Published online Nov 27, 2014. doi: 10.4254/wjh.v6.i11.825
Revised: August 27, 2014
Accepted: September 16, 2014
Published online: November 27, 2014
Processing time: 163 Days and 7 Hours
Intravenous epoprostenol is recommended for World Health Organization functional class (WHO-FC) IV patients with pulmonary arterial hypertension (PAH) in the latest guidelines. However, in portopulmonary hypertension (PoPH) patients, advanced liver dysfunction and/or thrombocytopenia often makes the use of intravenous epoprostenol challenging. Here we report the cases of two WHO-FC IV PoPH patients who were successfully treated with a combination of two oral vasodilators used to treat PAH: ambrisentan and tadalafil. Oral vasodilator therapy using a combination of ambrisentan and tadalafil may be a safe and effective therapeutic option for WHO-FC IV PoPH patients and should be considered for selected patients with severe and rapidly progressing PoPH.
Core tip: Advanced liver dysfunction and/or thrombocytopenia often hamper the use of intravenous epoprostenol in patients with portopulmonary hypertension (PoPH). However, recent progress in the oral treatment for pulmonary hypertension (PH) has enabled better clinical outcome in severe PH patients. Here we report two World Health Organization functional class IV patients with PoPH and thrombocytopenia who were successfully treated with ambrisentan and tadalafil. Oral vasodilator therapy using a combination of ambrisentan and tadalafil may be a safe and effective therapeutic option for patients with PoPH and advanced thrombocytopenia.
- Citation: Yamashita Y, Tsujino I, Sato T, Yamada A, Watanabe T, Ohira H, Nishimura M. Hemodynamic effects of ambrisentan-tadalafil combination therapy on progressive portopulmonary hypertension. World J Hepatol 2014; 6(11): 825-829
- URL: https://www.wjgnet.com/1948-5182/full/v6/i11/825.htm
- DOI: https://dx.doi.org/10.4254/wjh.v6.i11.825
Reportedly, 2%-6% of patients with portal hypertension also develop pulmonary hypertension (PH); this combined disorder is called portopulmonary hypertension (PoPH)[1-3]. According to the latest guidelines[4], PoPH is classified in the pulmonary arterial hypertension (PAH) spectrum. It is recommended that PoPH patients be managed similarly to those with other forms of PAH, while considering the presence of liver disease and its consequences[5]. Although intravenous epoprostenol treatment is recommended for World Health Organization functional class (WHO-FC) IV PAH patients, advanced liver dysfunction and/or thrombocytopenia often make such invasive management difficult in PoPH patients. Here we describe two WHO-FC IV PoPH patients who favorably responded to the combined use of ambrisentan and tadalafil, oral vasodilators that have been proven to be effective against PAH.
In September 2010, a 56-year-old man with hepatitis B virus-related cirrhosis, esophageal varix, and hepatocellular carcinoma was referred to our department with abnormal findings on electrocardiogram. Doppler echocardiography indicated increased systolic right ventricular pressure, estimated by a tricuspid regurgitation pressure gradient (TRPG) of 79 mmHg. Right heart catheterization (RHC) also revealed an increased mean pulmonary artery pressure (PAP) of 40 mmHg and an increased pulmonary vascular resistance (PVR) of 6.38 Wood units. PoPH was diagnosed; however, his clinical course (WHO-FC I) was modest. Accordingly, we decided to employ a careful wait-and-watch approach.
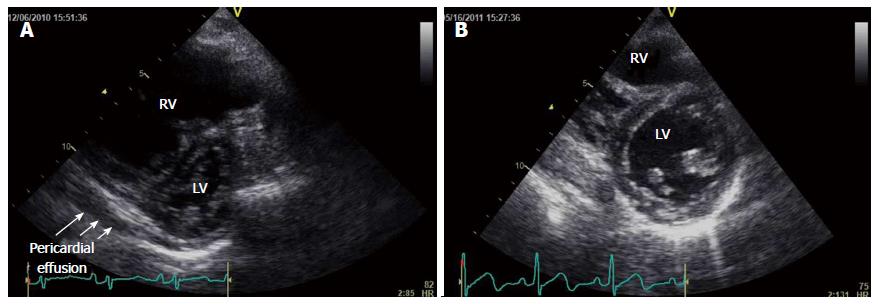
However, 3 mo later, the patient experienced rapid progression of exertional dyspnea, facial edema, and syncope, and he was consequently admitted to our department. He presented with jugular venous distention, pretibial pitting edema, and a pronounced pulmonary component of the second heart sound. Laboratory data revealed advanced thrombocytopenia (5.0 × 104/mL), an increased D-dimer level (9.38 μg/dL), an increased indirect bilirubin level (4.9 mg/dL), a mildly increased transaminase level (aspartate aminotransferase, 69 U/L; alanine aminotransferase, 40 U/L), and an increased plasma brain natriuretic peptide (BNP) level (2212 pg/mL). Transthoracic echocardiography revealed right ventricular dilatation and severe interventricular septal deviation toward the left ventricle, accompanied by pericardial effusion (Figure 1A). The pulmonary artery systolic pressure, estimated on the basis of TRPG, was 120 mmHg. Abdominal computed tomography (CT) revealed dilatation of the splenic, esophageal, and umbilical veins, suggestive of portal hypertension, whereas ventilation/perfusion lung scintigraphy revealed no significant mismatch. These results indicated rapid progression of PoPH; therefore, dobutamine (2 μg/kg per minute) was initiated. RHC revealed that the mean PAP was increased to 55 mmHg, cardiac index (CI) was decreased to 2.49 L/min per square, and PVR was increased to 10.9 Wood units.
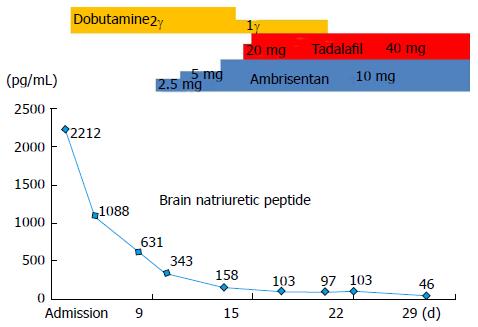
Because of comorbid advanced thrombocytopenia and hepatocellular carcinoma, we initiated ambrisentan at a dose of 2.5 mg once daily, added tadalafil at a dose of 20 mg once daily, and subsequently increased the dose of the two agents in turn to reach the maximum dose (ambrisentan, 10 mg/d; tadalafil, 40 mg/d) in 9 d. After a month, the patient’s heart failure symptoms and signs had improved and his plasma BNP level had decreased (Figure 2).
At a follow-up assessment 5 mo later, the patient’s WHO-FC had improved from IV to II and his plasma BNP level had decreased to 44.4 pg/mL. Echocardiography revealed a decrease in TRPG from 95 to 56.2 mmHg, a less pronounced interventricular septal shift toward the left ventricle, and no evidence of pericardial effusion (Figure 1B). Repeat RHC revealed a decrease in mean PAP (34 mmHg) and PVR (6.4 Wood units) and improvement in CI (4.27 L/min per square).
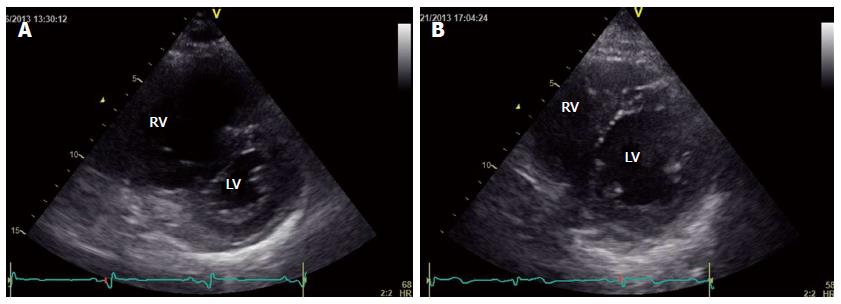
In April 2013, a 70-year-old man was referred to our department with rapid worsening of exertional dyspnea (WHO-FC IV) and suspected PH on echocardiography. He had been diagnosed with liver cirrhosis from excessive alcohol consumption, hepatocellular carcinoma, and esophageal varix approximately 9 years previously. He presented with jugular venous distention, hepatomegaly, and splenomegaly as well as a pronounced pulmonary component of the second heart sound and a third heart sound on chest auscultation. Laboratory data revealed advanced thrombocytopenia (3.5 × 104/mL), an increased D-dimer level (4.39 μg/dL), mild hepatic dysfunction (Child-Pugh B), and an increased plasma BNP level (988 pg/mL). Echocardiography revealed right ventricular dilatation and severe interventricular septal deviation toward the left ventricle accompanied by pericardial effusion (Figure 3A). Systolic PAP was 125 mmHg, as estimated by TRPG. CT suggested dilation of the esophageal vein. Ventilation/perfusion lung scintigraphy revealed a mismatch in the left upper lobe, although it was limited and not compatible with chronic thromboembolic PH. Cardiac magnetic resonance imaging indicated a dilated right ventricle and decreased right ventricular ejection fraction (RVEF; 25.8%). A hemodynamic study with oxygen at 5 L/min revealed an increased mean PAP (62 mmHg), a decreased CI (1.43 L/min per square), and an increased PVR (18.5 Wood units).
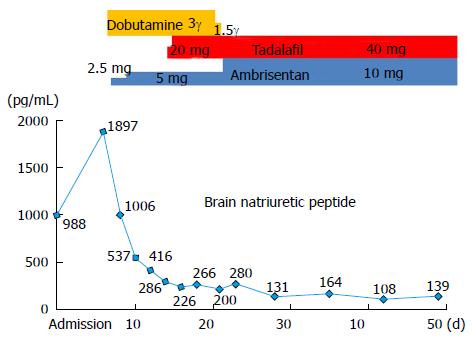
Based on findings indicative of portal hypertension and RHC findings, PoPH was diagnosed. We initiated dobutamine at 2 μg/kg per minute to support cardiac function. We then initiated ambrisentan at a dose of 2.5 mg once daily. and added tadalafil at a dose of 20 mg once daily. The doses of these two agents were then increased in turn to reach the maximum dose (ambrisentan, 10 mg/d; tadalafil, 40 mg/d) in 15 d.
Within a month and a half, the patient’s plasma BNP level had decreased, right heart failure signs had disappeared, and WHO-FC had decreased to III (Figure 4). At the follow-up assessment conducted 4 mo later, his WHO-FC was III and the plasma BNP level had decreased to 35 pg/mL. Echocardiography revealed that TRPG had decreased to 56.2 mmHg, the degree of interventricular septal deviation toward the left ventricle had decreased, and pericardial effusion was absent (Figure 3B). Cardiac magnetic resonance imaging-derived RVEF improved to 50.8%, and RHC revealed improvement in mean PAP (34 mmHg), CI (2.9 L/min per square), and PVR (4.7 Wood units).
Both patients gave their written informed consent prior to their inclusion in the present study. The present study complied with the Declaration of Helsinki.
In the latest guidelines for PH, the recommended treatment strategy for PoPH is similar to that for PAH, while liver transplantation is considered in a selected subset of patients[6]. Based on this strategy, intravenous epoprostenol would be considered for WHO-FC IV patients, including the present two cases. However, the successful use of oral agents effective against PAH has been reported for PoPH patients[7-9], suggesting a potentially important role of these drugs in PoPH treatment. In the two cases presented here, the use of intravenous epoprostenol was initially considered. However, it could not be used because of comorbid thrombocytopenia, which was thought to increase the risk during Hickman catheter implantation[10], and the probable further deterioration of thrombocytopenia caused by intravenous epoprostenol treatment itself[11]. In addition, both patients had been diagnosed with hepatocellular carcinoma, which further supported the use of a conservative treatment strategy. Considering the severe and rapid progressive state of the disease, we initiated combination treatment using ambrisentan, a dual endothelin receptor antagonist with limited liver toxicity, and tadalafil, a long-acting phosphodiesterase 5 inhibitor.
After the oral combination therapy, the patients’ signs and symptoms dramatically improved. In addition, pulmonary hemodynamics improved. In particular, PVR decreased by 41% in case 1 and by 75% in case 2. This degree of PVR reduction was comparable with or greater than that achieved by drugs effective against PAH[12]. Furthermore, WHO-FC, the plasma BNP level, and CI notably improved after treatment in both cases in the present study, suggesting a favorable clinical outcome[13].
Notably, some clinical features were unique to the two cases of PoPH presented here. One was the rapid progression of disease. Pathologically, PoPH cases reportedly show vascular remodeling, as observed in idiopathic PAH[10,11], which is likely to develop gradually. However, in these two cases, PH-related symptoms and signs rapidly progressed during the month prior to admission. In addition, CI is usually increased in PoPH, reflecting the portal systemic shunt. However, in the present two cases, CI decreased. One possible interpretation of these clinical features is a unique pathogenesis of PoPH in these two cases, such as vascular spasm, rather than the gradual progression of pulmonary vasculopathy typically observed in PoPH[14,15]. Such a rapid pathogenesis may explain why oral treatment dramatically improved the clinical features in the short term. In addition, decreased cardiac function, as represented by CI, may have been caused by an unusually rapid elevation of PAP/PVR, which quickly resolved after treatment.
In the Reveal registry, the survival rate of PoPH is reportedly worse than that of idiopathic PAH and familial PAH[16]. Two possible explanations have been proposed for the worse clinical outcome of PoPH. First, comorbid advanced liver diseases such as liver cirrhosis and cancer can negatively impact survival. Second, comorbid liver disease and its complications also impede the optimal use of drugs effective against PAH, such as endothelin receptor antagonists and intravenous epoprostenol. In the present two cases, long-term outcome must be evaluated, despite the short-term outcome (up to 5 mo) being favorable.
In conclusion, we presented two cases of severe PoPH with a favorable clinical response to a combination of ambrisentan and tadalafil. Although this approach cannot be generalized, this combination therapy should be considered in selected patients with severe and rapidly progressive PoPH. Further studies would be required to better understand the pathogenesis and establish optimal treatment strategies for PoPH patients.
Two male patients with liver cirrhosis and portal hypertension.
Rapidly progressive exertional dyspnea.
Progression of liver dysfunction, pulmonary and cardiovascular disease.
Case 1: thrombocytopenia (5.0 x 104/mL), and increased D-dimer (9.38 μg/dL), transaminases, and plasma BNP levels (2212 pg/mL); Case 2: thrombocytopenia (3.5 × 104/mL), and increased D-dimer (4.39 μg/dL) and plasma BNP levels (988 pg/mL).
Right ventricular dilatation and increase in the estimated systolic pulmonary artery pressure by transthoracic echocardiography in both cases. Abdominal CT scan revealed findings of portal hypertension, whereas ventilation/perfusion lung scintigraphy showed no significant mismatch in both cases.
Both patients were treated with ambrisentan–tadalafil combination therapy for rapidly progressive portopulmonary hypertension (PoPH).
Successful monotherapy using an oral agent effective against pulmonary artery hypertension has been recently reported for patients with PoPH.
PoPH is a subtype of pulmonary hypertension (defined as a mean pulmonary artery pressure equal to or greater than 25 mmHg) that develops in patients with portal hypertension.
A combination of ambrisentan and tadalafil may be a safe and effective therapeutic option for a certain subset of patients with PoPH and advanced thrombocytopenia.
It is a good paper for publication.
P- Reviewer: Feltracco P, Yao CL S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Hadengue A, Benhayoun MK, Lebrec D, Benhamou JP. Pulmonary hypertension complicating portal hypertension: prevalence and relation to splanchnic hemodynamics. Gastroenterology. 1991;100:520-528. [PubMed] |
| 2. | Castro M, Krowka MJ, Schroeder DR, Beck KC, Plevak DJ, Rettke SR, Cortese DA, Wiesner RH. Frequency and clinical implications of increased pulmonary artery pressures in liver transplant patients. Mayo Clin Proc. 1996;71:543-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Colle IO, Moreau R, Godinho E, Belghiti J, Ettori F, Cohen-Solal A, Mal H, Bernuau J, Marty J, Lebrec D. Diagnosis of portopulmonary hypertension in candidates for liver transplantation: a prospective study. Hepatology. 2003;37:401-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 209] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 4. | Galiè N, Simonneau G. The Fifth World Symposium on Pulmonary Hypertension. J Am Coll Cardiol. 2013;62:D1-D3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 5. | Galiè N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, Beghetti M, Corris P, Gaine S, Gibbs JS. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J. 2009;30:2493-2537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2304] [Cited by in RCA: 2259] [Article Influence: 141.2] [Reference Citation Analysis (0)] |
| 6. | Galiè N, Corris PA, Frost A, Girgis RE, Granton J, Jing ZC, Klepetko W, McGoon MD, McLaughlin VV, Preston IR. Updated treatment algorithm of pulmonary arterial hypertension. J Am Coll Cardiol. 2013;62:D60-D72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 468] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 7. | Hoeper MM, Halank M, Marx C, Hoeffken G, Seyfarth HJ, Schauer J, Niedermeyer J, Winkler J. Bosentan therapy for portopulmonary hypertension. Eur Respir J. 2005;25:502-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 121] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 8. | Reichenberger F, Voswinckel R, Steveling E, Enke B, Kreckel A, Olschewski H, Grimminger F, Seeger W, Ghofrani HA. Sildenafil treatment for portopulmonary hypertension. Eur Respir J. 2006;28:563-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 131] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 9. | Cartin-Ceba R, Swanson K, Iyer V, Wiesner RH, Krowka MJ. Safety and efficacy of ambrisentan for the treatment of portopulmonary hypertension. Chest. 2011;139:109-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 10. | Barst RJ, Rubin LJ, Long WA, McGoon MD, Rich S, Badesch DB, Groves BM, Tapson VF, Bourge RC, Brundage BH. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med. 1996;334:296-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1913] [Cited by in RCA: 1768] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 11. | Chin KM, Channick RN, de Lemos JA, Kim NH, Torres F, Rubin LJ. Hemodynamics and epoprostenol use are associated with thrombocytopenia in pulmonary arterial hypertension. Chest. 2009;135:130-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Channick RN, Simonneau G, Sitbon O, Robbins IM, Frost A, Tapson VF, Badesch DB, Roux S, Rainisio M, Bodin F. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomised placebo-controlled study. Lancet. 2001;358:1119-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1079] [Cited by in RCA: 990] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 13. | McLaughlin VV, Gaine SP, Howard LS, Leuchte HH, Mathier MA, Mehta S, Palazzini M, Park MH, Tapson VF, Sitbon O. Treatment goals of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D73-D81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 208] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 14. | Edwards BS, Weir EK, Edwards WD, Ludwig J, Dykoski RK, Edwards JE. Coexistent pulmonary and portal hypertension: morphologic and clinical features. J Am Coll Cardiol. 1987;10:1233-1238. [PubMed] |
| 15. | Jamison BM, Michel RP. Different distribution of plexiform lesions in primary and secondary pulmonary hypertension. Hum Pathol. 1995;26:987-993. [PubMed] |
| 16. | Krowka MJ, Miller DP, Barst RJ, Taichman D, Dweik RA, Badesch DB, McGoon MD. Portopulmonary hypertension: a report from the US-based REVEAL Registry. Chest. 2012;141:906-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 186] [Article Influence: 13.3] [Reference Citation Analysis (0)] |