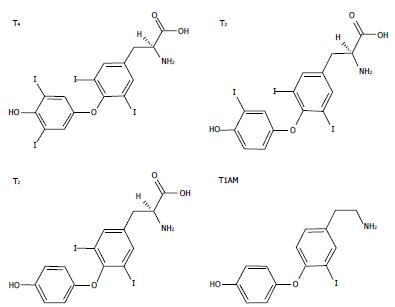
IODOTHYRONINES AND METABOLISM
Thyroid hormones (THs) secreted by the thyroid gland comprise two main iodothyronines: 3,5,3’,5’-tetraiodothyronine (thyroxine or T4) and 3,5,3’-triiodo-L-thyronine (T3) (Figure 1). T4 is the major form secreted by the thyroid and the most abundant TH in circulation, while T3, the active form, is mainly generated by peripheral deiodination of T4. T3 may be further deiodinated to yield different diiodothyronines such as 3,5-diiodo-L-thyronine (T2) (Figure 1). In the past, T3 was assumed to be the only active iodothyronine in vivo, but recent evidence suggested that other iodothyronines, such as 3’,5’,3-l-triiodothyronine (rT3) and T2, may be of biological relevance[1,2].
Figure 1 The chemical structures of three biologically active iodothyronines and one derivative: thyroxine, 3,3’,5-triiodothyronine, 3,5-diiodothyronine and 3-iodothyronamine.
T4: Thyroxine; T3: 3,3’,5-triiodothyronine; T2: 3,5-diiodothyronine; T1AM: 3-iodothyronamine.
THs influence a large number of physiological processes in vertebrates, including growth, development and differentiation. THs have stimulatory effects on metabolic activity, thus inducing thermogenesis (the so-called calorigenic effect) that represents a major component of the energy expenditure in endotherms. Of particular interest is the effect of THs on lipid metabolism resulting from the balance between stimulation of lipid synthesis and lipid oxidation (Figure 2). The liver represents one of the main target tissues of THs. At the hepatic level, T3 stimulates cholesterol synthesis and its metabolism into bile acids[3] and cholesterol uptake[4], and it induces lipogenic enzymes, including fatty acid synthase (FAS) and acetyl-CoA-carboxylase[5]. In addition to the lipogenic action, T3 also leads to a general reduction in the hepatic triglyceride (TAG) content, likely through stimulation of lipolytic pathways[6]. Moreover, it has been suggested that TH-stimulated lipogenesis/lipolysis “futile” cycle may contribute to the calorigenic effect[7].
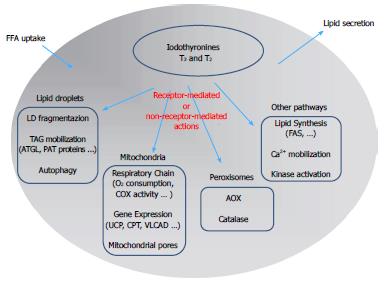
Figure 2 Schematic representation of the mechanisms underlying the control of lipid metabolism by iodothyronines in the hepatic cell: A summary of the possible signaling pathways involved in iodothyronine actions is presented.
The classic “receptor-mediated” pathway describes the action of iodothyronines through the thyroid hormone receptors (TR). The “non receptor-mediated” pathway occurs through the interaction of iodothyronines with different cellular targets. FFA: Free fatty acids; LD: Lipid droplet; AOX: Acyl-CoA oxidase; ATGL: Adipose triglyceride lipase; CPT: Carnitine palmitoyl-transferase 1; FAS: fatty acid synthase.
The metabolic effects of iodothyronines have long been investigated because of their potential use as drugs to treat obesity and lipid metabolism disorders[8]. However, due to the simultaneous undesirable side effects, such as the induction of a thyrotoxic state (tachycardia, muscle wasting, bone loss), the employment of T3 or T4 to stimulate body weight loss or treat metabolic syndrome has been limited. At the same time, the development of TH agonists/analogs retaining lipid-lowering and anti-obesity efficacies, while being devoid of thyrotoxic effects, has received great interest as a potential therapeutic advancement. The research of the last years has identified several iodothyronines other than T3 and T4 that display some thyromimetic activities. Among them, T2 assumed a great interest as it mimics several effects of T3 on energy metabolism[9-11] without inducing thyrotoxic effects[12]. A single dose of T2 (25 μg/100 g body wt) stimulated the resting metabolic rate (RMR) of hypothyroid rats and increased the liver oxidative capacity to the same extent as the same dose of T3[13]. Moreover, T2 significantly reduced serum triglyceride and cholesterol levels and increased liver oxygen consumption[10].
With regards to the calorigenic effects of THs, several cellular targets have been proposed but none has received universal acceptance. By virtue of their central role in the energy-transduction pathway, mitochondria are natural candidates to mediate the calorigenic activity of iodothyronines[14]. Single injections of T2 or T3 into hypothyroid rats stimulated RMR[15], in association with an increase in oxygen consumption[16]. It is widely accepted that iodothyronines may exert two kinds of effects on mitochondria: (1) a rapid stimulation of respiration (within minutes/hours); and (2) a long term effect leading to mitochondrial biogenesis and mitochondrial mass increase. The calorigenic activity of THs has long been ascribed to uncoupling of mitochondrial oxidative phosphorylation, but the mode by which they promote mitochondrial proton leak is still unresolved. Harper et al[17] related the T3-induced increase in mitochondrial proton leak to an increased permeability of the phospholipid bilayer due to a change in the lipid composition of the inner mitochondrial membrane. On the other hand, T2, and to a lesser extent T3, was shown to bind the Va subunit of the cytochrome oxidase complex, thus abolishing the allosteric inhibition due to ATP binding and stimulating enzyme activity[18]. Recently, Yehuda-Shnaidman et al[19] reported that mitochondrial uncoupling by T3 was transduced both in vivo (in rats) and in vitro (Jurkat cells) by gating of the mitochondrial permeability transition pore.
Interestingly, T2 administration has been demonstrated to be able to stimulate RMR and to also reduce body weight in humans. In a pilot study, two euthyroid subjects were treated with increasing doses of T2 (from 100 to 900 mcg/d, three times a day for 8 d) and for a further 3 weeks with 3000 mcg/d. A reduction in body weight of -4% was observed without effects at the cardiac level[20].
The pharmacological effects of some derivatives of thyronines called thyronamines have been also investigated. Scanlan et al[21] described the synthesis and biological properties of 3-iodothyronamine (T1AM), a novel thyronamine that was shown to be an endogenous component of biogenic amine extracts from rodents. T1AM has a carbon skeleton identical to that of T4 and theoretically, it could be produced from T4 by enzymatic decarboxylation and deiodination (Figure 1). T1AM treatment rapidly induced a hypometabolic state and hypothermia in rodents, with opposite effects compared with those typical of THs.
MECHANISMS OF ACTION OF IODOTHYRONINES
In the past, it was a common notion that TH actions were mediated by specific nuclear thyroid hormone receptors (TRs) acting as ligand-dependent transcription factors binding the “thyroid hormone response elements” (TREs) on the promoter region of thyroid hormone-responsive genes[22]. In the early 1960s, Tata and co-workers provided the first evidence for a “receptor-mediated” mechanism of T3 action on energy metabolism[23]. In the 1980s, two distinct genes, THRA and THRB, were identified in humans and rodents, each encoding a different TR isoform (TRα and TRβ, respectively). The THRA gene was originally identified in chicken[24], while THRB was cloned from human and rat cDNA libraries[25]. Each isoform shows alternative splice variants (TRα1, TRα2, TRβ1 and TRβ2) with specific and distinct functions and tissue localization.
Although the “receptor-mediated” mechanism accounts for several actions of THs, other effects independent of TRs have been described, suggesting an alternative model for their action. Effects of iodothyronines that are not initiated by binding to TRs are termed ‘non-receptor-mediated’ mechanisms[26] and could involve a multiplicity of signaling pathways, such as phosphorylation of effector proteins[27], binding to surface receptors[28], Ca2+ mobilization[29], alteration of mRNA stability[30], modification of membrane fluidity and permeability[2]. The possibility that TH action was mediated by interactions with membrane surface receptors was confirmed by using cell impermeant agarose-conjugated T3. The results clearly indicated that both free and conjugated hormones led to activation of extracellular signal-regulated kinase (ERK1/2s)[31] and affected Ca2+ homeostasis[32]. Moreover, THs were shown to interact with theαVβ3 integrin receptor triggering the ERK1/2 pathway[33]. Although the “non-receptor mediated” effects are sometimes called “non-genomic”, this term is rather confusing as these pathways may also in turn affect gene transcription [34].
In conclusion, it is now widely accepted that TH effects may result from a synergism between “receptor-mediated” and “non-receptor mediated” mechanisms. Moreover, we can distinguish between early and late effects of THs (also called “short-term” and “long-term” effects), the first being evident within minutes or a few hours, whereas the second occurs over several hours or days[34,35]. However, the latency of a response is not sufficient to discriminate between “receptor-mediated” and “non-receptor” mediated effects.
HEPATIC STEATOSIS: IN VITRO AND IN VIVO MODELS
With the rapidly growing prevalence of obesity throughout the Western countries, morbidity and mortality related to its complications are on the rise. Severe obesity is generally associated with TAG accumulation in non-adipose tissues like liver, muscle and pancreas and leads to a high risk of co-morbidities, including nonalcoholic fatty liver disease (NAFLD), cardiovascular disease and diabetes (for a review see[36]). NAFLD is a pathological condition associated with over-accumulation of TAGs in the liver and represents the most common of all hepatic disorders and the most frequent cause of chronic liver disease[37,38]. The earliest stage of NAFLD is hepatic steatosis characterized by the deposition of cytoplasmic TAGs as macro- and/or micro-vesicular lipid droplets in more than 5% of hepatocytes. Simple steatosis may progress to nonalcoholic steatohepatitis (NASH), cirrhosis and finally hepatocellular carcinoma[39]. NAFLD is now considered the hepatic manifestation of the metabolic syndrome and has insulin resistance as its hallmark. NAFLD is a syndrome with multifactorial etiology for which there is no effective treatment, although weight loss may halt disease progression and revert histological changes[36].
In hepatocytes, steatosis results from an imbalance between lipid availability (deriving from circulating lipid uptake or de novo lipid synthesis) and lipid disposal (through FFA oxidation or TAG secretion)[40]. Typically, the main cause of steatosis is an overflow of free fatty acids (FFAs) into the liver that may eventually trigger lipoperoxidative stress and hepatic injury[39,41]. In the liver, FFAs are stored as TAGs through their esterification with glycerol or, alternatively, catabolized by oxidation to generate adenosine triphosphate (ATP). Excess TAGs are accumulated inside lipid droplets (LDs) that regulate storage and traffic of lipids (for a review see[42]). Typically, LDs are composed of a core of neutral lipids surrounded by phospholipids and proteins of the PAT protein family (acronym referring to the first members identified)[43]. The main PAT proteins are the adipocyte differentiation-related protein (ADRP, also called PLIN2), the oxidative tissue-enriched PAT protein (OXPAT or PLIN5) and the tail-interacting protein (TIP47 or PLIN3)[44]. ADRP expression is increased in rat models of NAFLD and in isolated hepatocytes[45]. PAT proteins are under the control of peroxisome proliferator-activated receptors (PPARs), a subfamily of lipid-activated transcription factors[46] consisting of three members, PPARα, PPARγ and PPARδ, with distinct functional roles[47,48]. In the liver, PPARα enhances lipid catabolism and mobilization[49], PPARδ induces glycolysis/lipogenesis and PPARγ promotes lipid synthesis and LD formation[50]. In summary, PPARα and PPARδ mainly act in energy burning, whereas PPARγ regulates energy storage, although an overlapping in their function has been described[40-51]. Moreover, PAT proteins regulate action of hepatic lipases that mobilize TAGs stored in LDs towards oxidation or secretion[52], in particular, the adipose triglyceride lipase (ATGL) performs the first step in TAG hydrolysis.
In vivo models
Steatosis and steatohepatitis can be modeled in rodents by two main dietary protocols: a methionine and choline deficient (MCD) diet or a high-fat diet (HFD). Different dietary approaches produce different disease severities and work by specific mechanisms[53]. In rodents, a MCD diet quickly induces (2-4 wk) hepatic steatosis (mainly macrovesicular) that may progress to inflammation and fibrosis. MCD diet-induced NASH is reversible by switching to a diet with methionine and choline. Rodents fed MCD diets lose weight (due to the lower caloric intake) and do not show insulin resistance. By contrast, HFD increases body weight, body fat and induces insulin resistance in rodent models. In general, HFD feeding induces only mild steatosis (mainly microvesicular) and does not produce liver fibrosis. The term “HFD” encompasses a wide variety of diet formulas but in all of them about 30%-75% of total calories is derived from saturated fatty acids. This diet closely resembles the pathological and molecular alterations found in humans with NAFLD[53]. It can be emphasized that fatty liver is typically characterized by altered lipid metabolism, increased oxidative stress and abnormal pattern of cytokine production.
In vitro models
Hepatic steatosis in humans is typically associated with excess accumulation of oleic acid, a monounsaturated omega-9 fatty acid which represents the end product of de novo fatty acid synthesis. A number of studies using both primary cell cultures[54] and immortalized cell lines[55,56] proposed reliable cell models of hepatosteatosis in which the steatosis severity might be modulated and the TAG content was exactly quantifiable. These in vitro models represent a simple experimental system to investigate the mechanisms underlying the steatosis progression and the hepatocyte alterations by excluding the interference from the matrix and other non-hepatocytic cells. Over the past decade, several cellular models of hepatosteatosis have employed palmitate (C16:0) and oleate (C18:1) as exogenous fatty acids since these are common dietary long-chain FFAs and the most abundant FFAs in liver in both normal subjects and patients with NAFLD[57]. The human hepatoma cell line (HepG2) incubated with a mixture of oleate/palmitate (2:1 ratio) was used to study the cellular mechanisms involved in FFA-mediated lipotoxicity[55,58]. The same FFA mixture was used to induce steatosis in primary human hepatocytes[58]. In order to assess the different toxicity of saturated and unsaturated FFAs, primary mice hepatocytes and HepG2 cells were treated with various concentrations (0.05-0.5 mmol/L) of long chain FFAs with different degrees of saturation; exposure to monounsaturated fatty acids resulted in lipid accumulation without changes in hepatocyte viability; in contrast, saturated fatty acids significantly decreased cell viability[59]. The effect of increasing concentrations of oleate alone (0.1-2.0 mmol/L) was also evaluated in order to clarify the pathophysiological changes associated with NAFLD[60].
LIPID-LOWERING EFFECTS OF IODOTHYRONINES ON IN VIVO MODELS OF HEPATOSTEATOSIS
In 1994, a first study reported that a daily intraperitoneal (ip) injection of T3 (from 0 to 25 μg/100 g b.w.) to ob/ob mice decreased body weight and body fat and increased oxygen consumption and oxidative metabolism[61]. About ten years later, Goglia and coworkers described similar effects for T2[10]. They showed that a daily ip injection of T2 (25 μg/100 g b.w.) to rats simultaneously receiving HFD reduced both adiposity (about -50%) and body weight gain (about -13%) when compared with rats receiving HFD alone. Moreover, T2 administration resulted in an almost complete disappearance of fat accumulation in the liver, a reduction in serum TAG and cholesterol levels (-52% and -18%, respectively), and a stimulation (about +42%) of FFA oxidation rate without inducing thyrotoxicity [10]. The effects of T2 on liver metabolism seemed to involve mitochondria, even although peroxisomes are the main site for fat oxidation. In fact, long chain FFAs enter mitochondria through the activity of carnitine palmitoyl-transferase 1 (CPT1) that was stimulated by HFD and further increased by T2 [10].
Interestingly, dietary administration of T3 was also able to both prevent and reverse hepatic steatosis in rats[62]. In fact, concurrent dietary administration of T3 and MCD diet resulted in prevention of fatty liver and decrease in lipid peroxidation in rats fed a MCD diet for 10 weeks and then co-fed T3 for 1 week. Similar effects were observed using the potent TR selective agonist GC-1 [62].
The hepatic effects of T2 administration to HFD rats were investigated in more detail by Grasselli et al[63,64]. HFD feeding resulted in hepatic lipid accumulation under the form of numerous LDs, a condition resembling the microvesicular steatosis typical of NAFLD. Fat accumulation was associated with increased transcription of PPARα, a regulator for a number of genes involved in FFA catabolism, and of ATGL, a lipase mobilizing fat from LDs, together with a stimulation of anti-oxidant agents such as catalase and metallothioneins, in line with the increased production of reactive oxygen species (ROS) from mitochondria and peroxisomes as a consequence of fat accumulation[65]. In the liver of HFD rats, concomitant T2 administration was able to prevent lipid accumulation, but also oxidative stress conditions associated with the diet[63]. Moreover, T2 prevented the HFD-induced up-regulation of both PPARα and ATGL and stimulated - (AOX) expression, indicating a stimulation of peroxisomal FFA oxidation[64].
In addition to the above described reports demonstrating the ability of T2 to prevent liver steatosis when administered simultaneously to HFD, other studies demonstrated that T2 was also able to reverse hepatic steatosis after its induction through long term HFD and these effects were associated with a stimulation of mitochondrial uncoupling and a reduction in mitochondrial oxidative stress[66].
A recent paper investigated the changes in the rat liver proteome induced by T2 treatment. The proteomic approach allowed identification of which proteins were differentially expressed in the liver of HFD rats as a function of T2 treatment[67]. Upon T2 administration, the rat liver proteome resembled that typical of a non-steatotic condition. In particular, high-fat feeding led to changes in the expressions of enzymes involved in a multiplicity of pathways (i.e., lipid metabolism, antioxidant defense, respiratory chain, oxidative metabolism). Mitochondria, in particular, appeared as the major target for the metabolic/energy adaptations induced by lipid overload in the liver and showed the more marked changes in terms of proteome as a response to T2 treatment. In mitochondria from HFD rats, enhanced activities of complexes I and V and reduced activities of complexes II and IV were detected, even although the protein levels for all the complexes were increased. T2-treatment stimulated complexes I and II and normalized complex IV activity. On this basis, the authors suggest that the T2-induced enhancement of oxidative capacity may actually be based on a stimulation of the individual respiratory chain complexes (I, II and IV)[67].
LIPID LOWERING EFFECTS OF IODOTHYRONINES ON IN VITRO MODELS OF HEPATOSTEATOSIS
The above described in vivo studies could not distinguish between the direct antisteatosic effects of THs on the liver and their secondary effects due to upstream changes in endocrine or metabolic pathways. The employment of isolated hepatocytes allowed overcoming these problems.
Grasselli et al[51] assessed in vitro the direct effects of T2 and T3 (10-7-10-5 mol/L doses for 24 h) using primary cultures of rat hepatocytes overloaded of lipids (“steatotic” hepatocytes) by exposure to the classical oleate/palmitate (2:1 ratio) mixture. The use of supraphysiological doses of iodothyronines depends on both their rapid metabolism in vitro and on their binding to the high concentration (1%) of albumin present in the culture medium. In accordance with reports showing altered expression of PPARs in murine models developing fatty livers[47], isolated “steatotic” hepatocytes exhibited increased expression of both PPAR-γ and PPAR-δ, as well as of ADRP, a PPAR-regulated PAT protein. As in liver of HFD rats[64], also in isolated “steatotic” hepatocytes an increased activity of AOX, the enzyme catalyzing peroxisomal β-oxidation, as well as of SOD and catalase, two antioxidant enzymes protecting cells from the higher ROS production associated with FFA catabolism, was described. A reduction in the number and average sizes of LDs was observed after treatment with T2 or T3, suggesting that iodothyronines lead to dispersion/fragmentation of LDs, thus making the stored TAGs more accessible to enzymes acting on catabolism/secretion of FFAs. Moreover, both T2 and T3 were able to reduce the FFA-induced up-regulation of PPARγ and PPARδ, the stimulation of AOX, SOD and catalase activities. These results clearly indicate the lipid-lowering effect of iodothyronines mainly depends on a direct action on the hepatic cell[51].
The use of primary rat hepatocytes allowed verification that the lipid-lowering effect of iodothyronines was a direct action on the hepatocyte but the involvement of thyroid hormone receptors in mediating this action remained to be elucidated. To this end, the same experiments were repeated using the FaO rat hepatoma cell line defective for functional TRs. FaO cells were exposed to the classical oleate/palmitate (2:1) mixture and then treated with T2 or T3 for 24 h (10-7-10-5 mol/L doses). In FaO cells, TAG accumulation was associated with an increase in number and size of LDs and in PPARγ mRNA expression. The addition of T2 or T3 to “steatotic” cells reduced both the TAG content and the number and size of LDs and down-regulated expression of PPARα and PPARγ. Moreover, iodothyronines stimulated the fuel-induced O2 consumption. Since iodothyronines prevented the ADP-induced transient stimulation of O2 consumption, this indicated a mitochondrial uncoupling action. In conclusion, this study demonstrated that the lipid-lowering actions of both T2 and T3 on the hepatocyte occur via“non-receptor-mediated” mechanisms and involve a short-term action by stimulation of mitochondrial O2 consumption[68].
IODOTHYRONINES AND AUTOPHAGY OF LIPID DROPLETS
Despite the advances in the understanding of the effects of THs on cellular metabolism, little is known about the mechanisms by which THs regulate energy consumption within the cell. This is particularly true for the events involved in the delivery of FFAs to mitochondria, a necessary step in converting stored intracellular triglyceride fuel into ATP.
Autophagy is a stress-induced catabolic process involving lysosome fusion that is conserved in almost all eukaryotes. Autophagy of lipid droplets, termed “lipophagy”, has been shown to be a major pathway of lipid mobilization in hepatocytes[69] and its inhibition has been linked to development of fatty liver and insulin resistance[70]. The regulation of autophagy also appears to be important in the context of metabolic diseases, such as obesity. In a recent paper, Sinha et al[71] showed that T3 induced both lipophagy in cultured liver cell lines and hepatic autophagy in the mouse liver. The authors observed that the T3-stimulated autophagy of LDs depends on the presence of functional TRs and occurred before any stimulation of hepatic lipases or oxidation enzyme[71]. Moreover, in animals with impaired autophagy, the effect of THs on FFA oxidation was abolished. Therefore, they propose that T3 may increase the delivery of FFAs to mitochondria for β-oxidation through induction of autophagy of LDs. In this light, T3 or its analogs, through their proautophagic action, may be useful in the treatment or prevention of NAFLD and its associated complications.
CONCLUSION
In the last decades, extensive studies investigated the possible use of iodothyronines as pharmacological tools in the treatment of obesity, hyperlipidemia and dysmetabolic syndromes. The possible pharmacological use of the thyroid hormones T3 or T4 to stimulate body weight loss has found severe limitations because of the thyrotoxic effects associated with their long-term administration. For this reason, the identification of TH agonists/analogs retaining anti-obesity and hypolipemic efficacies, while being devoid of thyrotoxic effects, would represent a potential therapeutic advance.
Recent in vivo and in vitro studies have accumulated evidence on the lipid-lowering action of iodothyronines in the liver (Figure 2). The first studies showed that systemic administration of iodothyronines to rats receiving HFD resulted in a significant reduction in body weight gain and in the serum levels of triglycerides and cholesterol. At the organ level, the effects on the liver were very interesting, where iodothyronines could lower the excess lipid accumulation associated with HFD. These studies prosecuted by investigating the mechanisms of iodothyronine action. The development of in vitro models of hepatosteatosis using both primary cultures of rat hepatocytes and rat hepatoma cell lines allowed demonstration that the lipid lowering effects of iodothyronines depend on a direct interaction with the hepatic cell and is not mediated by thyroid hormone receptors. In conclusion, all the data summarized in this review clearly indicates that T2 is able to reduce the lipid content of “steatotic hepatocytes”, thus supporting the possible utilization of T2 as a pharmacological tool in the treatment of dysmetabolic syndromes, such as NAFLD, and also in the light of its lack of thyrotoxic effects.
Although a preliminary study on humans has been published, clinical trials are needed to translate these effects to the treatment of human obesity. If reproduced in humans, these results may offer an interesting perspective on the possible pharmacological approaches to the above mentioned lifestyle-related dysfunctions.
ACKNOWLEDGMENTS
The author wishes to thank Elena Grasselli, Fernando Goglia, Adriana Voci, Laura Canesi and Gabriella Gallo for valuable discussion and their contributions are cited in the article.
P- Reviewers: Goglia F, Hutz RJ, Kim JB S- Editor: Song XX L- Editor: Roemmele A E- Editor: Wu HL