In the co-infection cases, about 90% of HIV-infected population is seropositive for HBV where approximately 5%-40% individuals are chronically infected. In HIV co-infected individuals, liver-related mortality is estimated over 17 times higher than those with HBV mono-infection. The spectrum of HIV-induced liver diseases includes hepatitis, steatohepatitis, endothelialitis, necrosis, granulomatosis, cirrhosis and carcinoma. Moreover, HIV co-infection significantly alters the natural history of hepatitis B, and therefore complicates the disease management. Though several studies have demonstrated impact of HIV proteins on hepatocyte biology, only a few data is available on interactions between HBV and HIV proteins. Thus, the clinical spectrum as well as the complexity of the co-infection offers challenging fronts to study the underlying molecular mechanisms, and to design effective therapeutic strategies.
Core tip: The consequences of hepatitis B virus (HBV) and human immunodeficiency virus (HIV) co-infection on progression of severe liver diseases is a serious public health issue, worldwide. In HIV co-infected individuals, liver-related mortality is estimated over 17 times higher than those with HBV mono-infection. HIV co-infection significantly alters the natural history of hepatitis B, and therefore complicates the disease management. Thus, the clinical spectrum as well as the complexity of the HBV and HIV co-infection of liver offers challenging fronts to study the underlying molecular mechanisms, and to design effective therapeutic strategies.
- Citation: Parvez MK. HBV and HIV co-infection: Impact on liver pathobiology and therapeutic approaches. World J Hepatol 2015; 7(1): 121-126
- URL: https://www.wjgnet.com/1948-5182/full/v7/i1/121.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i1.121
Although inherently a non-hepatotropic virus, a direct cytopathic effect of human immunodeficiency virus-1 (HIV) on liver, independent of retroviral drug-toxicity or viral hepatitis is known, today. HIV co-infection with hepatitis B virus (HBV), and the consequences on progression of severe liver diseases further pose a serious health issue. HIV alone, infects nearly 40 million individuals leading to about 5% cases of mortality while HBV circulates in > 400 million persons causing about one million deaths, annually worldwide[1,2]. In the co-infection cases, approximately 90% of HIV-infected individuals have serological markers of HBV and approximately 5%-40% of them are clinically chronic[3,4]. As a consequence, HBV co-infection persists in almost 25% of HIV-infected adults and approximately 50%-90% of them acquire it at birth or in early age[5].
In HIV-infected patients, the CD4-independant tissue tropism, including liver and its impact on hepatopathogenesis is well established[6]. The spectrum of HIV-induced liver diseases includes hepatitis, alcohol-associated steatohepatitis, non-alcoholic steatohepatitis, endothelialitis, necrosis and granulomatosis[7]. Moreover, HIV co-infection leads to further complications in liver diseases compared to HBV mono-infection[8]. It is found that in HIV co-infected individuals, liver-related mortality is over 17 times higher than those infected with HBV, alone[2]. Also, with the introduction of “highly active anti-retroviral therapy (HAART)” in high HBV endemic areas, greater chances of progressive liver diseases is expected in the HIV co-infected individuals[9]. Moreover, HIV co-infection severely alters the natural history of hepatitis B[9]. As a result, management of chronic hepatitis B is hampered by the late and/or improper diagnosis as well as by effectiveness of nucleos(t)ide-based anti-viral therapy, and risks of emergence of drug-resistance. While it is very rare for HBV, transmittance of drug resistant-mutants accounts for up to 15% of new HIV infections[10,11].
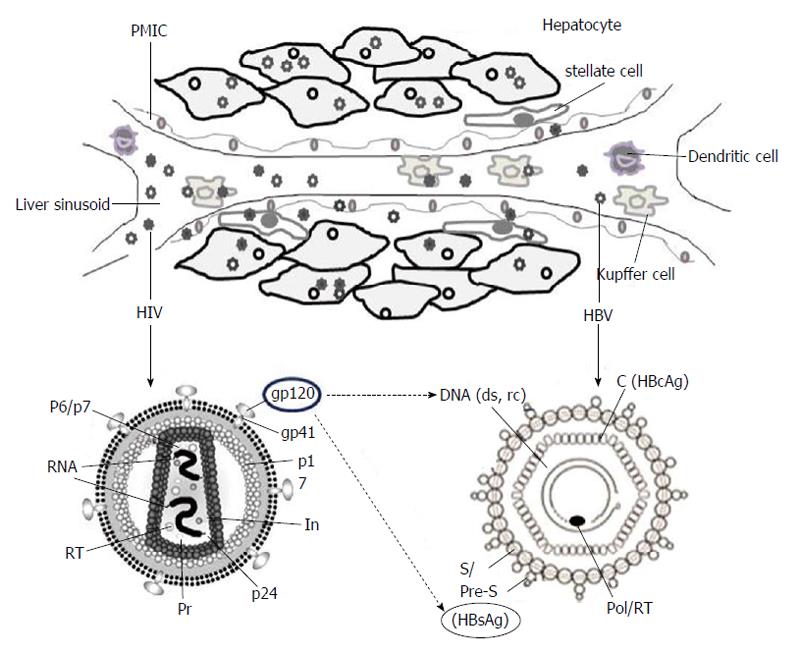
HIV infection requires interaction of the viral envelop protein (gp120) with cell surface receptor, CD4, aided by chemokine co-receptors, CCR5 and CXCR4[12,13]. While some HIV strains also require both of these co-receptors, few rare laboratory-adapted isolates are shown to utilize alternative co-receptors like, CCR1, CCR2b, CCR8, CXCR6, GPR1 and GPR15/Bob, in vitro[14]. Ample of reports has demonstrated cross-tissue tropism of HIV capable of infecting a variety of non-lymphoid tissues in vivo[15]. Further, HIV RNA, proviral-deoxyribonucleic acid (DNA) and capsid antigen (p24) have been detected in liver sinusoidal endothelial cells, Kupffer cells, portal mononuclear inflammatory cells as well as hepatocytes of infected patients (Figure 1)[16]. Very interestingly, Iser et al[17] have recently demonstrated the indispensability of CCR5 and CXCR4 in HIV entry into multiple liver cell lines. Nevertheless, the limited sensitiveness of the techniques employed as well as the sub-detectable levels of CD4 after several passages of cultured cells, cannot rule out the possibility of CD4 expressions on these cells. Therefore, in HIV-positive patients, the retroviral CD4 receptor-independent entry of hepatocytes still remains controversial. Nevertheless, another possibility of HIV to utilize an alternative liver-specific receptor is recently described[18].
The spectrum of HIV-induced liver diseases includes hepatitis, alcohol-associated steatohepatitis, non-alcoholic steatohepatitis, endothelialitis, necrosis and granulomatosis[7,19]. The consequences of HIV mono-infection is also linked to liver portal obliteration with nodular regenerative hyperplasia, responsible for non-cirrhotic portal hypertension[20,21]. In adult-acquired HBV infected individuals, the secondary infection with HIV leads to the chances of developing chronic hepatitis B by six-fold compared to HBV mono-infected patients[22,23]. Furthermore, in co-infection cases, HIV severely alters the natural history of hepatitis B[3,24]. HIV co-infection significantly decreases the rate of hepatitis B e antigen (HBeAg) clearance up to five-fold and increases the level of HBV replication[9,25]. Even co-infected persons who seroconvert to protective antibody to hepatitis B surface antigen (HBsAg) [anti-hepatitis B surface antibody (HBsAb)], remain at risk of reverse-seroconversion, and subsequent reactivation of HBV[26,27]. Also, while liver cirrhosis is more common in cases of HBV and HIV co-infections, a more severe progression and complexity in the development of hepatocellular carcinoma (HCC) is also reported[28].
Though the high HBV episomal DNA (cccDNA) accumulations are predictive of elevated hepatotoxicity and contrarily, the lower alanine amino transaminase (ALT) levels presenting less hepatic injury; the underlying mechanisms of HIV-induced liver pathology is largely unknown. Moreover, HBV core (C)/precore (pre-C) genetic variants, which can be more common in HIV co-infected individuals, are also suggested in disease progression[29,30]. In a closely related clinical study of HIV and hepatitis C virus co-infection cases, liver cirrhosis was found associated with higher systemic markers of gut-microbial translocation[31] that could be also true for HBV. Further, the activation of HBV-specific CD8+ cells, crucial in controlling HBV replication as well as the liver pathogenesis[32], is shown to be clearly impaired during HIV secondary infection. This might partially explain the tendency of progression of acute hepatitis B towards chronicity in HIV co-infection cases[33]. In co-infected patients with lower ALT levels compared to HBV mono-infection, the progression of cirrhosis and HCC is suggested to be related to lower CD4+ cell counts[34,35]. In addition to higher levels of HBV DNA and lower rates of spontaneous HBeAg seroconversion, severe flares of hepatitis can occur in HIV co-infected patients with low CD4+ counts who experience immune reconstitution after initiation of HAART[36]. Clinical studies in HBV and HIV co-infected patients have reported lower response rates to standard interferon (IFN)-α treatment than those with HBV mono-infection[34]. Responders tend to have a higher mean CD4+ cell count than nonresponders. It is expected that pegIFN-α will have similar or better efficacy than standard IFN-α. However, a detailed study of the mechanisms underlying adaptive immune responses in such cases is not available.
Furthermore, the CCR5 and CXCR4 co-receptors of hepatocytes as well as stellate cells are reported to involve in fibrogenic developments[36]. Very recently, the viral gp120 has been demonstrated to modulate hepatic chemokine receptors as well as stellate cell biology, and linked to liver fibrogenesis[37,38]. The gp120 binding of hepatic CXCR4 is also reported to up-regulate tumor necrosis factor-related TRAIL-R2 expression in apoptosing hepatocytes[39]. Another protein “R” of HIV is shown to modulate host transcription factors, like peroxisome proliferator-activated receptor-γ, crucial in lipid metabolism, insulin sensitivity, inflammatory processes and fibrogenesis[40].
Though several studies have demonstrated impact of HIV proteins on hepatocyte biology[20,37-40], only a few data is available on viral interactions, i.e., between HBV and HIV proteins (Figure 1). In the co-infection situation, the co-synthesized proteins of the two viruses might compete for host machinery involved in virion secretion pathways[41,42]. In ex vivo experimental set-up, the high retention of intrahepatic HBsAg had indicated its enhanced production or impaired secretion in the culture supernatant[43]. Moreover, in co-infected patients, exertion of gp120 on elevations and intracellular accumulation of HBV, DNA as well as HBsAg is proposed (Figure 1) to cause hepatotoxicity. In a clinical study, association of HBV and HIV co-infection with higher levels of HBV DNA has suggested that factors other than a direct virus-virus interaction might contribute to the increased HBV DNA levels[9]. While it has been shown that the HBV-X protein acts in alliance with HIV-tat in facilitating HIV replication[44], the synergistic effect of tat, if any, on HBV life cycle is not known.
The primary objective of hepatitis B treatment in HIV co-infection cases is to suppress HBV viral replication and minimize progressive liver damage. Notably, in co-infected patients, HIV severely alters the natural history of hepatitis B and therefore, complicates the diagnosis and disease management. HBsAg seronegativity and anti-HBsAb seroconversion that indicate resolution of active hepatitis B, are uncommon in HIV co-infection. Further, spontaneous reverse-seroconversion of anti-HBsAb can also occur in some co-infected patients and therefore, isolated anti-HBcAb test is recommended[45]. Since co-infected patients can have high levels of HBV DNA and hepatic necroinflammation with anti-HBc but not HBsAg, it is advised to test for both seromarkers first, and if either is positive, to test for HBV DNA[46].
Although, there is a limited therapeutic options for the treatment of chronic HBV, nearly twenty five approved drugs belonging to six classes [nucleos(t)ide RT-, protease-, nonnucleos(t)ide RT-, fusion-, integrase-, and CCR5-inhibitors] are available for HIV. In the co-infected individuals, the design of therapeutic regimens, like HAART are therefore, recommended to minimize the risk of hepatotoxicity. Also, due to association of continued anti-retroviral drug therapy with liver fibrogenesis and toxicity, it is prescribed for the necessity of balancing potential toxic effects with the need for increasing the CD4+ cell counts to control the two viruses[47]. Moreover, due to the resistance of highly stable, nuclear cccDNA to the currently available nucleos(t)ide analogs (e.g., Lamivudine, Adefovir, Emtricitabine, Tenofovir, etc.), a complete elimination of HBV has not been achieved. HBV therapy is recommended for all co-infected patients with abnormal aminotransferase values or HBV DNA levels of > 2000 IU/mL. Patients with an indication for HBV treatment should be started on fully active anti-retroviral regimen that contains nucleos(t)ide analogues, regardless of the CD4 cell count, to ensure that HIV is not partially treated[48]. Unlike in HBV mono-infection cases, combinatorial treatment with pegylated-Interferon plus Adefovir (preferred over Lamivudine resistance) has not led to any success in HIV co-infected individuals[49]. Further, because Entecavir can result in emergence of drug-resistant HIV muatants, its use is restricted in co-infected patients who are on a suppressive HIV regimen[50,51]. Although, Tenofovir is the most commonly used anti-viral analog in the co-infected patients, a few studies have examined the development of resistance in HIV as well as HBV. Since, in a proportion of Tenofovir treated patients, an undetectable serum HBV DNA still circulates, Tenofovir in combination with Emtricitabine is being preferred against the two viruses[52].
Though Lamivudine, Emtricitabine and Tenofovir are efficacious against both HBV and HIV, the rate of viral cross-resistance to Lamivudine, for example, in co-infected patients is high, reaching up to 90% at 4 years[53]. In view of this, the American Association of Study of Liver Diseases has the following guidelines for treatment of patients with HBV and HIV co-infection[54]: (1) Patients who meet criteria for chronic hepatitis B should be treated, and liver biopsy should be considered in those with fluctuating or mildly elevations in liver enzymes; (2) patients in whom treatment for both HBV and HIV is planned should receive therapies that are effective against both viruses-Lamivudine plus Tenofovir or Emtricitabine plus Tenofovir are preferred; (3) in co-infected patients with Lamivudine cross-resistance, Tenofovir or Adefovir should be added; and (4) when HAART regimens are altered, drug(s) that are effective against HBV should not be discontinued without substituting another drug that has activity against HBV, unless the patient has achieved anti-HBeAg seroconversion and has completed the prescribed course of treatment.
Furthermore, patients with HIV co-infection are less likely to develop protective HBsAb after HBV vaccination. Nevertheless, all HIV co-infected patients with serum HBsAb negativity and HBsAg positivity should be vaccinated for HBV[51,52]. Although low CD4 cell counts are associated with an impaired response to vaccination, HBV vaccination should not be deferred for patients with advanced HIV, as some individuals do develop protective antibody titers despite low CD4 cell counts. If feasible, it is recommended to vaccinate individuals with CD4 cell counts > 200/μL because response to vaccine is poor below this level. On the other hand, those with less CD4 counts should receive HAART first and HBV vaccine later when CD4 counts rise above 200/μL[55]. The patient’s HBsAb titre should be checked 1-2 mo post-vaccination, and re-vaccination should be considered for those who have not attained protective titers (≥ 10 IU/L)[47,56].
Co-infection of HBV and HIV, and the consequences on progression of severe liver diseases is a global public health issue. Despite the availability of extensive clinical data on the subject, the mechanisms of progressive hepatopathogenesis still remain inconclusive. The complexity of clinical manifestations in HBV and HIV co-infection cases therefore, offers challenging fronts to understand the basic pathobiology, and to formulate effective therapeutic strategies.
The author is very grateful to Dr. Jameel S (Welcome Trust-India Alliance, India) for his continued mentoring and support. Also, the author is thankful to Dr. Gallo R (Institute of Human Virology, Baltimore, United States) for giving opportunity to work on HIV pathobiology.
P- Reviewer: Kim YJ, Meng SD S- Editor: Gong XM L- Editor: A E- Editor: Liu SQ
| 1. | Thio CL. Hepatitis B and human immunodeficiency virus coinfection. Hepatology. 2009;49:S138-S145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 233] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 2. | Sun HY, Sheng WH, Tsai MS, Lee KY, Chang SY, Hung CC. Hepatitis B virus coinfection in human immunodeficiency virus-infected patients: a review. World J Gastroenterol. 2014;20:14598-14614. [PubMed] |
| 3. | Thio CL, Smeaton L, Saulynas M, Hwang H, Saravanan S, Kulkarni S, Hakim J, Nyirenda M, Iqbal HS, Lalloo UG. Characterization of HIV-HBV coinfection in a multinational HIV-infected cohort. AIDS. 2013;27:191-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 4. | McGovern BH. The epidemiology, natural history and prevention of hepatitis B: implications of HIV coinfection. Antivir Ther. 2007;12 Suppl 3:H3-13. [PubMed] |
| 5. | Yim HJ, Lok AS. Natural history of chronic hepatitis B virus infection: what we knew in 1981 and what we know in 2005. Hepatology. 2006;43:S173-S181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 372] [Article Influence: 19.6] [Reference Citation Analysis (1)] |
| 6. | Parvez MK. HIV heaptotropism and pathogenesis: new challenges. Fut Virol. 2013;8:1-3. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Bongiovanni M, Tordato F. Steatohepatitis in HIV-infected subjects: pathogenesis, clinical impact and implications in clinical management. Curr HIV Res. 2007;5:490-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Thio CL, Seaberg EC, Skolasky R, Phair J, Visscher B, Muñoz A, Thomas DL. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS). Lancet. 2002;360:1921-1926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 760] [Cited by in RCA: 774] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 9. | Chun HM, Mesner O, Thio CL, Bebu I, Macalino G, Agan BK, Bradley WP, Malia J, Peel SA, Jagodzinski LL. HIV outcomes in Hepatitis B virus coinfected individuals on HAART. J Acquir Immune Defic Syndr. 2014;66:197-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Geretti AM. Epidemiology of antiretroviral drug resistance in drug-naïve persons. Curr Opin Infect Dis. 2007;20:22-32. [PubMed] |
| 11. | Margeridon-Thermet S, Shafer RW. Comparison of the Mechanisms of Drug Resistance among HIV, Hepatitis B, and Hepatitis C. Viruses. 2010;2:2696-2739. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1667] [Cited by in RCA: 1649] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 13. | Dalgleish AG, Beverley PC, Clapham PR, Crawford DH, Greaves MF, Weiss RA. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984;312:763-767. [PubMed] |
| 14. | Berger EA, Doms RW, Fenyö EM, Korber BT, Littman DR, Moore JP, Sattentau QJ, Schuitemaker H, Sodroski J, Weiss RA. A new classification for HIV-1. Nature. 1998;391:240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 605] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 15. | Cao YZ, Friedman-Kien AE, Huang YX, Li XL, Mirabile M, Moudgil T, Zucker-Franklin D, Ho DD. CD4-independent, productive human immunodeficiency virus type 1 infection of hepatoma cell lines in vitro. J Virol. 1990;64:2553-2559. [PubMed] |
| 16. | Cao YZ, Dieterich D, Thomas PA, Huang YX, Mirabile M, Ho DD. Identification and quantitation of HIV-1 in the liver of patients with AIDS. AIDS. 1992;6:65-70. [PubMed] |
| 17. | Iser DM, Warner N, Revill PA, Solomon A, Wightman F, Saleh S, Crane M, Cameron PU, Bowden S, Nguyen T. Coinfection of hepatic cell lines with human immunodeficiency virus and hepatitis B virus leads to an increase in intracellular hepatitis B surface antigen. J Virol. 2010;84:5860-5867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Fromentin R, Tardif MR, Tremblay MJ. Human hepatoma cells transmit surface bound HIV-1 to CD4+ T cells through an ICAM-1/LFA-1-dependent mechanism. Virology. 2010;398:168-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Waisman J, Rotterdam H, Niedt GN, Lewin K, Racz P. AIDS: an overview of the pathology. Pathol Res Pract. 1987;182:729-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Mallet VO, Varthaman A, Lasne D, Viard JP, Gouya H, Borgel D, Lacroix-Desmazes S, Pol S. Acquired protein S deficiency leads to obliterative portal venopathy and to compensatory nodular regenerative hyperplasia in HIV-infected patients. AIDS. 2009;23:1511-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Vispo E, Morello J, Rodriguez-Novoa S, Soriano V. Noncirrhotic portal hypertension in HIV infection. Curr Opin Infect Dis. 2011;24:12-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Bodsworth NJ, Cooper DA, Donovan B. The influence of human immunodeficiency virus type 1 infection on the development of the hepatitis B virus carrier state. J Infect Dis. 1991;163:1138-1140. [PubMed] |
| 23. | Hadler SC, Judson FN, O’Malley PM, Altman NL, Penley K, Buchbinder S, Schable CA, Coleman PJ, Ostrow DN, Francis DP. Outcome of hepatitis B virus infection in homosexual men and its relation to prior human immunodeficiency virus infection. J Infect Dis. 1991;163:454-459. [PubMed] |
| 24. | Thio CL. Hepatitis B in the human immunodeficiency virus-infected patient: epidemiology, natural history, and treatment. Semin Liver Dis. 2003;23:125-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 90] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 25. | Gilson RJ, Hawkins AE, Beecham MR, Ross E, Waite J, Briggs M, McNally T, Kelly GE, Tedder RS, Weller IV. Interactions between HIV and hepatitis B virus in homosexual men: effects on the natural history of infection. AIDS. 1997;11:597-606. [PubMed] |
| 26. | Biggar RJ, Goedert JJ, Hoofnagle J. Accelerated loss of antibody to hepatitis B surface antigen among immunodeficient homosexual men infected with HIV. N Engl J Med. 1987;316:630-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 66] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Laukamm-Josten U, Müller O, Bienzle U, Feldmeier H, Uy A, Guggenmoos-Holzmann I. Decline of naturally acquired antibodies to hepatitis B surface antigen in HIV-1 infected homosexual men. AIDS. 1988;2:400-401. [PubMed] |
| 28. | Salmon-Ceron D, Rosenthal E, Lewden C, Bouteloup V, May T, Burty C, Bonnet F, Costagliola D, Jougla E, Semaille C. Emerging role of hepatocellular carcinoma among liver-related causes of deaths in HIV-infected patients: The French national Mortalité 2005 study. J Hepatol. 2009;50:736-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 29. | Revill PA, Littlejohn M, Ayres A, Yuen L, Colledge D, Bartholomeusz A, Sasaduesz J, Lewin SR, Dore GJ, Matthews GV. Identification of a novel hepatitis B virus precore/core deletion mutant in HIV/hepatitis B virus co-infected individuals. AIDS. 2007;21:1701-1710. [PubMed] |
| 30. | Warner N, Locarnini S. The antiviral drug selected hepatitis B virus rtA181T/sW172* mutant has a dominant negative secretion defect and alters the typical profile of viral rebound. Hepatology. 2008;48:88-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 186] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 31. | Balagopal A, Philp FH, Astemborski J, Block TM, Mehta A, Long R, Kirk GD, Mehta SH, Cox AL, Thomas DL. Human immunodeficiency virus-related microbial translocation and progression of hepatitis C. Gastroenterology. 2008;135:226-233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 216] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 32. | Chang JJ, Sirivichayakul S, Avihingsanon A, Thompson AJ, Revill P, Iser D, Slavin J, Buranapraditkun S, Marks P, Matthews G. Impaired quality of the hepatitis B virus (HBV)-specific T-cell response in human immunodeficiency virus type 1-HBV coinfection. J Virol. 2009;83:7649-7658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 33. | Thimme R, Wieland S, Steiger C, Ghrayeb J, Reimann KA, Purcell RH, Chisari FV. CD8(+) T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J Virol. 2003;77:68-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 705] [Cited by in RCA: 763] [Article Influence: 34.7] [Reference Citation Analysis (1)] |
| 34. | Di Martino V, Thevenot T, Colin JF, Boyer N, Martinot M, Degos F, Coulaud JP, Vilde JL, Vachon F, Degott C. Influence of HIV infection on the response to interferon therapy and the long-term outcome of chronic hepatitis B. Gastroenterology. 2002;123:1812-1822. [PubMed] |
| 35. | Clifford GM, Rickenbach M, Polesel J, Dal Maso L, Steffen I, Ledergerber B, Rauch A, Probst-Hensch NM, Bouchardy C, Levi F. Influence of HIV-related immunodeficiency on the risk of hepatocellular carcinoma. AIDS. 2008;22:2135-2141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 122] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 36. | Hong F, Tuyama A, Lee TF, Loke J, Agarwal R, Cheng X, Garg A, Fiel MI, Schwartz M, Walewski J. Hepatic stellate cells express functional CXCR4: role in stromal cell-derived factor-1alpha-mediated stellate cell activation. Hepatology. 2009;49:2055-2067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 124] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 37. | Svegliati-Baroni G, De Minicis S. HIV protein gp120 and chemokines receptor for liver fibrosis. Gut. 2010;59:428-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 38. | Bruno R, Galastri S, Sacchi P, Cima S, Caligiuri A, DeFranco R, Milani S, Gessani S, Fantuzzi L, Liotta F. gp120 modulates the biology of human hepatic stellate cells: a link between HIV infection and liver fibrogenesis. Gut. 2010;59:513-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 106] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 39. | Babu CK, Suwansrinon K, Bren GD, Badley AD, Rizza SA. HIV induces TRAIL sensitivity in hepatocytes. PLoS One. 2009;4:e4623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 40. | Shrivastav S, Kino T, Cunningham T, Ichijo T, Schubert U, Heinklein P, Chrousos GP, Kopp JB. Human immunodeficiency virus (HIV)-1 viral protein R suppresses transcriptional activity of peroxisome proliferator-activated receptor {gamma} and inhibits adipocyte differentiation: implications for HIV-associated lipodystrophy. Mol Endocrinol. 2008;22:234-247. [PubMed] |
| 41. | Lambert C, Döring T, Prange R. Hepatitis B virus maturation is sensitive to functional inhibition of ESCRT-III, Vps4, and gamma 2-adaptin. J Virol. 2007;81:9050-9060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 154] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 42. | Popov S, Popova E, Inoue M, Göttlinger HG. Human immunodeficiency virus type 1 Gag engages the Bro1 domain of ALIX/AIP1 through the nucleocapsid. J Virol. 2008;82:1389-1398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 43. | Xu Z, Yen TS. Intracellular retention of surface protein by a hepatitis B virus mutant that releases virion particles. J Virol. 1996;70:133-140. [PubMed] |
| 44. | Gómez-Gonzalo M, Carretero M, Rullas J, Lara-Pezzi E, Aramburu J, Berkhout B, Alcamí J, López-Cabrera M. The hepatitis B virus X protein induces HIV-1 replication and transcription in synergy with T-cell activation signals: functional roles of NF-kappaB/NF-AT and SP1-binding sites in the HIV-1 long terminal repeat promoter. J Biol Chem. 2001;276:35435-35443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 79] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 45. | Lacombe K, Boyd A, Gozlan J, Lavocat F, Girard PM, Zoulim F. Drug-resistant and immune-escape HBV mutants in HIV-infected hosts. Antivir Ther. 2010;15:493-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 46. | Soriano V, Puoti M, Bonacini M, Brook G, Cargnel A, Rockstroh J, Thio C, Benhamou Y. Care of patients with chronic hepatitis B and HIV co-infection: recommendations from an HIV-HBV International Panel. AIDS. 2005;19:221-240. [PubMed] |
| 48. | Ingiliz P, Valantin MA, Thibault V, Duvivier C, Dominguez S, Katlama C, Poynard T, Benhamou Y. Efficacy and safety of adefovir dipivoxil plus pegylated interferon-alpha2a for the treatment of lamivudine-resistant hepatitis B virus infection in HIV-infected patients. Antivir Ther. 2008;13:895-900. [PubMed] |
| 49. | McMahon MA, Jilek BL, Brennan TP, Shen L, Zhou Y, Wind-Rotolo M, Xing S, Bhat S, Hale B, Hegarty R. The HBV drug entecavir - effects on HIV-1 replication and resistance. N Engl J Med. 2007;356:2614-2621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 198] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 50. | Sasadeusz J, Audsley J, Mijch A, Baden R, Caro J, Hunter H, Matthews G, McMahon MA, Olender SA, Siliciano RF. The anti-HIV activity of entecavir: a multicentre evaluation of lamivudine-experienced and lamivudine-naive patients. AIDS. 2008;22:947-955. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 51. | Pessôa MG, Gazzard B, Huang AK, Brandão-Mello CE, Cassetti I, Mendes-Corrêa MC, Soriano V, Phiri P, Hall A, Brett-Smith H. Efficacy and safety of entecavir for chronic HBV in HIV/HBV coinfected patients receiving lamivudine as part of antiretroviral therapy. AIDS. 2008;22:1779-1787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 52. | Shire NJ, Welge JA, Sherman KE. Efficacy of inactivated hepatitis A vaccine in HIV-infected patients: a hierarchical bayesian meta-analysis. Vaccine. 2006;24:272-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 53. | Benhamou Y, Bochet M, Thibault V, Di Martino V, Caumes E, Bricaire F, Opolon P, Katlama C, Poynard T. Long-term incidence of hepatitis B virus resistance to lamivudine in human immunodeficiency virus-infected patients. Hepatology. 1999;30:1302-1306. [PubMed] |
| 54. | Kim HN, Harrington RD, Crane HM, Dhanireddy S, Dellit TH, Spach DH. Hepatitis B vaccination in HIV-infected adults: current evidence, recommendations and practical considerations. Int J STD AIDS. 2009;20:595-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 55. | Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45:507-539. [PubMed] |
| 56. | Mast EE, Weinbaum CM, Fiore AE, Alter MJ, Bell BP, Finelli L, Rodewald LE, Douglas JM, Janssen RS, Ward JW. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: immunization of adults. MMWR Recomm Rep. 2006;55:1-33; quiz CE1-4. [PubMed] |