Published online Oct 27, 2014. doi: 10.4254/wjh.v6.i10.716
Revised: July 14, 2014
Accepted: August 27, 2014
Published online: October 27, 2014
Processing time: 160 Days and 7.6 Hours
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related deaths worldwide. Only 30%-40% of the patients with HCC are eligible for curative treatments, which include surgical resection as the first option, liver transplantation and percutaneous ablation. Unfortunately, there is a high frequency of tumor recurrence after surgical resection and most HCC seem resistant to conventional chemotherapy and radiotherapy. Sorafenib, a multi-tyrosine kinase inhibitor, is the only chemotherapeutic option for patients with advanced hepatocellular carcinoma. Patients treated with Sorafenib have a significant increase in overall survival of about three months. Therefore, there is an urgent need to develop alternative treatments. Due to its role in cell growth and development, the insulin-like growth factor system is commonly deregulated in many cancers. Indeed, the insulin-like growth factor (IGF) axis has recently emerged as a potential target for hepatocellular carcinoma treatment. To this aim, several inhibitors of the pathway have been developed such as monoclonal antibodies, small molecules, antisense oligonucleotides or small interfering RNAs. However recent studies suggest that, unlike most tumors, HCC development requires increased signaling through insulin growth factor II rather than insulin growth factor I. This may have great implications in the future treatment of HCC. This review summarizes the role of the IGF axis in liver carcinogenesis and the current status of the strategies designed to target the IGF-I signaling pathway for hepatocellular carcinoma treatment.
Core tip: It is mandatory to develop alternative therapies for the successful treatment of hepatocellular carcinoma (HCC). One of the key drivers of hepatocarcinogenesis is the insulin-like growth factor (IGF) system. Therefore, several inhibitors of this pathway have been developed and their therapeutic potential is being tested in patients with HCC. However, recent studies suggest that IGF-II, a member of the pathway, may be more relevant for hepatocarcinogenesis than its close homologue IGF-I. The purpose of this review is to summarize these facts within a detailed description of the IGF axis and the alterations of the pathway that lead to HCC. The strategies designed to target the IGF-I signaling pathway for HCC treatment are also reviewed.
- Citation: Enguita-Germán M, Fortes P. Targeting the insulin-like growth factor pathway in hepatocellular carcinoma. World J Hepatol 2014; 6(10): 716-737
- URL: https://www.wjgnet.com/1948-5182/full/v6/i10/716.htm
- DOI: https://dx.doi.org/10.4254/wjh.v6.i10.716
The insulin-like growth factor system is formed by three ligands, three receptors and at least six high affinity binding proteins that work cooperatively to regulate cellular metabolism, proliferation, differentiation and apoptosis in most cells[1]. The three ligands are insulin, insulin growth factor (IGF)-I and -II. Each has the highest affinity for a specific receptor named after itself: insulin receptor (IR), IGF-I receptor (IGF-IR) and IGF-II receptor (IGF-IIR). Furthermore, the IGF axis is composed of IGF high affinity binding proteins (IGFBPs) and IGFBP proteases[2].
IGF-I and IGF-II are single chain polypeptides, approximately 7 kDa in size, that share 62% of the amino acid sequence[3]. They were first named somatomedins, because they mediate the activity of growth hormone (GH), also named somatotropin[4]. Later, they were renamed to highlight their similarity with insulin[5]. Insulin is a small hormone secreted by pancreatic beta-cells that maintains normal glucose levels in blood by regulating carbohydrate, protein and fat metabolism. Several excellent reviews on insulin and the insulin pathway have been published recently and therefore, this ligand will not be dealt with great detail in this article[6-8]. IGF ligands are bound with high affinity to IGFBPs. IGFBPs regulate the half-life and bioavailability of IGF-I and IGF-II and modulate their accessibility to the receptor[9]. IGFBP activities are closely regulated by post-translational modifications and IGFBP proteases. Most IGFBPs also have functions unrelated to the IGF system[10].
Most of the intracellular activity of IGF-I and IGF-II is mediated by the tyrosine kinase IGF-IR whereas insulin exerts its biologic functions mainly through IR[11]. These receptors are homologous because they derive from a common ancestor gene[12]. Despite the fact that IR and IGF-IR share most of their downstream mediators, it has been commonly accepted that IGF-IR activation promotes proliferation and differentiation and IR activation promotes metabolic signaling[11]. Surprisingly, the IGF-IIR differs largely from IR and IGF-IR, and sequesters IGF-II to internalize it for degradation[13].
IGF-I
IGF-I is the main mediator of GH function in normal embryonic development and postnatal growth[14]. GH is produced and secreted by the pituitary gland to induce body growth[15]. GH binds to the GH receptor in the liver and activates a signaling pathway that leads to transcription of several genes, including IGF-I[16]. Human IGF-I gene can be transcribed from two alternative promoters[17-19]. Furthermore, different mature IGF-I transcripts are produced by alternative splicing and polyadenylation[17,19-22]. These transcripts encode for different pre-proteins that undergo post-translational modifications and mature by proteolytic cleavage at both ends[23], resulting in a single polypeptide of 70 amino acids (7.5 kDa) cross-linked by 3 disulfide bonds[24,25]. Currently, the impact on IGF-I functionality of such a complex mRNA and protein processing is unclear.
IGF-I is produced by several tissues, including the liver, bone, muscle and brain[26]. The IGF-I produced in these organs acts locally, with the exception of the liver, which produces most of the secreted hormone[27]. Hepatocytes are the main producers of IGF-I in the liver while non-parenchymal cells make a minimal contribution[28]. Liver secretion is possible because IGF-I is not sequestered by liver IGF-IR, which is almost undetectable in healthy hepatocytes[29], and it is only expressed in the liver in non-abundant non-parenchymal cells such as Kupffer cells, hepatic stellate cells (HSCs) and myofibroblasts[28]. Circulating IGF-I levels increase from birth to puberty when they reach their maximum value and then decline with age thereafter[30]. When circulating IGF-I increases, it inhibits the synthesis of GH and IGF-I production is then controlled by negative feedback[31].
IGF-I has similar functions to insulin, since both regulate glucose uptake and their production is affected by nutritional status. IGF-I exerts its function by binding with high affinity to its principal receptor, IGF-IR (Figure 1). However, it can also bind to IR with 100-fold less affinity[32]. IGF-I binding to IGF-IR promotes anabolic processes such as DNA, RNA, protein and glycogen synthesis and results in proliferative and differentiating effects[33].
IGF-II
Unlike IGF-I, IGF-II expression is not regulated by GH[34]. In fact, the main regulator of IGF-II transcription is still unknown. The IGF-II gene is generally an imprinted gene expressed only from the paternal allele[35,36]. However, in the liver this control is only maintained at the fetal stage, as IGF-II expression becomes biallelic in the adult liver[37]. This is not due to a real loss of imprinting but to the activation of a biallelic adult liver specific promoter responsible for producing 50% of liver IGF-II[38]. The standard imprinted IGF-II promoters are still active in the adult liver and account for the remaining expression of IGF-II from the paternal allele[37,39]. The IGF-II gene encodes a pre-pro-IGF-II protein of 180 amino acids that transforms to a 156 amino acid-long pro-IGF-II upon peptide signal loss[40]. Most of the pro-IGF-II is cleaved and glycosylated to yield the 67 amino acid-long mature IGF-II[41].
IGF-II can be produced by several tissues, but most comes from both parenchymal and non-parenchymal liver cells[28]. IGF-II expression reaches maximal values during the fetal stage, as IGF-II plays a crucial role in fetal development[42]. After birth, IGF-II levels decrease to 400-600 ng/mL (about 4-fold higher than IGF-I) and remain constant for the rest of life[43]. Despite the higher amount of IGF-II than IGF-I, the function of IGF-II is gradually replaced by IGFI after birth[2]. Similar to IGF-I, IGF-II is able to bind with high affinity to IGF-IR, to regulate cell proliferation and differentiation (see below)[34]. Furthermore, IGF-II can bind to IGF-IIR, which induces IGF-II internalization and degradation[44]. Finally, IGF-II can also bind to insulin receptor subtype A (IR-A) to display mainly mitogenic effects[45,46]. Interestingly, both IR-A and IGF-II are upregulated in several tumors[47].
Synthesis and secretion of insulin is mainly regulated by glucose levels, but other stimuli can also influence these processes[6]. Upon binding to the IR, insulin plays a key role in maintaining normal glucose levels in blood by regulating carbohydrate, protein and fat metabolism[48]. While IR activation after insulin binding promotes mainly metabolic events, recent evidence supports the hypothesis that IR can also mediate mitogenic effects[49,50].
Six high affinity IGFBPs (IGFBP1-6) have been described which share 36% homology[51,52]. There are other IGFBP-related proteins (designated IGFBP-rP1-10) which bind IGF-I and IGF-II with lower affinity than classical IGFBPs[52]. IGFBP structure is composed of three domains. The N-terminal and the cysteine rich C-terminal domains are involved in IGF ligand binding and are common to all IGFBPs. The intermediate domain is different for each IGFBP and is probably involved in IGF-independent functions[10]. IGFBPs transcription is cell specific and is tightly regulated by hormones and by growth factors[2]. IGFBP levels are also controlled post-transcriptionally by proteolysis. There are three types of IGFBP proteases: serine proteinases, matrix-metalloproteinases (MMPs) and aspartyl proteinases[30]. IGFBP proteases are relatively specific for each IGFBP because the site of cleavage is inside the hyper-variable domain of the IGFBPs.
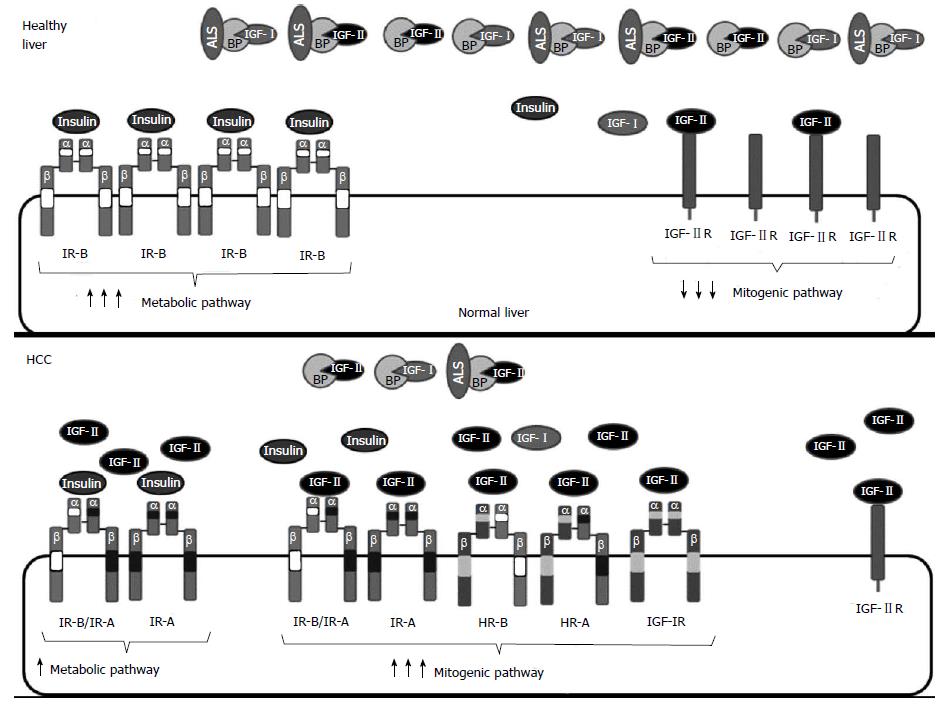
IGFBPs are widely expressed, but each tissue preferentially produces one or two classes[10]. The principal source of IGFBPs is the liver. There, hepatocytes express IGFBP1, 2 and 4 while non-parenchymal cells express IGFBP3[28]. After tissue secretion, IGFBPs circulate in blood and extravascular fluids and all of them bind IGF-I and IGF-II ligands with high affinity (10-10 M)[10]. IGFBP2, 5 and 6 have a special preference for IGF-II[53]. Interestingly, IGFBPs do not bind insulin because the specific amino acids that confer IGF binding affinity are not conserved in the insulin sequence[25,54]. Ninety-nine percent of circulating IGF-I is bound by IGFBPs[2]. This high efficiency of IGF-I binding is due to the excess of IGFBPs (50 times higher than IGF-I) and to the high binding affinity[55]. Note that the affinity of IGFBPs for IGF ligands is similar or even higher than the affinity of IGF ligands for their receptors[52].
IGF binding to IGFBPs increases ligand half-life but decreases IGF availability for signaling through IGF receptors. Both IGF-I and IGF-II are able to form binary complexes of approximately 50 kDa with IGFBPs or ternary complexes of approximately 150 kDa with IGFBP3 (or IGFBP5 to a lesser extend) and the acid-labile subunit (ALS) protein[9]. Almost 75% of the bound IGF forms ternary complexes[56]. When bound to IGFBP3, IGF-I half-life increases from 8 to 30 min and when bound to IGFBP3 and ALS, IGF-I half-life increases from 30 min to 15 h[57]. However, the IGF-I-IGFBP3-ALS ternary complex is too large to pass through the vascular endothelium to reach the IGF-IR[58]. Therefore, plasmatic proteases are required to break tertiary into binary complexes, able to cross the vasculature. Subsequent proteolysis of IGFBPs by plasmatic or tissue specific proteases releases IGFs and allows IGF signaling[55].
In general, it can be considered that IGFBP1, 3 and 5 activate IGF signaling while IGFBP2, 4 and 6 are inhibitory. However, the same IGFBP can potentiate or inhibit IGF signaling depending on post-translational modifications or binding to other factors[10,59-61].
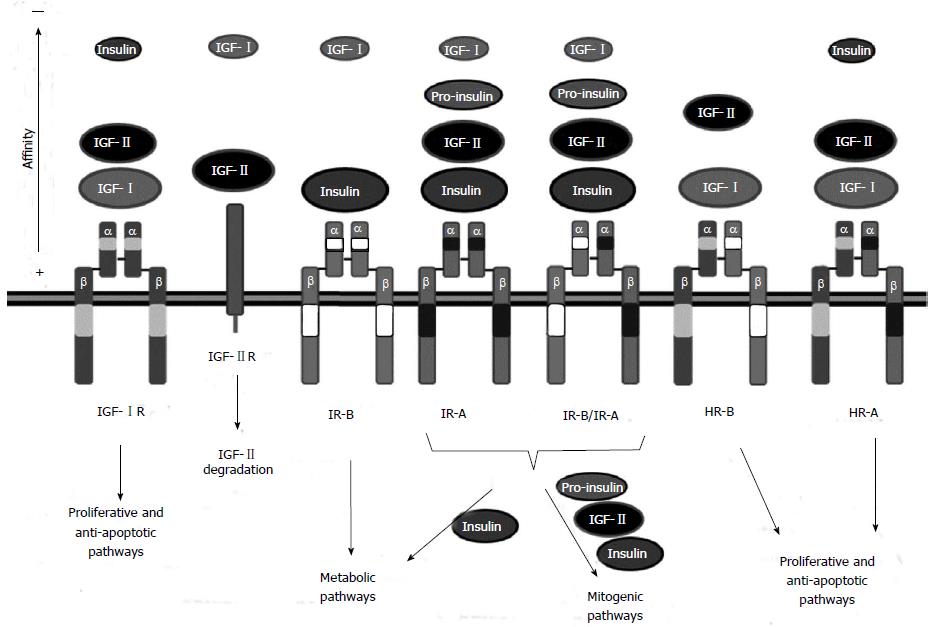
IGF-IR is a transmembrane tyrosine kinase receptor expressed ubiquitously. The mature receptor is composed of 2 homodimers, i.e., two α and two β subunits, cross-linked by disulfide bridges (Figure 1). The α subunit (130-135 kDa) is located extracellularly and contains the IGF binding domain, while the β subunit (90-97 kDa) crosses the membrane and reaches the cytoplasm where the tyrosine kinase domain is located[62].
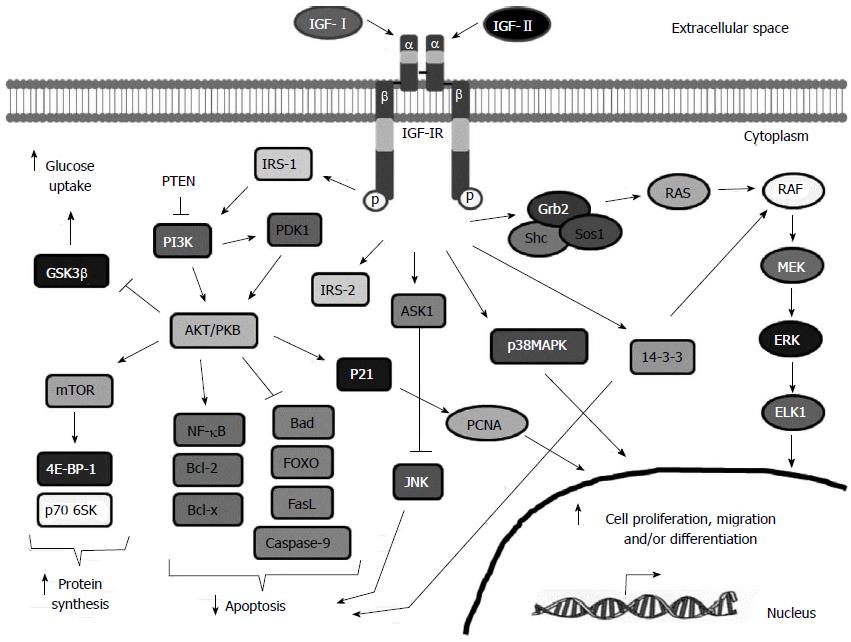
IGF-1R is mainly activated by IGF-I. However, it can also bind IGF-II and insulin with 2-5 fold and 100-1000 fold less affinity, respectively[63] (Figure 1). Following ligand binding, IGF-IR suffers a conformational change that activates the tyrosine kinase domain, leading to autophosphorylation of specific tyrosines and recruitment of specific docking proteins, including insulin-receptor substrate proteins (IRS-1 to -4) and Shc[64]. Thus, different signaling cascades are activated (Figure 2): (1) IRS-1 phosphorylation activates the phosphatidylinositol-3 kinase (PI3K)-AKT-mTOR pathway that leads to increased glucose uptake and protein synthesis, cell survival and apoptosis inhibition[65]. Following IRS-1 phosphorylation, PI3K is activated by phosphorylation, leading to activation of AKT/PKB[66]. AKT/PKB inhibits apoptosis by activating by phosphorylation anti-apoptotic proteins such as Bcl2, Bclx and NFκB, and by inhibiting by phosphorylation pro-apoptotic proteins such as the Bcl-2 family member Bad, members of the fork head transcription factor (FOXO) family, Fas ligand (FasL) and caspase 9[62,67]. Furthermore, AKT/PKB induces glucose uptake and glycogen synthesis through inhibition of glycogen synthase kinase-3 (GSK-3β) activity[68] by phosphorylation of the serine 9 residue[69,70]. Finally, AKT/PKB phosphorylates the DNA repair protein p21. Phosphorylated p21 does not bind PCNA leading to cell cycle progression[71]; and (2) Phosphorylation of the Shc protein activates the RAS-RAF-ERK pathway, related to cell differentiation, proliferation and migration[72]. Phosphorylated Shc complexed with Grb2 and SOS proteins leads to the activation of RAS[73]. RAS induces ERKs, which in turn inhibit apoptosis in a similar way to AKT and induces cell proliferation and migration[74].
Besides these major pathways, IGF-IR may also: (1) activate p38 mitogen-activated protein kinase (p38MAPK), leading to cellular growth and differentiation[75]; (2) bind apoptotic signal-regulated kinase 1 (ASK1), which impedes c-Jun N-terminal kinase (JNK) activation, that in turn, inhibits the apoptosis mediated by death-inducing receptors[76]; (3) lead to the 14-3-3-dependent mitochondrial translocation of Raf, maintaining the mitochondrial integrity, and thus protecting cells from apoptosis[77]; phosphorylate IRS-2 which influences integrin expression, that, together with the IRS-1-dependent decreased cell-cell contact, potentiates cell motility and anchorage independent growth[78]; and (4) affect JAK/STAT-3-mediated inhibition of apoptosis[79]. Thus, in summary, IGF-IR activation leads to differentiation or to increased cell proliferation and migration.
IGF-IIR
The human IGF-IIR gene is imprinted in rodents, where it shows maternal expression[80,81]. Surprisingly, expression in humans is polymorphic: most humans are biallelic but some show imprinted expression[82,83]. IGF-IIR gene expression results in a transmembrane protein of 2491 amino acids located in the Golgi apparatus (approximately 90%) and in the cell surface (approximately 10%)[84]. The extracellular domain consists of 15 homologous tandem repeats, able to bind with different affinities to mannose 6-phosphate (M6P)-containing proteins or M6P free factors[85]. M6P factors that bind IGF-IIR include leukemia inhibitory factor, cathepsin D and latent TGF. M6P free proteins bound by the receptor are urokinase-type plasminogen activator receptor (uPAR), retinoic acid and IGF-II[86]. It has been shown that IGF-I can also bind to IGF-IIR but with very low affinity, while insulin does not bind at all[87] (Figure 1).
The main function of IGF-IIR is to transport extracellular and Golgi derived-acid hydrolases and other ligands to lysosomes[86]. Upon IGF-II binding, the entire complex is internalized in clathrin-coated vesicles that travel to the endosomal compartment, where the ligand is degraded and the receptor is recycled to the cell membrane[88]. Therefore, in the IGF axis, IGF-IIR acts as a scavenger receptor lacking intrinsic signaling. Thus, several studies have demonstrated that IGF-IIR may act as a tumor suppressor gene[80,89,90].
Interestingly, some authors have described that IGF-IIR may be cleaved from the cell membrane to act as a truncated soluble form of 270-280 kDa[91]. This soluble receptor is detected at very low concentrations (0.1 nmol/L) in the serum and other fluids of several mammalian species[86]. However, it efficiently sequesters circulating IGF-II[92].
IR and IGF-IR share almost the same signaling pathway. Insulin binding to the IR induces a conformational change that results in the autophosphorylation of the IR and the recruitment of IRS proteins or Shc, leading, respectively, to the activation of PI3K-AKT-mTOR and metabolic effects or to the activation of RAS-RAF-ERK and mitogenic effects[6]. In fact there are two isoforms of the IR. The standard receptor is IR-B, expressed in adult liver, muscle and adipose tissue and involved in binding to insulin to regulate glucose homeostasis[62]. Alternative splicing regulation leads to a mature transcript that encodes for IR-A, which lacks 12 amino acids from exon 11 and is expressed by fetal tissues and some tumors[47]. IR-A can bind to insulin, IGF-II and proinsulin with different affinities to promote mainly proliferation, migration and inhibition of apoptosis, but also possess some metabolic activating capacity[62,93] (Figure 1). Insulin binding to IR-A seems to induce more metabolic effects than IGF-II binding[94,95]. IR-A binds insulin with 1.5 fold higher affinity than IR-B and possesses a higher dissociation and internalization rate[95]. Therefore, in cells with increased IR-A:IR-B ratios, most insulin signals through IR-A[7]. This is in line with the higher risk of HCC when insulin serum levels increase[96]. Proliferation can also result from IR-A binding to IGF-II, which interacts with 3-10 fold lower affinity than insulin, or to proinsulin[97].
IR-A and IR-B can form IR-A/IR-B heterodimers that behave similarly to IR-A[7]. It seems that insulin and IGF-II can bind IR-A/IR-B hybrids with the same affinity as IR-A homodimers (Figure 1). IGF-I also binds IR-A/IR-B hybrids with lower affinity[98]. When IR-A is overexpressed, most IR-B forms IR-A/IR-B hybrids, leading to decreased metabolic signaling and increased proliferation[7].
Due to the high homology between IGF-IR and IR, IR can also form heterodimers or hybrid receptors (HR) with IGF-IR[99,100]. The cellular content of HR depends only on the molar concentrations of each receptor because IGF-IR/IR heterodimers and homodimers are formed with similar efficiency[101]. Depending on the IR isoform, HR can be IGF-IR/IR-A (HR-A) or IGF-IR/IR-B (HR-B)[7]. HR-A is activated by IGF-I, IGF-II and, to a lesser extent, by insulin, while HR-B is activated mainly by IGF-I but also by IGF-II with lower affinity[102] (Figure 1). Functionally, HRs behave more like IGF-IR than like IR[48].
It is still unclear how the activation of the different IR isoforms, IGF-IR and HR by insulin, IGF-I and IGF-II leads to different biological effects despite the fact that they share most downstream mediators. Differences in ligand binding, internalization or dissociation rates, protein structure and the presence of cell or tissue specific factors could explain this phenomenon[7,11].
Given its role in cell proliferation, the IGF system is one of the pathways deregulated in cancer. As IGF-I protein is highly expressed in the liver, hepatocellular carcinoma (HCC) has been traditionally linked with increased IGF activity. HCC is the third cause of cancer-related deaths worldwide[103]. The most relevant risk factors in the development of HCC are those that induce liver cirrhosis, as 90% of HCC develops in a cirrhotic liver[104]. Liver cirrhosis is the result of chronic liver disease, due mainly to prolonged alcohol abuse, genetic predisposition, obesity and viral infections with HBV and HCV[105]. These agents induce chronic inflammation that leads to the death of hepatocytes and the activation of hepatic stellate cells (HSCs)[106]. Activated HSCs secrete collagen leading to liver fibrosis and ultimately, the breakdown of liver architecture and functionality. Hepatocyte death and proliferation results in the formation of regeneration nodules, characteristic of the cirrhosis stage[107]. The inflammation coupled with the high proliferation level of cirrhotic hepatocytes leads to the accumulation of mutations and to the loss of epigenetic control that may result in HCC initiation and progression[108]. The genetic and epigenetic events that lead to HCC include somatic mutations, telomere shortening, changes in gene expression profiles and RNA editing and genomic alterations[109,110]. These alterations result in a deregulation of several signaling pathways including PI3K/AKT/mTOR, RAS/RAF/MAPK, WNT, HGF/c-MET, EGFR, IGF-IR and PDGF, leading to hepatocarcinogenesis[103,111]. In the next section, the involvement of the IGF system in the development of HCC will be dealt with in detail. The contribution of other signaling pathways or the IGFI-R activated factors PI3K/AKT/mTOR and RAS/RAF/MAPK has been extensively reviewed by other authors and will not be described in this review[111-116].
Once an HCC is diagnosed, surgical resection is the primary curative treatment followed by liver transplantation and percutaneous ablation[117]. However, only 30%-40% of patients are eligible for these treatments. Moreover, there is a high frequency of tumor recurrence after surgical resection[118]. Unfortunately, most HCC seem resistant to conventional chemotherapy and radiotherapy[119]. The poor efficacy of antitumor agents is also due, at least in part, to the inefficient drug delivery and metabolism exerted by the cirrhotic liver that host the tumor[109]. In the clinical trials searching for alternative therapies for HCC, patients may suffer from unbearable drug toxicity and the treatment must be withdrawn leading to the therapeutic failure of compounds that are promising for the treatment of other tumors.
Thus, the development of novel therapies against HCC is urgently required. To this aim, the identification of oncogenic addiction loops or primary “gatekeeper” and “driver” mutations that would allow for HCC initiation and progression, respectively, is mandatory[109,111]. Furthermore, better therapeutic responses could be obtained after a correct patient stratification. Under the name of HCC there are tumors with different etiologies and tumors generated in response to a broad spectrum of deregulated pathways. Therefore, different HCC may respond differently to different therapies. There is a great need for establishing accurate HCC classifications not only for prognostic purposes but also to select the best therapeutic option for each HCC subtype.
To date, Sorafenib is the only drug approved by the FDA available for patients with advanced HCC. Sorafenib is a multikinase inhibitor that blocks PDGFR, VEGFR and RAF phosphorylation[120] resulting in decreased cell proliferation, activation of apoptosis and inhibition of angiogenesis[121]. In patients with advanced HCC, Sorafenib administration produces a statistically significant increase in the overall survival and a decrease in the time to progression of the disease[122]. Many other agents for HCC treatment are under development. Some drugs such as Sunitinib and Brivanib, showed negative results in phase III trials, as first-line or second-line therapies, respectively[109]. Other agents such as Tivantinib, a c-Met inhibitor against the HGF pathway, have shown promising results in patients with HCC[123]. Tivantinib, is particularly efficient in those HCC with high c-Met expression levels[124], highlighting the need for performing personalized medicine with proper HCC molecular analysis to aid in the choice of successful therapies. The combination of different therapies can also increase success. In fact, Sorafenib used in combination with other techniques or other molecules had synergistic effects in preclinical and clinical models of HCC[121,125]. In this review we will focus on therapies related to the IGF system, as other authors have recently reviewed therapies that affect different signaling pathways[114,125-127].
The IGF axis is one of the most commonly deregulated signaling pathways that contribute to cancer development. Alterations have been found in almost all members of the pathway. Here, we review the most important alterations that have been associated with hepatocarcinogenesis.
IGF-I
IGF-I is a mayor ligand of the IGF pathway, highly expressed in the liver and highly protumorigenic for several cancers. Surprisingly, several experiments suggest that IGF-I expression may be antitumorigenic in the case of HCC. Several results support this hypothesis: (1) In situations of chronic liver damage and functional insufficiency, such as liver cirrhosis, the secretion of liver derived molecules including IGF-I is reduced or even totally suppressed in the most severe cases[128]. As the cirrhotic liver is the substrate for HCC development, decreased IGF-I levels could contribute to hepatocarcinogenesis. In fact, in patients with chronic hepatitis, decreased levels of IGF-I are associated with HCC incidence[129]; (2) Patients with HCC also display lower levels of circulating IGF-I when compared with healthy controls[130]. In fact, the development of HCC is preceded by a significant reduction in IGF-I levels, independently of the degree of impairment of liver function. Thus, a precocious diagnosis of HCC could be performed based on a decrease in serum IGF-I levels[129]. Furthermore, transcriptome analysis reveals that IGF-I mRNA levels are decreased in HCC human samples compared to matching adjacent tissue[131]. This can also be observed when liver tumors develop in mouse models after a single exposure to DEN hepatotoxic. In this case, mouse HCC is induced in a non-cirrhotic liver; and (3) decreased levels of IGF-I are associated with higher tumor invasiveness and poor prognosis[132]. The combination of low IGF-I and high VEGF predicts median overall survival of 2.7 mo compared with 19 mo for patients with higher IGF-I and lower VEGF. Serum IGF1 levels also predict tumour progression and overall survival in patients with HCC who undergo transarterial chemoembolization[133]. Also, the lack of liver IGF-I mRNA increases the risk of HCC recurrence after curative resection[134,135].
IGF-II
Excessive IGF-IR signaling is a characteristic feature of liver tumors. Since IGF-I levels are reduced in most HCC, the ligand of the pathway should be insulin or IGF-II. In fact, overexpression of IGF-II has been estimated to occur in 16%-40% of human HCC[136]. Furthermore, in both in vivo and in vitro models of HCC, IGF-II overexpression correlates with higher cell proliferation[137,138] while IGF-II inhibition promotes apoptosis and decreases cellular proliferation[139,140]. Accordingly, miR-615-5p, a miRNA that targets IGF-II expression directly, induces a decrease in proliferation and migration of HuH7 and HepG2 human hepatoma cell lines[141]. In patients with HCC, increased intratumoral IGF-II mRNA levels are associated with higher metastatic potential whereas increased serum IGF-II levels correlate with the presence of extrahepatic metastasis[142,143].
Overexpression of IGF-II has been shown to be the result of increased transcription[143]. As IGF-II is required for fetal growth, it is expressed mainly during development by the potent paternally imprinted P3 promoter[144]. After birth, transcription of liver IGF-II is gradually shifted from initiation at the imprinted promoter to initiation at a biallelic less active P1 promoter. This maintains low levels of liver IGF-II throughout adulthood[144]. However, alteration in IGF-II imprinting has been described in many tumors[36,145-147]. In HCC, 50%-90% of human biopsies analyzed show a gain of IGF-II imprinting[37,148]. This imprinted phenotype results in increased transcription of IGF-II from the P3 promoter and decreased transcription from the P1 promoter by hypermethylation resulting in IGF-II overexpression[144,149,150]. Furthermore, IGF-II hypomethylation at exon 8-9 is found in 90% of HCV-cirrhotic patients analyzed and correlates with higher risk of developing HCC[151].
Other factors may also lead to IGF-II overexpression, such as Aflatoxin B1 (AFB1), a potent hepatocarcinogen present in food in developing countries[152]. The tumorigenic effect of AFB1 seems mediated by tumor suppressor genes such as p53 and by an overactive IGF signaling due to overexpression of IGF-IR and IGF-II[153,154]. Interestingly, p53mt249, a p53 mutant produced after AFB1 administration, can increase IGF-II transcription[154]. Also, the IGF-II polymorphism +3580AA, has been associated with higher serum levels of IGF-II and has been recently linked to higher risk of HCC in humans[51,155].
Little is known about the role of insulin in HCC development. It has been reported that increased insulin serum levels are associated with higher risk of cirrhosis[156] and HCC[96].
In general, IGFBPs limit bioavailability of IGF ligands, attenuating IGF-IR signaling. Thus, some IGFBPs exert antiproliferative effects in human hepatocarcinoma cell lines. The addition of IGFBP3 to the HepG2 hepatoma cell line is able to counteract the mitogenic effect induced by administration of exogenous IGF-I[157]. Similarly, the administration of IGFBP1-4 results in decreased PLC cell proliferation[158]. Accordingly, the expression of antiproliferative IGFBPs such as IGFBP1, 3 and 4, is downregulated in human HCC[159].
The levels of IGFBP3 are also reduced in cirrhotic patients, but not as much as in HCC samples. Unfortunately, IGFBP3 levels are unable to distinguish between different HCC stages[160]. ALS, which forms a trimeric complex with IGF-I and IGFBP3 incapable of passing through the vasculature and activating IGF-IR[161], has been recently found to be downregulated in human HCC due to genomic loss and hypermethylation[162]. Downregulation of IGFBP3 in human HCC samples has also been linked to promoter hypermethylation[163]. On the other hand, p53, a potent antiproliferative protein, increases the secretion of IGFBP3[164].
IGFBP-rP1, also known as IGFBP7, is a low affinity IGFBP that has been recently identified as a tumor suppressor gene in HCC[13]. Expression of IGFBP-rP1 is dramatically downregulated by astrocyte elevated gene-1 (AEG-1), a novel oncogene that is overexpressed in 90% of the HCC analyzed[165]. In some patients, there is a complete deletion of the IGFBP-rP1 gene[166]. In others, silencing of IGFBP-rP1 may result from promoter methylation, which might be used as a biomarker for HCC diagnosis[167]. When IGFBP-rP1 is overexpressed in human HCC cells or tumors, cell growth is inhibited. Interestingly, an inverse correlation between IGFBP-rP1 expression and HCC stage has been found[166].
However, not all IGFBPs display antitumor effects in HCC. Some IGFBPs, such as IGFBP2 and 5, are associated with IGF activation. Accordingly, elevated levels of IGFBP2 have been reported in HCC patients[168]. There are no data on IGFBP5 levels in patients with HCC but inhibition of IGFBP-5 expression exerts antiproliferative effects in the Huh-7 hepatoma cell line[169]. Similarly, as IGFBP proteases release IGF ligands from IGF-IGFBP complexes leading to overactivation of the IGF pathway, they contribute to HCC development. Therefore, increased plasma levels of Cathepsin D, an acidic protease that degrades IGFBP3, have been found in cirrhotic and HCC patients[170]. Moreover, TIMP-1, an inhibitor of MMPs, displays antitumor effects by inhibiting IGFBP3 degradation and IGF-II bioavailability[171].
Signaling through IGF-IR plays an important role in tumorigenesis because of its ability to promote proliferation, protect from apoptosis and potentiate cell migration[29]. IGF-IR overactivation is one of the hallmarks of HCC and can be mediated by increased levels of IGF-IR protein and/or excess of IGF ligands[172]. Healthy mature hepatocytes do not express IGF-IR. In liver cirrhosis the situation is unclear as some authors report that IGF-IR is upregulated while others claim it is downregulated[173]. Most hepatoma cell lines express detectable levels of IGF-IR mRNA and protein[28]. In HCC samples, upregulation of IGF-IR is one of the most common alterations occurring in 30% of the patients[174].
Expression of several downstream components of IGF-IR has been found altered in some HCC samples. IRS-1, the main substrate of IGF-IR activation, is implicated in hepatocarcinogenesis. In fact, 90% of HCC overexpress IRS-1 and IRS-1 overexpression correlates with tumor growth[175]. IRS-2 is also deregulated in HCC. Upregulation of IRS-2 has been found in early and late stages of hepatocarcinogenesis. IRS-2 and IRS-1 have overlapping and specific functions[176]. They are co-overexpressed in 76% of HCC samples and overexpressed independently in 24%[177]. Some studies suggest that a high IRS2/IRS1 ratio may correlate with tumor aggressiveness. In fact, it has been shown that AFB1 increases the levels of IRS-2 but decreases the levels of IRS-1, leading to increased cell migration[178].
IGF-IIR
Overactivation of IGF-IR signaling by excessive IGF-II molecules can be counteracted by IGF-IIR, which decreases IGF-II levels through lysosomal degradation[13]. In fact, increased levels of IGF-II may result from decreased expression of IGF-IIR. Indeed, tumor suppressor characteristics have been attributed to IGF-IIR in several tumor types. This has been the subject of a recent review[179]. Thus, inhibition of IGF-IIR expression increases cellular proliferation both in vitro and in vivo[180,181]. Conversely, overexpression of full length IGF-IIR into IGF-IIR deficient cells decreases cell growth and increases apoptosis in vitro[182,183] and decreases tumor growth in vivo[183,184]. However, overexpression of IGF-IIR has also been associated with an increase in cell number[185]. This is not surprising. It should be taken into consideration that IGF-II is not the only ligand for IGF-IIR. Overexpression of IGF-IIR may affect the signaling of other relevant molecules, such as TGFβ, resulting in proliferative effects[179]. Antiproliferative effects of overexpressed IGF-IIR may only occur in cell lines or tumors whose increased proliferation depends on increased IGF-II levels.
Given its role as a tumor suppressor protein, IGF-IIR is usually found downregulated in cancers, including HCC[186]. Low levels of IGF-IIR in HCC result from different alterations such as imprinting, loss of heterozygosity (LOH) and/or mutations[179,180,187-189]. There is a small subset of individuals carrying a paternally imprinted IGF-IIR allele[82]. Thus, they only have maternal expression of IGF-IIR, as happens with rodents. These individuals, together with rodents, should be more susceptible to developing HCC, as they only require mutations or decrease of gene expression from the active allele to suppress IGF-IIR functionality or production[190]. LOH caused by allelic deletion at the 6q26 locus, where the IGF-IIR gene locates, has been found in 54.5% of human HCC samples and in a smaller proportion of dysplastic liver lesions[179]. This LOH has also been observed in cirrhotic nodules suggesting that loss of the IGF-IIR gene could be an early event in hepatocarcinogenesis[187]. Furthermore, 55% of HCC with IGF-IIR LOH present mutations in the remaining allele[189]. Interestingly, while some mutations occur at the IGF-II binding site of IGF-IIR, most occur in repeats 9 or 10, which are important for M6P-binding[179]. This finding indicates that the binding of M6P-containing proteins to IGF-IIR may have antitumoral effects. In fact, the M6P-bearing protein CREG can inhibit cell proliferation by stimulation of lysosomal IGF-II degradation[191]. Generally, mutations in IGF-IIR lead to the formation of truncated proteins. Interestingly, using a truncated form of IGF-IIR derived from a reported splicing mutation[89], it has been demonstrated that truncated proteins can bind IGF-II and M6P-containing proteins and are able to form heterodimers with the full length IGF-IIR[188]. Surprisingly, these heterodimers are rapidly cleaved and liberated from the cell membrane by MMPs, leading to a great decrease in the amount IGF-IIR bound to the cell membrane[188]. These data indicate that truncated proteins can act as dominant negative regulators of IGF-IIR contributing to cancer development.
It has recently been published that the IR-A/IR-B expression ratio markedly increases in intratumoral HCC sections but not in adjacent tissues[7]. The relative abundance of IR-A, the fetal IR isoform, vs total IR mRNAs in normal liver is 5% while in hepatoma cell lines it reaches 50%-75%[192]. The increase of the aberrant splicing that leads to the IR-A isoform is a consequence of the activation of the EGF pathway, one of the most relevant dysregulated pathways in HCC[193]. Interestingly, production of IR-A after EGFR activation only occurs in transformed but not in healthy hepatocytes. High affinity binding of IR-A by IGF-II induces mitogenic and anti-apoptotic effects leading to HCC development. Tumors overexpressing both IR-A and IGF-II should be resistant to conventional therapies that target IGF-IR.
As many HCC overexpress IGF-IR and IR-A and HR formation depends on the concentration of each receptor, these HCC should display an increase in HR-A. Concomitantly, an increase in HR-B and in IR-A/IR-B, which promote proliferation through IGF ligands could also occur together with a decrease in IR-B/IR-B homodimers, responsible for insulin mediated metabolic activity[7]. Interestingly, some cancers have shown an increased fraction of hybrid receptors unrelated with their relative concentrations, suggesting that other factors may be implicated in hybrid receptor formation[194].
In summary, increased proliferation in HCC is generally characterized by an increase in the bioavailability of IGF-II, which signals through increased IGF-IR, IR-A homodimers and HR-A leading to increased proliferation and decreased apoptosis (Figure 3). The increase in available IGF-II may result from increased IGF-II gene expression but also from a decrease of IGF-II degradation by IGF-IIR or IGF-II sequestration by IGFBPs.
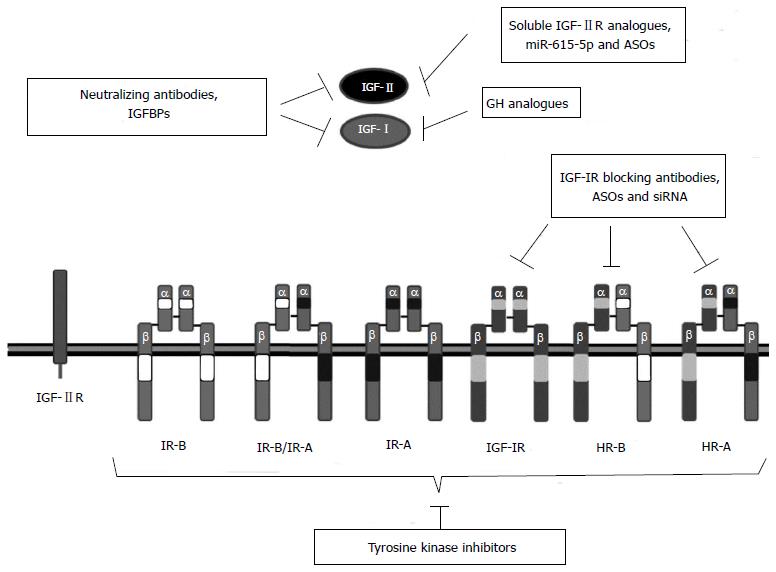
Compelling evidence exists to involve the IGF system in hepatocarcinogenesis. Therefore, several strategies that target different IGF components are being studied with the aim of developing new therapeutic drugs for HCC (Table 1)[28]. The most promising strategies include the inhibition of IGF-IR signaling using monoclonal antibodies against IGF-IR, IGF-II and/or IGF-I and small molecule tyrosine kinase inhibitors (TKIs) that inhibit IGF-IR activation and signaling (Figure 4). Other approaches such as the inhibition of IGF-IR or IGF-II expression using siRNAs or antisense oligonucleotides (ASO) and the modulation of the IGFBP activity are under preclinical investigation. Some strategies that target the IGF system may be effective as monotherapies but others may be more effective in combination with chemotherapy and can be used as chemosensitizing agents.
| Compound | Company | Description | Target | Treatment | Status | Ref./Clinical trial | Unaffected mitogenic IGF proteins/metabolic signaling status |
| Octreotide | Novartis Farmaceuticals | Somatostatin homologue | IGF-I | Monotherapy | Preclinical | [200,201] | IGF-II and insulin interaction with IGF-IR, IR-A, IR-B/IR-A and HRs/not affected |
| DX-2647 | Neutralizing antibody | IGF-II and proIGF-II | Monotherapy | Preclinical | [202] | Insulin interaction with IR-A, IR-B/IR-A and HR-A/not affected | |
| MEDI-573 | AstraZeneca (MedInmune) | Neutralizing antibody | IGF-II and IGF-I | MEDI-573 + Sorafenib | Phase I | NCT01498952; [192,203] | |
| Cixutumumab (IMC-A12) | ImClone Systems Inc | Blocking antibody | IGF-1R | Monotherapy | Phase II | NCT00639509; [53,215,216] | IGF-I, IGF-II and insulin interaction with IR-A, IR-B/IR-A and HRs/not affected |
| Cixutumumab + Sorafenib | Phase I | NCT01008566; NCT00906373 | |||||
| AVE1642 | Sanofi-Aventis | Blocking antibody | IGF-1R | Monotherapy | Phase I | NCT00791544; [125] | |
| AVE-1642 + Sorafenib | Phase II | NCT00791544 | |||||
| AVE-1642 + Erlotinib | Phase II | NCT00791544 | |||||
| BIIB022 | Biogen-Idec | Blocking antibody | IGF-1R | BIIB022 + Sorafenib | Phase I | NCT00956436; [53,223] | |
| Figitumumab (CP-751,871) | Pfizer | Blocking antibody | IGF-1R, HRs | Monotherapy | Preclinical | [224,225] | IGF-II and insulin interaction with IR-A, IR-B/IR-A/not affected |
| Linsitinib (OSI-906) | OSI Pharmaceuticals | TKI | IGF-1R, IR | Monotherapy | Phase II | NCT01101906; [226,227] | /impaired. |
| OSI-906 + Sorafenib | Phase II | NCT01334710 | |||||
| AG1024 (Tyrphostin) | TKI | IGF-1R, IR | Monotherapy | Preclinical | [231] | ||
| NVP-AEW541 | Novartis Farmaceuticals | TKI | IGF-1R, IR | Monotherapy | Preclinical | [232,233] | |
| BMS-536924 | Bristol-Myers Squibb | TKI | IGF-1R, IR | Monotherapy | Preclinical | [234] | |
| GSK1904529A | GlaxoSmithKline | TKI | IGF-1R, IR | Monotherapy | Preclinical | [235] |
These different strategies should be tested exclusively in HCC cells with an altered IGF-I axis. Furthermore, the expression and functionality of the different members of the pathway should be evaluated prior to treatment. This should ensure that the therapy is targeting an IGF pathway molecule relevant for the growth of the particular HCC to be treated. Such personalized medicine is essential to obtain therapeutic effects. To help in this analysis, an antibody array has been recently developed that detects ten members of the IGF system (IGF-I, IGF-IR, IGF-II, IGF-IIR, IGFBP1, IGFBP2, IGFBP3, IGFBP4, IGFBP6 and insulin)[195]. Unfortunately, antibodies for IR-A and IR-B isoforms are not included in the array, despite their importance in HCC development.
Targeting IGF-I
Despite the capability of IGF-I to activate IGF-IR signaling, IGF-I is decreased in liver tumors and in the serum of patients with HCC[130,131]. Furthermore, high circulating levels of IGF-I are associated with less aggressive HCC, with a better prognosis[132] and with a better outcome in patients with advanced HCC receiving Sorafenib[196]. The reason for this is unclear.
Alternatively, IGF-I could lead to increased differentiation in hepatocytes and decreased proliferation. In fact, IGF-I has a therapeutic role in human liver cirrhosis, the substrate of HCC development. In a pilot study, the intravenous administration of recombinant IGF-I in patients with advanced liver cirrhosis improved liver functionality[197]. Moreover, we have demonstrated that the administration of IGF-I from viral vectors before the induction of liver cirrhosis prevents the development of the disease in rat models[198]. Furthermore, administration of viral vectors expressing IGF-I into rat cirrhotic livers leads to the complete reversion of liver cirrhosis[198,199]. This therapeutic effect correlates with IGF-I mediated activation of an anti-inflammatory and anti-fibrogenic program. Furthermore, IGF-I expression increases differentiation of cirrhotic hepatocytes and liver functionality. Using our model, overexpression of IGF-I in healthy or cirrhotic livers for more than a year did not lead to detectable liver tumors. Given that IGF-I displays anti-inflammatory and hepatoprotective effects, IGF-I deficiency caused by liver cirrhosis may create an intrahepatic microenvironment that allows for hepatocyte dedifferentiation and facilitates HCC emergence. Alternatively, high IGF-I levels could mark for functional hepatocytes, which are more difficult to transform and easier to cure. Finally, it cannot be ruled out that IGF-I could have an unexpected antitumoral effect on its own. If this is the case, IGF-I and IGF-II signalling through IGF-IR should lead to different responses in HCC. This has never been shown experimentally. Therefore, even if IGF-I could exert some antitumoral effect, it would be risky to overexpress IGF-I once a HCC with altered IGF axis has developed.
Even if downregulation of IGF-I together with upregulation of IGF-IR, IR-A and IGF-II are common events in HCC, suggesting that IGF-I has a limiting role in hepatocarcinogenesis, there are ligand-based therapies that specifically target IGF-I without affecting IGF-II. Theoretically, blocking IGF-I could favor binding of IGF-II to IGF-IR and increase cell proliferation. This has not been observed with Octreotide, a cyclic octapeptide used as GH analogue in the treatment of several types of cancers. Octreotide competes with GH for binding to its receptor and inhibits GH-GHR interaction and signaling leading to a decrease in IGF-I synthesis by the liver, but also affecting the expression of many other molecules[200]. Furthermore, decreased IGF-I should result in increased GH levels. Octreotide treatment showed good results in prostate cancer, but not in HCC. In a recent meta-analysis of all randomized controlled clinical trials using Octreotide for HCC patients, there was an improvement in the overall survival of the treated patients compared with non-treated controls, which was not statistically significant when compared with placebo controls[201].
Targeting IGF-II
IGF-II is overexpressed in human HCC and it activates proliferation and migration through IGF-IR and IR-A receptors. IGF-II based therapies must be designed to decrease or normalize IGF-II levels and/or to inhibit its interaction with both receptors without altering insulin signaling. To this aim, blocking antibodies and strategies to decrease IGF-II expression are under development.
Human antibody DX-2647 binds to IGF-II and pro-IGF-II with high affinity impeding their interaction either with IGF-IR and IR-A and suppressing proliferation in several HCC cell lines. Moreover, DX-2647 administration delays tumor growth and inhibits angiogenesis in xenograft models of HCC. DX-2647 also reacts with IGF-I but with a 200-fold lower affinity[202]. This antibody remains in a preclinical status. Similarly, human monoclonal antibody MEDI-573 binds IGF-II and IGF-I (with 150-fold lower affinity than IGF-II) without interacting with insulin[203]. MEDI-573 impedes IGF binding to IGF-IR, IR-A, and IGFBP3[192]. MEDI-573 administration reduces proliferation in cells expressing either IGF-IR or IR-A receptors but also in mixed populations of cells expressing both receptors in which an IGF-IR-specific antibody was totally ineffective. These results were obtained in several mouse embryonic fibroblast cell lines overexpressing specific human proteins and were then validated in xenograft tumors[203]. Two phase I clinical trials designed to determine the effect of MEDI-573 administration on patients with solid tumors have recently finished but the results have not been yet published. Interestingly, a new clinical trial will test MEDI-573 administration in combination with Sorafenib in unresectable or metastatic HCC.
Strategies to decrease IGF-II expression, such as antisense (ASO) or methylated (MONs) oligonucleotides, are under preclinical investigation. ASOs are short single-stranded DNA molecules complementary to a chosen mRNA sequence. ASO binding to the target mRNA results in mRNA expression inhibition as a result of RNase H-mediated mRNA degradation and translation blockage[204]. Downregulation of IGF-II expression using ASOs that target IGF-II mRNA inhibits cellular growth in hepatoma cell lines, but only in those that overexpress IGF-II[205]. MONs that bind to the IGF-II P4 promoter results in target DNA methylation and in turn, downregulation of fetal IGF-II expression in the Hep3B human hepatoma cell line and in Hep3B derived tumors leading to enhanced survival[140]. Further development will be required to deliver these agents to most tumor cells.
Decreased IGF-II availability can also be achieved by increased IGF-IIR expression. The administration of the soluble form of IGF-IIR (sIGF-IIR) to myeloid cell lines leads to a decrease in proliferation and survival[206]. Moreover, IGF-II-induced DNA synthesis can be counteracted in hepatocytes and fibroblast using sIGF-IIR[92]. However, this soluble receptor can also bind other proteins and may have undesirable side effects. Therefore, therapeutic effects should be evaluated using a soluble form of IGF-IIR that only contains the IGF-II binding domain.
IGFBPs modulate IGF signaling by regulation of IGF bioavailability. Most IGFBPs inhibit IGF signaling by limiting ligand access to IGF receptors, with the exception of IGFBP2 and IGFBP5. Therapies based on the administration of inhibitory IGFBPs or the inhibition of activating IGFBPs could be developed. In fact, the effect of increased levels of IGFBP3 and IGFBP-rP1 has already been evaluated.
As IGFBP3 represents 90% of serum IGFBPs[53], IGFBP3 downregulation in cancer significantly increases IGF ligand bioavailability. It has already been shown that the administration of exogenous IGFBP3 inhibits cell proliferation in hepatoma cell lines[157,158]. Interestingly, IGFBP3 expression can be re-induced in liver cancer cells by histone deacetylase inhibitors such as Trichostatin A[207,208]. An ongoing phase I clinical trial combines Vorinostat, the first histone deacetylase inhibitor approved by the FDA, with different chemotherapy agents in patients with upper gastrointestinal cancers including liver cancer. Finally, as overexpression of IGFBP-rP1 (IGFBP7) decreases the tumorigenic potential of HCC cell lines[166], the antitumoral properties of an adenovirus expressing IGFBP7 have been recently demonstrated in both in vitro and in vivo models of HCC[209].
Different strategies have been described to block IGF-IR signaling including blocking antibodies, siRNAs, antisense oligonucleotides, small molecule inhibitors and tyrosine kinase inhibitors.
Monoclonal antibodies: The administration of monoclonal antibodies against IGF-IR induces apoptosis and decreases proliferation in HCC[210]. Some of the monoclonal antibodies that have demonstrated promising results in preclinical models are: cixutumumab or IMC-A12 is a human IgG1 monoclonal antibody that selectively binds to IGF-IR, preventing the binding of its natural ligands[211]. The antibody also activates internalization and degradation of IGF-IR, leading to decreased levels of this receptor. Thus, IMC-A12 treatment inhibits downstream signaling in several tumors without altering insulin signaling[212-214]. In vitro and in vivo studies using different HCC models showed that blockage of IGF-IR by IMC-A12 decreases cell proliferation and increases apoptosis, resulting in prolonged survival and delayed tumor growth[215]. On the basis of these results, a phase I clinical trial was performed in patients with advanced solid tumors. However, only partial responses were obtained[53]. In a subsequent phase II study, administration of IMC-A12 as monotherapy in patients with advanced HCC displayed no antitumoral activity[216]. Instead, half of the patients developed hyperglycemia and 62% of the patients required initiation or increase in active therapy for diabetes. Besides, several patients showed reduced liver function indicated by elevated transaminases and bilirubin and decreased albumin, suggesting that by blocking IGF-IR a protective effect of IGF-I on liver function had been lost. The mayor outcome of the study is that increased levels of IGFBP-1 correlated with progression free survival and with overall survival. The lack of therapeutic effect of IMC-A12 could be explained by the lack of IGF-IR in most of the patients, as IGF-IR expression could only be demonstrated in HCC samples obtained from 21% of the patients. However, the patients whose tumors were positive for IGF-IR did not show correlation with survival when compared with the IGF-IR-negative patients[216]. It needs to be determined whether IMC-A12 is more effective as a chemosensitizing molecule. Therefore, two clinical trials using combination of IMC-A12 and Sorafenib are ongoing.
AVE1642 is a humanized monoclonal antibody against IGF-IR that inhibits growth and metastasis in different human xenograft tumor models when used alone and/or in combination with chemotherapy[217-221]. AVE1642 was first tested in humans with advanced multiple myeloma yielding good tolerability but insufficient activity[222]. A posterior phase I/II clinical trial testing AVE1642 alone or in combination with Sorafenib or Erlotinib in patients with advanced or metastatic liver carcinoma supported the safety of AVE1642 in combination with active doses of Sorafenib[125].
BIIB022 is a human non-glycosylated IgG4.P antibody that blocks IGF-I and IGF-II binding to IGF-IR[223]. Preclinical data suggest that BIIB022 administration inhibits the growth of HepG2-derived tumors without induction of hyperglycemia. As BIIB022 lacks an Fc effector function, it displays less toxicity in normal IGF-IR expressing tissues[53]. A phase I study to evaluate the tolerability and safety of combinatorial therapy with Sorafenib has been completed but the results have not yet been published.
CP-751,871, also known as Figitumumab, is a human IgG2 antibody that inhibits IGF-I and IGF-II mediated autophosphorylation of IGF-IR but not IR, resulting in the internalization of the receptor[224]. It has been tested in 8 HCC cell lines, 2 of which, HepG2 and SNU368, were sensitive to the treatment in a dose-dependent manner. Administration of Figitumumab to HepG2 xenograft tumors leads to substantial growth inhibition[225]. Interestingly, in contrast to the other blocking antibodies, Figitumumab is able to inhibit hybrid receptor signaling. In fact, Figitumumab sensitivity has been associated with the levels of N-linked glycosylated IGF-IR/IR hybrids[225]. This compound has reach a phase III trial in multiple myeloma and non-small cell lung cancer, but it has not yet been tested for liver cancers.
Tyrosine kinase inhibitors: OSI-906 is a dual IGF-1R and IR Tyrosine kinase inhibitors (TKI) that displays antitumoral activity in several human cell lines and xenograft tumor models[226]. The mechanisms that mediate sensitivity to OSI-906 have been tested in a panel of 21 human HCC cell lines. In this study, higher responsiveness to OSI-906 was obtained in cell lines expressing high levels of IGF-II and IR[227]. Thus IGF-II and IR could be used as predictive markers for sensitivity to OSI-906 in HCC patients. OSI-906 evaluation in phase I dose escalation studies, alone or in combination with anti-cancer agents, resulted in good disease control rates and limited toxicity, including hyperglycemia, nauseas, vomiting and fatigue[8]. Two phase II clinical trials testing OSI-906 in patients with HCC have been carried out but were terminated due to the safety issues observed in the phase I study or to company policies. The partial results of the trials have not yet been published.
AG1024 (Tyrphostin) is a selective IGF-IR and IR TKI that is currently in preclinical development. Blockage of IGF-IR with AG1024 exerts antiproliferative and pro-apoptotic effects in several cancer cell lines[228-230]. Recently, AG1024 has been tested in two IGF-IR-expressing HCC, resulting in a significant decrease in cell invasiveness and a slight caspase-3 dependent proapoptotic effect[231].
NVP-AEW541 is a novel small molecule inhibitor of the IGF-IR tyrosine kinase activity. NVP-AEW541 has a 26-fold higher affinity for IGF-IR than for IR[232] and induces cell cycle arrest and apoptosis in HCC cell lines without cytotoxicity. When NVP-AEW541 was combined with chemotherapy, an additive antiproliferative effect was observed[233]. The effect of NVP-AEW541 remains to be tested in in vivo models of HCC.
BMS-536924 is a novel orally active, ATP-competitive, tyrosine kinase inhibitor of IGF-IR and IR. BMS-536924 antiproliferative activity has recently been described in HCC cell lines[234].
GSK1904529A is a tyrosine kinase inhibitor that blocks IGF-IR and IR phosphorylation. GSK1904529A has been tested in a wide range of cell lines and human xenograft tumor models resulting in low toxicity and strong antiproliferative and antitumoral effects. Although no HCC samples were included, the authors demonstrated that GSK1904529A inhibits the activity of the IR in liver tissues suggesting that it could be also effective in HCC[235].
The design of TKIs that target specifically IGF-IR signaling without altering IR signaling is difficult because of the high homology between these two receptors[62]. On one hand, targeting of both receptors can be advantageous since specific inhibition of IGF-IR was associated with higher IR signaling. On the other hand, targeting IR could lead to altered insulin signaling and unwanted secondary effects.
Antisense oligonucleotides: Phosphorothioate ASOs, which are more resistant to nuclease degradation than unmodified DNA, have been designed to target IGF-IR and have been evaluated in a model of HCC. In this study, inhibition of IGF-IR expression by ASOs results in a significant reduction of HepG2 proliferation. Systemic administration of IGF-IR ASOs in nude mice with orthotopic human HCC xenografts results in reduced tumor growth, recurrence and lung metastasis[236].
Deregulation of the IGF system is a common feature in HCC. Recent studies suggest that downregulation of IGF-I together with upregulation of IGF-II and overactivation of IGF-IR and IR-A are important events in HCC development. Thus, increased IGF-II bioavailability, caused by increased IGF-II expression or decreased regulation by IGF-IIR or IGFBPs, could be responsible for IGF-IR and IR-A overactivation. Furthermore, mutations in factors located downstream IGF receptors, such as Ras, PI3K or PTEN, could induce cell proliferation in tissues with normal IGF ligands or receptors. This has not been the subject of this review. Insulin and IR-B, by coupling to IGF-IR and IR-A, could also play a role in IGF pathway activation that leads to HCC. Little is known about the role of insulin in HCC development. Increased insulin serum levels have been associated with higher risk of HCC. This is probably caused by insulin binding to IR-A homo or heterodimeric receptors.
The role played by IGF-I in HCC should be studied in detail. It is unclear why IGF-I deregulation seems relevant for hepatocarcinogenesis. Experiments that address the effect of IGF-I overexpression or downregulation in HCC development should be performed in animal models. The results may be relevant for the management of HCC in humans. Also, efforts should be devoted to understand why the binding of IGF-I, IGF-II, or insulin to a specific receptor of the IGF pathway, such as IGF-IR or IR-A/IR-B derived receptors, results in the activation of different signals. It should be interesting to identify liver-specific factors that modify IGF-IR signaling according to the ligand that has been sensed by the receptor, either IGF-I or IGF-II.
Given the particular features of IGF deregulation in HCC, the most promising therapies to date for HCC are antibodies that block IGF-II or IGF-IR and tyrosine kinase inhibitors. The success of the treatment may depend on following personalized medicine protocols that first ensure that the IGF system is deregulated in the HCC to be treated. Furthermore, these protocols should evaluate the serum levels of IGF-I, IGF-II and insulin and the levels within the tumor of all the IGF ligands, receptors, binding proteins and signaling pathway factors. Such a detailed study of each tumor is essential to decide on a successful therapy. Thus, IGF-IR blocking antibodies are expected to be effective in tumors with increased IGF-IR and poor IR-A activation (Table 1). If this analysis is not performed, functional drugs may show no therapeutic effects. This may be the reason why IGF-IR antibodies display antitumoral effects in preclinical models but only partial responses in clinical trials. In the case of TKIs, as they are able to block IGF-IR and IR-A signaling, they are expected to be effective in all HCC with altered IGF ligands and receptors. However, TKI can also inhibit other tyrosine kinase receptors causing unwanted effects. TKI interaction with IR-B should lead to altered insulin metabolism.
Future therapies that target the IGF system should be developed for the treatment of HCC and other tumors. Novel specific antibodies or small molecules that affect the stability of IGF-II or impede IGF-II being sensed by IGF-IR and IRs should be developed. Similarly, design of functional TKI inhibitors or other molecules that affect IGF-IR and IR-A, but not IR-B is mandatory. Moreover, expression of key activators of the IGF pathway could be affected by antisense inhibitors or genome editing strategies. This will require the improvement of delivery techniques that allow the efficient delivery of the drugs to most tumor cells. Furthermore, present and future therapies need to take into consideration the altered drug metabolism of cirrhotic livers. As most HCC develop in a cirrhotic liver, it may be useful to stratify the patients according to liver functionality and liver fibrosis status before analyzing the therapeutic effects of a particular drug. Finally, even if the IGF system is altered in many HCC, it is not a unique tumor driver. It will be interesting to analyze the results of successful but also of non-successful trials to address if the blockade of IGF pathway was effective and whether other signaling pathways have been induced for tumor survival upon IGF system blockage. This may lead to rationalized combination therapies that may be essential for the successful treatment of HCC.
P- Reviewer: Gong ZJ, Sun XY, Nagy P S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell. 1993;75:59-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 64] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Yu H, Rohan T. Role of the insulin-like growth factor family in cancer development and progression. J Natl Cancer Inst. 2000;92:1472-1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 978] [Cited by in RCA: 975] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 3. | Marshman E, Streuli CH. Insulin-like growth factors and insulin-like growth factor binding proteins in mammary gland function. Breast Cancer Res. 2002;4:231-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Daughaday WH, Hall K, Raben MS, Salmon WD, van den Brande JL, van Wyk JJ. Somatomedin: proposed designation for sulphation factor. Nature. 1972;235:107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 566] [Cited by in RCA: 497] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 5. | Rinderknecht E, Humbel RE. The amino acid sequence of human insulin-like growth factor I and its structural homology with proinsulin. J Biol Chem. 1978;253:2769-2776. [PubMed] |
| 6. | Wilcox G. Insulin and insulin resistance. Clin Biochem Rev. 2005;26:19-39. [PubMed] |
| 7. | Belfiore A, Frasca F, Pandini G, Sciacca L, Vigneri R. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr Rev. 2009;30:586-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 719] [Cited by in RCA: 753] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 8. | Malaguarnera R, Belfiore A. The insulin receptor: a new target for cancer therapy. Front Endocrinol (Lausanne). 2011;2:93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Baxter RC. Insulin-like growth factor (IGF)-binding proteins: interactions with IGFs and intrinsic bioactivities. Am J Physiol Endocrinol Metab. 2000;278:E967-E976. [PubMed] |
| 10. | Mohan S, Baylink DJ. IGF-binding proteins are multifunctional and act via IGF-dependent and -independent mechanisms. J Endocrinol. 2002;175:19-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 305] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 11. | Sarfstein R, Werner H. Minireview: nuclear insulin and insulin-like growth factor-1 receptors: a novel paradigm in signal transduction. Endocrinology. 2013;154:1672-1679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Brogiolo W, Stocker H, Ikeya T, Rintelen F, Fernandez R, Hafen E. An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr Biol. 2001;11:213-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 908] [Cited by in RCA: 904] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 13. | Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1484] [Cited by in RCA: 1567] [Article Influence: 92.2] [Reference Citation Analysis (0)] |
| 14. | Yakar S, Liu JL, Stannard B, Butler A, Accili D, Sauer B, LeRoith D. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci USA. 1999;96:7324-7329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1007] [Cited by in RCA: 997] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 15. | Jansson JO, Edén S, Isaksson O. Sexual dimorphism in the control of growth hormone secretion. Endocr Rev. 1985;6:128-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 458] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 16. | Herrington J, Smit LS, Schwartz J, Carter-Su C. The role of STAT proteins in growth hormone signaling. Oncogene. 2000;19:2585-2597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 201] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 17. | Woelfle J, Billiard J, Rotwein P. Acute control of insulin-like growth factor-I gene transcription by growth hormone through Stat5b. J Biol Chem. 2003;278:22696-22702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 147] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 18. | LeRoith D, Roberts CT. Insulin-like growth factors. Ann N Y Acad Sci. 1993;692:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 62] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Lund PK. Insulin-like growth factor I: molecular biology and relevance to tissue-specific expression and action. Recent Prog Horm Res. 1994;49:125-148. [PubMed] |
| 20. | Zhang J, Whitehead RE, Underwood LE. Effect of fasting on insulin-like growth factor (IGF)-IA and IGF-IB messenger ribonucleic acids and prehormones in rat liver. Endocrinology. 1997;138:3112-3118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Pell JM, Saunders JC, Gilmour RS. Differential regulation of transcription initiation from insulin-like growth factor-I (IGF-I) leader exons and of tissue IGF-I expression in response to changed growth hormone and nutritional status in sheep. Endocrinology. 1993;132:1797-1807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Brameld JM, Atkinson JL, Saunders JC, Pell JM, Buttery PJ, Gilmour RS. Effects of growth hormone administration and dietary protein intake on insulin-like growth factor I and growth hormone receptor mRNA Expression in porcine liver, skeletal muscle, and adipose tissue. J Anim Sci. 1996;74:1832-1841. [PubMed] |
| 23. | Rotwein P, Pollock KM, Didier DK, Krivi GG. Organization and sequence of the human insulin-like growth factor I gene. Alternative RNA processing produces two insulin-like growth factor I precursor peptides. J Biol Chem. 1986;261:4828-4832. [PubMed] |
| 24. | Duguay SJ, Milewski WM, Young BD, Nakayama K, Steiner DF. Processing of wild-type and mutant proinsulin-like growth factor-IA by subtilisin-related proprotein convertases. J Biol Chem. 1997;272:6663-6670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 76] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Annunziata M, Granata R, Ghigo E. The IGF system. Acta Diabetol. 2011;48:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 137] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 26. | Cohick WS, Clemmons DR. The insulin-like growth factors. Annu Rev Physiol. 1993;55:131-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 370] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 27. | Ohlsson C, Mohan S, Sjögren K, Tivesten A, Isgaard J, Isaksson O, Jansson JO, Svensson J. The role of liver-derived insulin-like growth factor-I. Endocr Rev. 2009;30:494-535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 345] [Cited by in RCA: 311] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 28. | Scharf JG, Dombrowski F, Ramadori G. The IGF axis and hepatocarcinogenesis. Mol Pathol. 2001;54:138-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 125] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 29. | Baserga R. The insulin-like growth factor I receptor: a key to tumor growth? Cancer Res. 1995;55:249-252. [PubMed] |
| 30. | Lelbach A, Muzes G, Feher J. The insulin-like growth factor system: IGFs, IGF-binding proteins and IGFBP-proteases. Acta Physiol Hung. 2005;92:97-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Berelowitz M, Szabo M, Frohman LA, Firestone S, Chu L, Hintz RL. Somatomedin-C mediates growth hormone negative feedback by effects on both the hypothalamus and the pituitary. Science. 1981;212:1279-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 430] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 32. | Blakesley VA, Scrimgeour A, Esposito D, Le Roith D. Signaling via the insulin-like growth factor-I receptor: does it differ from insulin receptor signaling? Cytokine Growth Factor Rev. 1996;7:153-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 115] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 33. | Rao LV, Wikarczuk ML, Heyner S. Functional roles of insulin and insulinlike growth factors in preimplantation mouse embryo development. In Vitro Cell Dev Biol. 1990;26:1043-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16:3-34. [PubMed] |
| 35. | Brissenden JE, Ullrich A, Francke U. Human chromosomal mapping of genes for insulin-like growth factors I and II and epidermal growth factor. Nature. 1984;310:781-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 157] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 36. | Giannoukakis N, Deal C, Paquette J, Goodyer CG, Polychronakos C. Parental genomic imprinting of the human IGF2 gene. Nat Genet. 1993;4:98-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 302] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 37. | Wu J, Qin Y, Li B, He WZ, Sun ZL. Hypomethylated and hypermethylated profiles of H19DMR are associated with the aberrant imprinting of IGF2 and H19 in human hepatocellular carcinoma. Genomics. 2008;91:443-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 38. | Vu TH, Hoffman AR. Promoter-specific imprinting of the human insulin-like growth factor-II gene. Nature. 1994;371:714-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 160] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 39. | Sussenbach JS, Rodenburg RJ, Scheper W, Holthuizen P. Transcriptional and post-transcriptional regulation of the human IGF-II gene expression. Adv Exp Med Biol. 1993;343:63-71. [PubMed] |
| 40. | O’Dell SD, Day IN. Insulin-like growth factor II (IGF-II). Int J Biochem Cell Biol. 1998;30:767-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 207] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 41. | Sussenbach JS, Steenbergh PH, Holthuizen P. Structure and expression of the human insulin-like growth factor genes. Growth Regul. 1992;2:1-9. [PubMed] |
| 42. | Yaseen MA, Wrenzycki C, Herrmann D, Carnwath JW, Niemann H. Changes in the relative abundance of mRNA transcripts for insulin-like growth factor (IGF-I and IGF-II) ligands and their receptors (IGF-IR/IGF-IIR) in preimplantation bovine embryos derived from different in vitro systems. Reproduction. 2001;122:601-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 92] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 43. | Daughaday WH, Rotwein P. Insulin-like growth factors I and II. Peptide, messenger ribonucleic acid and gene structures, serum, and tissue concentrations. Endocr Rev. 1989;10:68-91. [PubMed] |
| 44. | Oka Y, Rozek LM, Czech MP. Direct demonstration of rapid insulin-like growth factor II Receptor internalization and recycling in rat adipocytes. Insulin stimulates 125I-insulin-like growth factor II degradation by modulating the IGF-II receptor recycling process. J Biol Chem. 1985;260:9435-9442. [PubMed] |
| 45. | Sacco A, Morcavallo A, Pandini G, Vigneri R, Belfiore A. Differential signaling activation by insulin and insulin-like growth factors I and II upon binding to insulin receptor isoform A. Endocrinology. 2009;150:3594-3602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 46. | Sciacca L, Prisco M, Wu A, Belfiore A, Vigneri R, Baserga R. Signaling differences from the A and B isoforms of the insulin receptor (IR) in 32D cells in the presence or absence of IR substrate-1. Endocrinology. 2003;144:2650-2658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 47. | Denley A, Wallace JC, Cosgrove LJ, Forbes BE. The insulin receptor isoform exon 11- (IR-A) in cancer and other diseases: a review. Horm Metab Res. 2003;35:778-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 102] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 48. | Varewijck AJ, Janssen JA. Insulin and its analogues and their affinities for the IGF1 receptor. Endocr Relat Cancer. 2012;19:F63-F75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 49. | LeRoith D, Werner H, Beitner-Johnson D, Roberts CT. Molecular and cellular aspects of the insulin-like growth factor I receptor. Endocr Rev. 1995;16:143-163. [PubMed] |
| 50. | Siddle K, Ursø B, Niesler CA, Cope DL, Molina L, Surinya KH, Soos MA. Specificity in ligand binding and intracellular signalling by insulin and insulin-like growth factor receptors. Biochem Soc Trans. 2001;29:513-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 104] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 51. | Rosenfeld RG, Lamson G, Pham H, Oh Y, Conover C, De Leon DD, Donovan SM, Ocrant I, Giudice L. Insulinlike growth factor-binding proteins. Recent Prog Horm Res. 1990;46:99-159; discussion 159-163. [PubMed] |
| 52. | Hwa V, Oh Y, Rosenfeld RG. The insulin-like growth factor-binding protein (IGFBP) superfamily. Endocr Rev. 1999;20:761-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 242] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 53. | Wu J, Zhu AX. Targeting insulin-like growth factor axis in hepatocellular carcinoma. J Hematol Oncol. 2011;4:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 54. | Clemmons DR. Insulin-like growth factor binding proteins and their role in controlling IGF actions. Cytokine Growth Factor Rev. 1997;8:45-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 345] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 55. | Rajaram S, Baylink DJ, Mohan S. Insulin-like growth factor-binding proteins in serum and other biological fluids: regulation and functions. Endocr Rev. 1997;18:801-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 216] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 56. | Baxter RC. Insulin-like growth factor (IGF) binding proteins: the role of serum IGFBPs in regulating IGF availability. Acta Paediatr Scand Suppl. 1991;372:107-114; discussion 115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 76] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 57. | Rechler MM, Clemmons DR. Regulatory Actions of Insulin-like Growth Factor-binding Proteins. Trends Endocrinol Metab. 1998;9:176-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 69] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 58. | Guler HP, Zapf J, Schmid C, Froesch ER. Insulin-like growth factors I and II in healthy man. Estimations of half-lives and production rates. Acta Endocrinol (Copenh). 1989;121:753-758. [PubMed] |
| 59. | Clemmons DR. Role of insulin-like growth factor binding proteins in controlling IGF actions. Mol Cell Endocrinol. 1998;140:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 267] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 60. | Arai T, Busby W, Clemmons DR. Binding of insulin-like growth factor (IGF) I or II to IGF-binding protein-2 enables it to bind to heparin and extracellular matrix. Endocrinology. 1996;137:4571-4575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 61. | Jones JI, D’Ercole AJ, Camacho-Hubner C, Clemmons DR. Phosphorylation of insulin-like growth factor (IGF)-binding protein 1 in cell culture and in vivo: effects on affinity for IGF-I. Proc Natl Acad Sci USA. 1991;88:7481-7485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 173] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 62. | Riedemann J, Macaulay VM. IGF1R signalling and its inhibition. Endocr Relat Cancer. 2006;13 Suppl 1:S33-S43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 211] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 63. | Roth RA, Kiess W. Insulin-like growth factor receptors: recent developments and new methodologies. Growth Regul. 1994;4 Suppl 1:31-38. [PubMed] |
| 64. | Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4:505-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1039] [Cited by in RCA: 1043] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 65. | Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261-1274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4880] [Cited by in RCA: 4817] [Article Influence: 267.6] [Reference Citation Analysis (0)] |
| 66. | Brazil DP, Yang ZZ, Hemmings BA. Advances in protein kinase B signalling: AKTion on multiple fronts. Trends Biochem Sci. 2004;29:233-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 676] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 67. | Kooijman R. Regulation of apoptosis by insulin-like growth factor (IGF)-I. Cytokine Growth Factor Rev. 2006;17:305-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 146] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 68. | Buller CL, Loberg RD, Fan MH, Zhu Q, Park JL, Vesely E, Inoki K, Guan KL, Brosius FC. A GSK-3/TSC2/mTOR pathway regulates glucose uptake and GLUT1 glucose transporter expression. Am J Physiol Cell Physiol. 2008;295:C836-C843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 191] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 69. | Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3826] [Cited by in RCA: 4055] [Article Influence: 135.2] [Reference Citation Analysis (0)] |
| 70. | van Weeren PC, de Bruyn KM, de Vries-Smits AM, van Lint J, Burgering BM. Essential role for protein kinase B (PKB) in insulin-induced glycogen synthase kinase 3 inactivation. Characterization of dominant-negative mutant of PKB. J Biol Chem. 1998;273:13150-13156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 281] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 71. | Rössig L, Jadidi AS, Urbich C, Badorff C, Zeiher AM, Dimmeler S. Akt-dependent phosphorylation of p21(Cip1) regulates PCNA binding and proliferation of endothelial cells. Mol Cell Biol. 2001;21:5644-5657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 279] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 72. | Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocr Relat Cancer. 2009;16:1103-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 765] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 73. | Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3:11-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2345] [Cited by in RCA: 2381] [Article Influence: 108.2] [Reference Citation Analysis (0)] |
| 74. | Shelton JG, Steelman LS, White ER, McCubrey JA. Synergy between PI3K/Akt and Raf/MEK/ERK pathways in IGF-1R mediated cell cycle progression and prevention of apoptosis in hematopoietic cells. Cell Cycle. 2004;3:372-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 75. | Héron-Milhavet L, Karas M, Goldsmith CM, Baum BJ, LeRoith D. Insulin-like growth factor-I (IGF-I) receptor activation rescues UV-damaged cells through a p38 signaling pathway. Potential role of the IGF-I receptor in DNA repair. J Biol Chem. 2001;276:18185-18192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 76. | Galvan V, Logvinova A, Sperandio S, Ichijo H, Bredesen DE. Type 1 insulin-like growth factor receptor (IGF-IR) signaling inhibits apoptosis signal-regulating kinase 1 (ASK1). J Biol Chem. 2003;278:13325-13332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 77. | Peruzzi F, Prisco M, Dews M, Salomoni P, Grassilli E, Romano G, Calabretta B, Baserga R. Multiple signaling pathways of the insulin-like growth factor 1 receptor in protection from apoptosis. Mol Cell Biol. 1999;19:7203-7215. [PubMed] |
| 78. | Chaves J, Saif MW. IGF system in cancer: from bench to clinic. Anticancer Drugs. 2011;22:206-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 79. | Yadav A, Kalita A, Dhillon S, Banerjee K. JAK/STAT3 pathway is involved in survival of neurons in response to insulin-like growth factor and negatively regulated by suppressor of cytokine signaling-3. J Biol Chem. 2005;280:31830-31840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 110] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 80. | Mills JJ, Falls JG, De Souza AT, Jirtle RL. Imprinted M6p/Igf2 receptor is mutated in rat liver tumors. Oncogene. 1998;16:2797-2802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 81. | Barlow DP, Stöger R, Herrmann BG, Saito K, Schweifer N. The mouse insulin-like growth factor type-2 receptor is imprinted and closely linked to the Tme locus. Nature. 1991;349:84-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 661] [Cited by in RCA: 638] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 82. | Xu Y, Goodyer CG, Deal C, Polychronakos C. Functional polymorphism in the parental imprinting of the human IGF2R gene. Biochem Biophys Res Commun. 1993;197:747-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 162] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 83. | Kalscheuer VM, Mariman EC, Schepens MT, Rehder H, Ropers HH. The insulin-like growth factor type-2 receptor gene is imprinted in the mouse but not in humans. Nat Genet. 1993;5:74-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 156] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 84. | Killian JK, Jirtle RL. Genomic structure of the human M6P/IGF2 receptor. Mamm Genome. 1999;10:74-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 85. | Morgan DO, Edman JC, Standring DN, Fried VA, Smith MC, Roth RA, Rutter WJ. Insulin-like growth factor II receptor as a multifunctional binding protein. Nature. 1987;329:301-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 600] [Cited by in RCA: 635] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 86. | Gary-Bobo M, Nirdé P, Jeanjean A, Morère A, Garcia M. Mannose 6-phosphate receptor targeting and its applications in human diseases. Curr Med Chem. 2007;14:2945-2953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 130] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 87. | Braulke T. Type-2 IGF receptor: a multi-ligand binding protein. Horm Metab Res. 1999;31:242-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 105] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 88. | Espelund U, Bruun JM, Richelsen B, Flyvbjerg A, Frystyk J. Pro- and mature IGF-II during diet-induced weight loss in obese subjects. Eur J Endocrinol. 2005;153:861-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 89. | De Souza AT, Hankins GR, Washington MK, Orton TC, Jirtle RL. M6P/IGF2R gene is mutated in human hepatocellular carcinomas with loss of heterozygosity. Nat Genet. 1995;11:447-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 258] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 90. | De Souza AT, Hankins GR, Washington MK, Fine RL, Orton TC, Jirtle RL. Frequent loss of heterozygosity on 6q at the mannose 6-phosphate/insulin-like growth factor II receptor locus in human hepatocellular tumors. Oncogene. 1995;10:1725-1729. [PubMed] |
| 91. | Clairmont KB, Czech MP. Extracellular release as the major degradative pathway of the insulin-like growth factor II/mannose 6-phosphate receptor. J Biol Chem. 1991;266:12131-12134. [PubMed] |
| 92. | Costello M, Baxter RC, Scott CD. Regulation of soluble insulin-like growth factor II/mannose 6-phosphate receptor in human serum: measurement by enzyme-linked immunosorbent assay. J Clin Endocrinol Metab. 1999;84:611-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 93. | Denley A, Bonython ER, Booker GW, Cosgrove LJ, Forbes BE, Ward CW, Wallace JC. Structural determinants for high-affinity binding of insulin-like growth factor II to insulin receptor (IR)-A, the exon 11 minus isoform of the IR. Mol Endocrinol. 2004;18:2502-2512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 158] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 94. | Morrione A, Valentinis B, Xu SQ, Yumet G, Louvi A, Efstratiadis A, Baserga R. Insulin-like growth factor II stimulates cell proliferation through the insulin receptor. Proc Natl Acad Sci USA. 1997;94:3777-3782. [PubMed] |
| 95. | Frasca F, Pandini G, Scalia P, Sciacca L, Mineo R, Costantino A, Goldfine ID, Belfiore A, Vigneri R. Insulin receptor isoform A, a newly recognized, high-affinity insulin-like growth factor II receptor in fetal and cancer cells. Mol Cell Biol. 1999;19:3278-3288. [PubMed] |
| 96. | Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer. 2012;12:159-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 771] [Cited by in RCA: 839] [Article Influence: 64.5] [Reference Citation Analysis (0)] |
| 97. | Malaguarnera R, Sacco A, Voci C, Pandini G, Vigneri R, Belfiore A. Proinsulin binds with high affinity the insulin receptor isoform A and predominantly activates the mitogenic pathway. Endocrinology. 2012;153:2152-2163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 98. | Blanquart C, Achi J, Issad T. Characterization of IRA/IRB hybrid insulin receptors using bioluminescence resonance energy transfer. Biochem Pharmacol. 2008;76:873-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 99. | Soos MA, Field CE, Siddle K. Purified hybrid insulin/insulin-like growth factor-I receptors bind insulin-like growth factor-I, but not insulin, with high affinity. Biochem J. 1993;290:419-426. [PubMed] |
| 100. | Soos MA, Whittaker J, Lammers R, Ullrich A, Siddle K. Receptors for insulin and insulin-like growth factor-I can form hybrid dimers. Characterisation of hybrid receptors in transfected cells. Biochem J. 1990;270:383-390. [PubMed] |
| 101. | Bailyes EM, Navé BT, Soos MA, Orr SR, Hayward AC, Siddle K. Insulin receptor/IGF-I receptor hybrids are widely distributed in mammalian tissues: quantification of individual receptor species by selective immunoprecipitation and immunoblotting. Biochem J. 1997;327:209-215. [PubMed] |
| 102. | Pandini G, Frasca F, Mineo R, Sciacca L, Vigneri R, Belfiore A. Insulin/insulin-like growth factor I hybrid receptors have different biological characteristics depending on the insulin receptor isoform involved. J Biol Chem. 2002;277:39684-39695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 355] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 103. | Cornellà H, Alsinet C, Villanueva A. Molecular pathogenesis of hepatocellular carcinoma. Alcohol Clin Exp Res. 2011;35:821-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 104. | Seitz HK, Stickel F. Risk factors and mechanisms of hepatocarcinogenesis with special emphasis on alcohol and oxidative stress. Biol Chem. 2006;387:349-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 204] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 105. | Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3381] [Cited by in RCA: 4118] [Article Influence: 205.9] [Reference Citation Analysis (3)] |
| 106. | Reeves HL, Friedman SL. Activation of hepatic stellate cells--a key issue in liver fibrosis. Front Biosci. 2002;7:d808-d826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 338] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 107. | Brenner DA. Molecular pathogenesis of liver fibrosis. Trans Am Clin Climatol Assoc. 2009;120:361-368. [PubMed] |
| 108. | Zhang DY, Friedman SL. Fibrosis-dependent mechanisms of hepatocarcinogenesis. Hepatology. 2012;56:769-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 322] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 109. | Villanueva A, Hernandez-Gea V, Llovet JM. Medical therapies for hepatocellular carcinoma: a critical view of the evidence. Nat Rev Gastroenterol Hepatol. 2013;10:34-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 253] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 110. | Colnot S, Fortes P. Editing liver tumours. Gut. 2014;63:709-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 111. | Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Kinzler KW. Cancer genome landscapes. Science. 2013;339:1546-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5906] [Cited by in RCA: 5595] [Article Influence: 466.3] [Reference Citation Analysis (0)] |
| 112. | Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6:674-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1461] [Cited by in RCA: 1568] [Article Influence: 82.5] [Reference Citation Analysis (0)] |
| 113. | Thomas MB, Abbruzzese JL. Opportunities for targeted therapies in hepatocellular carcinoma. J Clin Oncol. 2005;23:8093-8108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 129] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 114. | Höpfner M, Schuppan D, Scherübl H. Growth factor receptors and related signalling pathways as targets for novel treatment strategies of hepatocellular cancer. World J Gastroenterol. 2008;14:1-14. [PubMed] |
| 115. | McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M, Tafuri A. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007;1773:1263-1284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1793] [Cited by in RCA: 1763] [Article Influence: 97.9] [Reference Citation Analysis (0)] |
| 116. | McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Montalto G, Cervello M, Nicoletti F, Fagone P, Malaponte G, Mazzarino MC. Mutations and deregulation of Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR cascades which alter therapy response. Oncotarget. 2012;3:954-987. [PubMed] |
| 117. | Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30:1434-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1331] [Cited by in RCA: 1270] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 118. | Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907-1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3241] [Cited by in RCA: 3282] [Article Influence: 149.2] [Reference Citation Analysis (0)] |
| 119. | Avila MA, Berasain C, Sangro B, Prieto J. New therapies for hepatocellular carcinoma. Oncogene. 2006;25:3866-3884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 295] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 120. | Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008;7:3129-3140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1149] [Cited by in RCA: 1109] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 121. | Gauthier A, Ho M. Role of sorafenib in the treatment of advanced hepatocellular carcinoma: An update. Hepatol Res. 2013;43:147-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 172] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 122. | Zhang T, Ding X, Wei D, Cheng P, Su X, Liu H, Wang D, Gao H. Sorafenib improves the survival of patients with advanced hepatocellular carcinoma: a meta-analysis of randomized trials. Anticancer Drugs. 2010;21:326-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 123. | Santoro A, Rimassa L, Borbath I, Daniele B, Salvagni S, Van Laethem JL, Van Vlierberghe H, Trojan J, Kolligs FT, Weiss A. Tivantinib for second-line treatment of advanced hepatocellular carcinoma: a randomised, placebo-controlled phase 2 study. Lancet Oncol. 2013;14:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 463] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 124. | Trojan J, Zeuzem S. Tivantinib in hepatocellular carcinoma. Expert Opin Investig Drugs. 2013;22:141-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 125. | Cervello M, McCubrey JA, Cusimano A, Lampiasi N, Azzolina A, Montalto G. Targeted therapy for hepatocellular carcinoma: novel agents on the horizon. Oncotarget. 2012;3:236-260. [PubMed] |
| 126. | Yang JD, Nakamura I, Roberts LR. The tumor microenvironment in hepatocellular carcinoma: current status and therapeutic targets. Semin Cancer Biol. 2011;21:35-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 290] [Cited by in RCA: 314] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 127. | Tanaka S, Arii S. Molecularly targeted therapy for hepatocellular carcinoma. Cancer Sci. 2009;100:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 128. | Fernández-Rodriguez CM, Prada I, Andrade A, Moreiras M, Guitián R, Aller R, Lledó JL, Cacho G, Quiroga J, Prieto J. Disturbed synthesis of insulinlike growth factor I and its binding proteins may influence renal function changes in liver cirrhosis. Dig Dis Sci. 2001;46:1313-1320. [PubMed] |
| 129. | Mazziotti G, Sorvillo F, Morisco F, Carbone A, Rotondi M, Stornaiuolo G, Precone DF, Cioffi M, Gaeta GB, Caporaso N. Serum insulin-like growth factor I evaluation as a useful tool for predicting the risk of developing hepatocellular carcinoma in patients with hepatitis C virus-related cirrhosis: a prospective study. Cancer. 2002;95:2539-2545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 130. | Su WW, Lee KT, Yeh YT, Soon MS, Wang CL, Yu ML, Wang SN. Association of circulating insulin-like growth factor 1 with hepatocellular carcinoma: one cross-sectional correlation study. J Clin Lab Anal. 2010;24:195-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 131. | Su TS, Liu WY, Han SH, Jansen M, Yang-Fen TL, P’eng FK, Chou CK. Transcripts of the insulin-like growth factors I and II in human hepatoma. Cancer Res. 1989;49:1773-1777. [PubMed] |
| 132. | Kaseb AO, Morris JS, Hassan MM, Siddiqui AM, Lin E, Xiao L, Abdalla EK, Vauthey JN, Aloia TA, Krishnan S. Clinical and prognostic implications of plasma insulin-like growth factor-1 and vascular endothelial growth factor in patients with hepatocellular carcinoma. J Clin Oncol. 2011;29:3892-3899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 133. | Cho E, Kim HC, Lee JH, Jeong-Ju Yoo WM, Cho YY, Lee MJ, Cho Y, Lee DH, Lee YB, Yu SJ. Serum insulin-like growth factor-1 predicts disease progression and survival in patients with hepatocellular carcinoma who undergo transarterial chemoembolization. PLoS One. 2014;9:e90862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 134. | Hoshida Y, Villanueva A, Kobayashi M, Peix J, Chiang DY, Camargo A, Gupta S, Moore J, Wrobel MJ, Lerner J. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med. 2008;359:1995-2004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1044] [Cited by in RCA: 1005] [Article Influence: 59.1] [Reference Citation Analysis (0)] |
| 135. | Villanueva A, Hoshida Y, Toffanin S, Lachenmayer A, Alsinet C, Savic R, Cornella H, Llovet JM. New strategies in hepatocellular carcinoma: genomic prognostic markers. Clin Cancer Res. 2010;16:4688-4694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 106] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 136. | Whittaker S, Marais R, Zhu AX. The role of signaling pathways in the development and treatment of hepatocellular carcinoma. Oncogene. 2010;29:4989-5005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 608] [Cited by in RCA: 680] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 137. | Schirmacher P, Held WA, Yang D, Chisari FV, Rustum Y, Rogler CE. Reactivation of insulin-like growth factor II during hepatocarcinogenesis in transgenic mice suggests a role in malignant growth. Cancer Res. 1992;52:2549-2556. [PubMed] |
| 138. | Breuhahn K, Vreden S, Haddad R, Beckebaum S, Stippel D, Flemming P, Nussbaum T, Caselmann WH, Haab BB, Schirmacher P. Molecular profiling of human hepatocellular carcinoma defines mutually exclusive interferon regulation and insulin-like growth factor II overexpression. Cancer Res. 2004;64:6058-6064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 101] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 139. | Lund P, Schubert D, Niketeghad F, Schirmacher P. Autocrine inhibition of chemotherapy response in human liver tumor cells by insulin-like growth factor-II. Cancer Lett. 2004;206:85-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 140. | Yao X, Hu JF, Daniels M, Shiran H, Zhou X, Yan H, Lu H, Zeng Z, Wang Q, Li T. A methylated oligonucleotide inhibits IGF2 expression and enhances survival in a model of hepatocellular carcinoma. J Clin Invest. 2003;111:265-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 141. | El Tayebi HM, Hosny KA, Esmat G, Breuhahn K, Abdelaziz AI. miR-615-5p is restrictedly expressed in cirrhotic and cancerous liver tissues and its overexpression alleviates the tumorigenic effects in hepatocellular carcinoma. FEBS Lett. 2012;586:3309-3316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 142. | Dong ZZ, Yao DF, Yao DB, Wu XH, Wu W, Qiu LW, Jiang DR, Zhu JH, Meng XY. Expression and alteration of insulin-like growth factor II-messenger RNA in hepatoma tissues and peripheral blood of patients with hepatocellular carcinoma. World J Gastroenterol. 2005;11:4655-4660. [PubMed] |
| 143. | Qian J, Yao D, Dong Z, Wu W, Qiu L, Yao N, Li S, Bian Y, Wang Z, Shi G. Characteristics of hepatic igf-ii expression and monitored levels of circulating igf-ii mRNA in metastasis of hepatocellular carcinoma. Am J Clin Pathol. 2010;134:799-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 144. | Yun K, Jinno Y, Sohda T, Niikawa N, Ikeda T. Promoter-specific insulin-like growth factor 2 gene imprinting in human fetal liver and hepatoblastoma. J Pathol. 1998;185:91-98. [PubMed] |
| 145. | Cui H, Cruz-Correa M, Giardiello FM, Hutcheon DF, Kafonek DR, Brandenburg S, Wu Y, He X, Powe NR, Feinberg AP. Loss of IGF2 imprinting: a potential marker of colorectal cancer risk. Science. 2003;299:1753-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 514] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 146. | Kim HT, Choi BH, Niikawa N, Lee TS, Chang SI. Frequent loss of imprinting of the H19 and IGF-II genes in ovarian tumors. Am J Med Genet. 1998;80:391-395. [PubMed] |
| 147. | van Roozendaal CE, Gillis AJ, Klijn JG, van Ooijen B, Claassen CJ, Eggermont AM, Henzen-Logmans SC, Oosterhuis JW, Foekens JA, Looijenga LH. Loss of imprinting of IGF2 and not H19 in breast cancer, adjacent normal tissue and derived fibroblast cultures. FEBS Lett. 1998;437:107-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 148. | Poirier K, Chalas C, Tissier F, Couvert P, Mallet V, Carrié A, Marchio A, Sarli D, Gicquel C, Chaussade S. Loss of parental-specific methylation at the IGF2 locus in human hepatocellular carcinoma. J Pathol. 2003;201:473-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 149. | Li X, Nong Z, Ekström C, Larsson E, Nordlinder H, Hofmann WJ, Trautwein C, Odenthal M, Dienes HP, Ekström TJ. Disrupted IGF2 promoter control by silencing of promoter P1 in human hepatocellular carcinoma. Cancer Res. 1997;57:2048-2054. [PubMed] |
| 150. | Fiorentino M, Grigioni WF, Baccarini P, D’Errico A, De Mitri MS, Pisi E, Mancini AM. Different in situ expression of insulin-like growth factor type II in hepatocellular carcinoma. An in situ hybridization and immunohistochemical study. Diagn Mol Pathol. 1994;3:59-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 151. | Couvert P, Carrié A, Pariès J, Vaysse J, Miroglio A, Kerjean A, Nahon P, Chelly J, Trinchet JC, Beaugrand M. Liver insulin-like growth factor 2 methylation in hepatitis C virus cirrhosis and further occurrence of hepatocellular carcinoma. World J Gastroenterol. 2008;14:5419-5427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 152. | Williams JH, Phillips TD, Jolly PE, Stiles JK, Jolly CM, Aggarwal D. Human aflatoxicosis in developing countries: a review of toxicology, exposure, potential health consequences, and interventions. Am J Clin Nutr. 2004;80:1106-1122. [PubMed] |
| 153. | Ubagai T, Kikuchi T, Fukusato T, Ono Y. Aflatoxin B1 modulates the insulin-like growth factor-2 dependent signaling axis. Toxicol In Vitro. 2010;24:783-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 154. | Lee YI, Lee S, Das GC, Park US, Park SM, Lee YI. Activation of the insulin-like growth factor II transcription by aflatoxin B1 induced p53 mutant 249 is caused by activation of transcription complexes; implications for a gain-of-function during the formation of hepatocellular carcinoma. Oncogene. 2000;19:3717-3726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 72] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 155. | Weng CJ, Hsieh YH, Tsai CM, Chu YH, Ueng KC, Liu YF, Yeh YH, Su SC, Chen YC, Chen MK. Relationship of insulin-like growth factors system gene polymorphisms with the susceptibility and pathological development of hepatocellular carcinoma. Ann Surg Oncol. 2010;17:1808-1815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 156. | Chao LT, Wu CF, Sung FY, Lin CL, Liu CJ, Huang CJ, Tsai KS, Yu MW. Insulin, glucose and hepatocellular carcinoma risk in male hepatitis B carriers: results from 17-year follow-up of a population-based cohort. Carcinogenesis. 2011;32:876-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 157. | Huynh H, Chow PK, Ooi LL, Soo KC. A possible role for insulin-like growth factor-binding protein-3 autocrine/paracrine loops in controlling hepatocellular carcinoma cell proliferation. Cell Growth Differ. 2002;13:115-122. [PubMed] |
| 158. | Scharf JG, Schmidt-Sandte W, Pahernik SA, Ramadori G, Braulke T, Hartmann H. Characterization of the insulin-like growth factor axis in a human hepatoma cell line (PLC). Carcinogenesis. 1998;19:2121-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 159. | Alexia C, Fallot G, Lasfer M, Schweizer-Groyer G, Groyer A. An evaluation of the role of insulin-like growth factors (IGF) and of type-I IGF receptor signalling in hepatocarcinogenesis and in the resistance of hepatocarcinoma cells against drug-induced apoptosis. Biochem Pharmacol. 2004;68:1003-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 136] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 160. | Aleem E, Elshayeb A, Elhabachi N, Mansour AR, Gowily A, Hela A. Serum IGFBP-3 is a more effective predictor than IGF-1 and IGF-2 for the development of hepatocellular carcinoma in patients with chronic HCV infection. Oncol Lett. 2012;3:704-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 161. | Domené HM, Hwa V, Argente J, Wit JM, Camacho-Hübner C, Jasper HG, Pozo J, van Duyvenvoorde HA, Yakar S, Fofanova-Gambetti OV. Human acid-labile subunit deficiency: clinical, endocrine and metabolic consequences. Horm Res. 2009;72:129-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 162. | Neumann O, Kesselmeier M, Geffers R, Pellegrino R, Radlwimmer B, Hoffmann K, Ehemann V, Schemmer P, Schirmacher P, Lorenzo Bermejo J. Methylome analysis and integrative profiling of human HCCs identify novel protumorigenic factors. Hepatology. 2012;56:1817-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 163. | Hanafusa T, Yumoto Y, Nouso K, Nakatsukasa H, Onishi T, Fujikawa T, Taniyama M, Nakamura S, Uemura M, Takuma Y. Reduced expression of insulin-like growth factor binding protein-3 and its promoter hypermethylation in human hepatocellular carcinoma. Cancer Lett. 2002;176:149-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 92] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 164. | Buckbinder L, Talbott R, Velasco-Miguel S, Takenaka I, Faha B, Seizinger BR, Kley N. Induction of the growth inhibitor IGF-binding protein 3 by p53. Nature. 1995;377:646-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 608] [Cited by in RCA: 612] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 165. | Yoo BK, Emdad L, Su ZZ, Villanueva A, Chiang DY, Mukhopadhyay ND, Mills AS, Waxman S, Fisher RA, Llovet JM. Astrocyte elevated gene-1 regulates hepatocellular carcinoma development and progression. J Clin Invest. 2009;119:465-477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 277] [Cited by in RCA: 281] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 166. | Chen D, Yoo BK, Santhekadur PK, Gredler R, Bhutia SK, Das SK, Fuller C, Su ZZ, Fisher PB, Sarkar D. Insulin-like growth factor-binding protein-7 functions as a potential tumor suppressor in hepatocellular carcinoma. Clin Cancer Res. 2011;17:6693-6701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 167. | Li F, Fan YC, Gao S, Sun FK, Yang Y, Wang K. Methylation of serum insulin-like growth factor-binding protein 7 promoter in hepatitis B virus-associated hepatocellular carcinoma. Genes Chromosomes Cancer. 2014;53:90-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 168. | Ranke MB, Maier KP, Schweizer R, Stadler B, Schleicher S, Elmlinger MW, Flehmig B. Pilot study of elevated levels of insulin-like growth factor-binding protein-2 as indicators of hepatocellular carcinoma. Horm Res. 2003;60:174-180. [PubMed] |
| 169. | Umemura A, Itoh Y, Itoh K, Yamaguchi K, Nakajima T, Higashitsuji H, Onoue H, Fukumoto M, Okanoue T, Fujita J. Association of gankyrin protein expression with early clinical stages and insulin-like growth factor-binding protein 5 expression in human hepatocellular carcinoma. Hepatology. 2008;47:493-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 170. | Brouillet JP, Hanslick B, Maudelonde T, Pivat MT, Grenier J, Blanc F, Rochefort H. Increased plasma cathepsin D concentration in hepatic carcinoma and cirrhosis but not in breast cancer. Clin Biochem. 1991;24:491-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 171. | Martin DC, Fowlkes JL, Babic B, Khokha R. Insulin-like growth factor II signaling in neoplastic proliferation is blocked by transgenic expression of the metalloproteinase inhibitor TIMP-1. J Cell Biol. 1999;146:881-892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 76] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 172. | Aleem E, Nehrbass D, Klimek F, Mayer D, Bannasch P. Upregulation of the insulin receptor and type I insulin-like growth factor receptor are early events in hepatocarcinogenesis. Toxicol Pathol. 2011;39:524-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 173. | Kasprzak A, Adamek A. The insulin-like growth factor (IGF) signaling axis and hepatitis C virus-associated carcinogenesis (review). Int J Oncol. 2012;41:1919-1931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 174. | Faivre S, Bouattour M, Raymond E. Novel molecular therapies in hepatocellular carcinoma. Liver Int. 2011;31 Suppl 1:151-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 175. | Tanaka S, Wands JR. Insulin receptor substrate 1 overexpression in human hepatocellular carcinoma cells prevents transforming growth factor beta1-induced apoptosis. Cancer Res. 1996;56:3391-3394. [PubMed] |
| 176. | Boissan M, Beurel E, Wendum D, Rey C, Lécluse Y, Housset C, Lacombe ML, Desbois-Mouthon C. Overexpression of insulin receptor substrate-2 in human and murine hepatocellular carcinoma. Am J Pathol. 2005;167:869-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 177. | Cantarini MC, de la Monte SM, Pang M, Tong M, D’Errico A, Trevisani F, Wands JR. Aspartyl-asparagyl beta hydroxylase over-expression in human hepatoma is linked to activation of insulin-like growth factor and notch signaling mechanisms. Hepatology. 2006;44:446-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 123] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 178. | Ma Y, Kong Q, Hua H, Luo T, Jiang Y. Aflatoxin B1 up-regulates insulin receptor substrate 2 and stimulates hepatoma cell migration. PLoS One. 2012;7:e47961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 179. | Martin-Kleiner I, Gall Troselj K. Mannose-6-phosphate/insulin-like growth factor 2 receptor (M6P/IGF2R) in carcinogenesis. Cancer Lett. 2010;289:11-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 180. | O’Gorman DB, Costello M, Weiss J, Firth SM, Scott CD. Decreased insulin-like growth factor-II/mannose 6-phosphate receptor expression enhances tumorigenicity in JEG-3 cells. Cancer Res. 1999;59:5692-5694. [PubMed] |
| 181. | Chen Z, Ge Y, Landman N, Kang JX. Decreased expression of the mannose 6-phosphate/insulin-like growth factor-II receptor promotes growth of human breast cancer cells. BMC Cancer. 2002;2:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 182. | Souza RF, Wang S, Thakar M, Smolinski KN, Yin J, Zou TT, Kong D, Abraham JM, Toretsky JA, Meltzer SJ. Expression of the wild-type insulin-like growth factor II receptor gene suppresses growth and causes death in colorectal carcinoma cells. Oncogene. 1999;18:4063-4068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 183. | Probst OC, Puxbaum V, Svoboda B, Leksa V, Stockinger H, Mikula M, Mikulits W, Mach L. The mannose 6-phosphate/insulin-like growth factor II receptor restricts the tumourigenicity and invasiveness of squamous cell carcinoma cells. Int J Cancer. 2009;124:2559-2567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 184. | Li J, Sahagian GG. Demonstration of tumor suppression by mannose 6-phosphate/insulin-like growth factor 2 receptor. Oncogene. 2004;23:9359-9368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 185. | Schaffer BS, Lin MF, Byrd JC, Park JH, MacDonald RG. Opposing roles for the insulin-like growth factor (IGF)-II and mannose 6-phosphate (Man-6-P) binding activities of the IGF-II/Man-6-P receptor in the growth of prostate cancer cells. Endocrinology. 2003;144:955-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 186. | Sue SR, Chari RS, Kong FM, Mills JJ, Fine RL, Jirtle RL, Meyers WC. Transforming growth factor-beta receptors and mannose 6-phosphate/insulin-like growth factor-II receptor expression in human hepatocellular carcinoma. Ann Surg. 1995;222:171-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 187. | Kishimoto Y, Morisawa T, Kitano M, Shiota G, Horie Y, Suou T, Ito H, Kawasaki H, Hasegawa J. Loss of heterozygosity of the mannose 6-phosphate/insulin-like growth factor II receptor and p53 genes in human hepatocellular carcinoma. Hepatol Res. 2001;20:68-83. [PubMed] |
| 188. | Kreiling JL, Montgomery MA, Wheeler JR, Kopanic JL, Connelly CM, Zavorka ME, Allison JL, Macdonald RG. Dominant-negative effect of truncated mannose 6-phosphate/insulin-like growth factor II receptor species in cancer. FEBS J. 2012;279:2695-2713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 189. | Yamada T, De Souza AT, Finkelstein S, Jirtle RL. Loss of the gene encoding mannose 6-phosphate/insulin-like growth factor II receptor is an early event in liver carcinogenesis. Proc Natl Acad Sci USA. 1997;94:10351-10355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 143] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 190. | De Souza AT, Yamada T, Mills JJ, Jirtle RL. Imprinted genes in liver carcinogenesis. FASEB J. 1997;11:60-67. [PubMed] |
| 191. | Han YL, Guo P, Sun MY, Guo L, Luan B, Kang J, Yan CH, Li SH. Secreted CREG inhibits cell proliferation mediated by mannose 6-phosphate/insulin-like growth factor II receptor in NIH3T3 fibroblasts. Genes Cells. 2008;13:977-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 192. | Gao J, Chesebrough JW, Cartlidge SA, Ricketts SA, Incognito L, Veldman-Jones M, Blakey DC, Tabrizi M, Jallal B, Trail PA. Dual IGF-I/II-neutralizing antibody MEDI-573 potently inhibits IGF signaling and tumor growth. Cancer Res. 2011;71:1029-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 193. | Chettouh H, Fartoux L, Aoudjehane L, Wendum D, Clapéron A, Chrétien Y, Rey C, Scatton O, Soubrane O, Conti F. Mitogenic insulin receptor-A is overexpressed in human hepatocellular carcinoma due to EGFR-mediated dysregulation of RNA splicing factors. Cancer Res. 2013;73:3974-3986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 194. | Pandini G, Vigneri R, Costantino A, Frasca F, Ippolito A, Fujita-Yamaguchi Y, Siddle K, Goldfine ID, Belfiore A. Insulin and insulin-like growth factor-I (IGF-I) receptor overexpression in breast cancers leads to insulin/IGF-I hybrid receptor overexpression: evidence for a second mechanism of IGF-I signaling. Clin Cancer Res. 1999;5:1935-1944. [PubMed] |
| 195. | Zhou Q, Mao YQ, Jiang WD, Chen YR, Huang RY, Zhou XB, Wang YF, Shi Z, Wang ZS, Huang RP. Development of IGF signaling antibody arrays for the identification of hepatocellular carcinoma biomarkers. PLoS One. 2012;7:e46851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 196. | Shao YY, Huang CC, Lin SD, Hsu CH, Cheng AL. Serum insulin-like growth factor-1 levels predict outcomes of patients with advanced hepatocellular carcinoma receiving antiangiogenic therapy. Clin Cancer Res. 2012;18:3992-3997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 197. | Conchillo M, de Knegt RJ, Payeras M, Quiroga J, Sangro B, Herrero JI, Castilla-Cortazar I, Frystyk J, Flyvbjerg A, Yoshizawa C. Insulin-like growth factor I (IGF-I) replacement therapy increases albumin concentration in liver cirrhosis: results of a pilot randomized controlled clinical trial. J Hepatol. 2005;43:630-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 198. | Vera M, Sobrevals L, Zaratiegui M, Martinez L, Palencia B, Rodríguez CM, Prieto J, Fortes P. Liver transduction with a simian virus 40 vector encoding insulin-like growth factor I reduces hepatic damage and the development of liver cirrhosis. Gene Ther. 2007;14:203-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 199. | Sobrevals L, Rodriguez C, Romero-Trevejo JL, Gondi G, Monreal I, Pañeda A, Juanarena N, Arcelus S, Razquin N, Guembe L. Insulin-like growth factor I gene transfer to cirrhotic liver induces fibrolysis and reduces fibrogenesis leading to cirrhosis reversion in rats. Hepatology. 2010;51:912-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 200. | Slijkhuis WA, Stadheim L, Hassoun ZM, Nzeako UC, Kremers WK, Talwalkar JA, Gores GJ. Octreotide therapy for advanced hepatocellular carcinoma. J Clin Gastroenterol. 2005;39:333-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 201. | Ji XQ, Ruan XJ, Chen H, Chen G, Li SY, Yu B. Somatostatin analogues in advanced hepatocellular carcinoma: an updated systematic review and meta-analysis of randomized controlled trials. Med Sci Monit. 2011;17:RA169-RA176. [PubMed] |
| 202. | Dransfield DT, Cohen EH, Chang Q, Sparrow LG, Bentley JD, Dolezal O, Xiao X, Peat TS, Newman J, Pilling PA. A human monoclonal antibody against insulin-like growth factor-II blocks the growth of human hepatocellular carcinoma cell lines in vitro and in vivo. Mol Cancer Ther. 2010;9:1809-1819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 203. | Gao J, Chang YS, Jallal B, Viner J. Targeting the insulin-like growth factor axis for the development of novel therapeutics in oncology. Cancer Res. 2012;72:3-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 204. | Dias N, Stein CA. Antisense oligonucleotides: basic concepts and mechanisms. Mol Cancer Ther. 2002;1:347-355. [PubMed] |
| 205. | Lin SB, Hsieh SH, Hsu HL, Lai MY, Kan LS, Au LC. Antisense oligodeoxynucleotides of IGF-II selectively inhibit growth of human hepatoma cells overproducing IGF-II. J Biochem. 1997;122:717-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 206. | Duplomb L, Chaigne-Delalande B, Vusio P, Raher S, Jacques Y, Godard A, Blanchard F. Soluble mannose 6-phosphate/insulin-like growth factor II (IGF-II) receptor inhibits interleukin-6-type cytokine-dependent proliferation by neutralization of IGF-II. Endocrinology. 2003;144:5381-5389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 207. | Gray SG, Kytola S, Lui WO, Larsson C, Ekstrom TJ. Modulating IGFBP-3 expression by trichostatin A: potential therapeutic role in the treatment of hepatocellular carcinoma. Int J Mol Med. 2000;5:33-41. [PubMed] |
| 208. | Regel I, Eichenmüller M, Joppien S, Liebl J, Häberle B, Müller-Höcker J, Vollmar A, von Schweinitz D, Kappler R. IGFBP3 impedes aggressive growth of pediatric liver cancer and is epigenetically silenced in vascular invasive and metastatic tumors. Mol Cancer. 2012;11:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 209. | Chen D, Siddiq A, Emdad L, Rajasekaran D, Gredler R, Shen XN, Santhekadur PK, Srivastava J, Robertson CL, Dmitriev I. Insulin-like growth factor-binding protein-7 (IGFBP7): a promising gene therapeutic for hepatocellular carcinoma (HCC). Mol Ther. 2013;21:758-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 210. | Zhang YC, Wang XP, Zhang LY, Song AL, Kou ZM, Li XS. Effect of blocking IGF-I receptor on growth of human hepatocellular carcinoma cells. World J Gastroenterol. 2006;12:3977-3982. [PubMed] |
| 211. | Rowinsky EK, Youssoufian H, Tonra JR, Solomon P, Burtrum D, Ludwig DL. IMC-A12, a human IgG1 monoclonal antibody to the insulin-like growth factor I receptor. Clin Cancer Res. 2007;13:5549s-5555s. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 136] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 212. | Wu KD, Zhou L, Burtrum D, Ludwig DL, Moore MA. Antibody targeting of the insulin-like growth factor I receptor enhances the anti-tumor response of multiple myeloma to chemotherapy through inhibition of tumor proliferation and angiogenesis. Cancer Immunol Immunother. 2007;56:343-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 213. | Wu JD, Odman A, Higgins LM, Haugk K, Vessella R, Ludwig DL, Plymate SR. In vivo effects of the human type I insulin-like growth factor receptor antibody A12 on androgen-dependent and androgen-independent xenograft human prostate tumors. Clin Cancer Res. 2005;11:3065-3074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 128] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 214. | Burtrum D, Zhu Z, Lu D, Anderson DM, Prewett M, Pereira DS, Bassi R, Abdullah R, Hooper AT, Koo H. A fully human monoclonal antibody to the insulin-like growth factor I receptor blocks ligand-dependent signaling and inhibits human tumor growth in vivo. Cancer Res. 2003;63:8912-8921. [PubMed] |
| 215. | Tovar V, Alsinet C, Villanueva A, Hoshida Y, Chiang DY, Solé M, Thung S, Moyano S, Toffanin S, Mínguez B. IGF activation in a molecular subclass of hepatocellular carcinoma and pre-clinical efficacy of IGF-1R blockage. J Hepatol. 2010;52:550-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 187] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 216. | Abou-Alfa GK, Capanu M, O’Reilly EM, Ma J, Chou JF, Gansukh B, Shia J, Kalin M, Katz S, Abad L. A phase II study of cixutumumab (IMC-A12, NSC742460) in advanced hepatocellular carcinoma. J Hepatol. 2014;60:319-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 217. | Maloney EK, McLaughlin JL, Dagdigian NE, Garrett LM, Connors KM, Zhou XM, Blättler WA, Chittenden T, Singh R. An anti-insulin-like growth factor I receptor antibody that is a potent inhibitor of cancer cell proliferation. Cancer Res. 2003;63:5073-5083. [PubMed] |
| 218. | Sachdev D, Zhang X, Matise I, Gaillard-Kelly M, Yee D. The type I insulin-like growth factor receptor regulates cancer metastasis independently of primary tumor growth by promoting invasion and survival. Oncogene. 2010;29:251-262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 219. | Spiliotaki M, Markomanolaki H, Mela M, Mavroudis D, Georgoulias V, Agelaki S. Targeting the insulin-like growth factor I receptor inhibits proliferation and VEGF production of non-small cell lung cancer cells and enhances paclitaxel-mediated anti-tumor effect. Lung Cancer. 2011;73:158-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 220. | Geoerger B, Brasme JF, Daudigeos-Dubus E, Opolon P, Venot C, Debussche L, Vrignaud P, Vassal G. Anti-insulin-like growth factor 1 receptor antibody EM164 (murine AVE1642) exhibits anti-tumour activity alone and in combination with temozolomide against neuroblastoma. Eur J Cancer. 2010;46:3251-3262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 221. | Descamps G, Gomez-Bougie P, Venot C, Moreau P, Bataille R, Amiot M. A humanised anti-IGF-1R monoclonal antibody (AVE1642) enhances Bortezomib-induced apoptosis in myeloma cells lacking CD45. Br J Cancer. 2009;100:366-369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 222. | Macaulay VM, Middleton MR, Protheroe AS, Tolcher A, Dieras V, Sessa C, Bahleda R, Blay JY, LoRusso P, Mery-Mignard D. phase I study of humanized monoclonal antibody AVE1642 directed against the type 1 insulin-like growth factor receptor (IGF-1R), administered in combination with anticancer therapies to patients with advanced solid tumors. Ann Oncol. 2013;24:784-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 223. | Rodon J, DeSantos V, Ferry RJ, Kurzrock R. Early drug development of inhibitors of the insulin-like growth factor-I receptor pathway: lessons from the first clinical trials. Mol Cancer Ther. 2008;7:2575-2588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 132] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 224. | Cohen BD, Baker DA, Soderstrom C, Tkalcevic G, Rossi AM, Miller PE, Tengowski MW, Wang F, Gualberto A, Beebe JS. Combination therapy enhances the inhibition of tumor growth with the fully human anti-type 1 insulin-like growth factor receptor monoclonal antibody CP-751,871. Clin Cancer Res. 2005;11:2063-2073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 265] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 225. | Kim JG, Kang MJ, Yoon YK, Kim HP, Park J, Song SH, Han SW, Park JW, Kang GH, Kang KW. Heterodimerization of glycosylated insulin-like growth factor-1 receptors and insulin receptors in cancer cells sensitive to anti-IGF1R antibody. PLoS One. 2012;7:e33322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 226. | Mulvihill MJ, Cooke A, Rosenfeld-Franklin M, Buck E, Foreman K, Landfair D, O’Connor M, Pirritt C, Sun Y, Yao Y. Discovery of OSI-906: a selective and orally efficacious dual inhibitor of the IGF-1 receptor and insulin receptor. Future Med Chem. 2009;1:1153-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 238] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 227. | Zhao H, Desai V, Wang J, Epstein DM, Miglarese M, Buck E. Epithelial-mesenchymal transition predicts sensitivity to the dual IGF-1R/IR inhibitor OSI-906 in hepatocellular carcinoma cell lines. Mol Cancer Ther. 2012;11:503-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 228. | Camirand A, Zakikhani M, Young F, Pollak M. Inhibition of insulin-like growth factor-1 receptor signaling enhances growth-inhibitory and proapoptotic effects of gefitinib (Iressa) in human breast cancer cells. Breast Cancer Res. 2005;7:R570-R579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 87] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 229. | Deutsch E, Maggiorella L, Wen B, Bonnet ML, Khanfir K, Frascogna V, Turhan AG, Bourhis J. Tyrosine kinase inhibitor AG1024 exerts antileukaemic effects on STI571-resistant Bcr-Abl expressing cells and decreases AKT phosphorylation. Br J Cancer. 2004;91:1735-1741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 230. | Qi HW, Shen Z, Fan LH. Combined inhibition of insulin-like growth factor-1 receptor enhances the effects of gefitinib in a human non-small cell lung cancer resistant cell line. Exp Ther Med. 2011;2:1091-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 231. | Yao WF, Liu JW, Sheng GL, Huang DS. Blockade of IGF-IR exerts anticancer effects in hepatocellular carcinoma. Mol Med Rep. 2011;4:719-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 232. | García-Echeverría C, Pearson MA, Marti A, Meyer T, Mestan J, Zimmermann J, Gao J, Brueggen J, Capraro HG, Cozens R. In vivo antitumor activity of NVP-AEW541-A novel, potent, and selective inhibitor of the IGF-IR kinase. Cancer Cell. 2004;5:231-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 431] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 233. | Höpfner M, Huether A, Sutter AP, Baradari V, Schuppan D, Scherübl H. Blockade of IGF-1 receptor tyrosine kinase has antineoplastic effects in hepatocellular carcinoma cells. Biochem Pharmacol. 2006;71:1435-1448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 90] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 234. | Chang AY, Wang M. In-vitro growth inhibition of chemotherapy and molecular targeted agents in hepatocellular carcinoma. Anticancer Drugs. 2013;24:251-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 235. | Sabbatini P, Rowand JL, Groy A, Korenchuk S, Liu Q, Atkins C, Dumble M, Yang J, Anderson K, Wilson BJ. Antitumor activity of GSK1904529A, a small-molecule inhibitor of the insulin-like growth factor-I receptor tyrosine kinase. Clin Cancer Res. 2009;15:3058-3067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 236. | Lin RX, Wang ZY, Zhang N, Tuo CW, Liang QD, Sun YN, Wang SQ. Inhibition of hepatocellular carcinoma growth by antisense oligonucleotides to type I insulin-like growth factor receptor in vitro and in an orthotopic model. Hepatol Res. 2007;37:366-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |