Published online Apr 27, 2014. doi: 10.4254/wjh.v6.i4.251
Revised: January 10, 2014
Accepted: February 20, 2014
Published online: April 27, 2014
Processing time: 204 Days and 10.5 Hours
AIM: To investigate the hepatic hemodynamics in the Budd-Chiari syndrome (BCS) using per-rectal portal scintigraphy (PRPS) and liver angioscintigraphy (LAS).
METHODS: Fourteen consecutive patients with BCS were evaluated by PRPS between 2003 and 2012. Ten of them underwent LAS and liver scan (LS) with Tc-99m colloid. Eleven patients had clinical manifestations and three were asymptomatic, incidentally diagnosed at PRPS. The control group included 15 healthy subjects. We used new parameters at PRPS, the liver transit time of portal inflow and the blood circulation time between the right heart and liver. PRPS offered information on the hepatic areas missing venous outflow or portal inflow, length and extent of the lesions, open portosystemic shunts (PSS), involvement of the caudate lobe (CL) as an intrahepatic shunt and flow reversal in the splenic vein. LAS was useful in the differential diagnosis between the BCS and portal obstructions, highlighting the hepatic artery buffer response and reversed portal flow. LS offered complementary data, especially on the CL.
RESULTS: We described three hemodynamic categories of the BCS with several subtypes and stages, based on the finding that perfusion changes depend on the initial number and succession in time of the hepatic veins (HVs) obstructions. Obstruction of one hepatic vein (HV) did not cause opening of PSS. The BCS debuted by common obstruction of two HVs had different hemodynamic aspects in acute and chronic stages after subsequent obstruction of the third HV. In chronic stages, obstruction of two HVs resulted in opening of PSS. The BCS, determined by thrombosis of the terminal part of the inferior vena cava, presented in the acute stage with open PSS with low speed flow. At least several weeks are required in the obstructions of two or three HVs for the spontaneous opening of dynamically efficient PSS. The CL seems to have only a transient important role of intrahepatic shunt in several types of the BCS.
CONCLUSION: Dynamic nuclear medicine investigations assess the extent and length of hepatic venous obstructions, open collaterals, areas without portal inflow, hemodynamic function of the CL and reverse venous flow.
Core tip: Per-rectal portal scintigraphy (PRPS) and liver angioscintigraphy (LAS) are reliable investigations of the liver hemodynamics in the Budd-Chiari syndrome (BCS). Diagnosis of the number, length and succession in time of hepatic vein obstructions allows identification of hemodynamic varieties and stages of the BCS. Our new PRPS parameters, liver transit time and right heart to liver time, are used to diagnose obstructed hepatic veins, areas missing venous outflow or portal inflow, open collaterals, reverse splenic vein flow and hemodynamic role of the caudate lobe. LAS is useful in the differential diagnosis with portal occlusions, highlighting arterial-venous shunts and reverse portal flow.
- Citation: Dragoteanu M, Balea IA, Piglesan CD. Nuclear medicine dynamic investigations in the diagnosis of Budd-Chiari syndrome. World J Hepatol 2014; 6(4): 251-262
- URL: https://www.wjgnet.com/1948-5182/full/v6/i4/251.htm
- DOI: https://dx.doi.org/10.4254/wjh.v6.i4.251
The Budd-Chiari syndrome (BCS) is determined by hepatic venous obstruction localized from the small hepatic veins (HVs) to the terminal part of the inferior vena cava (IVC), resulting in increased sinusoidal pressure, hepatic congestion and portal hypertension (PHT)[1]. The natural outcome is poor in many cases, with a three year survival rate of about 10%[2,3]. Clinical manifestations are extremely varied and include ascites, jaundice, hepatomegaly, splenomegaly, collateral veins and upper right abdominal quadrant pain[4]. Ascites fluid has a characteristic high protein concentration (> 2.5 g/dL).
A widely accepted classification is based on etiology, site of obstruction, manifestations and duration of the disease[5]. Primary BCS is produced by thrombosis, its sequels or web obstruction[6]. Secondary forms are determined by malignant or parasitic obstruction of the lumen or by extrinsic compression, commonly produced by tumors[7]. The site involved by the obstruction and the affected HVs are commonly diagnosed through non-invasive imaging [ultrasound (US), magnetic resonance imaging (MRI), computed tomography (CT)] or by using venography[8].
Clinical approach accounts for the severity of disease (fulminant/non-fulminant) and its duration (acute, subacute or chronic). Subacute forms show signs or symptoms for less than six months and no evidence of liver cirrhosis. Chronic evolution is characterized by onset over six months, with evidence of PHT and cirrhosis[9]. However, the severity of liver damage may be inconsistent with apparent symptomatology. Recent clinical onset may be discordant with advanced liver fibrosis, suggesting a long course without clinical symptoms. Asymptomatic disease (10%-15% of cases) is usually associated with the obstruction of only one HV but also with spontaneous development of dynamically efficient intrahepatic collaterals and extrahepatic portosystemic shunts (PSS), incidentally diagnosed by imaging at surgery or necropsy[10]. For the patients with acute clinical onset but with advanced histological damage, a recent hepatic venous obstruction added to older obstructions of other HVs has to be suspected. In acute forms, the liver area without physiological outflow is hypertrophied and PHT occurs. Because of the higher pressure (35 mmHg) in the hepatic artery (HA) than in the portal vein (PV) branches (3-6 mmHg), the portal flow may be reversed.
Chronic alterations include parenchyma atrophy and extended fibrosis, gaining a cirrhotic appearance and considerably reducing both portal and arterial inflow. Splenomegaly is found in a third of patients[11].
Thrombosis is the usual cause for the occlusion of large HVs, while the obstructions of the IVC or of the small HVs are rarely thrombotic[12].
The anatomical varieties of the HVs have to be accounted for, both for the diagnosis and surgical treatment. A common opening into the IVC of the left hepatic vein (LHV) and middle hepatic vein (MHV) was reported in around 55%-60% of cases[13]. Other varieties, such as a common trunk of the MHV and right hepatic vein (RHV), separate opening of the three HVs into the IVC or the existence of an accessory RHV, were identified in various percentages[14-16]. The caudate lobe (CL) hemodynamic status is important as it may be an anastomosis between the obstructed HVs and the IVC. CL hypertrophy is described in 65%-75% of cases, with good sensitivity but not specificity[17]. A caliber of the CL vein higher than 3 mm is considered diagnostic for the BCS[18].
US usually demonstrates altered HVs and hypertrophy of the CL[19]. Duplex Doppler sonography is widely used, offering a good assessment of the blood flow through the HVs[20,21]. Color Doppler sonography allows a more reliable identification of abnormalities of the HVs than conventional sonography and detects collateral vessels not visible with other techniques. The lack of flow signals in the HVs, intrahepatic and extrahepatic collaterals, together with reverse, slow or turbulent portal flow, are characteristic findings. US techniques should be the first line investigations, due to a low cost and a diagnostic sensitivity of more than 75%. There are, however, cases where the occlusion of HVs is difficult to demonstrate by US, even by color Doppler imaging[22].
CT scans offer a good assessment of thrombosis of the HVs or IVC, global liver enlargement, abnormalities of liver structure, size and direction of the venous flow. The contrast-enhanced helical CT allows a good dynamic visualization of HVs[23]. MRI provides useful images of the hepatic venous outflow and thrombosis of HVs, as second line investigations together with CT[24,25]. It can be difficult to diagnose spontaneous intrahepatic anastomoses and prominent azygos and hemiazygos veins (especially in IVC thrombosis) on MRI[26]. The liver biopsy has limited value due to the inhomogeneous distribution of liver lesions in the BCS[27].
Per-rectal portal scintigraphy (PRPS) was used over the last decades to investigate PHT and PSS in chronic liver disease (CLD) by evaluating a per-rectal portal shunt index[28,29]. Detailed information may be acquired about liver hemodynamics by using the parameters introduced by us in the interpretation of PRPS dynamic curves - liver transit time (LTT) and right heart to liver time (RHLT)[30].
Liver angioscintigraphy (LAS) evaluates the contribution of arterial inflow to the total liver perfusion. It is especially useful in the differential diagnosis between the BCS and obstructions of the portal branches. Liver areas missing portal inflow have compensatory increased arterial inflow due to the hepatic artery buffer response (HABR)[31] and a characteristic pattern at LAS, with abrupt arterial entry and a flattened portal segment.
Liver scan (LS) with Tc-99m labeled colloid performed after LAS may show changes in radiotracer capture by liver, spleen and spine. Increased radioactivity on the CL area is considered a characteristic finding in the BCS[32].
The aim of this study is to underline the diagnostic possibilities in the BCS of the PRPS and LAS, with auxiliary use of the LS. The goals are assessment of liver areas missing venous drainage, length and order of the appearance of lesions, arterial and portal perfusion changes, existence of dynamically efficient collaterals and of supplementary drainage through the CL.
We evaluated 14 consecutive patients with the BCS between 2003 and 2012, 9 females and 5 males, between 20 and 63 years old. Eleven patients had clinically manifested BCS and 3 were asymptomatic, incidentally identified at PRPS among over 400 patients explored for chronic liver disease (CLD) staging. The control group included 15 healthy subjects from the laboratory casework, 7 men and 8 women, between 19 and 67 years old. All 14 patients with BCS underwent PRPS. LAS and LS were performed in 10 of them. Anonymity of the patients was respected. All persons gave their informed consent prior to the investigations, accepting inclusion in research studies. The study was realized as part of routine clinical practice.
All the investigations were performed by using a single photon emission computed tomography (SPECT) Orbiter Siemens gamma-camera with high resolution, low-energy, parallel collimator, connected to a Power Macintosh computer, using ICON dedicated software. We used Tc-99m sodium pertechnetate, eluted from Drygen generators (General Electric, Amersham, United Kindom) and Fyton colloid (Institute of Isotopes, Budapest, Hungary).
PRPS was performed using the method developed by Shiomi et al[33]. A solution containing 2 mL (296-370 MBq/8-10 mCi) of Tc-99m sodium pertechnetate was instilled at PRPS into the upper part of the rectum through a Nelaton tube, followed by 15 mL of air under pressure. Serial scintigrams were recorded every 2 s for 5 min. Radioactivity curves were built thereafter by computer on liver and heart areas to analyze the dynamics of the radiotracer absorbed from the rectum. The patients were told to fast from the evening preceding the test. Two enemas were performed in each patient, the first the previous evening and the the second one 2 h prior to the PRPS. The patients were placed in a supine position, with the camera detector in the anterior view, including in the field the liver and heart areas.
LTT and RHLT allow detailed assessment of liver hemodynamics in the BCS. These time parameters can be assessed for particular hepatic areas or for the whole liver. The portal inflow is missing in those areas where the tracer arrives (through the HA) with a delay equal to RHLT after entering into the right heart (RH). LTT increased between 25-40 s shows a higher resistance opposed to the portal inflow. Values over 50 s of the time interval between the entering of tracer into the liver and its arrival to the RH may occur in the acute stage of the BCS produced by obstruction of the terminal part of the IVC. This high delay is determined by the slow flow through the PSS open to the superior vena cava. LTT between 15-23 s reflects a decrease of the resistance opposed by the liver to the portal inflow due to the enlargement of intrahepatic small size shunts between terminal portal branches and small HVs. Open extrahepatic PSS of high flow are emphasized at PRPS by arrival of the portal tracer to the RH before entering into the liver. PRPS also detects alterations of portal and arterial perfusion in the liver areas which maintain their physiological venous outflow, highlighting the changes determined by the redistribution of flow from the affected areas.
The arterial upward inflexion (AUI) of the PRPS curve on a liver area is placed at a time interval equal to RHLT after the moment when the tracer entered into the RH. The beginning of the AUI segment on our figures is noted HA, marking the arrival of tracer to the liver through the HA. The moments when the portal tracer enters into the different areas of the liver are noted as Co, Lo, Mo and Ro. The entering of tracer into the RH is noted as Ho. The slope of the AUI gives information about the amplitude of arterial inflow. The portal segment of the PRPS curve (before the AUI) offers information about the portal inflow and the dynamic resistance encountered. Summed images at PRPS resulting from the overlapping of all the sequential images may highlight the normal aspect, increased accumulation or low quantity of tracer in a liver area. The presence of the spleen on the summed image highlights reverse flow in the splenic vein.
LAS was performed by rapid bolus injection of 8 to 15 mCi (300-450 MBq) of Tc-99m labeled colloid in a volume smaller than 0.5 mL, followed by computer dynamic recording of sequential images during 1 min at a 1 s rate. The detector’s area included the heart, liver and kidneys. Six patients underwent LAS in the posterior-anterior view (P-A) and four in the anterior-posterior view (A-P). Patients were asked to fast 12 h before the LAS. Dynamic time-radioactivity curves for the liver, spleen and left or right kidney were built by computer. The moment of the peak of the kidney curve corresponds on the liver curve to the upward inflection point when the portal inflow of tracer adds to the arterial inflow. The interval of 8 s on the liver curve before the kidney curve’s peak corresponds to the arterial segment, while the 8 s interval after the peak represents the portal segment[34]. The shape of the LAS curve built on a liver area may highlight amplitude changes of the arterial flow, a higher resistance opposed to the blood flow or open arterial-venous shunts. Hepatic perfusion index (HPI) calculated at LAS is used to estimate the ratio between the arterial inflow and total liver perfusion[35]. HPI > 45% on an area of the right liver lobe (RLL) without tumors diagnoses a decrease in portal inflow, with reactive increase in the arterial flow determined by the HABR[36]. HPI > 100% emphasizes reverse flow in the portal vein. HPI can be accounted for the RLL only, as the left liver lobe (LLL) normally has an increased arterial inflow.
LS images were acquired beginning at least 15 min after the LAS, based on the same administration of Tc-99m labeled colloid. P-A, A-P and right lateral (RL) views were used. Time consuming SPECT was not considered to bring significantly more information on hemodynamic status than planar LS and was not performed.
We highlighted three hemodynamic categories of the BCS using PRPS and LAS: (1) BCS debuted by obstruction of one HV: asymptomatic, incidentally diagnosed and old obstruction of one HV followed by recent obstruction of the other two HVs; (2) BCS started by obstruction of two HVs: old obstruction of two HVs, followed by recent obstruction of the third HV, and old obstruction of two HVs, also followed by old obstruction of the third HV; and (3) BCS with acute onset due to simultaneous obstruction of all three HVs caused by obstruction of the terminal part of the IVC.
We had three cases of asymptomatic BCS in our study, two with obstruction of the MHV and one with obstruction of the RHV. All three were incidentally identified at PRPS.
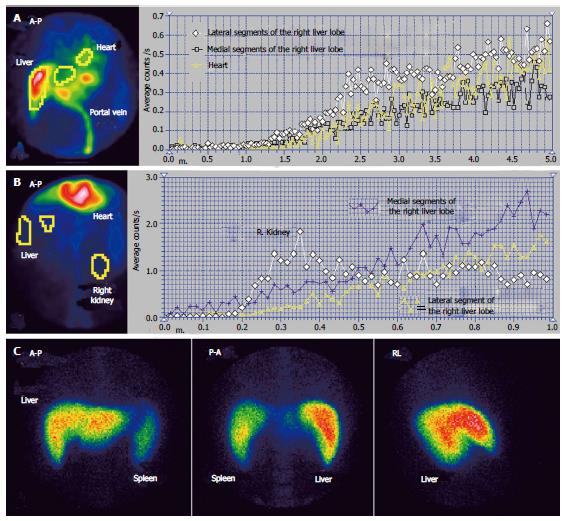
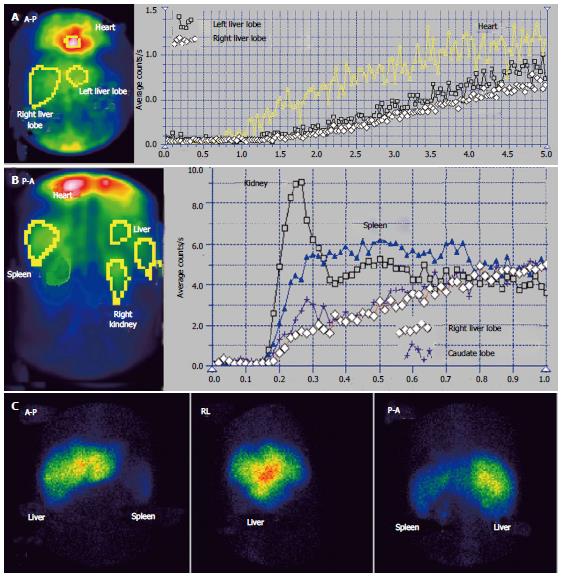
The case presented in Figures 1 and 2 had MHV obstruction. The patient was a 36-year-old male, known to have chronic alcoholic hepatitis. US described inhomogeneous liver with regenerative nodules and dilated portal branches.
Summed image at PRPS (Figure 1A) reveals a decreased amount of radiotracer in the medial part of the RLL. The dynamic PRPS curves built on the RLL show highly different slopes on the medial and lateral segments. The arrival of portal tracer to the medial area was delayed about 30 s after the entrance into the lateral segments. Initial segment of the PRPS curve built on the medial area of the RLL has a slow slope, denoting moderately increased resistance opposed to the portal inflow (Figure 2A). The PRPS curve built on the lateral part of the RLL has an increased slope, showing a rise of its portal inflow (Figure 2D). The dynamic curve built on the CL has a low initial slope, indicating that the portal flow blocked due to the obstruction of the MHV was not drained through the CL. The LLL has a simultaneous entry of tracer as the CL and as the lateral area of the RLL, with a normal initial slope of the PRPS curve.
LTT is prolonged to 30 s, most likely due to the subjacent CLD, with increased resistance opposed to the portal inflow. The LS images confirm the diagnosis of alcoholic CLD, showing splenomegaly and hypertrophy of the LLL, with a normal aspect of the spine. Normal LS aspect of the medial area of the RLL suggests a subacute stage of the BCS, without cirrhotic changes of the affected parenchyma. PSS were not open, as the tracer arrived at the RH by passing through the liver. The LAS curve on the medial segments of the RLL is significant for arterial-venous shunts. The differential diagnosis accounted for the value of LTT and missing of the HABR, excluding a portal branch obstruction.
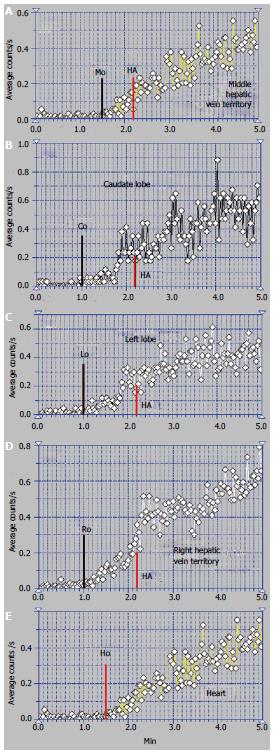
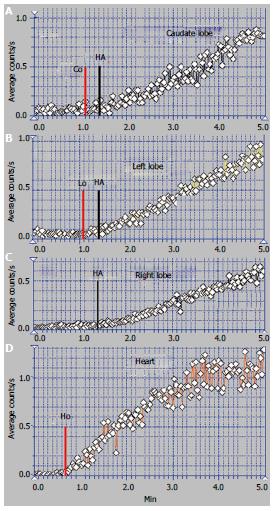
The patient presented in Figures 3 and 4 was a 32-year-old male, without a previous diagnosis of CLD. The patient complained of flatulence, vomiting and severe abdominal pain which had started several weeks earlier. US highlighted ascites and venous dilations. No blood flow in the HVs and no portal thrombosis were observed at the US, while numerous intrahepatic arterial-venous and venous-venous collaterals were described. Esophageal varices and gastric injuries were not seen on endoscopy.
The summed image at PRPS presents a sharply increased radioactivity on the CL area (Figure 3A). The LLL only has arterial inflow, receiving tracer after the RH with a delay equal to RHLT (Figure 4B). This suggests an old obstruction of the LHV, with chronic alteration of perfusion of the LLL. The clinical onset in this case resulted from a recent obstruction of the RHV and MHV, while the LHV had been obstructed for a long time. The PRPS curve on the RLL has low slopes on the initial portal segment and on the AUI, showing that its portal and arterial inflows were decreased. The differential diagnosis excluded portal obstruction, accounting for small arterial inflow of the two liver lobes and missing the HABR.
Arrival of tracer to the RH at 30 s after entering into the CL shows that PSS were not open due to the earlier obstruction of one HV and the recent obstruction of the other two HVs (Figure 4). The tracer was detected in the RLL about 12 s after the CL, a slow portal inflow to the RLL being maintained.
Altered flow of the RLL was highlighted at the LAS (Figure 3B). After a short initial interval of about 1 s with normal arterial input, the LAS curve flattens on the rest of the arterial segment and has a low slope on the portal segment. The flattened curve on the RLL highlights the higher dynamic resistance encountered by the arterial inflow.
The ASH curve of the CL has an initial abrupt and high segment, due to the increased arterial inflow, followed by a flat portal segment and then by fluctuations of amplitude suggesting arterial-venous shunts (Figure 3B). The CL has a very high entering of portal tracer at the PRPS (Figure 4A). LTT on the CL is increased to 30 s due to the dynamic resistance opposed by the supplementary arterial and portal flows redirected from the RLL and LLL. The high amplitude of the PRPS curve on the CL was determined by the increased and slowed portal inflow. LS (Figure 3C) revealed increased radioactivity in the CL, inhomogeneous liver parenchyma and slightly increased radioactivity in the spleen.
The perfusion changes in the LLL and RLL suggest a subacute stage of the BCS. We underline that the old obstruction of the LLL together with more recent occlusion of the other two HVs did not open PSS. The arterial and portal blood flow of the whole liver was drained to the IVC through the CL.
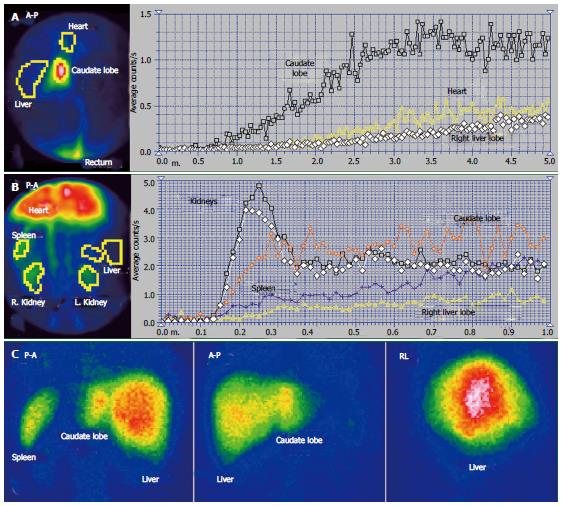
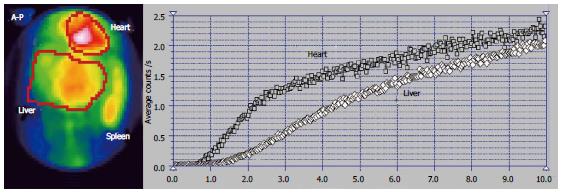
The scintigraphy investigations in a patient with BCS with an old obstruction of the MHV and RHV and subsequent but also old obstruction of the LHV are presented in Figures 5 and 6. This 20 year old woman, a user of an oral contraceptive, was hospitalized with an impaired general condition and abdominal pain. The first symptoms appeared two and a half years earlier when the BCS was diagnosed. US at admission showed abundant ascites and PV dilation to 15 mm. Doppler US could not detect flow in the HVs.
The RLL received tracer at PRPS at a time interval equal to RHLT after the RH, emphasizing that the portal inflow was missing and the blood supply of the RLL came from the HA only (Figures 6C, D).
The tracer entered the LLL and the CL (Figure 6A, B) at about 25 s after reaching the RH, showing that their perfusion was mainly (but not completely) arterial. The initial low slopes of the CL and LLL curves were caused by increased resistance opposed to the portal inflow. The high slope of the AUI on the CL and LLL curves was determined by the large quantity of tracer that arrived through the HA. The slope of the AUI is smaller on the RLL than on the LLL and CL curves with about 50% (Figure 6C). Due to the older impairment of the RLL, its arterial perfusion per unit of area became lower than for the LLL and CL.
PSS were open, allowing the tracer absorbed from rectum to arrive faster to the RH than to the liver. The presence of the IVC on the summed PRPS image suggests the existence of open per-rectal PSS.
The ASH curve on the CL has a steep and biphasic entrance during the arterial segment. The portal phase has a drop in amplitude and subsequent fluctuations (Figure 5B). Increased arterial perfusion and existence of arterial-venous shunts are highlighted in the CL. The high arterial inflow suggests that the CL maintained part of its role of intrahepatic shunt to the IVC for the arterial inflow redirected from the rest of the liver. The ASH curve on the RLL shows increased arterial perfusion and reverse portal flow, with HPI = 115%.
The CL is visible on the LS, contrasting to the low colloid capture in the RLL (Figure 5C). The normal LS aspect of the spine and spleen argues against alcoholic or viral cirrhosis.
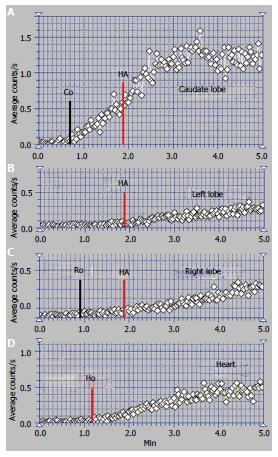
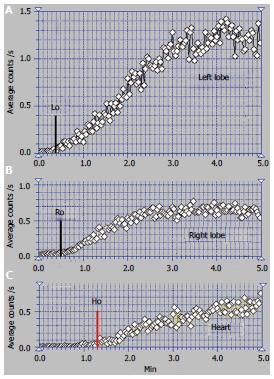
We encountered one case of the BCS syndrome with old RHV and MHV obstruction and very recent occlusion of the LHV. One of these patients was a 32-year-old woman, an oral contraceptive user, with ascites in high quantity, increasing abdominal girth and without viral or alcoholic cirrhosis.
The time interval at PRPS (Figure 7) between the dynamic curves built on the heart and on the whole liver is equal to RHLT, showing that both the LLL and RLL missed portal inflow and the extrahepatic PSS of high flow were open. The high initial slope of the PRPS curve on the heart confirms that the PSS were hemodynamically efficient.
The highly increased radioactivity in the congested LLL on the summed PRPS image suggests that its deprivation of physiological venous drainage was very recent, while the obstruction of the MHV and RHV was old. The CL cannot be specifically distinguished on the PRPS summed image or by building dynamic curves on its area, therefore not playing a specific hemodynamic role. The image of the spleen on the summed PRPS image suggests inversion of flow in the splenic vein, while presence of the IVC suggests open per-rectal shunts.
Two of the BCS patients in our study had fulminant symptoms. US and CT scans argued for obstruction of the terminal portion of the IVC in both of them. The data shown in Figure 8 belong to a 39-year-old woman with myeloproliferative disease, admitted for recently appeared severe pain in the upper right abdominal quadrant, with vomiting and encephalopathy signs. US and CT scans showed abundant ascites and significant dilation of the HVs.
PRPS highlights a sharp increase of the transit time of the tracer from entering into the liver to reaching the RH, up to values over 50 s. The amount of tracer passing through the heart during the first 30 s after its arrival was very low, with a flattened PRPS cardiac curve. The normal slope of the PRPS curves built on the liver lobes show that the dynamic resistance encountered by the portal flow was not significantly increased. The very late arrival to the RH of a small quantity of tracer was determined by the slow transit through the PSS open to the superior vena cava. PRPS highlights the completely blocked venous outflow on the physiological pathway between the liver and the RH, involving all the HVs. The slow flow through the PSS suggested that the obstruction of the terminal portion of the IVC had been recently installed.
We propose a new method to assess the liver hemodynamics in the BCS by performing the PRPS and LAS, with auxiliary use of the LS. PRPS is the main investigation, based on the parameters introduced by us, LTT and RHLT, offering information about the portal and arterial flows and the effects of the venous outflow obstruction.
The liver areas missing portal inflow with only arterial perfusion are highlighted at PRPS based on the time interval equal to RHLT between the origins of cardiac and liver curves. The increased arterial perfusion of a liver area causes a high slope of the AUI at PRPS and HPI > 45% at LAS. In old obstructions of the HVs, PRPS shows decreased portal and arterial flows of the parenchyma without venous outflow. In recent obstructions of the HVs, PRPS detects small or inverted portal flow, increased arterial inflow and accumulation of radioactivity on the summed image in the affected area. A high quantity of radiotracer appears on the summed PRPS image in the CL of the patients with recent common occlusion of two HVs. Inverted flow in the splenic vein is suggested by a visible spleen on the summed PRPS image.
ASH is useful in the differential diagnosis between the BCS and portal obstructions and also in highlighting arterial-venous shunts and increased resistance opposed to the arterial flow. Reverse portal flow is emphasized by HPI > 100%. LS was performed after ASH, showing increased radioactivity in the CL in particular types of BCS. LS aspect of the liver lobes, spleen and spine is useful when viral or alcoholic CLD is suspected.
Several hemodynamic varieties and stages of the BCS were described by using PRPS and LAS. Liver perfusion status is closely related to the initial number of obstructed HVs and to the lengths of occlusions. The category of the BCS debuted by obstruction of one HV included the patients actually affected by one obstructed HV and the patients affected by an old obstruction of one HV followed by recent obstruction of the other two HVs. For the BCS started by obstruction of two HVs we found different perfusion patterns in acute and chronic stages after the obstruction of the third HV. The BCS with acute onset due to the simultaneous obstruction of all the three HVs is commonly caused by obstruction of the terminal part of the IVC and specifically presents in the acute stage highly prolonged LTT at PRPS. Different hemodynamic patterns of the liver flows related to various types of hepatic venous occlusions underline the autonomous regulation of the perfusion of the two liver lobes.
It is important to know in all the varieties and stages of the BCS if PSS are open. PRPS highlighted that PSS were not open in patients with occlusion of one HV. The CL did not play a significant hemodynamic role in the subacute stage of such patients, suggesting that the blood flow redirected from the area without physiological venous outflow was drained through the unaffected HVs. PSS were not open even in the acute stage after common obstruction of two HVs following an old obstruction of the other HV.
Open PSS were emphasized in our cases with old obstruction of two HVs. This finding suggests that the outflow of two HVs is too high to be redirected in chronic stages only through the unaffected HV and through the CL, with having to leave the liver through extrahepatic PSS.
In acute stages after the occlusion of terminal part of the IVC, the PSS draining the blood to the superior vena cava allowed only a low speed flow. Our data suggest that spontaneous effective drainage through PSS requires at least several weeks to open after the obstruction of two or three HVs.
Hypertrophy of the CL is currently underlined in the diagnosis of the BCS. However, the hemodynamic role of the CL looks to be unimportant or transient in several varieties and stages of the BCS. The CL was not involved as an intrahepatic shunt in asymptomatic patients with obstruction of one HV and in IVC obstructions. In the patients with chronic obstruction of two HVs, the CL had a reduced role of a hemodynamic shunt for part of the arterial flow redistributed from affected areas, presenting arterial-venous collaterals. The CL was highly active in the subacute stage after the obstruction of two HVs following an old occlusion of the third HV. Our study suggests that the CL usually plays an efficient hemodynamic role of intrahepatic shunt to the IVC in acute or subacute stages and in particular varieties of the BCS. The importance in each case of the caliber and morphology of the CL venous drainage for its functioning as hemodynamic shunt has also to be accounted for.
Due to the rarity of the disease, we did not explore several other theoretical varieties of the BCS so our classification will have to be detailed. Common obstruction of the LHV and MHV, acute stage after the debut of the BCS by obstruction of one or two HVs, and chronic stage after the occlusion of the terminal part of the IVC may bring more information about the opening of PSS and the role of the CL in draining the redirected flows.
To conclude, PRPS associated with LAS are able to play a useful role as second line investigations in the BCS, adding important data to the US, CT or MRI findings. These scintigraphic procedures have reliable costs, are non-invasive and easily reproducible. The accuracy of the method, however, is dependent on the operator’s expertise.
We are grateful to Professors Sabin Cotul and Doru Dejica, to Dr. Liliana Dina and Crina Briciu for their contribution to the development of PRPS and ASH investigations in our laboratory. We are also grateful to the colleagues from the 3rd Medical and Surgical Clinics of Cluj-Napoca and especially to the Ultrasound department for their diagnosis in the BCS patients.
Impaired liver perfusion in the Budd-Chiari syndrome is determined by the obstruction of the hepatic blood outflow. Portal and arterial altered flows may be properly explored by combined use of two nuclear medicine dynamic investigations, per-rectal portal scintigraphy and liver angioscintigraphy. Radioisotope techniques allow a more precise diagnosis in different types and stages of the Budd-Chiari syndrome and highlight the changes of perfusion patterns during evolution of the disease.
Scintigraphy investigations proposed by us to explore the Budd-Chiari syndrome highlight open portosystemic shunts, liver areas without portal inflow, hemodynamic involvement of the caudate lobe, inverted flow in the splenic or portal vein and length of the obstructions of the hepatic veins or the terminal portion of the inferior vena cava. The authors described three hemodynamic categories of the Budd-Chiari syndrome with several subtypes and stages, based on the finding that perfusion changes depend on the initial number and succession in time of the hepatic veins obstructions.
The authors previously described the use of dynamic nuclear medicine investigations to evaluate portal hypertension and portosystemic shunts in chronic liver disease. Clinical applications of the liver angioscintigraphy are commonly related to hepatic tumors evaluation.
The authors introduced a new method of interpretation for the per-rectal portal scintigraphy by proposing two new parameters, the transit time of the portal inflow through the liver and the transit time of the blood from the right heart to the liver. These time parameters allow an accurate description of hepatic hemodynamic changes determined by venous obstructions. The authors used liver angioscintigraphy in the differential diagnosis between the Budd-Chiari syndrome and portal obstructions, highlighting the absence of the hepatic artery buffer response in the Budd-Chiari syndrome. The authors showed that portosystemic shunts are not open after the obstruction of one hepatic vein, while at least several weeks are required in the obstructions of two or three hepatic veins for the spontaneous opening of dynamically efficient portosystemic shunts.
The hemodynamic data offered by per-rectal portal scintigraphy and angioscintigraphy of the liver are especially important for surgery and TIPS mounting. Diagnosis of the number, length and succession in time of hepatic veins obstructions allow identification of hemodynamic varieties and stages of the Budd-Chiari syndrome and support an adequate therapeutic approach.
Per-rectal portal scintigraphy is a dynamic procedure performed by instillation into the rectum of a small quantity of radioactive tracer followed by recording its dynamics through the portal vein, liver and heart areas. Liver angioscintigraphy is performed by rapid antecubital intravenous bolus injection of a small quantity of radiotracer followed by recording its entrance into the liver through the hepatic artery and portal vein, allowing the assessment of an arterial to total liver perfusion (arterial plus portal) ratio. The Budd-Chiari syndrome is a vascular liver disease determined by hepatic venous obstruction localized from the small hepatic veins to the terminal part of the inferior vena cava, resulting in increased sinusoidal pressure, hepatic congestion and portal hypertension.
This is a well thought and comprehensive manuscript on the relevance of using PRPS and liver angioscintigraphy techniques to investigate the liver hemodynamics in Budd-Chiari syndrome. The manuscript provides useful and interesting information. It also has potential for clinical application.
P- Reviewers: Das UN, Verma S, Yu HP S- Editor: Song XX L- Editor: Roemmele A E- Editor: Wu HL
| 1. | Mukund A, Gamanagatti S. Imaging and interventions in Budd-Chiari syndrome. World J Radiol. 2011;3:169–177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 2. | Plessier A, Valla DC. Budd-Chiari syndrome. Semin Liver Dis. 2008;28:259-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 101] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 3. | Orloff MJ, Daily PO, Orloff SL, Girard B, Orloff MS. A 27-year experience with surgical treatment of Budd-Chiari syndrome. Ann Surg. 2000;232:340-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 100] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Darwish Murad S, Plessier A, Hernandez-Guerra M, Fabris F, Eapen CE, Bahr MJ, Trebicka J, Morard I, Lasser L, Heller J, Hadengue A, Langlet P, Miranda H, Primignani M, Elias E, Leebeek FW, Rosendaal FR, Garcia-Pagan JC, Valla DC, Janssen HL; EN-Vie (European Network for Vascular Disorders of the Liver). Etiology, management, and outcome of the Budd-Chiari syndrome. Ann Intern Med. 2009;151:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 339] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 5. | Janssen HL, Garcia-Pagan JC, Elias E, Mentha G, Hadengue A, Valla DC. Budd-Chiari syndrome: a review by an expert panel. J Hepatol. 2003;38:364-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 329] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 6. | Valla DC. Primary Budd-Chiari syndrome. J Hepatol. 2009;50:195-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 207] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 7. | De Stefano V, Za T, Ciminello A, Betti S, Rossi E. Causes of adult splanchnic vein thrombosis in the mediterranean area. Mediterr J Hematol Infect Dis. 2011;3:e2011063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Virmani V, Khandelwal N, Kang M, Gulati M, Chawla Y. MDCT venography in the evaluation of inferior vena cava in Budd-Chiari syndrome. Indian J Gastroenterol. 2009;28:17-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Loomes DE, Chang A, Webber D, Scudamore CH, Yoshida EM. Acute Budd-Chiari syndrome. Can J Gastroenterol. 2011;25:302-303. [PubMed] |
| 10. | Hadengue A, Poliquin M, Vilgrain V, Belghiti J, Degott C, Erlinger S, Benhamou JP. The changing scene of hepatic vein thrombosis: recognition of asymptomatic cases. Gastroenterology. 1994;106:1042-1047. [PubMed] |
| 11. | Powell-Jackson PR, Ede RJ, Williams R. Budd-Chiari syndrome presenting as fulminant hepatic failure. Gut. 1986;27:1101–1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Ludwig J, Hashimoto E, McGill DB, van Heerden JA. Classification of hepatic venous outflow obstruction: ambiguous terminology of the Budd-Chiari syndrome. Mayo Clin Proc. 1990;65:51-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 138] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Fang CH, You JH, Lau WY, Lai EC, Fan YF, Zhong SZ, Li KX, Chen ZX, Su ZH, Bao SS. Anatomical variations of hepatic veins: three-dimensional computed tomography scans of 200 subjects. World J Surg. 2012;36:120-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Buhe S, Miyaki T, Saito T, Sawuti A, Terayama H, Naito M, Yi SQ, Itoh M. A study of the accessory hepatic vein to segments VI and VII with a morphological reconsideration of the human liver. Surg Radiol Anat. 2008;30:201-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Kamel IR, Lawler LP, Fishman EK. Variations in anatomy of the middle hepatic vein and their impact on formal right hepatectomy. Abdom Imaging. 2003;28:668-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Ming YZ, Niu Y, Shao MJ, She XG, Ye QF. Hepatic veins anatomy and piggy-back liver transplantation. Hepatobiliary Pancreat Dis Int. 2012;11:429-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Abdalla EK, Vauthey JN, Couinaud C. The caudate lobe of the liver: implications of embryology and anatomy for surgery. Surg Oncol Clin N Am. 2002;11:835-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 79] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Bargalló X, Gilabert R, Nicolau C, García-Pagán JC, Bosch J, Brú C. Sonography of the caudate vein: value in diagnosing Budd-Chiari syndrome. AJR Am J Roentgenol. 2003;181:1641-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Boozari B, Bahr MJ, Kubicka S, Klempnauer J, Manns MP, Gebel M. Ultrasonography in patients with Budd-Chiari syndrome: diagnostic signs and prognostic implications. J Hepatol. 2008;49:572-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 20. | Chawla Y, Kumar S, Dhiman RK, Suri S, Dilawari JB. Duplex Doppler sonography in patients with Budd-Chiari syndrome. J Gastroenterol Hepatol. 1999;14:904-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Zwiebel WJ. Sonographic diagnosis of hepatic vascular disorders. Semin Ultrasound CT MR. 1995;16:34-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Ralls PW, Johnson MB, Radin DR, Boswell WD, Lee KP, Halls JM. Budd-Chiari syndrome: detection with color Doppler sonography. AJR Am J Roentgenol. 1992;159:113-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Mathieu D, Vasile N, Menu Y, Van Beers B, Lorphelin JM, Pringot J. Budd-Chiari syndrome: dynamic CT. Radiology. 1987;165:409-413. [PubMed] |
| 24. | Wang L, Lu JP, Wang F, Liu Q, Wang J. Diagnosis of Budd-Chiari syndrome: three-dimensional dynamic contrast enhanced magnetic resonance angiography. Abdom Imaging. 2011;36:399-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Kane R, Eustace S. Diagnosis of Budd-Chiari syndrome: comparison between sonography and MR angiography. Radiology. 1995;195:117-121. [PubMed] |
| 26. | Soyer P, Rabenandrasana A, Barge J, Laissy JP, Zeitoun G, Hay JM, Levesque M. MRI of Budd-Chiari syndrome. Abdom Imaging. 1994;19:325-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Tang TJ, Batts KP, de Groen PC, van Hoek B, Haagsma EB, Hop WC, Janssen HL. The prognostic value of histology in the assessment of patients with Budd-Chiari syndrome. J Hepatol. 2001;35:338-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 59] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Shiomi S, Sasaki N, Habu D, Takeda T, Nishiguchi S, Kuroki T, Tanaka T, Ochi H. Natural course of portal hemodynamics in patients with chronic liver diseases, evaluated by per-rectal portal scintigraphy with Tc-99m pertechnetate. J Gastroenterol. 1998;33:517-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Dragoteanu M, Cotul SO, Tamas S, Piglesan C. Nuclear medicine dynamic investigations of diffuse chronic liver diseases and portal hypertension. Rom J Gastroenterol. 2004;13:351-357. [PubMed] |
| 30. | Dragoteanu M, Balea IA, Dina LA, Piglesan CD, Grigorescu I, Tamas S, Cotul SO. Staging of portal hypertension and portosystemic shunts using dynamic nuclear medicine investigations. World J Gastroenterol. 2008;14:3841-3848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Dragoteanu M, Cotul SO, Pîgleşan C, Tamaş S. Liver angioscintigraphy: clinical applications. Rom J Gastroenterol. 2004;13:55-63. [PubMed] |
| 32. | Gupta S, Barter S, Phillips GW, Gibson RN, Hodgson HJ. Comparison of ultrasonography, computed tomography and 99mTc liver scan in diagnosis of Budd-Chiari syndrome. Gut. 1987;28:242-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 39] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Shiomi S, Kuroki T, Ueda T, Takeda T, Ikeoka N, Nishiguchi S, Nakajima S, Kobayashi K, Ochi H. Clinical usefulness of evaluation of portal circulation by per rectal portal scintigraphy with technetium-99m pertechnetate. Am J Gastroenterol. 1995;90:460-465. [PubMed] |
| 34. | Bouvard G, Bouvard N, Dao T, el Fadel S, Fernandez Y. Value of hepatic angioscintigraphy combined to pulsed Echo-Doppler: application to the study of portal hypertension of cirrhotic patients. Apropos of 148 cases. Ann Radiol (Paris). 1991;34:362-368. [PubMed] |
| 35. | Leveson SH, Wiggins PA, Giles GR, Parkin A, Robinson PJ. Deranged liver blood flow patterns in the detection of liver metastases. Br J Surg. 1985;72:128-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 89] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 36. | Lautt WW. Hepatic Circulation in Health and Disease. NY: Raven Press 1981; 210-212. |